Abstract
Background: Heavy metals, such as lead (Pb), mercury (Hg), and cadmium (Cd), are known toxicants, but their associations with the thyroid axis have not been well quantified at U.S. background levels.
Objectives: We investigated the relationships between thyroid hormones (total and free thyroxine [TT4 and FT4], total and free triiodothyronine [TT3 and FT3], thyroid-stimulating hormone [TSH], and thyroglobulin [Tg]) and levels of Pb, Hg, and Cd in blood and Cd in urine.
Methods: We separately analyzed a sample of 1,109 adolescents (12–19 years of age) and a sample of 4,409 adults from the U.S. National Health and Nutrition Examination Survey (NHANES) 2007–2008. We estimated associations after adjusting for age, sex, race, urinary iodine, body mass index, and serum cotinine.
Results: The geometric mean (GM) levels of blood Pb (BPb), total Hg, and Cd were 0.81 µg/dL, 0.47 µg/L, and 0.21 µg/L in adolescents and 1.43 µg/dL, 0.96 µg/L, and 0.38 µg/L in adults, respectively. The GMs of urinary Cd were 0.07 and 0.25 µg/g creatinine in adolescents and adults, respectively. No consistent pattern of metal and thyroid hormone associations was observed in adolescents. In adults, blood Hg was inversely related to TT4, TT3, and FT3 and urinary Cd was positively associated with TT4, TT3, FT3, and Tg, but there were no associations with Pb. Associations were relatively weak at an individual level, with about 1–4% change in thyroid hormones per interquartile range increase in Hg or Cd.
Conclusions: Our analysis suggests an inverse association between Hg exposure and thyroid hormones, and a positive association between Cd exposure and thyroid hormones in adults.
Keywords: cadmium, heavy metals, lead, mercury, thyroid hormones
Thyroid hormones (THs) play a critical role in the functions of nervous, reproductive, and cardiovascular systems in both children and adults (Danzi and Klein 2012; Williams 2008; Yazbeck and Sullivan 2012). The hypothalamus–pituitary–thyroid (HPT) axis regulates thyroid function through thyrotropin releasing hormone, thyroid-stimulating hormone (TSH), and the THs [thyroxine (T4) and triiodothyronine (T3)]. Circulating T4 and T3 are mostly bound to thyroxine-binding globulin, transthyretin, and albumin; < 1% is unbound and biologically active. In peripheral tissues, T4 is converted to T3 by type 1 and type 2 deiodinases; T3 in turn binds thyroid receptors α and β and initiates target gene expression (Stathatos 2012). Disruption of TH synthesis, transport, deiodination, and metabolism can result in clinical or subclinical thyroid diseases (Cooper and Biondi 2012). Circulating TSH and THs, even at levels within reference ranges, are significantly associated with effects in neurodevelopment (Ghassabian et al. 2011; Pop et al. 2003), blood pressure (Asvold et al. 2007), cholesterol, triglycerides, and insulin resistance (Roos et al. 2007).
Environmental chemicals might alter TH levels via several mechanisms, including disruption of iodine (I) transport, thyroid peroxidase, TH-binding proteins, hepatic catabolism, deiodinases, and receptor binding (Miller et al. 2009). Studies of human populations have focused primarily on chemicals that are structurally similar to T4, such as polychlorinated biphenyls (PCBs), polybrominated diphenyl ethers, and bisphenol A (BPA), with little attention on heavy metals (Boas et al. 2006; Pearce and Braverman 2009). Lead (Pb), mercury (Hg), and cadmium (Cd) are known environmental toxicants, but only a few studies have examined associations with total and free T4 (TT4, FT4), total and free T3 (TT3, FT3), or TSH (Dundar et al. 2006; Jin et al. 2006; Lamb et al. 2008; Pearce and Braverman 2009; Robins et al. 1983; Schell et al. 2008).
Pb is known to have adverse neurological, hematological, renal, and gastrointestinal effects (Bellinger 2004; Gurer-Orhan et al. 2004); however, associations with THs have been inconsistent (Meeker et al. 2009). Pb exposure [mean 15 µg/dL blood Pb (BPb) level] was negatively correlated with transthyretin levels in cerebrospinal fluid samples from human patients (Zheng et al. 2001). Previous studies of populations with high exposure to Pb (indicated by BPb levels of > 20 µg/dL) suggested negative associations with circulating T4, FT4, or T3 (López et al. 2000; Robins et al. 1983; Singh et al. 2000; Tuppurainen et al. 1988); however, associations were not evident in other similar study populations (Erfurth et al. 2001; Schumacher et al. 1998; Siegel et al. 1989). Fewer studies have investigated associations of BPb levels of < 10 µg/dL with THs. Dundar and colleagues reported a negative association between BPb and FT4 levels in adolescents with mean BPb of 7 µg/dL (Dundar et al. 2006). A recent study (Meeker et al. 2009) has suggested an inverse association between BPb (median, 1.5 µg/dL) and TSH levels in men of the couples presenting at infertility clinics. Another study, in the lakeside communities of Quebec, Canada, found no association between BPb (median, 3.1 µg/dL) and THs in men, but identified a positive association with T3 and an inverse association with TSH in females with median BPb of 1.7 µg/dL (Abdelouahab et al. 2008).
Hg has adverse effects on a variety of systems that vary with the level, length of exposure, and time window of exposure (Tan et al. 2009). Proposed mechanisms of Hg-related TH disruption involve selective binding to sulfhydryl (SH)-containing ligands in the thyroid, reduced TSH production, and inhibition of deiodination (Soldin et al. 2008; Tan et al. 2009). FT3 levels were reduced in association with occupational exposure to Hg vapor among chloralkali plant workers (Barregard et al. 1994; Ellingsen et al. 2000). Studies of populations with environmental exposure, for example, from fish consumption and from dental amalgams, have had mixed findings (Abdelouahab et al. 2008; Meeker et al. 2009; Schell et al. 2008; Takser et al. 2005). A study in a Canadian lakeside community with exposure levels slightly higher than reported for the U.S. National Health and Nutrition Examination Survey (NHANES) [median total Hg, 2.25 µg/L in men and 1.50 µg/L in women compared with median total Hg, 0.8 µg/L in both men and women for NHANES 2003–2006 (Caldwell et al. 2009)] suggested a positive association between Hg and TSH in men only, and no associations with TT3 and TT4 (Abdelouahab et al. 2008). Hg was not associated with TSH or FT4 in 232 Akwesasne Mohawk adolescents with a geometric mean (GM) Hg level of 1 µg/L (Schell et al. 2008).
Cd affects the renal, skeletal, and respiratory systems and is classified as a Group 1 carcinogen by the International Agency for Research on Cancer (Järup and Åkesson 2009). Cd exposure in animal studies has been related to decreased serum TT4 levels, and interference with deiodination has been suggested as a possible mechanism (Hammouda et al. 2008; Mori et al. 2006). A Japanese study comparing residents of the Cd-polluted Kakehashi River basin with residents of a nonpolluted area reported lower FT4 levels in exposed females but higher TT3 levels in both sexes (Nishijo et al. 1994). Studies of neonates and children with environmental exposures have reported inconsistent results (Iijima et al. 2007; Maervoet et al. 2007; Osius et al. 1999). Blood Cd (median, 0.2 µg/L) was not associated with TSH in male infertility clinic patients (Meeker et al. 2009).
Many previous studies have had fairly small sample sizes or have been based on populations with occupational exposures that may not be relevant to the general public. In addition, many studies have measured blood Cd, which is a good biomarker for recent exposure, but urinary Cd is a better indicator of long-term exposure (Järup and Åkesson 2009). In this study, we analyzed NHANES data from 2007–2008 to estimate associations of Pb, Hg, and Cd with TH levels in a large U.S. population with background levels of exposure.
Methods
We used study subjects’ data from NHANES 2007–2008 [National Center for Health Statistics (NCHS) 2009a] to examine the association between heavy metals and TH levels in the general population with environmental exposure levels. NHANES is conducted in a nationally representative sample of the U.S. civilian population by the Centers for Disease Control and Prevention (CDC; Atlanta, GA). In 2007–2008, a sample of 10,149 subjects was included in this complex multistage, stratified cluster survey. Of those participants, TH levels were measured in 6,260 subjects ≥ 12 years of age. We excluded subjects with no BPb, Hg, or Cd measures (n = 5), those who had been told by a doctor or health professional that they have thyroid problems or were currently taking thyroid medications (n = 520) (Belin et al. 2004), and those currently pregnant or taking steroid hormones (i.e, estrogen, androgen) that might alter TH or thyroxine-binding globulin levels (n = 317). The analytical sample for this analysis was 5,418, including 1,009 adolescents (12–19 years of age) and 4,409 adults (20–80 years of age). After consideration of sampling weights, this analytic sample represents 26,770,162 adolescents and 159,282,838 adults in the general U.S. population who had no reported thyroid diseases, thyroid medications, pregnancy, and sex steroid medications. The analysis was exempt from review by the University of Cincinnati Institutional Review Board, but each subject had provided written informed consent to participate in the NHANES study.
Heavy metals. In the NHANES 2007–2008 cycle, metal assays were conducted in whole blood or urine samples at the Division of Laboratory Sciences, National Center for Environmental Health of the CDC. BPb, total Hg, and Cd levels were determined by inductively coupled plasma mass spectrometry (ICP-MS; CDC method no. ITB0001A) with modification from a published method (Nixon et al. 1999) with limits of detection (LOD) of 0.25 µg/dL for Pb, 0.33 µg/L for total Hg, and 0.2 µg/L for Cd (NCHS 2009d). Inorganic Hg in whole blood was measured using Flow Injection Mercury System Cold Vapor Atomic Absorption (PerkinElmer, Norwalk, CT), with an LOD of 0.35 µg/L. In the data set provided by the CDC (2009), levels < LOD were imputed as being the metal-specific LOD divided by the square root of 2 (Hornung and Reed 1990).
Only 6 participants had BPb levels < LOD. In the United States, organic Hg accounts for the majority of total blood Hg (Mahaffey et al. 2004). Therefore, if the total Hg level was < LOD (n = 884), we assumed that organic Hg was equal to the imputed total Hg level (0.2 µg/L). If the total Hg level was > LOD, we calculated organic Hg as the difference between total and inorganic Hg. In this data set, 4,062 subjects (75%) had inorganic Hg levels < LOD; therefore, we did not test for associations between inorganic Hg and TH levels. Blood Cd levels were < LOD in 1,282 subjects. In addition to whole blood samples, a one-third-sample subset of participants in the NHANES 2007–2008 had urine samples tested for Cd (n = 1,767) using ICP-MS. Among them, 106 had urine Cd levels < LOD (< 0.042 µg/L). We calculated creatinine-adjusted urinary Cd levels to account for urine dilution.
TH levels. Serum TH and thyroid antibody levels were determined in the Department of Laboratory Medicine at the University of Washington (Seattle, WA) (NCHS 2009b). Access HYPERsensitive hTSH assay (Beckman Coulter Inc., Brea, CA) was used to assay TSH. Competitive binding immunoenzymatic assay was used to determine TT4, FT4, TT3, and FT3. In addition, NHANES 2007–2008 samples were assayed for thyroglobulin (Tg), Tg antibody (TgAb), and thyroid peroxidase antibody (TPOAb) using immunoenzymatic assays.
Statistical analyses. In this study, we performed separate analyses for adolescents and adults. In samples from both adolescent (12–19 years of age) and adult subjects (≥ 20 years of age), we first examined the association between heavy metals and TH levels using linear regression models. Because both the exposure and outcome variables were not normally distributed, we used natural log transformation to analyze the data. We examined associations of BPb, blood total Hg, blood organic Hg, blood Cd, and urinary Cd with TT4, FT4, TT3, FT3, TSH, and Tg, using separate regression models for each exposure–outcome association. Second, we categorized exposures into quintiles and estimated differences in mean values for the 2nd, 3rd, 4th, and 5th quintiles compared with the first quintile. Third, we examined the proportion of subjects with high levels of TgAb (> 4 IU/mL) or TPOAb (> 9 IU/mL), an indicator of immunologic disturbance of thyroid tissue functions, based on NHANES laboratory method references (NCHS 2009c). Logistic regression was used to estimate odds ratios (ORs) and 95% confidence intervals (CIs) for metal exposure. Because this analytical sample was thyroid disease–free and medication-free (based on self-report) and the percentage of subjects with clinical and subclinical hyperthyroidism or hypothyroidism was < 2% according to NHANES reference values (TSH 0.34–5.6 µIU/mL) (NCHS 2009c), we did not examine hyperthyroidism or hypothyroidism as dichotomous outcomes. Fourth, we did a subset analysis restricted to women of reproductive age (15–44 years of age, n = 1,095) to examine whether metal exposure levels in nonpregnant U.S. women had a discernible association with TH levels during reproductive age. Fifth, we summarized significant findings in adolescents and adults by calculating percentage change in TH levels with an interquartile range (IQR) increase in metal exposure levels. Sixth and last, we examined associations between TH levels and multiple metal exposures by categorizing adult BPb, total Hg, and Cd by their corresponding medians (1.39 µg/dL, 0.88 µg/L, 0.33 µg/dL) and modeling the eight possible comparison groups, using the group with levels of all three metals < median as the reference group.
In the regression models, we adjusted for a priori covariates (Caldwell et al. 2009; Hollowell et al. 2002; Muntner et al. 2005; Tellez-Plaza et al. 2012): age (continuous), sex (male, female), race/ethnicity (white, black, Hispanic/other), natural log transformed creatinine–adjusted urinary iodine (measured by ICP-MS at the CDC), body mass index (BMI; age- and sex-specific z-score in adolescent models, original value in adult models), and serum cotinine levels [measured by high performance liquid chromatography tandem mass spectrometry at the CDC; < 1 ng/mL as nonsmoking, 1–9.9 ng/mL as environmental tobacco smoke (ETS) exposure, ≥ 10 ng/mL as active smoking, dummy variables used] (CDC 2009). Because NHANES is a complex multistage sampling survey, we used PROC SURVEYREG and PROC SURVEYLOGISTIC in SAS version 9.2 (SAS Institute Inc., Cary, NC) to calculate regression parameters and 95% CIs after accounting for sampling weights and survey methods. The significance level was set at α = 0.05 for two-sided tests.
Results
In the adolescent subjects sample, the mean age was 15.5 years, with 55% male, 60% white, 15% black, and 25% Hispanic and other ethnicity. Twelve percent were exposed to ETS, and 15% were active smokers. The mean BMI z-score was 0.54. The GM of urinary iodine was 140 µg/g creatinine. For the adult subjects sample, the mean age was 46.4 years, with 55% male, 68% white, 11% black, and 22% Hispanic and other ethnicity. Adults had 28% active smoking percentage, with 5% exposed to ETS. The mean adult BMI was 28.5 kg/m2. The GM of adult urinary iodine was 156 µg/g creatinine. The covariates used in adjusted regression models, including age, sex, race and ethnicity, smoking status, BMI, urinary iodine, were associated with metal exposures and TH levels in most models (detailed data not shown). We also observed increased Pb and Hg levels with age, higher Cd levels in smokers versus nonsmokers and in females versus males, and lower TSH levels in smokers overall.
Table 1 displays the means, ranges, and GMs for Pb, Hg, Cd, and TH levels in both adolescents and adults. Adults had statistically significant higher levels of metal exposures than adolescents.
Table 1.
Blood and urinary Pb, Hg, and Cd levels and TH levels in the NHANES 2007–2008.
| Adolescents | Adults | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean | Range | GM | n | Mean | Range | GM | ||
| Metal | |||||||||
| Blood Pb (µg/dL) | 1,009 | 0.93 | 0.18–9.20 | 0.81 | 4,409 | 1.75 | 0.18–33.1 | 1.43 | |
| Total Hg (µg/L) | 1,009 | 0.68 | 0.20–6.98 | 0.47 | 4,409 | 1.62 | 0.20–43.9 | 0.96 | |
| Organic Hg (µg/L) | 1,005 | 0.49 | 0.01–6.73 | 0.21 | 4,404 | 1.31 | 0.01–42.9 | 0.61 | |
| Blood Cd (µg/L) | 1,009 | 0.29 | 0.14–4.70 | 0.21 | 4,409 | 0.55 | 0.14–8.81 | 0.38 | |
| Urinary Cd (µg/g creatinine) | 312 | 0.08 | 0.01–1.60 | 0.07 | 1,455 | 0.34 | 0.02–4.04 | 0.25 | |
| TH | |||||||||
| TT4 (µg/dL) | 1,009 | 7.46 | 1.50–18.50 | 7.34 | 4,409 | 7.63 | 2.00–27.6 | 7.48 | |
| FT4 (ng/dL) | 1,009 | 0.79 | 0.10–1.50 | 0.78 | 4,409 | 0.77 | 0.30–4.80 | 0.76 | |
| TT3 (ng/dL) | 1,009 | 130.37 | 74.00–241.00 | 128.36 | 4,409 | 112.50 | 37.0–632.0 | 110.4 | |
| FT3 (pg/mL) | 1,009 | 3.64 | 2.20–6.00 | 3.61 | 4,409 | 3.21 | 1.90–20.70 | 3.18 | |
| TSH (µIU/mL) | 1,009 | 1.84 | 0.01–280.76 | 1.47 | 4,409 | 2.01 | 0.002–80.97 | 1.60 | |
| Tg (ng/mL) | 1,009 | 10.83 | 0.07–353.56 | 7.64 | 4,409 | 15.62 | 0.07–4461.00 | 9.56 | |
| GM, geometric mean. | |||||||||
Statistically significant negative associations between blood total Hg and TT4 and FT3 were observed in adolescents (Table 2). Blood Cd was positively associated with FT3 and urinary Cd was positively associated with FT4. Table 3 shows that in adults, BPb exposure was not associated with any TH levels. Both total and organic Hg had significant negative associations with TT4, TT3, and FT3. Blood Cd was positively associated with FT4 and Tg; urinary Cd was positively associated with TT4, TT3, FT3, and Tg. In the analyses of quintiles of metal exposures and TH levels in adolescents, the dose responses were not evident [see Supplemental Material, Table S1 (http://dx.doi.org/10.1289/ehp.1205239)]. However, in adults, the dose–response patterns consistent with the modeling of continuous exposure were evident for total Hg, organic Hg, and urinary Cd (see Supplemental Material, Table S2). Figure 1 shows that total blood Hg levels were associated with lower TT4 and TT3, with the 5th quintile of exposure (≥ 2.16 µg/dL) showing the strongest associations. Urinary Cd levels were positively associated with TT4 and TT3. In contrast, no consistent patterns were found for BPb levels (see Supplemental Material, Table S2).
Table 2.
Adjusted regression coefficients (95% CIs) of blood and urinary Pb, Hg, and Cd in relation to THs in adolescents.
| Metal | lnTT4 | lnFT4 | lnTT3 | lnFT3 | lnTSH | lnTg |
|---|---|---|---|---|---|---|
| lnBPb | 0.01 (–0.02, 0.04) | 0.01 (–0.01, 0.04) | 0.01 (–0.01, 0.04) | 0.02 (–0.002, 0.04) | –0.05 (–0.18, 0.07) | 0.05 (–0.13, 0.24) |
| lnTotal Hg | –0.02 (–0.04, –0.001)* | 0.005 (–0.01, 0.02) | –0.02 (–0.03, 0.001) | –0.01 (–0.02, –0.003)* | 0.02 (–0.04, 0.08) | –0.05 (–0.20, 0.10) |
| lnOrganic Hg | –0.01 (–0.03, 0.004) | 0.01 (–0.01, 0.02) | –0.01 (–0.02, 0.01) | –0.01 (–0.02, –0.001)* | –0.01 (–0.06, 0.04) | –0.06 (–0.17, 0.04) |
| lnBlood Cd | –0.01 (–0.06, 0.03) | –0.01 (–0.05, 0.04) | –0.01 (–0.03, 0.02) | 0.02 (0.0003, 0.03)* | –0.11 (–0.27, 0.04) | 0.07 (–0.15, 0.28) |
| lnUrinary Cd | –0.0003 (–0.03, 0.03) | 0.04 (0.002, 0.08)* | 0.02 (–0.02, 0.06) | 0.02 (–0.01, 0.04) | –0.03 (–0.24, 0.17) | 0.08 (–0.13, 0.30) |
| Adjusted for age, sex, race/ethnicity, creatinine-adjusted urinary iodine, BMI z–score, and serum cotinine level. *p < 0.05. | ||||||
Table 3.
Adjusted regression coefficients (95% CIs) of blood and urinary Pb, Hg, and Cd in relation to THs in adults.
| Metal | lnTT4 | lnFT4 | lnTT3 | lnFT3 | lnTSH | lnTg |
|---|---|---|---|---|---|---|
| lnBPb | –0.01 (–0.02, 0.01) | 0.01 (–0.01, 0.02) | –0.0004 (–0.02, 0.02) | 0.01 (–0.001, 0.02) | –0.01 (–0.06, 0.04) | 0.01 (–0.03, 0.06) |
| lnTotal Hg | –0.02 (–0.02, –0.01)* | –0.005 (–0.01, 0.004) | –0.03 (–0.04, –0.01)* | –0.01 (–0.02, –0.01)* | 0.004 (–0.02, 0.03) | –0.01 (–0.05, 0.03) |
| lnOrganic Hg | –0.01 (–0.02, –0.004)* | –0.004 (–0.01, 0.001) | –0.02 (–0.03, –0.01)* | –0.01 (–0.01, –0.004)* | 0.01 (–0.01, 0.03) | 0.001 (–0.02, 0.03) |
| lnBlood Cd | 0.005 (–0.01, 0.02) | 0.01 (0.001, 0.02)* | 0.004 (–0.01, 0.02) | 0.0002 (–0.01, 0.01) | –0.02 (–0.07, 0.03) | 0.10 (0.05, 0.16)* |
| lnUrinary Cd | 0.02 (0.001, 0.03)* | 0.01 (–0.01, 0.03) | 0.03 (0.02, 0.05)* | 0.01 (0.002, 0.02)* | –0.04 (–0.09, 0.02) | 0.15 (0.23, 0.25)* |
| Adjusted for age, sex, race/ethnicity, creatinine-adjusted urinary iodine, BMI value, and serum cotinine level. *p < 0.05. | ||||||
Figure 1.
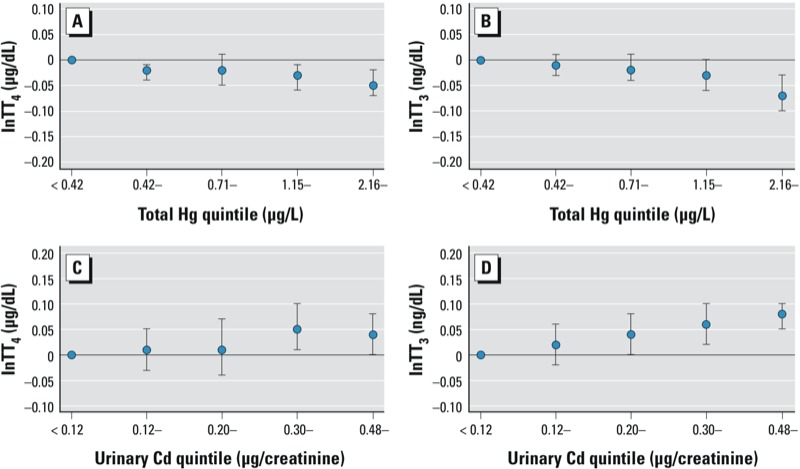
Estimated thyroid hormone levels according to natural log blood total Hg or urinary Cd exposure quintiles in adults, NHANES 2007–2008. (A) TT4 by blood total Hg quintiles, (B) TT3 by blood total Hg quintiles, (C) TT4 by urinary Cd quintiles, (D) TT3 by urinary Cd quintiles.
The percentages of high thyroid antibody levels were slightly higher in adults than adolescents (5.77% vs. 4.79% for TgAb; 8.83% vs. 6.04% for TPOAb, Table 4). However, in neither adolescents nor adults were blood or urinary Pb, Hg, Cd levels significantly associated with high TgAb or TPOAb levels.
Table 4.
Adjusted ORs (95% CIs) of high TgAb (> 4 IU/mL) and high TPOAb (> 9 IU/mL) per unit change in ln metal exposure in adolescents and adults.
| Metal | Adolescentsa | Adultsb | |||
|---|---|---|---|---|---|
| High TgAb | High TPOAb | High TgAb | High TPOAb | ||
| Percent | 4.79 | 6.04 | 5.77 | 8.83 | |
| lnBPb | 0.77 (0.37–1.59) | 1.16 (0.58–2.33) | 1.20 (0.87–1.64) | 1.09 (0.82–1.46) | |
| lnTotal Hg | 1.17 (0.64–2.13) | 1.15 (0.68–1.95) | 0.94 (0.78–1.13) | 0.91 (0.80–1.04) | |
| lnOrganic Hg | 1.29 (0.85–1.96) | 1.06 (0.66–1.72) | 0.95 (0.81–1.11) | 0.96 (0.87–1.06) | |
| lnBlood Cd | 1.18 (0.56–2.51) | 1.44 (0.71–2.95) | 1.09 (0.74–1.60) | 1.14 (0.85–1.51) | |
| lnUrinary Cd | 0.99 (0.49–2.02) | 0.97 (0.41–2.28) | 0.84 (0.47–1.48) | 1.20 (0.75–1.93) | |
| aAdjusted for age, sex, race/ethnicity, creatinine-adjusted urinary iodine, BMI z-score, and serum cotinine level. bAdjusted for age, sex, race/ethnicity, creatinine-adjusted urinary iodine, BMI value, and serum cotinine level. | |||||
Subset analyses of continuous exposures among women of reproductive age were generally consistent with associations in the adult population as a whole [see Supplemental Material Table S3 (http://dx.doi.org/10.1289/ehp.1205239)]. Urinary Cd levels were positively related to TT4, but associations with other TH levels did not reach statistical significance.
Table 5 gives the estimated percentage difference in TH levels per IQR increase in exposures that were significantly associated with TH levels when modeled as continuous variables. Overall estimated differences in mean levels were small, at 1–4%. However, we estimated a 12% increase in Tg associated with blood Cd at 0.61 vs. 0.21 µg/L, and an 18% increase in Tg associated with urinary Cd of 0.41 vs. 0.14 µg/g creatinine in adults.
Table 5.
Interpretation of the observed association per IQR change in exposure on TH levels.
| Population/metal exposure | IQR (P25 to P75) or LOD/√–2a to P75 | lnP75–lnP25 | Association with THs | Regression estimate for IQR change (95% CI) | Percentage difference in THs (95% CI) for exposure at P75 compared with P25 or LOD/√–2 if > 25% of subjects had exposure < LOD |
|---|---|---|---|---|---|
| Adolescents | |||||
| Total Hg (µg/L) | 0.20a–0.82 | 1.41 | ↓TT4 | –0.03 (–0.05, –0.001) | –2.6 (–5.0, –0.1) |
| ↓FT3 | –0.02 (–0.03, –0.004) | –1.6 (–2.7, –0.4) | |||
| Organic Hg (µg/L) | 0.20a–0.53 | 0.97 | ↓FT3 | –0.01 (–0.02, –0.001) | –0.8 (–1.6, –0.1) |
| Blood Cd (µg/L) | 0.14a–0.25 | 0.58 | ↑FT3 | 0.01 (0.0001, 0.02) | 0.9 (0.01, 1.9) |
| Urinary Cd (µg/g creatinine) | 0.04–0.11 | 1.01 | ↑FT4 | 0.04 (0.002, 0.08) | 4.3 (0.2, 8.5) |
| Adults | |||||
| Total Hg (µg/L) | 0.49–1.80 | 1.30 | ↓TT4 | –0.02 (–0.03, –0.008) | –2.0 (–3.0, –0.8) |
| ↓TT3 | –0.03 (–0.05, –0.02) | –3.3 (–5.0, –1.5) | |||
| ↓FT3 | –0.02 (–0.02, –0.01) | –1.7 (–2.2, –1.2) | |||
| Organic Hg (µg/L) | 0.21–1.39 | 1.89 | ↓TT4 | –0.02 (–0.03, –0.01) | –2.0 (–3.2, –0.8) |
| ↓TT3 | –0.03 (–0.05, –0.01) | –3.2 (–5.2, –1.1) | |||
| ↓FT3 | –0.01 (–0.02, –0.01) | –1.4 (–2.1, –0.7) | |||
| Blood Cd (µg/L) | 0.21–0.61 | 1.07 | ↑FT4 | 0.01 (0.001, 0.02) | 1.0 (0.1, 1.9) |
| ↑Tg | 0.11 (0.06, 0.17) | 11.9 (6.0, 18.1) | |||
| Urinary Cd (µg/g creatinine) | 0.14–0.41 | 1.07 | ↑TT4 | 0.02 (0.001, 0.03) | 1.9 (0.1, 3.6) |
| ↑TT3 | 0.04 (0.02, 0.05) | 3.6 (1.8, 5.4) | |||
| ↑FT3 | 0.01 (0.002, 0.02) | 1.0 (0.2, 1.9) | |||
| ↑Tg | 0.16 (0.07, 0.25) | 17.5 (7.7, 28.3) | |||
| Abbreviations: P75, 75th percentile; P25, 25th percentile. aInstead of first quartile, the interval starts from < LOD (value replaced with LOD divided by the square root of 2) to reflect > 25% participants with exposure < LOD. | |||||
In the three-metal analysis in adults, the negative association between total Hg and TT4 and TT3 was evident with and without exposures to Cd or Pb above median levels, and the positive association between blood Cd and Tg was evident for all combinations with exposure to Cd above the median, regardless of exposure to Hg or Pb [see Supplemental Material, Table S4 (http://dx.doi.org/10.1289/ehp.1205239)].
Discussion
In adults, Hg exposure was negatively associated with TH levels, whereas Cd exposure was positively associated with TH levels and the pre-hormone Tg. TSH levels were not consistently associated with Hg or Cd exposure, suggesting that these exposures may not affect pituitary function. In women of reproductive age, the inverse associations between Hg and TH levels persisted, whereas the associations between Cd and TH levels were mostly positive but not statistically significant.
The lack of association between BPb and TH levels suggests current exposure levels experienced by the U.S. population do not adversely affect TH synthesis and regulation, though effects at higher environmental exposure levels cannot be ruled out. Occupational Pb exposure has been associated with significant reductions in TH levels (Robins et al. 1983), and a recent animal study also noted an effect of Pb on TH levels in rats (Wu et al. 2011).
Negative associations observed between Hg and TH levels are consistent with proposed mechanisms for Hg toxicity in which Hg accumulates in the thyroid and reduces iodide uptake at the sodium/iodide symporter by binding to iodide (Nishida et al. 1986) and inhibits TH deiodinase function in peripheral tissues (Soldin et al. 2008; Tan et al. 2009). In the Abdelouahab et al. (2008) study, TSH was positively associated with hair and blood Hgs, but we did not find an association between TSH and blood Hg in our population. A recent analysis of NHANES 2007–2008 data suggested an increase in the prevalence of TgAb in women with blood Hg > 1.8 µg/L versus ≤ 0.4 µg/L (Gallagher and Meliker 2012), but that analysis did not exclude subjects that had thyroid disease or were taking medications to treat thyroid disease. We did not evaluate inorganic Hg, which has been associated with a higher FT4/FT3 ratio in two occupational studies (Barregard et al. 1994; Ellingsen et al. 2000). Prior research has suggested that PCBs may influence TH levels, and effects of PCB could therefore confound associations with Hg because both may be consumed in fish (Hagmar 2003). However, we did not have data on PCB exposures.
Studies in experimental animals suggest lower TH levels in Cd-exposed mice and rats (Gupta and Kar 1997, 1998; Hammouda et al. 2008; Yoshizuka et al. 1991), in contrast with our finding of positive associations between Cd and TH levels. This discrepancy could be due to species differences or to higher exposure doses being used in animal studies, although we cannot rule out the possibility that the associations we observed were due to chance or bias. TT3 was increased in residents of a Cd-polluted region (GM urinary Cd levels, 6.6 and 9.2 µg/g creatinine in males and females, respectively) compared with TT3 levels in residents of a control region (GMs of 2.6 and 4.4 µg/g creatinine, respectively) (Nishijo et al. 1994). In the present study population, GM urinary Cd levels were an order of magnitude lower, but we observed higher TH levels with increased urinary Cd. Additional research is needed to clarify the potential effects of Cd exposure on thyroid function in humans.
The observed associations between Hg and Cd exposures and TH levels were relatively weak on the individual level, with an IQR increase in exposure associated with a 1–4% change in hormone levels. The HPT axis is precisely regulated and it is plausible that the low environmental exposures experienced by the U.S. population do not substantially influence individual thyroid profiles. However, exposures at the very high end (e.g., ≥ 5th quintile compared with the 1st quintile) may be associated with a TH level change of > 5%, such as total Hg at ≥ 2.16 µg/L versus < 0.42 µg/L and TT3 in adults as shown in Supplemental Material, Table S2 (http://dx.doi.org/10.1289/ehp.1205239). This may be related to significant health effects in individuals who already have compromised thyroid functions. More research is needed for Hg and Cd exposure at the higher end of exposures, for example, in specific populations such as people who eat a large amount of fish or live close to Cd-contaminated areas.
This analysis has limitations related to the cross-sectional design of the NHANES. The associations cannot be interpreted as causation, and the results are more relevant to background exposure in the general population. Although we did analyze levels of BPb, Hg, and Cd as well as urinary Cd, the latter is only available in a subset of one-third of the total sample population. The research was only assessing one time point and we lacked longitudinal data. We performed multiple comparisons in the analysis and may have encountered the problem of false positive findings. Instead of adopting a strict Bonferroni correction, we mainly focused the patterns (relation to more than one TH), dose response (significance for more than one quintile), and consistency between exposures (total and organic Hg, blood and urinary Cd). In spite of these limitations, we were able to test the associations between metals and FT4 and FT3, which often were not measured in prior studies. We stratified the analysis by adolescents and adults, and replicated results in women of reproductive age. We completed the analysis using continuous exposure variable and exposure quintiles, and we summarized percentage change in hormone levels by IQR of exposure.
Conclusions
In the general adult U.S. population, we observed inverse associations between Hg and TH levels and positive associations between Cd and TH levels. Research is needed to quantify the associations at higher levels of exposure and to examine potential mechanisms of Hg and Cd thyroid toxicity.
Supplemental Material
Footnotes
This work was supported in part by the Center for Environmental Genetics (grant P30ES006096) and by the National Institute of Environmental Health Sciences (grant RC4ES09755).
The authors declare they have no actual or potential competing financial interests.
References
- Abdelouahab N, Mergler D, Takser L, Vanier C, St-Jean M, Baldwin M, et al. Gender differences in the effects of organochlorines, mercury, and lead on thyroid hormone levels in lakeside communities of Quebec (Canada). Environ Res. 2008;107(3):380–392. doi: 10.1016/j.envres.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Asvold BO, Bjoro T, Nilsen TI, Vatten LJ. Association between blood pressure and serum thyroid-stimulating hormone concentration within the reference range: a population-based study. J Clin Endocrinol Metab. 2007;92(3):841–845. doi: 10.1210/jc.2006-2208. [DOI] [PubMed] [Google Scholar]
- Barregard L, Lindstedt G, Schutz A, Sallsten G. Endocrine function in mercury exposed chloralkali workers. Occup Environ Med. 1994;51(8):536–540. doi: 10.1136/oem.51.8.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin RM, Astor BC, Powe NR, Ladenson PW. Smoke exposure is associated with a lower prevalence of serum thyroid autoantibodies and thyrotropin concentration elevation and a higher prevalence of mild thyrotropin concentration suppression in the third National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2004;89(12):6077–6086. doi: 10.1210/jc.2004-0431. [DOI] [PubMed] [Google Scholar]
- Bellinger DC. Lead. Pediatrics. 2004;113(4) suppl:1016–1022. [PubMed] [Google Scholar]
- Boas M, Feldt-Rasmussen U, Skakkebaek NE, Main KM. Environmental chemicals and thyroid function. Eur J Endocrinol. 2006;154(5):599–611. doi: 10.1530/eje.1.02128. [DOI] [PubMed] [Google Scholar]
- Caldwell KL, Mortensen ME, Jones RL, Caudill SP, Osterloh JD. Total blood mercury concentrations in the U.S. population: 1999–2006. Int J Hyg Environ Health. 2009;212(6):588–598. doi: 10.1016/j.ijheh.2009.04.004. [DOI] [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention) Fourth National Report on Human Exposure to Environmental Chemicals. 2009. Available: http://www.cdc.gov/exposurereport/pdf/fourthreport.pdf [accessed 7 December 2012]
- Cooper DS, Biondi B. Subclinical thyroid disease. Lancet. 2012;379(9821):1142–1154. doi: 10.1016/S0140-6736(11)60276-6. [DOI] [PubMed] [Google Scholar]
- Danzi S, Klein I. Thyroid hormone and the cardiovascular system. Med Clin North Am. 2012;96(2):257–268. doi: 10.1016/j.mcna.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Dundar B, Oktem F, Arslan MK, Delibas N, Baykal B, Arslan C, et al. The effect of long-term low-dose lead exposure on thyroid function in adolescents. Environ Res. 2006;101(1):140–145. doi: 10.1016/j.envres.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Ellingsen DG, Efskind J, Haug E, Thomassen Y, Martinsen I, Gaarder PI. Effects of low mercury vapour exposure on the thyroid function in chloralkali workers. J Appl Toxicol. 2000;20(6):483–489. doi: 10.1002/1099-1263(200011/12)20:6<483::aid-jat722>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Erfurth EM, Gerhardsson L, Nilsson A, Rylander L, Schutz A, Skerfving S, et al. Effects of lead on the endocrine system in lead smelter workers. Arch Environ Health. 2001;56(5):449–455. doi: 10.1080/00039890109604481. [DOI] [PubMed] [Google Scholar]
- Gallagher CM, Meliker JR. Mercury and thyroid autoantibodies in U.S. women, NHANES 2007–2008. Environ Int. 2012;40:39–43. doi: 10.1016/j.envint.2011.11.014. [DOI] [PubMed] [Google Scholar]
- Ghassabian A, Bongers-Schokking JJ, Henrichs J, Jaddoe VW, Visser TJ, Visser W, et al. Maternal thyroid function during pregnancy and behavioral problems in the offspring: the generation R study. Pediatr Res. 2011;69(5 Pt 1):454–459. doi: 10.1203/PDR.0b013e3182125b0c. [DOI] [PubMed] [Google Scholar]
- Gupta P, Kar A. Role of testosterone in ameliorating the cadmium induced inhibition of thyroid function in adult male mouse. Bull Environ Contam Toxicol. 1997;58(3):422–428. doi: 10.1007/s001289900351. [DOI] [PubMed] [Google Scholar]
- Gupta P, Kar A. Role of ascorbic acid in cadmium-induced thyroid dysfunction and lipid peroxidation. J Appl Toxicol. 1998;18(5):317–320. doi: 10.1002/(sici)1099-1263(1998090)18:5<317::aid-jat514>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Gurer-Orhan H, Sabir HU, Ozgunes H. Correlation between clinical indicators of lead poisoning and oxidative stress parameters in controls and lead-exposed workers. Toxicology. 2004;195(2–3):147–154. doi: 10.1016/j.tox.2003.09.009. [DOI] [PubMed] [Google Scholar]
- Hagmar L. Polychlorinated biphenyls and thyroid status in humans: a review. Thyroid. 2003;13(11):1021–1028. doi: 10.1089/105072503770867192. [DOI] [PubMed] [Google Scholar]
- Hammouda F, Messaoudi I, El Hani J, Baati T, Said K, Kerkeni A. Reversal of cadmium-induced thyroid dysfunction by selenium, zinc, or their combination in rat. Biol Trace Elem Res. 2008;126(1–3):194–203. doi: 10.1007/s12011-008-8194-8. [DOI] [PubMed] [Google Scholar]
- Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, et al. Serum TSH, T4, and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 2002;87(2):489–499. doi: 10.1210/jcem.87.2.8182. [DOI] [PubMed] [Google Scholar]
- Hornung RW, Reed LD. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg. 1990;5:46–51. [Google Scholar]
- Iijima K, Otake T, Yoshinaga J, Ikegami M, Suzuki E, Naruse H, et al. Cadmium, lead, and selenium in cord blood and thyroid hormone status of newborns. Biol Trace Elem Res. 2007;119(1):10–18. doi: 10.1007/s12011-007-0057-1. [DOI] [PubMed] [Google Scholar]
- Järup L, Åkesson A. Current status of cadmium as an environmental health problem. Toxicol Appl Pharmacol. 2009;238(3):201–208. doi: 10.1016/j.taap.2009.04.020. [DOI] [PubMed] [Google Scholar]
- Jin Y, Liao Y, Lu C, Li G, Yu F, Zhi X, et al. Health effects in children aged 3–6 years induced by environmental lead exposure. Ecotoxicol Environ Saf. 2006;63(2):313–317. doi: 10.1016/j.ecoenv.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Lamb MR, Janevic T, Liu X, Cooper T, Kline J, Factor-Litvak P. Environmental lead exposure, maternal thyroid function, and childhood growth. Environ Res. 2008;106(2):195–202. doi: 10.1016/j.envres.2007.09.012. [DOI] [PubMed] [Google Scholar]
- López CM, Piñeiro AE, Núñez N, Avagnina AM, Villaamil EC, Roses OE. Thyroid hormone changes in males exposed to lead in the Buenos Aires area (Argentina). Pharmacol Res. 2000;42(6):599–602. doi: 10.1006/phrs.2000.0734. [DOI] [PubMed] [Google Scholar]
- Maervoet J, Vermeir G, Covaci A, Van Larebeke N, Koppen G, Schoeters G, et al. Association of thyroid hormone concentrations with levels of organochlorine compounds in cord blood of neonates. Environ Health Perspect. 2007;115:1780–1786. doi: 10.1289/ehp.10486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahaffey KR, Clickner RP, Bodurow CC. Blood organic mercury and dietary mercury intake: National Health and Nutrition Examination Survey, 1999 and 2000. Environ Health Perspect. 2004;112:562–570. doi: 10.1289/ehp.6587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Rossano MG, Protas B, Diamond MP, Puscheck E, Daly D, et al. Multiple metals predict prolactin and thyrotropin (TSH) levels in men. Environ Res. 2009;109(7):869–873. doi: 10.1016/j.envres.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MD, Crofton KM, Rice DC, Zoeller RT. Thyroid-disrupting chemicals: interpreting upstream biomarkers of adverse outcomes. Environ Health Perspect. 2009;117:1033–1041. doi: 10.1289/ehp.0800247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori K, Yoshida K, Hoshikawa S, Ito S, Yoshida M, Satoh M, et al. Effects of perinatal exposure to low doses of cadmium or methylmercury on thyroid hormone metabolism in metallothionein-deficient mouse neonates. Toxicology. 2006;228(1):77–84. doi: 10.1016/j.tox.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Muntner P, Menke A, DeSalvo KB, Rabito FA, Batuman V. Continued decline in blood lead levels among adults in the United States: the National Health and Nutrition Examination Surveys. Arch Intern Med. 2005;165(18):2155–2161. doi: 10.1001/archinte.165.18.2155. [DOI] [PubMed] [Google Scholar]
- NCHS (National Center for Health Statistics) National Health and Nutrition Examination Survey 2007–2008. 2009a. Available: http://www.cdc.gov/nchs/nhanes/nhanes2007-2008/nhanes07_08.htm [accessed 15 June 2012]
- NCHS (National Center for Health Statistics) National Health and Nutrition Examination Survey 2007–2008 Data Documentation, Codebook, and Frequencies Thyroid Profile (THYROD_E). 2009b. Available: http://www.cdc.gov/nchs/nhanes/nhanes2007-2008/THYROD_E.htm [accessed 15 June 2012]
- NCHS (National Center for Health Statistics) National Health and Nutrition Examination Survey 2007–2008 Lab Methods. 2009c. Available: http://www.cdc.gov/nchs/nhanes/nhanes2007-2008/lab_methods_07_08.htm [accessed 15 June 2012]
- NCHS (National Center for Health Statistics) NHANES 2007–2008 Laboratory Files. 2009d. Available: http://www.cdc.gov/nchs/nhanes/nhanes2007-2008/lab07_08.htm [accessed 15 June 2012]
- Nishida M, Yamamoto T, Yoshimura Y, Kawada J. Subacute toxicity of methylmercuric chloride and mercuric chloride on mouse thyroid. J Pharmacobiodyn. 1986;9(4):331–338. doi: 10.1248/bpb1978.9.331. [DOI] [PubMed] [Google Scholar]
- Nishijo M, Nakagawa H, Morikawa Y, Tabata M, Senma M, Miura K, et al. Nihon Eiseigaku Zasshi. 1994;49(2):598–605. doi: 10.1265/jjh.49.598. [A study of thyroid hormone levels of inhabitants of the cadmium-polluted Kakehashi River basin] [in Japanese] [DOI] [PubMed] [Google Scholar]
- Nixon DE, Burritt MF, Moyer TP. The determination of mercury in whole blood and urine by inductively coupled plasma mass spectrometry. Spectrochim Acta Part B At Spectrosc. 1999;54(8):1141–1153. [Google Scholar]
- Osius N, Karmaus W, Kruse H, Witten J. Exposure to polychlorinated biphenyls and levels of thyroid hormones in children. Environ Health Perspect. 1999;107:843–849. doi: 10.1289/ehp.99107843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce EN, Braverman LE. Environmental pollutants and the thyroid. Best Pract Res Clin Endocrinol Metab. 2009;23(6):801–813. doi: 10.1016/j.beem.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Pop VJ, Brouwers EP, Vader HL, Vulsma T, van Baar AL, de Vijlder JJ. Maternal hypothyroxinaemia during early pregnancy and subsequent child development: a 3-year follow-up study. Clin Endocrinol (Oxf) 2003;59(3):282–288. doi: 10.1046/j.1365-2265.2003.01822.x. [DOI] [PubMed] [Google Scholar]
- Robins JM, Cullen MR, Connors BB, Kayne RD. Depressed thyroid indexes associated with occupational exposure to inorganic lead. Arch Intern Med. 1983;143(2):220–224. [PubMed] [Google Scholar]
- Roos A, Bakker SJ, Links TP, Gans RO, Wolffenbuttel BH. Thyroid function is associated with components of the metabolic syndrome in euthyroid subjects. J Clin Endocrinol Metab. 2007;92(2):491–496. doi: 10.1210/jc.2006-1718. [DOI] [PubMed] [Google Scholar]
- Schell LM, Gallo MV, Denham M, Ravenscroft J, DeCaprio AP, Carpenter DO. Relationship of thyroid hormone levels to levels of polychlorinated biphenyls, lead, p,p’-DDE, and other toxicants in Akwesasne Mohawk youth. Environ Health Perspect. 2008;116:806–813. doi: 10.1289/ehp.10490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher C, Brodkin CA, Alexander B, Cullen M, Rainey PM, van Netten C, et al. Thyroid function in lead smelter workers: absence of subacute or cumulative effects with moderate lead burdens. Int Arch Occup Environ Health. 1998;71(7):453–458. doi: 10.1007/s004200050305. [DOI] [PubMed] [Google Scholar]
- Siegel M, Forsyth B, Siegel L, Cullen MR. The effect of lead on thyroid function in children. Environ Res. 1989;49(2):190–196. doi: 10.1016/s0013-9351(89)80064-7. [DOI] [PubMed] [Google Scholar]
- Singh B, Chandran V, Bandhu HK, Mittal BR, Bhattacharya A, Jindal SK, et al. Impact of lead exposure on pituitary–thyroid axis in humans. Biometals. 2000;13(2):187–192. doi: 10.1023/a:1009201426184. [DOI] [PubMed] [Google Scholar]
- Soldin OP, O’Mara DM, Aschner M. Thyroid hormones and methylmercury toxicity. Biol Trace Elem Res. 2008;126(1–3):1–12. doi: 10.1007/s12011-008-8199-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stathatos N. Thyroid physiology. Med Clin North Am. 2012;96(2):165–173. doi: 10.1016/j.mcna.2012.01.007. [DOI] [PubMed] [Google Scholar]
- Takser L, Mergler D, Baldwin M, de Grosbois S, Smargiassi A, Lafond J. Thyroid hormones in pregnancy in relation to environmental exposure to organochlorine compounds and mercury. Environ Health Perspect. 2005;113:1039–1045. doi: 10.1289/ehp.7685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan SW, Meiller JC, Mahaffey KR. The endocrine effects of mercury in humans and wildlife. Crit Rev Toxicol. 2009;39(3):228–269. doi: 10.1080/10408440802233259. [DOI] [PubMed] [Google Scholar]
- Tellez-Plaza M, Navas-Acien A, Caldwell KL, Menke A, Muntner P, Guallar E. Reduction in cadmium exposure in the United States population, 1988–2008: the contribution of declining smoking rates. Environ Health Perspect. 2012;120:204–209. doi: 10.1289/ehp.1104020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuppurainen M, Wagar G, Kurppa K, Sakari W, Wambugu A, Froseth B, et al. Thyroid function as assessed by routine laboratory tests of workers with long-term lead exposure. Scand J Work Environ Health. 1988;14(3):175–180. doi: 10.5271/sjweh.1934. [DOI] [PubMed] [Google Scholar]
- Williams GR. Neurodevelopmental and neurophysiological actions of thyroid hormone. J Neuroendocrinol. 2008;20(6):784–794. doi: 10.1111/j.1365-2826.2008.01733.x. [DOI] [PubMed] [Google Scholar]
- Wu CY, Liu B, Wang HL, Ruan DY. Levothyroxine rescues the lead-induced hypothyroidism and impairment of long-term potentiation in hippocampal CA1 region of the developmental rats. Toxicol Appl Pharmacol. 2011;256(2):191–197. doi: 10.1016/j.taap.2011.08.010. [DOI] [PubMed] [Google Scholar]
- Yazbeck CF, Sullivan SD. Thyroid disorders during pregnancy. Med Clin North Am. 2012;96(2):235–256. doi: 10.1016/j.mcna.2012.01.004. [DOI] [PubMed] [Google Scholar]
- Yoshizuka M, Mori N, Hamasaki K, Tanaka I, Yokoyama M, Hara K, et al. Cadmium toxicity in the thyroid gland of pregnant rats. Exp Mol Pathol. 1991;55(1):97–104. doi: 10.1016/0014-4800(91)90021-o. [DOI] [PubMed] [Google Scholar]
- Zheng W, Lu YM, Lu GY, Zhao Q, Cheung O, Blaner WS. Transthyretin, thyroxine, and retinol-binding protein in human cerebrospinal fluid:effect of lead exposure. Toxicol Sci. 2001;61(1):107–114. doi: 10.1093/toxsci/61.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


