Abstract
Epidemiological studies report a negative association between circulating bilirubin concentrations and the risk for cancer and cardiovascular disease. Structurally related tetrapyrroles also possess in vitro anti-genotoxic activity and may prevent mutation prior to malignancy. Furthermore, few data suggest that tetrapyrroles exert anti-carcinogenic effects via induction of cell cycle arrest and apoptosis. To further investigate whether tetrapyrroles provoke DNA-damage in human cancer cells, they were tested in the single cell gel electrophoresis assay (SCGE). Eight tetrapyrroles (unconjugated bilirubin, bilirubin ditaurate, biliverdin, biliverdin-/bilirubin dimethyl ester, urobilin, stercobilin and protoporphyrin) were added to cultured Caco2 and HepG2 cells and their effects on comet formation (% tail DNA) were assessed. Flow cytometric assessment (apoptosis/necrosis, cell cycle, intracellular radical species generation) assisted in revealing underlying mechanisms of intracellular action. Cells were incubated with tetrapyrroles at concentrations of 0.5, 5 and 17 μM for 24 h. Addition of 300 μM tertiary-butyl hydroperoxide to cells served as a positive control. Tetrapyrrole incubation mostly resulted in increased DNA-damage (comet formation) in Caco2 and HepG2 cells. Tetrapyrroles that are concentrated within the intestine, including protoporphyrin, urobilin and stercobilin, led to significant comet formation in both cell lines, implicating the compounds in inducing DNA-damage and apoptosis in cancer cells found within organs of the digestive system.
Abbreviations: BP(s), bile pigment(s); BR, unconjugated bilirubin; BR-DME, Bilirubin dimethyl ester; BRf, free bilirubin; BRDT, bilirubin ditaurate; BV, biliverdin; BV-DME, biliverdin dimethyl ester; PRO, protoporphyrin; SCGE, single cell gel electrophoresis; TP(s), tetrapyrrole(s); SB, stercobilin; UB, urobilin
Keywords: Stercobilin, Urobilin, Protoporphyrin, SCGE, Comet
Highlights
► DNA-damaging effects of bile pigments have been rarely investigated. ► Thus, eight tetrapyrroles were tested for DNA-damaging effects in the comet assay. ► To assess DNA damage, cancer cells were used, and flow cytometry parameters were measured. ► Especially protoporphyrin, urobilin and stercobilin increased DNA strand breaks significantly. ► Mechanisms could include oxidative stress, cell cycle arrest and apoptosis.
Introduction
Bile pigments (BPs) such as bilirubin (BR), biliverdin (BV) and structurally related tetrapyrroles (TPs) are formed naturally within the human body. Derived from heme within hemoglobin and heme-containing enzymes, endogenous TPs possess porphyrin structure and carry a conjugated system of double-bonds [1]. Heme catabolism requires the action of heme oxygenase (HMOX-1/-2) and biliverdin reductase (BLVRA), within certain organs including the liver and intestine. Tetrapyrroles derived from this process are found in the bile (mainly conjugated BR), and in the intestinal milieu (mainly stercobilin SB, urobilin UB and protoporphyrin PRO) [2,3]. The literature reports anti-mutagenic, antioxidant as well as potentially anti-carcinogenic activities of BR and BV in vitro [4–8]. Further studies suggest an important role for mildly elevated circulating BR in preventing disease in human subjects [9–13] by antio idant mechanisms [9,11–13], and emphasize a protective physiological role for unconjugated BR in protecting against gastrointestinal and colorectal cancers [14]. These effects might also be related to BR’s intestinal abundance [14], with recent evidence indicating the potential efficacy of intestinal absorption [15,16]. Consequently, physiologically abundant TPs could play significant health promoting roles in the organs where they are absorbed/accumulated including the liver, gall bladder and intestine [17] in addition to the urinary tract and the circulatory system [16].
Thus far, the scientific focus has mainly been directed toward in vitro anti-genotoxic properties of BR and BV [5,18], with little attention focused on structurally related metabolites. Unconjugated BR induces cell cycle arrest, apoptosis and cytostasis in vitro in multiple cell lines [19]. Besides, BR may be defending against cancer by interfering with pro-carcinogenic signaling pathways, and therefore potently inhibit tumor cell proliferation in vivo [19]. Furthermore, BR induces mitochondrial depolarization in colon cancer cells, as it diffuses across the outer mitochondrial membrane, and thereby activates the intrinsic apoptotic pathway [17]. However, data concerning the effects of related tetrapyrrolic compounds on cancer cell biology are entirely lacking, with the only exception being PRO which is successfully applied in the clinic [20]. Despite the use of PRO and derivatives in the photodynamic treatment of skin cancer [21,22], in vitro comet data and evidence on pro-apoptotic, anti-carcinogenic and anti-proliferative effects of PRO in different cell culture models are rare [22–26]. Also the DNA-damaging effects of BR in cancer cells have been investigated only once [27], when applying the single cell gel electrophoresis (SCGE/comet) assay.
Stress stimuli such as DNA-damage provoke cellular responses including oxidative stress, cell cycle arrest and apoptosis, which is mainly controlled by the action of tumor suppressors [21,22,26,28]. Many chemotherapeutics including purine and pyrimidine analogs as well as alkylating agents induce DNA-damage within rapidly proliferating cells in an attempt to selectively target malignant cells. To assess whether TPs exert comparable effects on cancer cell lines (e.g. induce free radical formation), five essentially untested TPs (BR-/BV-dimethyl ester (BR-/BV-DME), UB, SB, PRO) were investigated together with BR, BRDT and BV in human colorectal adenocarcinoma (Caco2) and hepatocellular carcinoma (HepG2) cells. These cell lines represent meaningful models for TP in vitro testing, since both the liver and intestine represent central organs for BP metabolism [29]. To elucidate cellular regulatory mechanisms in response to TP exposure, flow cytometry analyses (apoptosis/necrosis, intracellular radical species (ROS), cell cycle) were conducted to reveal underlying mechanisms of TPs action, relevant to cancer cell biology [21,22,26,28,30], while the comet assay was applied to determine the extent of DNA damage.
Materials and methods:
Chemicals
Unconjugated bilirubin IXα (BR) [CAS# 635–65–4], bilirubin conjugate (ditaurate; disodium; BRDT) [CAS# 635–65–4], biliverdin IXα (BV) [CAS# 55482–27–4], bilirubin dimethyl ester (BR-DME) [CAS# 19792–68–8], biliverdin dimethyl ester (BV-DME) [CAS# 10035–62–8], protoporphyrin IX (PRO) [CAS# 553–12–8] as well as urobilin (UB) [CAS# 28925–89–5] and stercobilin (SB) [CAS# 34217–90–8] were purchased from Frontier Scientific, UK, and were dissolved in DMSO. Solubility was tested spectrophotometrically and purity via HPLC [15,31]. DMSO final concentrations in media did not exceed 0.1%. Test compounds were stored in airtight and lightproof containers at −80 °C until use, and were protected from light throughout all test procedures using foil-covered containers. Other chemicals were purchased from Sigma Aldrich, Austria (unless otherwise noted), were of the highest analytical grade available and were stored and used according to instructions.
Single cell gel electrophoresis assay
The comet assay measures DNA single- and double-strand breaks in eukaryotic cells embedded in 1% low melting agarose (LMA; Invitrogen Austria), fixed on agarose pre-coated microscope slides (1% normal melting agarose, NMA; Invitrogen Austria). After cell lysis at pH 10 and 20 min of DNA unwinding (as well as 300 μM tertiary-butyl hydroperoxide (tert-BOOH) treatment for positive controls), cells were exposed to a directed electric field (Electrophoresis CSL-10M40, Biozym Austria; pH >13). After ethidium bromide staining (20 μl of 20 μg/ml/gel), DNA migration/comet formation was evaluated using a fluorescent microscope (Zeiss Germany) equipped with a camera (Hitachi Austria). Komet 5.5. software (Andor Technology, GB) assessed comet tail DNA content which was expressed as a percentage of total cellular DNA (% tail DNA). The method of Azqueta et al. [32] was used to measure both DNA single- and double-strand breaks. Human lymphocytes were replaced by cancer cells, using 1×106 cells/ml. Eight gels (two per slide; per compound and concentration) were prepared for statistical and quality assurance analysis, six gels of which at minimum were randomly counted (50 cells/gel).
Human Cancer cell lines
Cytotoxicity was assessed in two cell lines (Caco2 and HepG2) that were originally derived from primary human tumors as reported by the provider (source as below). Cell viability and cell counts were assessed using a trypan blue assay-based automated cell counter (Countess; Invitrogen, Austria).
Cell culture media
Cells were obtained from the German Collection of Microorganisms and Cell Cultures (Leibniz Institute, DSMZ), and were maintained using standard culture techniques. Caco2 cells were cultivated in antibiotic-free Dulbecco’s modified essential medium (DMEM with high glucose; PAA Austria), and HepG2 cells in Eagle’s minimum essential medium with Earle's salts (MEM; PAA Austria) in sterile 25 cm2 filter cap flasks (SPL Life Sciences Inc., Austria). Media were supplemented with 10% (v/v) fetal bovine serum (FBS; PAA Austria), 5 ml of non-essential amino acids, 1 ml of Na-pyruvate (HepG2) or 20% FBS (v/v) and 1 ml of 100 mM Na-pyruvate (Caco2). Experiments were conducted between passages 17–37 for Caco2, and 34–54 for HepG2 cells. Media were changed every second day and on the days before and after cell splitting, which was performed at 70–80% confluence, using 1 ml per flask of Accutase solution (PAA Austria). For TP incubation, 4.5×105 cells/ml were seeded in sterile 24 well plates (SPL Life Sciences Inc., Austria) and were allowed to adhere for 24 h. All cultures were maintained at 37 °C in a humidified atmosphere of 95% air, 5% CO2.
Flow cytometric analyses
After 24 h of TP incubation, cells were harvested from 24 well plates and transferred to 5 ml FACS tubes (BD Biosciences, Austria). After treatment including centrifugation, washing and staining steps, cell suspensions were analyzed using a BD FACSCalibur flow cytometer (BD Biosciences, Austria).
Apoptosis/necrosis assay
The applied detection kit (r-phycoerythrin (PE) annexin V apoptosis detection kit I, BD Pharmingen Austria) was used to measure apoptosis and necrosis via flow cytometry. This assay requires the Ca2+-dependent affinity of annexin V to phosphatidyl serine as well as the specificity of 7-aminoactinomycin-d (7-AAD) for guanine–cytosine DNA-base pairs for the assessment of apoptosis and necrosis. Briefly, 1×106 cells/ml were washed in Ca2+/Mg2+-free 1× phosphate buffered saline (PBS; PAA Austria) and re-suspended in binding buffer, were doubly labeled with PE-stained annexin V (exmax: 496, emmax: 575 nm; FL-2), and 7-AAD (exmax: 546, emmax: 647 nm; FL-3) and were incubated for 15 min in the dark prior to cytometric analyses [33,34].
Intracellular radical species
To detect intracellular reactive oxygen species (ROS), 10 μM final concentration dihydroethidium (DHE) and 25 μM final concentration dihydrofluorescein diacetate (DCFH-DA), both in DMSO, were added individually to incubated cells. Dihydroethidium is cell-permeable and reacts with O2-. to form oxyethidium. This product interacts with nucleic acids and emits red fluorescence, which can be measured cytometrically (exmax: 329, emmax: 373 nm; FL-2) [35]. When DCFH-DA enters the cell, it is cleaved by intracellular esterases to form DCFH, which is then non-specifically oxidized by (hydro)peroxides to fluorescent 7′-dichlorodihydrofluorescein (DCF) (exmax: 498, emmax: 522 nm; FL-1) [35]. After washing in Ca2+/Mg2+-free 1×PBS and prior to measurement, 1×106 cells/ml were stained with DHE (in FACS tubes after harvesting) or with DCFH (in 24 wells prior to harvesting) and were left to incubate for 20 min in the dark [36].
Cell cycle assay
The cell cycle assay measures the percentage number of cells in the cell cycle phases G0/1, S, G2/M, as well as apoptotic cells (sub G0/1), according to fluorescence intensity, based on the number of DNA copies within cells. Propidium iodide (PI; exmax: 535, emmax: 617 nm; FL-2) binds to DNA by intercalating between the bases with a stoichiometry of one PI molecule per 4.5 DNA base pairs. After washing, 1×106 cells/ml were fixed with 75% ice-cold absolute ethanol and were left to incubate for 30 min on ice. Cells were re-suspended in 500 μl of phosphate buffer (96 ml 0.2 M Na2HPO4+4 ml 0.1 M C6H8O7), and after centrifugation, 50 μM of 100 μg/ml RNase and 200 μl of 50 μg/ml PI (both in dH2O) were added [37,38]. Cell suspensions were measured immediately.
Statistical analyses
To evaluate DNA-damaging effects of TPs in the comet assay, 50 cells/gel were counted. As a measure of DNA-damage, mean percentages of total DNA (% tail DNA) were calculated in Microsoft Excel. Statistical analysis was completed using IBM SPSS 19 for Windows. Normal distribution of the data was assessed using the K–S test. To calculate differences in DNA-damage between groups, ANOVA was performed on parametric data and Kruskal–Wallis H-test on non-parametric data, followed by the Dunnett-T3 post-hoc test, assuming non-homogeneous variances. Pearson bivariate correlations were performed on parametric data, and Spearman rho on non-parametric data. A p-value ≤0.05 was considered significant.
Free BR concentrations (BRf) were calculated as published earlier [39], by using the Microsoft Excel’s Solver function.
Results
DNA-damage/comet formation in Caco2 and HepG2 cell lines:
Protoporphyrin, urobilin, stercobilin
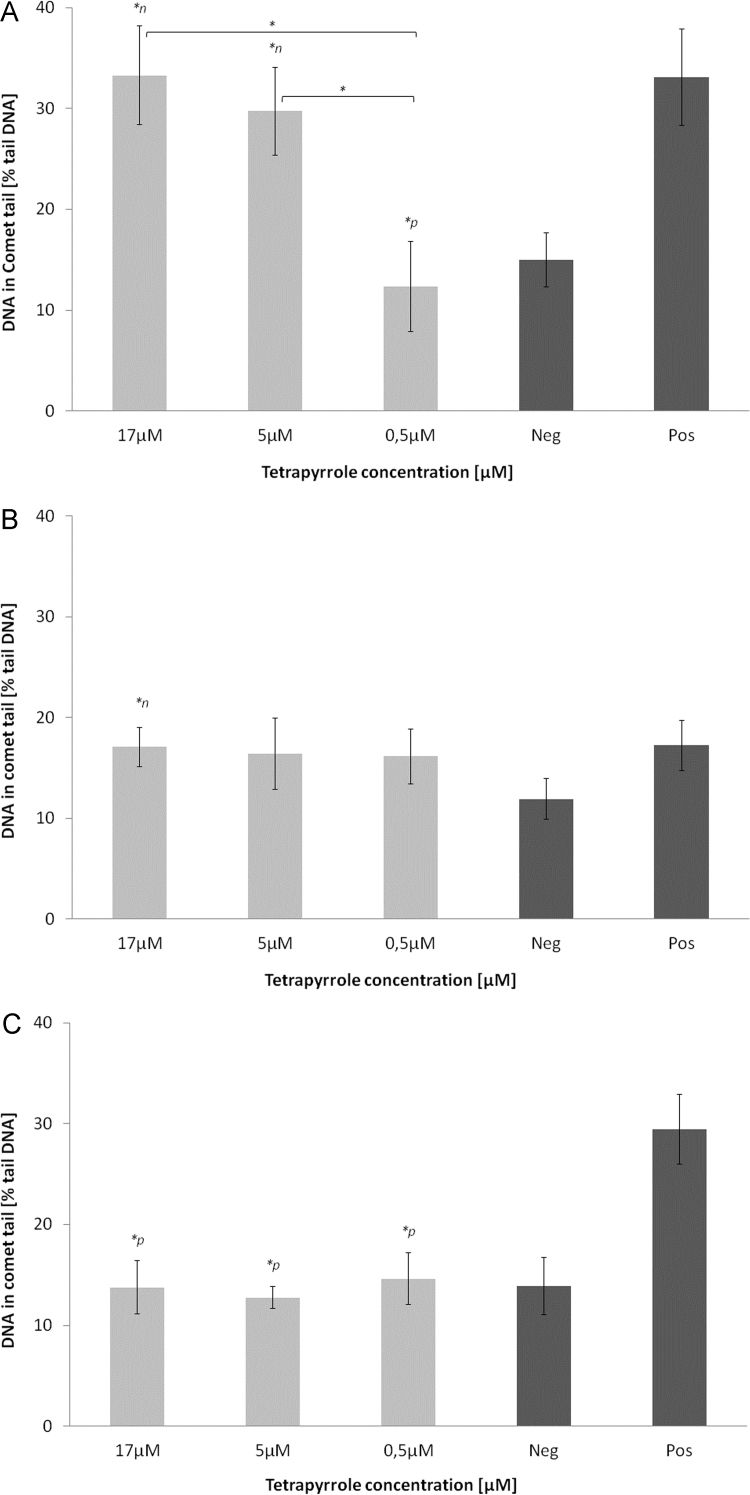
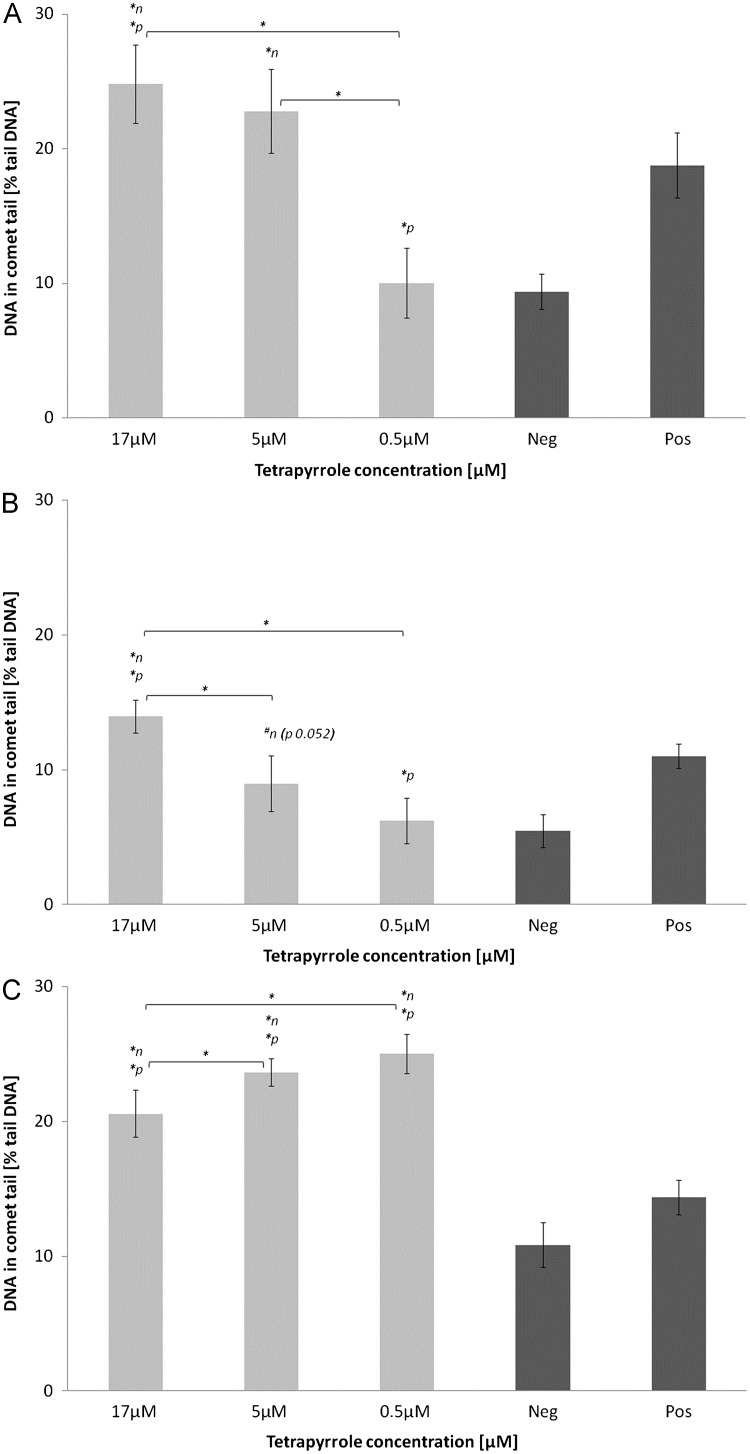
In both cell lines, PRO at the highest and lowest tested concentrations induced significant DNA-damage versus negative control (p<0.05), as was the case in HepG2 cells, also in comparison to positive control (p<0.05; Figs. 1 and 2A). Following UB incubation in both cell lines, DNA-damaging effects tended to be or were significantly increased compared to negative control (p<0.05; Figs. 1 and 2B). In HepG2 cells, SB elevated DNA-damage compared to negative and positive controls (p<0.05; Fig. 2C).
Fig. 1.
DNA-damaging effects of (A) protoporphyrin, (B) urobilin and (C) stercobilin in Caco2 cells. ⁎n: significantly different to negative control (p≤0.05), ⁎p: significantly different to positive control (p≤0.05).
Fig. 2.
DNA-damaging effects of (A) protoporphyrin, (B) urobilin and (C) stercobilin in HepG2 cells. ⁎n: significantly different to negative control, ⁎p: significantly different to positive control, #n: not significantly different to negative control, however strong trend (p≤0.1).
Bilirubin, bilirubin ditaurate, and bilirubin dimethyl ester
In Caco2 cells, the highest tested BR concentration led to significantly reduced comet formation compared to negative control (p<0.05; Table 1). Somewhat in contrast, conjugated BRDT at 0.5 μM showed significantly elevated DNA-damage versus negative control in Caco2 cells (p<0.05; Table 1). In HepG2 cells, however, BRDT did not induce DNA-damage compared to negative or positive control (p<0.05; Table 1). Bilirubin dimethyl ester incubation with Caco2 and HepG2 cells showed no DNA-damaging effects.
Table 1.
Mean % tail DNA in Caco2 and HepG2 cells (± SD) after 24 h TP incubations, compared to positive and negative controls.
|
Caco2 | |||
|---|---|---|---|
| Tetrapyrrole (μM) | % tail DNA±SD | % tail DNA pos control±SD | % tail DNA neg control±SD |
| BR 0.5 | 18.7±3p | 36.5±4 | 21.3±3 |
| BR 5 | 21.3±3.8p | ||
| BR 17 | 13.8±1.9np | ||
| BRDT 0.5 | 10.8±2.6np | 29.1±2.7 | 7.7±2.0 |
| BRDT 5 | 11.7±2.6p | ||
| BRDT 17 | 18.2±4.1p | ||
| BR-DME 0.5 | 8.7±2.7 | 21.9±3.9 | 9.9±4.0 |
| BR-DME 5 | 11.5±2.3p | ||
| BR-DME 17 | 15.0±6.9p | ||
| BV 0.5 | 20.9±4.3p | 30.1±5.0 | 16.5±2.6 |
| BV 5 | 20.8±2 | ||
| BV 17 | 18.4±2.7 | ||
| BV-DME 0.5 | 15.6±3.5p | 26.6±2.9 | 15.5±2.5 |
| BV-DME 5 | 15.3±3.2p | ||
| BV-DME 17 | 12.9±2.2p | ||
| HepG2 | |||
| Tetrapyrrole | % tail DNA±SD | % tail DNA pos control±SD | % tail DNA neg control±SD |
| BR 0.5 | 10.8±2.8 | 18.5±3.9 | 9.3±1.0 |
| BR 5 | 9.2±2.9 | ||
| BR 17 | 9.7±1.3 | ||
| BRDT 0.5 | 8.2±0.8p | 13.3±0.9 | 7.4±2.0 |
| BRDT 5 | 8.8±1.4p | ||
| BRDT 17 | 8.5±1.1p | ||
| BR-DME 0.5 | 8.8±2.5p | 13.4±2.5 | 6.8±2.3 |
| BR-DME 5 | 9.3±1.3p | ||
| BR-DME 17 | 8.7±2.5 | ||
| BV 0.5 | 17.2±1.4n | 16.8±0.7 | 12.3±1.0 |
| BV 5 | 15.1±0.9np | ||
| BV 17 | 16.6±1.3n | ||
| BV-DME 0.5 | 17.0±2.2np | 21.6±2.1 | 12.3±1.6 |
| BV-DME 5 | 14.6±2.0p | ||
| BV-DME 17 | 17.8±1.4np | ||
- p: significantly different to positive control (p≤0.05).
- n: significantly different to negative control (p≤0.05).
- np: significantly different to negative and positive controls (p≤0.05).
Biliverdin, biliverdin dimethyl ester
In Caco2 cells, BV and BV-DME did not significantly increase DNA-damage versus negative control (p<0.05; Table 1). However, BV incubation in HepG2 cells caused significantly elevated DNA-damage at all tested concentrations compared to the negative control (p<0.05; Table 1). Also BV-DME at the lowest and highest tested concentrations led to significantly elevated DNA-damage compared to negative control (p<0.05; Table 1).
Correlations between DNA-damage/comet formation and flow cytometry parameters:
Protoporphyrin, urobilin, and stercobilin
In HepG2 cells, a positive relationship between PRO-induced comet formation and intracellular ROS (superoxide) production was determined (p<0.05; Table 2).Superoxide formation after PRO incubation in HepG2 cells was positively correlated to apoptosis (r 0.529, p<0.05).
Table 2.
Correlations (r) of comet tail DNA percentages (% tail DNA) and flow cytometry parameters (cell death, ROS formation) in Caco2 and HepG2 cells.
| Caco2 |
DNA damage (% tail DNA) |
||||||
|---|---|---|---|---|---|---|---|
| BR | BRDT | BR-DME | BV | BV-DME | SB | PRO | |
| Necrosis | −0.726 p=0.011 | ||||||
| Hydroperoxide | 0.727 p=0.011 | 0.904 p=0.001 | |||||
| HepG2 |
DNA damage (% tail DNA) |
||||||
| BR | BRDT | BV-DME | UB | SB | PRO | ||
| Apoptosis | 0.644 p=0.010 | ||||||
| Superoxide | 0.547 p=0.053# | 0.583 p=0.036 | |||||
BR: unconjugated bilirubin, BRDT: bilirubin ditaurate, BR-DME: bilirubin dimethyl ester, BV: biliverdin, BV-DME: biliverdin dimethyl ester, UB: urobilin, SB: stercobilin, PRO: protoporphyrin.#non-significant trend.
Bilirubin, bilirubin ditaurate, and bilirubin dimethyl ester
For BR, a positive correlation was determined between comet and intracellular ROS formations (superoxide and hydroperoxide) in both Caco2 and HepG2 cell lines (p<0.05; Table 2). ROS (superoxide) formation in HepG2 cells after BR incubation was positively related to apoptosis (r 0.704, p<0.01).
Biliverdin, biliverdin dimethyl ester
Following BV incubation in Caco2 cells a positive correlation was determined between DNA-damaging effects and ROS formation (Table 2). In HepG2 cells BV-DME-based comet formation significantly correlated with apoptosis (p<0.05; Table 2). In HepG2 cells ROS formation after BV incubation non-significantly correlated with apoptosis (r 0.488, p 0.077).
Discussion
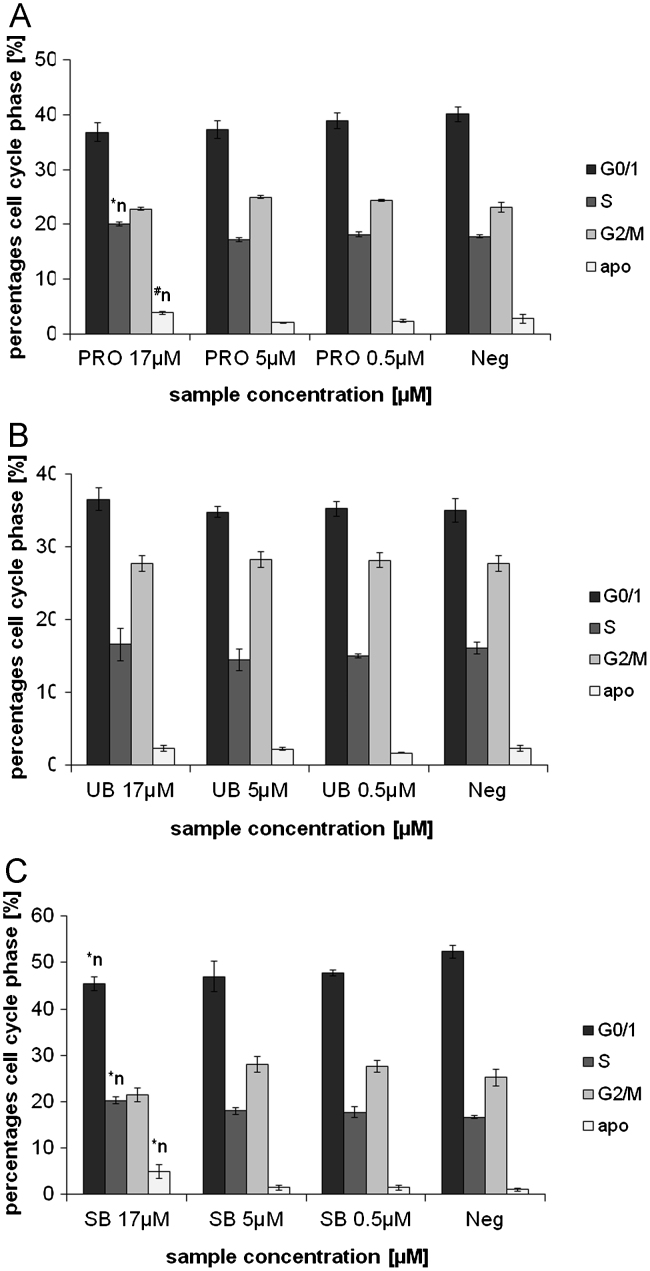
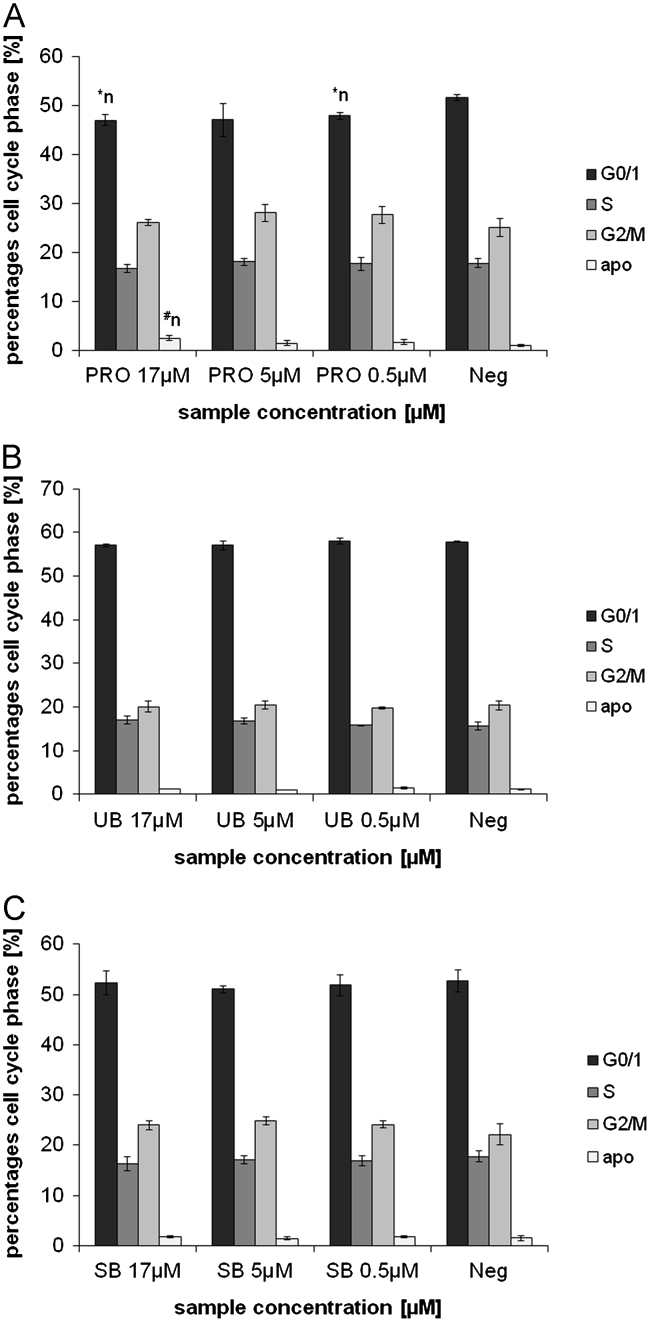
In this study, a range of physiologically abundant TPs (BR, BRDT, BV, UB, SB, PRO) were investigated with non-physiological BR- and BV-DME, to test their effects on ROS accumulation, DNA-damage (comet formation) and apoptosis in HepG2 and Caco2 cell lines. Most of the investigated TPs were found to induce DNA-damage in Caco2 and HepG2 cells, and effects appeared to be most pronounced with intestinal TPs (SB, UB, PRO; Figs. 1 and 2A–C). Cell viability data (for all tests between 86 and 99% viability; not shown) showed no significant differences between the tested TP concentrations and negative controls. Therefore,it can be concluded that comet formation was not due to acute cytotoxic effects, but was predicated by mild TP-induced DNA damage. This was confirmed in both cell lines by elevated levels of apoptosis after BR, PRO and SB incubations (Supplementary material 2; Figs. 3 and 4A–C), as well as by the lack of positive correlation between DNA-damage and necrosis (Table 2). These data suggest that intestinal BPs induce non-acute toxicity in malignant hepatic and intestinal cancer cells in vitro.
Fig. 3.
Effects of 24 h PRO (A), UB (B) and SB (C) incubation in Caco2 cells, on cell cycle progression (PI-staining). PRO: protoporphyrin, UB: urobilin, SB: stercobilin; ⁎n: significantly different to negative control (p≤0.05), #n: tends to differ from negative control (p≤0.1).
Fig. 4.
Effects of 24 h PRO (A), UB (B) and SB (C) incubation in HepG2 cells, on cell cycle progression (PI-staining). PRO: protoporphyrin, UB: urobilin, SB: stercobilin; ⁎n: significantly different to negative control (p≤0.05), #n: tends to differ from negative control (p≤0.1).
Single- and double-strand DNA breaks represent critical damage to healthy cells [30]. Whilst mild impairment can yet activate innate repair mechanisms, severe nucleotide breaks can trigger cell death including apoptosis and/or tumorigenesis [40,41], the latter resulting from genetic amplification or translocation processes leading to oncogene activation [30]. In contrast, radiation [42] and chemotherapeutic treatment can overwhelm cellular repair systems by inducing the production of ROS, which attack and fragment cancer cell DNA, and can cause apoptosis in tumor cells [43]. Cell survival after imperfect repair of DNA-damage on the other hand, can lead to carcinogenesis through mutations, and the formation of many tumors has been associated with the inhibition of apoptosis [44,45].
Bile pigments (particularly BR) are known for their antioxidant activity at low to moderate concentrations; however, they can possess pro-oxidant activity at high concentrations [6,46]. Furthermore it is important to note that the ratio of BR to albumin represents an important determinant of ‘free’ BR [47]. This concentration influences the degree to which unbound BR can diffuse into cells [48] and interact with the mitochondrial membrane, where it may induce depolarization leading to apoptosis [17]. The average fecal BR concentration is estimated at 42 μM, a considerable proportion of which however is assumingly unavailable for cellular absorption due to binding and precipitation [17]. It is currently unknown whether also related TPs interact with albumin and how they interact with cells within the human organism. Clearly, further pharmacokinetic and bio-distribution studies are required to reveal the in vivo fate and consequences of these molecules [15,16].
In the present study, BR provoked intracellular ROS accumulation (Table 2). This result had been observed previously [27], and BR’s anti-cancer behavior had been discussed with reference to a pro-oxidant effect. Particularly with regard to BR, observed toxic or pro-oxidant effects in HepG2 cells seen in the present study (Table 2; online Supplementary material 2) can probably be explained by a relatively higher “free” BR concentration in the cell culture media administered to HepG2 cells (10% FBS addition), compared to the higher albumin binding capacity present in Caco2 cell media (20% FBS addition). It is likely that the mildly elevated “free” bilirubin concentration resulted in BR absorption [48]into the mitochondrial membrane, leading to mitochondrial dysfunction and increased superoxide and hydrogen peroxide production, by interfering with mitochondrial cytochrome function [49]. At a BR concentration of 17 μM, calculated BRf levels in both cell lines (10% and 20% FBS addition; data not shown) clearly exceeded 70 nM (by orders of 2.3–60). Concentrations above this are associated with the induction of apoptosis [39]. Therefore, we hypothesize that BR, despite its known antioxidant potential, cannot neutralize increased production of reactive oxygen species, during mitochondrial depolarization/dysfunction.
As initially stated, DNA-damage is frequently associated with increased ROS production. With relevance to ROS-induced effects, correlations between DNA-damage and ROS formation (superoxide) were found in PRO and BR conditions in HepG2, as well as in BV and BR conditions in Caco2 cells (hydroperoxide). This emphasizes the compounds’ potential role in causing ROS-mediated cell death in cancer cells. Elevated oxidative stress after PRO administration in rat hepatocytes had also been reported earlier, in which elevated superoxide formation had been assumed based upon an elevated superoxide dismutase (SOD) activity [50]. Elevated superoxide radical formation has also been detected in vitro after PRO administration using spin trapping techniques [51].
With the remaining TPs (BRDT, UB, SB, and BR-/BV-DME) no relationships with ROS formation were found in this study. These data suggest that these compounds either induced DNA-damage directly by interfering with DNA or associated molecules, or that radical species other than those measured, account for the observed DNA-damage. In some cases (PRO and BR in HepG2 cells), ROS formation was correlated to apoptosis, which supports a role for the induction of oxidation by certain BPs, leading to cancer cell death via activation of apoptotic pathways.
Information regarding the regulation of cell cycle (particularly G0/1; Figs. 3 and 4A–C) provides further important data for mechanistically explaining cytotoxic results reported in the comet assay. Comet formation in both cell lines, and specifically in HepG2 cells after SB, UB and PRO incubations (Figs. 1 and 2A–C) are supported by an accumulation of cells in G0/1 phase and an increase in apoptosis, measured using annexin V-staining (Figs. 3 and 4A–C). This conclusion is particularly evident for SB-induced DNA-damage, which is supported by increased percentage of cells in G0/1 and sub G0/1 (apoptosis) phases. With reference to these data, SB had the strongest effects on G0/1 cell cycle accumulation and apoptosis in both cell lines. In general, SB and PRO-induced more pronounced effects especially in Caco2 cells, whereas UB’s effects on apoptosis were comparatively mild (Fig. 3A–C).
In summary, the current results indicate that BPs such as PRO, BR and BV induce radical formation that is related to DNA-damage, which was associated with increased apoptosis (PRO, BR). Since correlations with ROS formation (superoxide and hydroperoxide) were not found in all test conditions, it is also possible that other radical species had been formed such as carbon/nitrogen-centered radicals, which were not detected by the assays utilized in this study. Generally speaking, DNA-damage was induced by TPs leading to elevated ROS production, which agrees with a possible mechanism of DNA damage proposed by Goodsell [43]. In this model, reactive forms of oxygen attack and differently fragment cancer cell DNA involving single- and double-strand breaks, and lead to cell death via cell cycle arrest and subsequent apoptosis induction.
In normally proliferating lymphocyte cultures, used as a model of healthy human tissue, no increased micronuclei [13] and comet formations (submitted elsewhere) in the presence of mildly elevated circulating BR have been found recently by our work-group. These data further support the possibility of toxicity and DNA-damaging effects of BPs/derivatives specifically in cancer cells, and emphasize the role moderately elevated BR levels play in (gastrointestinal) carcinogenesis, as was reported in a representative epidemiological US study [14]. A 1 mg/dl increment in serum BR was associated with a reduced lifetime prevalence of gastrointestinal and colorectal cancer, with odds ratios of 0.186 in women and 0.295 in men [14].
Conclusions
Bile pigments with structurally related TPs are reported to possess anti-mutagenic and antioxidant effects in vitro, and to be of physiological importance by inhibiting oxidative stress in vivo and thereby preventing chronic disease [9]. However, data concerning the potential toxic effects of these compounds in cancer cells (beyond BR and PRO) are lacking. This study revealed substantial DNA-damaging effects of tetrapyrrolic compounds in human cancer cells, particularly for PRO, UB and SB in HepG2 and for PRO in Caco2 cell lines. From this evidence, a chemopreventive effect of the compounds might exist within the liver and intestine, as has been suggested in epidemiological studies [14,52,53]. Possible underlying mechanisms of DNA-damage (e.g. including ROS formation), leading to cell cycle arrest and subsequent apoptosis induction, might be responsible for the toxicity observed in malignant cells.
Subsequent future experiments could focus on clarifying the effects of TPs also in non-malignant cells as well as the pigments’ DNA-damaging potential in a range of various other cancer cell lines. Furthermore, exploring the effects of TPs also on chromosomal stability (e.g. prevention of micronuclei formation) could represent a valuable future scientific approach.
Declaration of interest
The authors declare that there are no conflicts of interest.
Funding and Acknowledgments
This project (Grant no. P21162-B11) was supported by the Austrian Science Fund (FondszurFörderung der wissenschaftlichen Forschung, FWF). The authors would like to thank Dr. Silvia Gazzin and Prof. Claudio Tiribelli for sharing their expertise and resources in calculating BRf levels.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.yexcr.2012.12.003.
Appendix A. Supplementary materials
Figure S1.
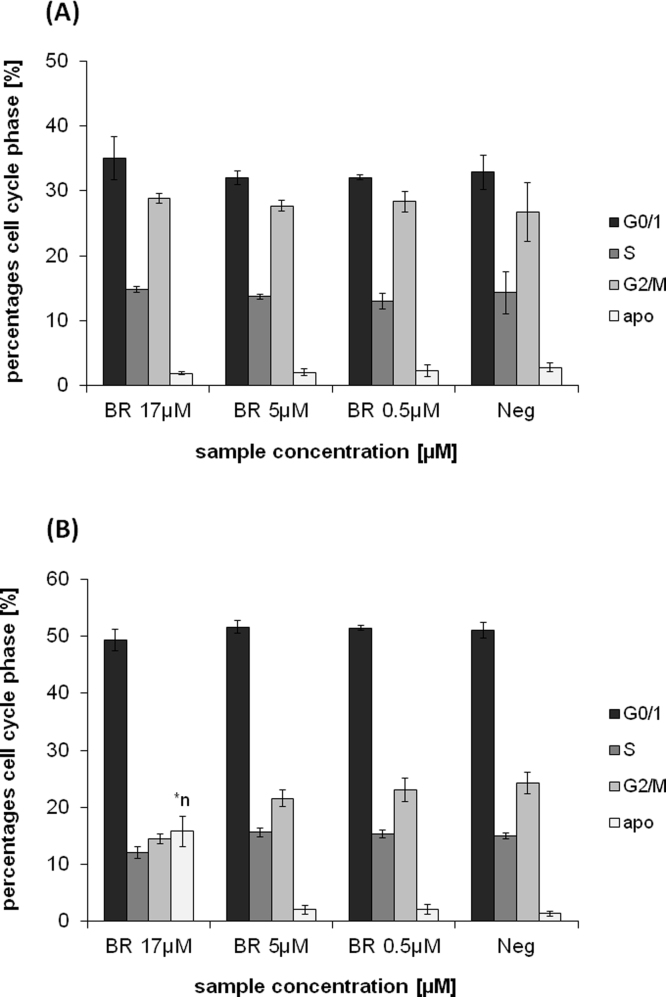
Effects of 24 h BR incubation in Caco2 (A) and HepG2 (B) cell lines, on cell cycle progression (PI-staining). BR: unconjugated bilirubin; *n: significantly different to negative control (p<0.05).
Figure S2.
Chemical structures of the test compounds.
References
- 1.Odin A.P. Antimutagenicity of the porphyrins and non-enzyme porphyrin-containing proteins. Mutat. Res. 1997;387:55–68. doi: 10.1016/s1383-5742(97)00023-9. [DOI] [PubMed] [Google Scholar]
- 2.Blanc D. Vol. 12. Blackwell Publishing Ltd; Paris, France: 1987. (Abstracts from the 13th Annual Meeting of the Society for Cutaneous Ultrastructure Research and the European Society for Comparative Skin Biology, -Part I, 13th Annual Meeting of the Society for Cutaneous Ultrastructure Research and the European Society for Comparative Skin Biology). pp. 224–237. [Google Scholar]
- 3.Klatskin G. Bile pigment metabolism. Annu. Rev. Med. 1961;12:211–250. doi: 10.1146/annurev.me.12.020161.001235. [DOI] [PubMed] [Google Scholar]
- 4.Bulmer A.C., Ried K., Blanchfield J.T., Wagner K.H. The anti-mutagenic properties of bile pigments. Mutat. Res. 2008;658:28–41. doi: 10.1016/j.mrrev.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Bulmer A.C., Ried K., Coombes J.S., Blanchfield J.T., Toth I., Wagner K.H. The anti-mutagenic and antioxidant effects of bile pigments in the Ames Salmonella test. Mutat. Res. 2007;629:122–132. doi: 10.1016/j.mrgentox.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 6.Stocker R., Glazer A.N., Ames B.N. Antioxidant activity of albumin-bound bilirubin. Proc. Natl. Acad. Sci. U.S.A. 1987;84:5918–5922. doi: 10.1073/pnas.84.16.5918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mölzer C., Huber H., Diem K., Wallner M., Bulmer A.C., Wagner K.H. Extracellular and intracellular anti-mutagenic effects of bile pigments in the Salmonella typhimurium reverse mutation assay. Toxicol. in Vitro. 2012 doi: 10.1016/j.tiv.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mölzer C., Huber H., Steyrer A., Ziesel G., Wallner M., Ertl A., Plavotic A., Bulmer A., Wagner K.-H. In vitro antioxidant capacity and anti-genotoxic properties of protoporphyrin and structurally related tetrapyrroles. Free Radical Res. 2012 doi: 10.3109/10715762.2012.715371. [DOI] [PubMed] [Google Scholar]
- 9.Boon A.C., Hawkins C.L., Bisht K., Coombes J.S., Bakrania B., Wagner K.H., Bulmer A.C. Reduced circulating oxidized LDL is associated with hypocholesterolemia and enhanced thiol status in Gilbert syndrome. Free. Radical Biol. Med. 2012;52:2120–2127. doi: 10.1016/j.freeradbiomed.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bulmer A.C., Blanchfield J.T., Toth I., Fassett R.G., Coombes J.S. Improved resistance to serum oxidation in Gilbert’s syndrome: a mechanism for cardiovascular protection. Atherosclerosis. 2008;199:390–396. doi: 10.1016/j.atherosclerosis.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 11.Horsfall L.J., Rait G., Walters K., Swallow D.M., Pereira S.P., Nazareth I., Petersen I. Serum bilirubin and risk of respiratory disease and death. JAMA. 2011;305:691–697. doi: 10.1001/jama.2011.124. [DOI] [PubMed] [Google Scholar]
- 12.Vitek L. Impact of serum bilirubin on human diseases. Pediatrics. 2005;115:1411–1412. doi: 10.1542/peds.2004-1796. [DOI] [PubMed] [Google Scholar]
- 13.Wallner M., Blassnigg S.M., Marisch K., Pappenheim M.T., Müllner E., Mölzer C., Nersesyan A., Marculescu R., Doberer D., Knasmüller S., Bulmer A.C., Wagner K.-H. Effects of unconjugated bilirubin on chromosomal damage in individuals with Gilbert’s syndrome measured with the micronucleus cytome assay. Mutagenesis. 2012 doi: 10.1093/mutage/ges039. [DOI] [PubMed] [Google Scholar]
- 14.Zucker S.D., Horn P.S., Sherman K.E. Serum bilirubin levels in the U.S. population: gender effect and inverse correlation with colorectal cancer. Hepatology. 2004;40:827–835. doi: 10.1002/hep.20407. [DOI] [PubMed] [Google Scholar]
- 15.Bulmer A.C., Blanchfield J.T., Coombes J.S., Toth I. In vitro permeability and metabolic stability of bile pigments and the effects of hydrophilic and lipophilic modification of biliverdin. Bioorg. Med. Chem. 2008;16:3616–3625. doi: 10.1016/j.bmc.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 16.Bulmer A.C., Coombes J.S., Blanchfield J.T., Toth I., Fassett R.G., Taylor S.M. Bile pigment pharmacokinetics and absorption in the rat: therapeutic potential for enteral administration. Br. J. Pharmacol. 2011;164:1857–1870. doi: 10.1111/j.1476-5381.2011.01413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keshavan P., Schwemberger S.J., Smith D.L., Babcock G.F., Zucker S.D. Unconjugated bilirubin induces apoptosis in colon cancer cells by triggering mitochondrial depolarization. Int. J. Cancer. 2004;112:433–445. doi: 10.1002/ijc.20418. [DOI] [PubMed] [Google Scholar]
- 18.Stocker R., Yamamoto Y., McDonagh A.F., Glazer A.N., Ames B.N. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- 19.Ollinger R., Kogler P., Troppmair J., Hermann M., Wurm M., Drasche A., Konigsrainer I., Amberger A., Weiss H., Ofner D., Bach F.H., Margreiter R. Bilirubin inhibits tumor cell growth via activation of ERK. Cell Cycle. 2007;6:3078–3085. doi: 10.4161/cc.6.24.5022. [DOI] [PubMed] [Google Scholar]
- 20.Ethirajan M., Chen Y., Joshi P., Pandey R.K. The role of porphyrin chemistry in tumor imaging and photodynamic therapy. Chem. Soc. Rev. 2011;40:340–362. doi: 10.1039/b915149b. [DOI] [PubMed] [Google Scholar]
- 21.Sznarkowska A., Malenczyk K., Kadzinski L., Bielawski K.P., Banecki B., Zawacka-Pankau J. Targeting of p53 and its homolog p73 by protoporphyrin IX. FEBS Lett. 2011;585:255–260. doi: 10.1016/j.febslet.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 22.Zawacka-Pankau J., Kowalska A., Issaeva N., Burcza A., Kwiek P., Bednarz N., Pramanik A., Banecki B., Podhajska A.J. Tumor suppressor Fhit protein interacts with protoporphyrin IX in vitro and enhances the response of HeLa cells to photodynamic therapy. J. Photochem. Photobiol. B. Biol. 2007;86:35–42. doi: 10.1016/j.jphotobiol.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 23.Bednarz N., Zawacka-Pankau J., Kowalska A., Protoporphyrin IX. induces apoptosis in HeLa cells prior to photodynamic treatment. Pharmacol. Rep.: PR. 2007;59:474–479. [PubMed] [Google Scholar]
- 24.Grimm S., Mvondo D., Grune T., Breusing N. The outcome of 5-ALA-mediated photodynamic treatment in melanoma cells is influenced by vitamin C and heme oxygenase-1. Biofactors. 2011;37:17–24. doi: 10.1002/biof.129. [DOI] [PubMed] [Google Scholar]
- 25.Hovhannisyan G., Aroutiounian R., Ghazaryan R., Gevorgyan A., Margaryan K., Haroutiunian S. DNA damage induced by new porphyrins of different chemical structure. Korean J. Environ. Biol. 2005:379–382. [Google Scholar]
- 26.Zawacka-Pankau J., Issaeva N., Hossain S., Pramanik A., Selivanova G., Podhajska A.J., Protoporphyrin IX. interacts with wild-type p53 protein in vitro and induces cell death of human colon cancer cells in a p53-dependent and -independent manner. J. Biol. Chem. 2007;282:2466–2472. doi: 10.1074/jbc.M608906200. [DOI] [PubMed] [Google Scholar]
- 27.Rao P., Suzuki R., Mizobuchi S., Yamaguchi T., Sasaguri S. Bilirubin exhibits a novel anti-cancer effect on human adenocarcinoma. Biochem. Biophys. Res. Commun. 2006;342:1279–1283. doi: 10.1016/j.bbrc.2006.02.074. [DOI] [PubMed] [Google Scholar]
- 28.Hedstrom E., Issaeva N., Enge M., Selivanova G. Tumor-specific induction of apoptosis by a p53-reactivating compound. Exp. Cell Res. 2009;315:451–461. doi: 10.1016/j.yexcr.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 29.Vitek L., Ostrow J.D. Bilirubin chemistry and metabolism; harmful and protective aspects. Curr. Pharm. Des. 2009;15:2869–2883. doi: 10.2174/138161209789058237. [DOI] [PubMed] [Google Scholar]
- 30.Mills K.D., Ferguson D.O., Alt F.W. The role of DNA breaks in genomic instability and tumorigenesis. Immunol. Rev. 2003;194:77–95. doi: 10.1034/j.1600-065x.2003.00060.x. [DOI] [PubMed] [Google Scholar]
- 31.Brower J.O., Lightner D.A., McDonagh A.F. Aromatic congeners of bilirubin: synthesis, stereochemistry, glucuronidation and hepatic transport. Tetrahedron. 2001;57:7813–7827. [Google Scholar]
- 32.Azqueta A., Shaposhnikov S., Collins A.R. DNA oxidation: investigating its key role in environmental mutagenesis with the comet assay. Mutat. Res. 2009;674:101–108. doi: 10.1016/j.mrgentox.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 33.Schmid I., Krall W.J., Uittenbogaart C.H., Braun J., Giorgi J.V. Dead cell discrimination with 7-amino-actinomycin D in combination with dual color immunofluorescence in single laser flow cytometry. Cytometry. 1992;13:204–208. doi: 10.1002/cyto.990130216. [DOI] [PubMed] [Google Scholar]
- 34.Vermes I., Haanen C., Steffens-Nakken H., Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labeled Annexin V. J. Immunol. Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 35.Cai L., Wang J., Li Y., Sun X., Wang L., Zhou Z., Kang Y.J. Inhibition of superoxide generation and associated nitrosative damage is involved in metallothionein prevention of diabetic cardiomyopathy. Diabetes. 2005;54:1829–1837. doi: 10.2337/diabetes.54.6.1829. [DOI] [PubMed] [Google Scholar]
- 36.Zhou X., Ferraris J.D., Cai Q., Agarwal A., Burg M.B. Increased reactive oxygen species contribute to high NaCl-induced activation of the osmoregulatory transcription factor TonEBP/OREBP. Am. J. Physiol. Renal Physiol. 2005;289:F377–385. doi: 10.1152/ajprenal.00463.2004. [DOI] [PubMed] [Google Scholar]
- 37.H. Shapiro, Practical Flow Cytometry, Alan, R, New York, 1988.
- 38.Darzynkiewicz Z. Wiley & Sons; New York: 1997. Current Protocols in Cytometry. [Google Scholar]
- 39.Ostrow J.D., Pascolo L., Tiribelli C. Reassessment of the unbound concentrations of unconjugated bilirubin in relation to neurotoxicity in vitro. Pediatr. Res. 2003;54:926. doi: 10.1203/01.PDR.0000103388.01854.91. [DOI] [PubMed] [Google Scholar]
- 40.Migliore L., Coppede F. Genetic and environmental factors in cancer and neurodegenerative diseases. Mutat. Res. 2002;512:135–153. doi: 10.1016/s1383-5742(02)00046-7. [DOI] [PubMed] [Google Scholar]
- 41.Pages V., Fuchs R.P., How DNA. lesions are turned into mutations within cells? Oncogene. 2002;21:8957–8966. doi: 10.1038/sj.onc.1206006. [DOI] [PubMed] [Google Scholar]
- 42.Park I., Avraham H.K. Cell cycle-dependent DNA damage signaling induced by ICRF-193 involves ATM, ATR, CHK2, and BRCA1. Exp. Cell. Res. 2006;312:1996–2008. doi: 10.1016/j.yexcr.2006.02.029. [DOI] [PubMed] [Google Scholar]
- 43.Goodsell D.S. The molecular perspective: double-stranded DNA breaks. The Oncologist. 2005;10:361–362. doi: 10.1634/theoncologist.10-5-361. [DOI] [PubMed] [Google Scholar]
- 44.Behn M., Qun S., Pankow W., Havemann K., Schuermann M. Frequent detection of ras and p53 mutations in brush cytology samples from lung cancer patients by a restriction fragment length polymorphism-based enriched PCR technique. Clin. Cancer Res. 1998;4:361–371. [PubMed] [Google Scholar]
- 45.Eisinger F., Jacquemier J., Charpin C., Stoppa-Lyonnet D., Bressac-de Paillerets B., Peyrat J.P., Longy M., Guinebretiere J.M., Sauvan R., Noguchi T., Birnbaum D., Sobol H. Mutations at BRCA1: the medullary breast carcinoma revisited. Cancer Res. 1998;58:1588–1592. [PubMed] [Google Scholar]
- 46.Chen Y.H., Hung S.C., Tarng D.C. Serum bilirubin links UGT1A1⁎28 polymorphism and predicts long-term cardiovascular events and mortality in chronic hemodialysis patients, Clin. J. Am. Soc. Nephrol.: CJASN. 2011;6:567–574. doi: 10.2215/CJN.06130710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Calligaris S.D., Bellarosa C., Giraudi P., Wennberg R.P., Ostrow J.D., Tiribelli C. Cytotoxicity is predicted by unbound and not total bilirubin concentration. Pediatr. Res. 2007;62:576–580. doi: 10.1203/PDR.0b013e3181568c94. [DOI] [PubMed] [Google Scholar]
- 48.Zucker S.D., Goessling W., Hoppin A.G. Unconjugated bilirubin exhibits spontaneous diffusion through model lipid bilayers and native hepatocyte membranes. J. Biol. Chem. 1999;274:10852–10862. doi: 10.1074/jbc.274.16.10852. [DOI] [PubMed] [Google Scholar]
- 49.Mustafa M.G., Cowger M.L., King T.E. Effects of bilirubin on mitochondrial reactions. J. Biol. Chem. 1969;244:6403–6414. [PubMed] [Google Scholar]
- 50.Afonso S., Vanore G., Batlle A. Protoporphyrin IX and oxidative stress. Free Radical Res. 1999;31:161–170. doi: 10.1080/10715769900300711. [DOI] [PubMed] [Google Scholar]
- 51.Buettner G.R., Oberley L.W., Leuthauser S.W. The effect of iron on the distribution of superoxide and hydroxyl radicals as seen by spin trapping and on the superoxide dismutase assay. Photochem. Photobiol. 1978;28:693–695. doi: 10.1111/j.1751-1097.1978.tb07001.x. [DOI] [PubMed] [Google Scholar]
- 52.Jiraskova A., Novotny J., Novotny L., Vodicka P., Pardini B., Naccarati A., Schwertner H.A., Hubacek J.A., Puncocharova L., Smerhovsky Z., Vitek L. Association of serum bilirubin and promoter variations in HMOX1 and UGT1A1 genes with sporadic colorectal cancer. Int. J. Cancer. 2012;131:1549–1555. doi: 10.1002/ijc.27412. [DOI] [PubMed] [Google Scholar]
- 53.Ko W.-F., Helzlsouer K.J., Comstock G.W. Serum Albumin, bilirubin, and uric acid and the anatomic site-specific incidence of colon cancer. J. Nat. Cancer Inst. 1994;86:1874–1875. doi: 10.1093/jnci/86.24.1874. [DOI] [PubMed] [Google Scholar]