Abstract
Objective
Minimum radiographic joint space width (mJSW) represents the FDA standard for demonstrating structural therapeutic benefits for knee osteoarthritis (KOA), but only shows moderate responsiveness (sensitivity to change). We directly compare the responsiveness of MRI-based cartilage thickness and JSW measures from fixed-flexion radiography (FFR) and explore the correlation of region-matched changes between both methods.
Methods
967 knees of Osteoarthritis Initiative participants with radiographic KOA were studied: 445 over one year with coronal FLASH MRI and FFR, and 375/522 over one/two years with sagittal DESS MRI and FFR. Standardized response means (SRM) of cartilage thickness and mJSW were compared using the sign-test.
Results
With FLASH MRI, SRM was −0.28 for medial compartment (MFTC) cartilage loss vs. −0.15 for mJSW, and −0.32 vs. −0.22 for the most sensitive MRI subregion (central MFTC) vs. the most sensitive fixed location JSW(X=0.25). With DESS MRI, one-year SRM was −0.34 for MFTC vs. −0.22 for mJSW and −0.44 vs. −0.28 for central MFTC vs. JSW(X=0.225). Over two years, the SRM was significantly greater for MFTC than for mJSW (−0.43 vs. −0.31, p=0.017) and for central MFTC than for JSW(X=0.225) (−0.51 vs. −0.44, p<0.001). Correlations between changes in spatially matched MRI subregions and fixed location JSW were not consistently higher (r=0.10–0.51) than those between non-matched locations (r=0.15–0.50).
Conclusions
MRI displays greater responsiveness in KOA than JSW FFR-based JSW, with the greatest SRM observed in the central medial femorotibial compartment. Fixed-location radiographic measures appear not capable of determining the spatial distribution of femorotibial cartilage loss.
Keywords: Sensitivity to change, Radiography, Fixed Flexion, Magnetic resonance imaging, Knee osteoarthritis
INTRODUCTION
Quantification of structural disease progression in knee osteoarthritis (OA) is of great importance for evaluating risk factors for OA progression 1–3 and for evaluating the response to pharmacological and non-pharmacological treatment. Magnetic resonance imaging (MRI)–based measurement of cartilage morphology (e.g. cartilage volume, thickness, and subregional thickness) has been suggested to be more sensitive to change than radiographic measures of progression (e.g. increase in joint space narrowing [JSN] scores and reduction in joint space width [JSW]), and hence to be a more powerful tool for identifying risk factors and for evaluating therapeutic intervention. MRI is considered to be more specific to (regional) cartilage loss 4,5 than radiography as there is evidence that JSW change is strongly associated with meniscal pathology 6–8. Further, sensitivity to change in radiography critically depends on achieving optional medial tibia plateau alignment, which poses a considerable challenge in clinical studies 9–13.
The 1999 Food and Drug Administration (FDA) draft guidance, which has not been revised to date, considers radiographic JSW the reference standard for demonstrating benefits of therapeutic intervention in OA 14. Recently, the Osteoarthritis Research Society International (OARSI) published a series of articles as a response to questions raised by the FDA for revising the 1999 draft guidance document 15. As part of this OARSI FDA initiative15, responsiveness to change and reliability of radiographic JSW in knee OA was reviewed using the standardized response mean (SRM = mean change/standard deviation [SD] of change) as a measure of responsiveness to change 13 An overall pooled SRM of 0.33 (95% confidence interval [CI] 0.26/0.41, positive SRM values defined as sensitivity to decrease in JSW) was reported for 43 estimates with variable follow-up (mean sample size = 100). Responsiveness (SRM) was 0.24 for studies with less than 1 year follow-up, 0.25 for those with 1–2 years of follow-up, and 0.57 for those with >2 years of follow-up. In parallel, the responsiveness of MRI was reviewed 16 and a pooled SRM for quantitative cartilage morphometry of the medial femorotibial compartment of −0.86 (95% CI −1.26/−0.46, negative SRM values defined as sensitivity to decrease in cartilage thickness) was reported from 31 estimates with variable follow-up (mean sample size = 92). Substantial differences in SRMs were noted between earlier (published before 2007) and more recent studies (2007 to 2009), and between different cartilage regions of the knee16.
The direct comparability of SRMs between radiography and MRI from these reviews is limited, because both the cohort composition (radiographic stage of knee OA) and the follow-up time, which differ between studies, critically impact the observed rates of change and SRMs 17. Only few studies have directly compared the rate of change and the sensitivity to change between radiography and MRI in the same knees and over the same observation period18–20 and these have been conducted in rather small samples. Further, there have been recent innovations in the standardization of radiographic acquisition techniques 9–11, computerized and standardized (location-specific) JSW measurement of radiographs 21, MRI sequence and magnet development 11,22, and subregional measures of cartilage change with MRI 23–25, that have not been accounted for in the OARSI FDA initiative literature review, which included literature of up to 2009.
The objective of the current study therefore was to directly compare the responsiveness of minimum and location-specific JSW measures of standardized fixed-flexion radiographs (FFR) with compartment-level and subregional cartilage thickness measures obtained from 3Tesla MRI sequences in the medial compartment of the same knees selected from the Osteoarthritis Initiative (OAI). Specifically, we stratified the relative responsiveness of MRI versus FFR between different follow-up periods (one- and two years), radiographic disease stages (Kellegren Lawrence grades 26), and MRI acquisition protocols (coronal FLASH, sagittal DESS). Further, we studied the correlation between location-specific JSW FFR measurements and anatomically corresponding subregional cartilage thickness change from MRI, to explore whether radiography is capable of assessing the spatial distribution of cartilage loss within the medial femorotibial joint space.
METHODS
Sample selection
OAI participants were aged 45 to 79 years at study start, had no contraindications to 3T MR imaging, had at most unilateral end-stage knee OA, had no rheumatoid or other inflammatory arthritis, and were able to walk without aids. Please see http://oai.epi-ucsf.org/datarelease/docs/StudyDesignProtocol.pdf for detailed inclusion and exclusion criteria of the OAI.
We used knees with definite radiographic OA (defined equivalent to a Kellgren and Lawrence grade [KLG] 26 >=2 as definite tibiofemoral osteophyte with or without joint space narrowing,) for which quantitative measurements of medial JSW (funded by the OAI for public use) and quantitative MRI measurements were available. Knees analysed at baseline and year one using coronal FLASH MRI were previously selected as part of a consortium-initiative of private sponsors focusing on knees at advanced stages of radiographic OA (please see 27,28 for a detailed description of the selection criteria). The analysis of knees using sagittal DESS MRI was funded by the OAI. Knees in the DESS sample were selected by the OAI coordinating center from the OAI progression subcohort to form a “core image assessment cohort” and included only knees with frequent symptoms and KLG 2 or 3 in site readings at baseline 17,28,29. Baseline cartilage measurements for the FLASH sample, baseline and follow-up cartilage measurements for the DESS sample, and quantitative JSW measurements for both samples are available at http://oai.ucsf.edu/datarelease/. The analyses in the present study classified the baseline OA status of knees using the KL grades from the OAI-sponsored central radiographic readings30 instead of the KL readings performed at the OAI clinical sites during enrollment.
A complete set of semi-quantitative radiographic readings at baseline 31, medial JSW measurements, and subregional cartilage thickness measurements (MRI) were available for a total of 1080 knees from the OAI progression subcohort (520 with FLASH MRI [baseline and one year follow-up] and 560 with DESS MRI [baseline and two year follow-up, and 508 of these also with one-year follow-up]). Knees were excluded if the length of the observation periods differed by ≥ 45 days (one year follow-up, FLASH: n=75 or DESS sample: n=101) or by ≥ 90 days (two year follow-up, DESS sample: n=29) between radiography and MR imaging. When data on both knees from the same participant was available, the knee with the less severe KL grade or the left knee was excluded (DESS sample one/two year follow-up: n=8/9), because longitudinal changes in both knees of the same participant may not be independent. This selection resulted in a total of 967 knees (445 FLASH with one year, 522 DESS with two year, of which 375 DESS also had one year follow-up).
Imaging
As part of the OAI image acquisitions, bilateral FFR was performed annually using a SynaFlexer™ frame (Synarc, Inc., San Francisco, CA) 32–34. The OAI knee MRI protocol included sagittal DESS (in-plane resolution: 0.37 × 0.46mm interpolated to 0.37 × 0.37mm, slice thickness: 0.7mm, repetition time: 16.3ms, echo time: 4.7ms, flip angle: 25°) and coronal FLASH MRI data (in-plane resolution: 0.31 × 0.31mm, slice thickness: 1.5mm, repetition time: 20ms, echo time: 7.57ms, flip angle: 12°), both with water excitation, that were acquired annually using 3 Tesla MRI scanners (Siemens Trio, Siemens, Erlangen, Germany) and quadrature transmit-receive knee coils 22,35. The MR sequences were planned parallel to the long axis of the femoral diaphysis and either parallel (coronal FLASH) or perpendicular (sagittal DESS) to the line tangent to the posterior cortices of the femoral condyles 22,29.
Image analysis
The minimum JSW in the medial femorotibial compartment was measured in the digitized bilateral baseline, one, and two year follow-up FFRs using an automated software application 19,21,36. In addition, fixed distance measures of the JSW were obtained between the external and internal border of the medial femorotibial compartment. To that end, the software automatically determined the tangent lines to the femoral condyles, which represented the x-axis (external to internal, internal = adjacent to the intercondylar notch) of the coordinate system. The medial and lateral borders of the knee were marked manually, perpendicular to this x-axis and tangential to the greatest prominence of the medial and the lateral femoral epicondyles (Figure 1). After normalization to the range between 0 (medial epicondyle) and 1 (lateral epicondyle), the x-axis was used to define the fixed locations, and JSW(x) measurements were performed between x=0.15 (external) and x=0.30 (internal) for the medial femorotibial compartment according to a coordinate system defined elsewhere19. The output was verified by an expert reader (JD), and corrected if needed. Because lateral JSW measurements were only available for parts of the cohort and because JSW measurements were reported to reliably measure the cartilage thickness in the medial but not in the lateral compartment 37, this study included only medial compartment measurements.
Figure 1.
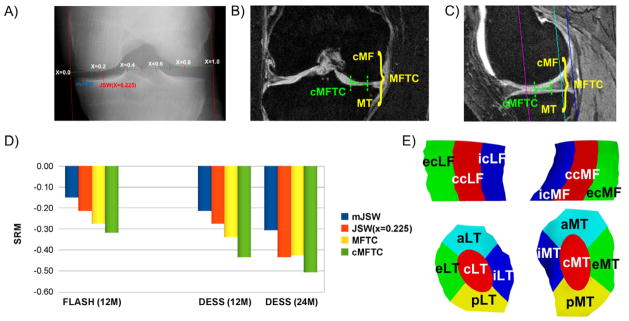
A) Illustration showing the radiography-based measurement of the minimal joint space width (mJSW) and of the joint space width at the central fixed location JSW(x=0.225). B) Illustration showing the coronal FLASH MRI-based measurement in the medial femorotibial compartment (MFTC) and in the most sensitive subregion of the MFTC (cMFTC = central subregion of MFTC). C) Illustration showing the sagittal DESS MRI-based measurement in the MFTC and in the most sensitive subregion of the MFTC (cMFTC). D) Bar graph showing the sensitivity to change (standardized response mean = SRM = mean change/standard deviation of the change) for mJSW, MFTC, and the most sensitive fixed location JSW(x) in the FLASH sample over 12 months (12M, JSW(x)=JSW(x=0.25)), in the DESS sample over 12M (JSW(x)=JSW(x=0.225)), and in the DESS sample over 24 months (24M, JSW(x)=JSW(x=0.225)). The DESS sample over 12 months is a sub-sample of the 24 months cohort, for which a complete set of 12 months measurements was available. E) Illustration showing the central (c), external (e), internal (i), anterior (a), and posterior subregions (p) computed in the medial (MT) and lateral tibia (LT) and in the central, weight-bearing part if the medial (cMF) and lateral (cLF) femoral condyle (only central, external, and internal subregions).
MRI-based cartilage thickness measurements were computed from segmentations of the weight-bearing femorotibial cartilage plates that were performed by fourteen experienced operators with blinding to the time of acquisition and to the baseline radiographic readings 38 (Figure 1). All segmentations underwent quality control by an expert reader and were corrected by the operators, if necessary. Cartilage thickness over the total area of subchondral bone (ThCtAB) was computed in the medial femorotibial cartilage plates (MT and cMF), the medial femorotibial compartment (MFTC=MT+cMF), and in 8 medial femorotibial subregions (5 in MT and 3 in cMF; Figure 1) 24. In addition to the individual subregions (Figure 1), cartilage thickness measurements in central, external and internal subregions of the medial tibia and central, external and internal (internal = adjacent to the intercondylar notch) subregions of the central, weight-bearing part of the medial femoral condyle were added to combined central (cMFTC), external (eMFTC), and internal (iMFTC) femorotibial subregions. Based on previous findings that reported similar responsiveness 39, the current analysis relied on the segmentation of every second slice of the DESS (1.4mm intervals).
Statistical analysis
The mean change (MC), standard deviation (SD) of change, and 95% confidence intervals of change were determined for JSW (FFR) and cartilage thickness measures (MRI). Percent changes were computed as 100 * MC [μm]/(Mean baseline value [μm]) for each sample. The standardized response mean (SRM=MC/SD of change) was used to describe the responsiveness (=sensitivity to change), because it has been widely used in quantitative OA studies and can therefore be easily compared between studies. The correlation of changes between the two imaging methodologies was calculated using parametric (Pearson r) correlation coefficients. For the analysis of location-specific correlations, the seven fixed-distance measures between JSW(x=0.150) and JSW(x=0.300) were partitioned into two external (JSW(x=0.150) and JSW(x=0.175)), three central (JSW(x=0.200), JSW(x=0.225), and JSW(x=0.250)), and two internal (JSW(x=0.275) and JSW(x=0.300)) measures. The maximum correlation observed between matched locations (external JSW vs. eMFTC, central JSW vs. cMFTC, internal JSW vs. iMFTC) was compared to the maximum correlation observed for non-matched locations.
To assess whether the SRM differed significantly between FFR-based JSW and MRI-based cartilage thickness, the observed changes in each knee were scaled by the standard deviation of the changes among all knees in each of the DESS and FLASH subsamples. A two-sided sign-test was then applied to the difference between these standardized changes (MRI cartilage thickness – radiographic JSW), to determine whether the number of positive or negative differences was significantly greater than expected by chance. Because this result depends on the estimated SD, bootstrapped (n=100,000) samples of both cohorts were generated and randomization tests (randomly inverting the sign of the scaled differences) were carried out to account for the uncertainty in the estimate of the SD of change in testing for differences between SRM of change in JSW and cartilage thickness. P-values were estimated as the proportion of p-values from bootstrapped sign tests as small as the one computed from the observed results. These tests were applied to compare the responsiveness between FFR-based mJSW and MRI-based cartilage thickness in MFTC, and between the most sensitive fixed location measure (FFR) and the most sensitive subregion (MRI) within each sample. The required significance level (p<0.05) was adjusted (p<0.025) to account for these two parallel comparisons within each sample (mJSW vs MFTC and most sensitive fixed location (JSW) vs. most sensitive subregion (MRI)). Because the objective of the study was not to test for significant differences in either one of the two samples, but to see whether the results were consistent in both samples, we did not correct for the analysis for two samples. Further, no correction was made for analyzing two observation periods, because these were considered complimentary and results were not interpreted independently or in isolation.
RESULTS
The FLASH sample comprised 445 knees from 281 women and 164 men (age [mean±SD]: 63.2±9.4 years, BMI: 30.1±4.7kg/m2). Of these knees, 255 were KLG 2, 135 KLG 3, and 55 KLG 4 (Table 1). The DESS two year sample comprised 522 knees from 300 women and 222 men (age: 61.2±8.8 years, BMI: 30.3±5.0 kg/m2). Of these knees, 256 were KLG 2, 261 KLG 3, and 5 KLG 4 (Table 1). The participants in the DESS sample for whom one-year follow-up data were available, contained 375 knees from 209 women and 166 men (age: 61.0±8.9 years, BMI: 30.2±5.1 kg/m2, Table 1). When compared to the entire OAI progression cohort (Table 1), the FLASH sample contained a larger proportion of female participants (63% vs. 57%) and the participants in the FLASH sample were on average 1.8 years older, 1.4 cm shorter and of lower weight (−1.6 kg). There were only marginal differences between the DESS sample and the entire OAI progression cohort with regard to gender, age, BMI, height, or weight (Table 1).
Table 1.
Baseline (BL) demographics, observation period, Kellgren and Lawrence grades (KLG), and joint-space narrowing scores (JSN) in the medial femorotibial compartment (MFTC) for the coronal FLASH sample, the sagittal DESS sample, and the entire OAI progression cohort as reference.
| FLASH sample | DESS sample (12M) | DESS sample (24M) | OAI Progression Cohort | ||
|---|---|---|---|---|---|
| N | 445 | 375 | 522 | 1390 | |
| Gender | Women (%) | 281 (63.1%) | 209 (55.7%) | 300 (57.5%) | 793 (57.1%) |
| Men (%) | 164 (36.9%) | 166 (44.3%) | 222 (42.5%) | 597 (42.9%) | |
| Side | Left (%) | 2 (0.4%) | 186 (49.6%) | 261 (50.0%) | |
| Right (%) | 443 (99.6%) | 189 (50.4%) | 261 (50.0%) | ||
| Age (years) | Average | 63.2 | 61.0 | 61.2 | 61.4 |
| SD | 9.4 | 8.9 | 8.8 | 9.1 | |
| BMI (kg/m^2) | Average | 30.1 | 30.2 | 30.3 | 30.2 |
| SD | 4.7 | 5.1 | 5.0 | 4.9 | |
| Height (cm) | Average | 167.3 | 168.6 | 168.7 | 168.7 |
| SD | 8.8 | 9.0 | 9.2 | 9.3 | |
| Weight (kg) | Average | 84.6 | 86.1 | 86.3 | 86.2 |
| SD | 16.0 | 16.1 | 16.0 | 16.3 | |
| KLG | 0 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 275.0 (21.5%) |
| 1 | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 173.0 (13.5%) | |
| 2 | 255 (57.3%) | 182 (48.5%) | 256 (49.0%) | 479.0 (37.5%) | |
| 3 | 135 (30.3) | 188 (50.1%) | 261 (50.0%) | 357.0 (27.9%) | |
| 4 | 55 (12.4%) | 5 (1.3%) | 5 (1.0%) | 106.0 (8.3%) | |
| medial JSN | 0 | 150 (33.7%) | 130 (34.7%) | 181 (34.7%) | 707.0 (55.3%) |
| 1 | 154 (34.6%) | 101 (26.9%) | 140 (26.8%) | 346.0 (27.1%) | |
| 2 | 102 (22.9%) | 141 (37.6%) | 198 (37.9%) | 266.0 (20.8%) | |
| 3 | 39 (8.8%) | 3 (0.8%) | 3 (0.6%) | 71 (5.6%) | |
| BL mJSW (mm) | Average | 3.7 | 3.9 | 3.9 | 4.0 |
| SD | 1.6 | 1.5 | 1.5 | 1.5 | |
| BL ThC (mm) | Average | 3.3 | 3.4 | 3.4 | N/A |
| SD | 0.8 | 0.7 | 0.7 | N/A |
All knees in the FLASH and the DESS sample had definite radiographic OA at baseline (BL), defined as Kellgren and Lawrence grade (KLG) >= 2 (obtained from central readings). SD = standard deviation. BMI = Body Mass Index. mJSW = minimum joint space width, ThC = Thickness of cartilage. 12M = 12 months follow-up, 24M = 24 months follow-up.
Height measurements were missing for ten participants from the FLASH sample and for ten participants from the DESS sample. Weight and BMI measurements were missing for one participant from the FLASH sample and for one participant from the DESS sample. FFR = fixed-flexion radiography
Mean change (in %), the SD of change (in %), and the SRMs for FFR-based JSW and MRI-based cartilage thickness are shown in Tables 2 and 3. Mean change (in μm), SD of change (in μm), and 95% confidence intervals of change (in μm) are shown in Online Tables 1 and 2 (in μm). The fixed location measure JSW(X=0.25) was the most responsive FFR measure in the FLASH sample, whereas JSW(X=0.225) was the most responsive measure for FFR in the DESS sample; cMFTC was consistently the most responsive MRI subregion.
Table 2.
Mean change (MC in %), standard deviation (SD in %) of the change and responsiveness (sensitivity to change; SRM = standardized response mean) in cartilage thickness (ThCtAB) and medial joint space width (JSW) in the coronal FLASH sample (n=445 knees) over 12 months.
| All knees | KLG 2 | KLG 3 | KLG 4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MC % | SD % | SRM | MC % | SD % | SRM | MC % | SD % | SRM | MC % | SD % | SRM | |
| MFTC | −1.1 | 3.9 | −0.28 | −0.6 | 3.2 | −0.18 | −1.9 | 4.7 | −0.41 | −1.9 | 5.6 | −0.34 |
| eMFTC | −1.4 | 6.5 | −0.22 | −0.8 | 4.6 | −0.17 | −2.7 | 9.1 | −0.30 | −2.5 | 11.2 | −0.22 |
| cMFTC | −1.6 | 5.0 | −0.32 | −0.9 | 4.3 | −0.22 | −2.7 | 5.6 | −0.49 | −2.8 | 8.2 | −0.34 |
| iMFTC | −0.7 | 3.4 | −0.22 | −0.3 | 2.9 | −0.11 | −1.3 | 3.7 | −0.35 | −1.5 | 5.0 | −0.30 |
| MT | −0.7 | 3.4 | −0.21 | −0.2 | 2.9 | −0.07 | −1.5 | 4.0 | −0.37 | −1.5 | 4.4 | −0.35 |
| cMF | −1.5 | 5.7 | −0.26 | −0.9 | 4.8 | −0.20 | −2.5 | 6.9 | −0.36 | −2.3 | 8.8 | −0.26 |
| eMT | −1.6 | 7.2 | −0.23 | −0.9 | 4.7 | −0.19 | −3.1 | 10.6 | −0.30 | −2.8 | 14.8 | −0.19 |
| cMT | −1.2 | 4.9 | −0.23 | −0.3 | 4.1 | −0.09 | −2.4 | 5.2 | −0.46 | −2.4 | 8.1 | −0.29 |
| iMT | −0.5 | 4.0 | −0.12 | −0.2 | 3.7 | −0.05 | −0.7 | 4.1 | −0.18 | −1.3 | 4.9 | −0.27 |
| aMT | −0.1 | 5.0 | −0.02 | 0.5 | 4.5 | 0.11 | −0.9 | 5.3 | −0.17 | −0.9 | 6.2 | −0.15 |
| pMT | −0.6 | 4.4 | −0.13 | −0.4 | 4.1 | −0.10 | −0.7 | 4.5 | −0.17 | −1.0 | 5.7 | −0.17 |
| ecMF | −1.3 | 8.1 | −0.16 | −0.7 | 6.4 | −0.11 | −2.3 | 10.4 | −0.22 | −2.1 | 13.0 | −0.16 |
| ccMF | −2.1 | 7.6 | −0.28 | −1.6 | 6.7 | −0.23 | −3.2 | 8.5 | −0.38 | −3.5 | 12.3 | −0.28 |
| icMF | −1.0 | 5.0 | −0.20 | −0.5 | 3.9 | −0.12 | −1.9 | 5.9 | −0.32 | −1.7 | 7.8 | −0.22 |
| mJSW | −2.3 | 15.2 | −0.15 | −1.9 | 14.1 | −0.13 | −4.2 | 16.0 | −0.26 | 0.3 | 21.2 | 0.02 |
| JSW(x=0.150) | −2.0 | 14.2 | −0.14 | −1.7 | 12.8 | −0.13 | −3.1 | 15.3 | −0.20 | −1.0 | 22.5 | −0.04 |
| JSW(x=0.175) | −2.1 | 13.2 | −0.16 | −1.7 | 11.7 | −0.14 | −3.5 | 14.5 | −0.24 | −0.6 | 22.2 | −0.03 |
| JSW(x=0.200) | −2.3 | 12.9 | −0.18 | −1.8 | 11.4 | −0.16 | −3.9 | 14.7 | −0.27 | −0.7 | 21.0 | −0.03 |
| JSW(x=0.225) | −2.3 | 11.7 | −0.20 | −1.9 | 10.4 | −0.18 | −3.5 | 12.6 | −0.28 | −1.9 | 21.0 | −0.09 |
| JSW(x=0.250) | −2.2 | 10.3 | −0.22 | −1.9 | 9.1 | −0.21 | −2.8 | 11.4 | −0.24 | −2.7 | 16.4 | −0.17 |
| JSW(x=0.275) | −1.8 | 9.8 | −0.19 | −1.5 | 8.6 | −0.17 | −1.8 | 11.1 | −0.17 | −4.6 | 14.9 | −0.31 |
| JSW(x=0.300) | −1.0 | 9.9 | −0.10 | −0.8 | 8.9 | −0.09 | −0.6 | 10.7 | −0.05 | −3.4 | 14.6 | −0.23 |
All knees had definite radiographic OA, defined as Kellgren and Lawrence grade (KLG) >= 2 (obtained from central readings; KLG 2: n=255, KLG 3: n=135, KLG 4: n=55). e/c/iMFTC = external/central/internal subregion of the medial femorotibial compartment; MT = medial tibia; a/pMT anterior/posterior subregion of the medial tibia; cMF = central, weight-bearing part of the medial femoral condyle; mJSW = minimal joint space width, JSW(X) = joint space width at fixed location measures in the medial femorotibial compartment (0.15 – 0.3).
Table 3.
Mean change (MC in %), standard deviation (SD in %) of the change and responsiveness (sensitivity to change; SRM = standardized response mean) in cartilage thickness (ThCtAB) and medial joint space width (JSW) in the sagittal DESS sample over 12 (n=375) and 24 months (n=522).
| All knees | KLG 2 | KLG 3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MC % | SD % | SRM | MC % | SD % | SRM | MC % | SD % | SRM | ||
| 12 months: | MFTC | −1.6 | 4.6 | −0.34 | −0.5 | 3.6 | −0.14 | −2.7 | 5.5 | −0.49 |
| eMFTC | −2.3 | 8.2 | −0.28 | −0.8 | 6.5 | −0.13 | −4.1 | 10.0 | −0.41 | |
| cMFTC | −2.7 | 6.2 | −0.44 | −0.8 | 4.4 | −0.18 | −4.9 | 7.5 | −0.65 | |
| iMFTC | −0.6 | 4.8 | −0.12 | −0.2 | 4.4 | −0.04 | −0.8 | 5.0 | −0.15 | |
| MT | −1.1 | 4.5 | −0.25 | −0.4 | 3.4 | −0.12 | −1.8 | 5.4 | −0.33 | |
| cMF | −2.0 | 6.6 | −0.31 | −0.6 | 5.2 | −0.11 | −3.6 | 7.8 | −0.46 | |
| eMT | −3.2 | 10.6 | −0.30 | −1.4 | 7.4 | −0.19 | −5.6 | 14.4 | −0.39 | |
| cMT | −2.4 | 6.6 | −0.36 | −0.8 | 5.1 | −0.15 | −4.1 | 7.8 | −0.53 | |
| iMT | 0.4 | 6.4 | 0.05 | 0.3 | 6.2 | 0.04 | 0.7 | 6.5 | 0.10 | |
| aMT | −0.4 | 5.8 | −0.07 | 0.1 | 5.0 | 0.02 | −0.8 | 6.5 | −0.12 | |
| pMT | −0.3 | 5.3 | −0.06 | −0.3 | 4.9 | −0.06 | −0.2 | 5.6 | −0.04 | |
| ecMF | −1.3 | 9.1 | −0.15 | −0.3 | 7.9 | −0.03 | −2.6 | 10.5 | −0.25 | |
| ccMF | −3.1 | 8.8 | −0.35 | −0.8 | 5.9 | −0.13 | −5.9 | 11.4 | −0.51 | |
| icMF | −1.6 | 6.4 | −0.24 | −0.7 | 5.7 | −0.12 | −2.3 | 7.0 | −0.33 | |
| mJSW | −3.3 | 15.3 | −0.22 | −1.1 | 12.3 | −0.09 | −6.2 | 18.3 | −0.34 | |
| JSW(x=0.150) | −3.3 | 13.6 | −0.24 | −1.2 | 9.7 | −0.12 | −5.8 | 17.0 | −0.34 | |
| JSW(x=0.175) | −3.3 | 12.5 | −0.26 | −1.2 | 8.8 | −0.13 | −5.7 | 15.8 | −0.36 | |
| JSW(x=0.200) | −3.2 | 11.6 | −0.28 | −1.0 | 8.4 | −0.12 | −5.6 | 14.4 | −0.39 | |
| JSW(x=0.225) | −3.1 | 11.0 | −0.28 | −0.7 | 7.9 | −0.09 | −5.5 | 13.4 | −0.41 | |
| JSW(x=0.250) | −2.6 | 10.0 | −0.26 | −0.7 | 7.4 | −0.09 | −4.5 | 11.9 | −0.38 | |
| JSW(x=0.275) | −2.5 | 9.4 | −0.26 | −0.7 | 7.2 | −0.09 | −4.3 | 11.0 | −0.39 | |
| JSW(x=0.300) | −1.9 | 9.8 | −0.20 | −0.7 | 8.1 | −0.09 | −3.0 | 10.6 | −0.28 | |
| 24 months | MFTC | −2.6 | 6.1 | −0.43 | −0.9 | 4.4 | −0.20 | −4.5 | 7.3 | −0.62 |
| eMFTC | −3.9 | 11.4 | −0.34 | −1.1 | 8.4 | −0.14 | −7.5 | 14.3 | −0.52 | |
| cMFTC | −4.2 | 8.2 | −0.51 | −1.6 | 5.5 | −0.29 | −7.1 | 10.0 | −0.71 | |
| iMFTC | −1.1 | 5.1 | −0.22 | −0.5 | 4.5 | −0.11 | −1.6 | 5.6 | −0.28 | |
| MT | −1.9 | 5.4 | −0.35 | −0.6 | 3.9 | −0.16 | −3.1 | 6.5 | −0.48 | |
| cMF | −3.3 | 8.4 | −0.40 | −1.1 | 6.2 | −0.18 | −5.8 | 10.1 | −0.58 | |
| eMT | −5.2 | 14.3 | −0.37 | −2.0 | 9.7 | −0.21 | −9.5 | 19.1 | −0.49 | |
| cMT | −3.5 | 8.0 | −0.43 | −1.3 | 5.4 | −0.24 | −5.8 | 9.7 | −0.59 | |
| iMT | −0.1 | 6.8 | −0.01 | 0.0 | 6.9 | −0.01 | 0.1 | 6.7 | 0.01 | |
| aMT | −0.8 | 6.2 | −0.13 | 0.0 | 5.3 | −0.01 | −1.4 | 6.8 | −0.21 | |
| pMT | −0.7 | 7.1 | −0.10 | 0.1 | 6.0 | 0.01 | −1.3 | 7.9 | −0.16 | |
| ecMF | −2.7 | 12.2 | −0.22 | −0.3 | 10.0 | −0.03 | −5.6 | 14.2 | −0.40 | |
| ccMF | −4.9 | 11.4 | −0.43 | −1.9 | 8.0 | −0.24 | −8.7 | 14.5 | −0.60 | |
| icMF | −2.2 | 6.9 | −0.32 | −1.0 | 5.8 | −0.17 | −3.4 | 7.6 | −0.45 | |
| mJSW | −5.4 | 17.5 | −0.31 | −3.0 | 14.8 | −0.20 | −9.1 | 20.7 | −0.44 | |
| JSW(x=0.150) | −5.2 | 15.3 | −0.34 | −3.5 | 13.2 | −0.27 | −7.8 | 17.6 | −0.44 | |
| JSW(x=0.175) | −5.4 | 14.1 | −0.38 | −3.5 | 11.8 | −0.29 | −8.2 | 16.4 | −0.50 | |
| JSW(x=0.200) | −5.2 | 13.2 | −0.39 | −3.1 | 11.1 | −0.28 | −8.1 | 15.2 | −0.53 | |
| JSW(x=0.225) | −5.3 | 12.1 | −0.44 | −3.3 | 10.2 | −0.32 | −7.9 | 13.8 | −0.57 | |
| JSW(x=0.250) | −4.9 | 11.4 | −0.43 | −3.5 | 9.6 | −0.36 | −6.8 | 13.2 | −0.51 | |
| JSW(x=0.275) | −4.6 | 10.8 | −0.43 | −3.3 | 8.9 | −0.36 | −6.4 | 12.6 | −0.51 | |
| JSW(x=0.300) | −3.7 | 10.5 | −0.35 | −2.6 | 9.2 | −0.28 | −5.0 | 12.0 | −0.42 | |
All knees had definite radiographic OA, defined as Kellgren and Lawrence grade (KLG) >= 2 (obtained from central readings; 12 months: KLG 2: n=182, KLG 3: n=188, KLG 4: n=5 [data not shown separately]; 24 months: KLG 2: n=256, KLG 3: n=261, KLG 4: n=5 [data not shown separately]). e/c/iMFTC = external/central/internal subregion of the medial femorotibial compartment; MT = medial tibia; a/pMT anterior/posterior subregion of the medial tibia; cMF = central, weight-bearing part of the medial femoral condyle; mJSW = minimal joint space width, JSW(X) = joint space width at fixed location measures in the medial femorotibial compartment (0.15 – 0.3). The DESS sample analyzed over 12 months is a sub-sample of the 24 months cohort, for which a complete set of 12 months measurements was available.
In the FLASH sample, the SRM observed for MFTC (−0.28) was greater than that for mJSW (−0.15), but the difference did not reach statistical significance (p=0.08). This also applied to the difference between the most sensitive MRI and FFR measures in the FLASH sample (cMFTC: −0.32 vs. JSW(X=0.250): −0.22; p=0.36). In the DESS sample, the SRMs observed over one year were greater for MFTC than for mJSW (−0.34 vs −0.22), and for the most sensitive MRI subregion than for the most sensitive FFR measure (cMFTC: −0.44 vs. JSW(x=0.225): −0.28), but again the differences did not reach statistical significance after correcting for multiple comparisons (p=0.034 and p=0.213, respectively), whereas over two years, the SRM in the DESS sample was significantly greater for MFTC than for mJSW (−0.43 vs −0.31; p=0.017) and significantly greater for the most sensitive MRI measure than for the most sensitive FFR measure (cMFTC: −0.51 vs. JSW(0.225): −0.44; p=0.0006).
Rates of change and SRMs for strata with different KL grades are shown in Tables 2 and 3, and in Online Tables 1 and 2. With MRI, the absolute longitudinal changes (in μm) and SRMs were greatest in KLG3 and smallest in KLG2 knees, and were in between for KLG4 knees (FLASH sample). With FFR, JSW measures showed a smaller rate of change and responsiveness in KLG 4 knees than in KLG 3 and KLG2 knees, except for the more internally located fixed location measures (FLASH sample, Online Table 1). Because only 5 knees were graded as KLG 4 in the DESS sample, no separate results were reported for these knees.
There was a significant (positive) correlation between mJSW and MFTC (ThCtAB) changes in both the FLASH (r=0.42) and the DESS sample (12/24 months: r=0.24/0.42, Online Table 3). The correlations in the FLASH sample were higher between mJSW and MRI-based measures than between fixed location measures and MRI, while the correlation between mJSW and MRI did not exceed the correlations observed between fixed location measures and MRI in the DESS sample. Changes in the weight-bearing femur (cMF) tended to have larger Pearson’s correlation coefficients with JSW changes (FLASH r ≤ 0.42, DESS 12M/24M: r ≤ 0.26/0.47) than changes in the tibia (FLASH r ≤ 0.28, DESS 12M/24M: r ≤ 0.23/0.34, Online Table 3).
Amongst combined femorotibial subregions and FFR fixed location measures, changes showed larger correlation values for external and central than for internal measures (Online Table 3). However, correlation coefficients between fixed locations in radiographs and anatomically matched subregions in MRI were not consistently higher than non-location-matched correlation coefficients (Online Table 3).
DISCUSSION
Given the availability of newer and potentially more responsive imaging measures of cartilage, we directly compared the responsiveness of location-specific JSW measures from standardized fixed-flexion radiographs with subregional cartilage thickness measures from MRI in two large samples of the OAI progression subcohort. Key findings are that location-specific measures of JSW display greater responsiveness than minimum JSW, that the central femorotibial compartment is the most sensitive MRI subregion, and that MRI measures display a greater responsiveness than both location-specific and minimum JSW measures. Whereas moderate correlations were observed between JSW and MRI-based cartilage thickness changes, location-matched JSW and MRI measures did not exhibit consistently stronger correlations than non-location-matched comparisons, indicating that radiography is incapable of determining the regional distribution pattern (internal to external) of femorotibial cartilage loss in the medial compartment.
Previous studies that have directly compared radiography and MRI generally encompassed much smaller samples 18,20,40–42 and only one recent study compared fixed-location measures of JSW with 3T MRI measurements (based on 150 knees from the OAI)19. Whereas some of these studies observed a greater responsiveness for MRI than FFR18,40,41, the more recent study 19 found greater SRMs for the new FFR-based fixed location measures than for mJSW and concluded the responsiveness of the fixed location measures to be comparable to global cartilage plate measures in 3T MRI. Using subregional measures of cartilage loss in MRI and a much larger sample, the current study more clearly demonstrated the superiority of MRI in terms of sensitivity to structural change. Further, end stage (KLG4) knees displayed substantial rates and sensitivity to change with MRI, but not with FFR, and MRI does not apply ionizing radiation. However, given the particular context and goal of a study, JSW still is a useful measure, because radiographic image acquisition and image analysis is less expensive and provides less burden on patient time. However, fixed-flexion radiography requires larger samples and/or longer observation times due to the somewhat lower sensitivity.
A greater sensitivity to change of JSW was reported by Hellio Le Graverand et al. for fluoroscopically acquired Lyon Schuss radiography than for FFR 18,43. In the same study, Lyon Schuss also was more sensitive to change than MRI-based cartilage thickness change in MFTC 18,43. A reason for this observation is likely related to optimal alignment of the tibia plateau when using fluoroscopic control 44 or the modified Lyon Schuss technique 45.
The SRMs reported here for MRI are smaller than those from the FDA OARSI meta-analysis of published evidence between 2002 and 2009 16. However, they are in the same range of other reports from the OAI 46,47 and other recent longitudinal studies 18,42,48. In the FDA OARSI initiative meta-analysis, a trend was noted for earlier MRI studies having reported greater SRMs, potentially due to insufficient technology for effectively blinding readers to time points of image acquisitions. Further, SRMs in the OAI may be lower due to the relatively broad inclusion criteria, whereas smaller studies may have had more selective inclusion criteria in terms of risk factors for progression, and greater percentages of participants with advanced radiographic knee OA. As observed in previous studies 28,49, we found that knees with advanced radiographic OA (KLG3) showed substantially greater rates of change and SRMs than those with KLG2, both with MRI and with FFR, and KLG3 knees may therefore be of particular interest for inclusion in clinical trials.
A limitation of the current study is that the knees analyzed using the sagittal DESS and the coronal FLASH protocol were not identical and that the results from these two protocols cannot be compared directly. However, a previous study directly compared longitudinal changes in 80 knees from the progression subcohort between these two protocols and has reported a similar rate and sensitivity to change for coronal FLASH and sagittal DESS MRI39. Moreover, the knees analyzed in the FLASH and DESS sample were not selected specifically for the purpose of this study, which is reflected in the somewhat heterogeneous selection criteria. In contrast to a previous publication on the FLASH cohort 28, however, the current study used a KL classification provided by a central group of readers, who also provided the central KL readings in the DESS sample 29. Knees without definite radiographic OA (KLG ≤ 2) were excluded, to obtain samples that should be representative of clinical trial populations, to which the direct comparison between SRMs from JSW and MRI is particularly relevant.
Another limitation of the current study is that alignment readings are not yet available for the OAI cohort and we were hence unable to exclude knees with valgus malalignment, who are known to loose less cartilage medially than laterally 19,48,50. Still, the lack of exclusion of valgus knees does not limit the comparability between medial compartment measures from radiography and MRI.
Finally, given its generality, the non-parametric sign-test was employed to test whether the sensitivity to change of MRI- and FFR-based measures differed. Further work is needed to explore whether this test can be replaced by other, more sensitive, parametric or non-parametric statistical approaches.
Most of the previous studies reported weak to moderate correlations between MRI-based cartilage loss and JSW changes in radiography 20,23,41,42 but did not attempt to correlate spatially matched measures. The correlations between changes in anatomically matched FFR and MRI locations explored here were not generally greater than those between non-matched locations. A likely reason is that the medial JSW in radiographs does not correspond with the summed cartilage thickness of MT and cMF at each location and only provides an indirect measure, particularly for the internal regions adjacent to the intercondylar area. Whereas focal loss of cartilage thickness will affect (subregional) MRI measures of cartilage thickness, it may not impact the JSW assessed by FFR in situations where the adjacent cartilage or the meniscus maintains the JSW in the compartment and the area of focal cartilage loss is not in direct contact with the opposite joint surface during imaging. Moreover, several studies found meniscus position and integrity to be strongly associated with JSN and JSW: In a study including 233 subjects with symptomatic OA and 58 asymptomatic controls, Gale at al. found a significant association between meniscal subluxation scored on MR and the severity of JSN in knees with symptomatic OA8. Hunter at al. reported that the meniscus position and its degeneration not only account for a substantial proportion of the variance JSW, but also found that changes in meniscal position cause a substantial proportion of change in JSW 6. Further, radiographic JSW in the medial compartment may also be influenced by cartilage and meniscus status in the lateral compartment (and vice versa), because pseudo-widening of the medial JSW may occur in knees with lateral JSN when load is shifted to the lateral compartment. Therefore, whereas radiography provides a 2D depiction of the joint space width and a composite measure of cartilage thickness, meniscus integrity and extrusion 6, MRI directly depicts the articular cartilage (and other structures) in 3D. Studies interested in measuring subregional changes of cartilage thickness with great sensitivity to change hence profit from selecting high-resolution MRI as an imaging modality.
In conclusion, location-specific measures of JSW display superior responsiveness to measurement of minimum JSW from fixed-flexion radiographs, the central femorotibial compartment is the most responsive subregion of MRI-based cartilage thickness change, and MRI-based measures display superior responsiveness to (location-specific and minimum) JSW. Location-matched radiographic and MRI subregions did not exhibit stronger correlations than non-location-matched comparison, suggesting that radiographic measures of JSW are not sensitive to regional differences in the pattern of medial femorotibial cartilage loss.
Supplementary Material
Online Table 1: Mean change (MC in μm), standard deviation (SD in μm) of the change, and 95% confidence intervals (CI, in μm) of the change in cartilage thickness (ThCtAB) and medial joint space width (JSW) in the coronal FLASH sample (n=445 knees) over 12 months.
Online Table 2: Mean change (MC in μm), standard deviation (SD in μm) of the change, and 95% confidence intervals (CI, in μm) of the change in cartilage thickness (ThCtAB) and medial joint space width (JSW) in the sagittal DESS sample over 12 (n=375) and 24 months (n=522).
Online Table 3: Pearson correlation coefficients between change in joint space width (JSW) and cartilage thickness (ThCtAB) in the medial femorotibial compartment (MFTC) of knees from the coronal FLASH (n=445 knees) and the sagittal DESS (24 months: n=522 knees; 12 months: n=375 knees) sample.
Acknowledgments
We would like to thank the Chondrometrics readers Gudrun Goldmann, Linda Jakobi, Tanja Killer, Manuela Kunz, Dr. Susanne Maschek, Jana Matthes, Tina Matthes, Sabine Mühlsimer, Julia Niedermeier, Annette Thebis, Dr. Barbara Wehr, Dr. Gabriele Zeitelhack for dedicated data segmentation, Dr. Martin Hudelmaier for the quality control readings of the DICOM datasets, and Dr. Susanne Maschek for the quality control readings of all segmented MRI data. This manuscript has received the approval of the OAI Publications Committee based on a review of its scientific content and data interpretation
FUNDING SOURCE
The study and image acquisition was funded by the Osteoarthritis Initiative, a public-private partnership comprised of five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262). The Osteoarthritis Initiative is funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. Private funding partners include Merck Research Laboratories; Novartis Pharmaceuticals Corporation, GlaxoSmithKline; and Pfizer, Inc. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health. The image analysis of the MRI component of this study was funded by an industry consortium consisting of Pfizer Inc., Eli Lilly & Co, Merck Serono SA, Glaxo Smith Kline, Wyeth Research, Centocor, and Novartis Pharma AG, and by the coordinating center of the OAI at UCSF. The image analysis of the X-ray component of the study was funded by the coordinating center of the OAI at UCSF.
ROLE OF THE FUNDING SOURCES
Marie-Pierre Hellio Le Graverand, Michael Nevitt, and Markus John were involved in the discussion and interpretation of the data and in the editing of this manuscript.
Footnotes
CONTRIBUTORS
WW and FE were involved in the conception and the analysis of the study, RB was involved in the analysis of the study. All authors were involved in the data interpretation, writing and critically revising the article.
COMPETING INTERESTS
Wolfgang Wirth has a part time appointment with Chondrometrics GmbH, is also shareholder of Chondrometrics GmbH, and provides consulting services to MerckSerono. Marie-Pierre Hellio Le Graverand is a full-time employee of Pfizer, Markus John is a full-time employee of Novartis. Robert Buck is owner of StatAnswers Consulting LLC, a companing providing statistical consulting services. Felix Eckstein is share-holder and CEO of Chondrometrics GmbH, a company providing MR image analysis services to academic researchers and to industry. He provides consulting services to MerckSerono, Novartis, and Sanofi Aventis, has received speaker honoraria from Merck, GlaxoSmithKline, Genzyme, Medtronic, and Synthes, and has received research support from Pfizer, Eli Lilly, MerckSerono, Glaxo Smith Kline, Centocor R&D, Wyeth, Novartis, and Stryker. Michael Nevitt and Jeff Duryea have no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jeff Duryea, Email: jduryea@bwh.harvard.edu.
Marie-Pierre Hellio Le Graverand, Email: helliomp@pfizer.com.
Markus R. John, Email: markus.john@novartis.com.
Michel Nevitt, Email: mnevitt@psg.ucsf.edu.
Robert Buck, Email: robert.j.buck@gmail.com.
Felix Eckstein, Email: felix.eckstein@pmu.ac.at.
References
- 1.Belo JN, Berger MY, Reijman M, Koes BW, Bierma-Zeinstra SM. Prognostic factors of progression of osteoarthritis of the knee: a systematic review of observational studies. Arthritis Rheum. 2007;57(1):13–26. doi: 10.1002/art.22475. [DOI] [PubMed] [Google Scholar]
- 2.Neogi T, Felson D, Niu J, Nevitt M, Lewis CE, Aliabadi P, et al. Association between radiographic features of knee osteoarthritis and pain: results from two cohort studies. BMJ. 2009;339:b2844. doi: 10.1136/bmj.b2844.():b2844–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Y, Niu J, Felson DT, Choi HK, Nevitt M, Neogi T. Methodologic challenges in studying risk factors for progression of knee osteoarthritis. Arthritis Care Res (Hoboken ) 2010;62(11):1527–1532. doi: 10.1002/acr.20287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peterfy C, Kothari M. Imaging osteoarthritis: magnetic resonance imaging versus x-ray. Curr Rheumatol Rep. 2006;8(1):16–21. doi: 10.1007/s11926-006-0020-8. [DOI] [PubMed] [Google Scholar]
- 5.Peterfy CG. Imaging of the disease process. Curr Opin Rheumatol. 2002;14(5):590–596. doi: 10.1097/00002281-200209000-00020. [DOI] [PubMed] [Google Scholar]
- 6.Hunter DJ, Zhang YQ, Tu X, LaValley M, Niu JB, Amin S, et al. Change in joint space width: hyaline articular cartilage loss or alteration in meniscus? Arthritis Rheum. 2006;54(8):2488–2495. doi: 10.1002/art.22016. [DOI] [PubMed] [Google Scholar]
- 7.Adams JG, McAlindon T, Dimasi M, Carey J, Eustace S. Contribution of meniscal extrusion and cartilage loss to joint space narrowing in osteoarthritis. Clin Radiol. 1999;54(8):502–506. doi: 10.1016/s0009-9260(99)90846-2. [DOI] [PubMed] [Google Scholar]
- 8.Gale DR, Chaisson CE, Totterman SM, Schwartz RK, Gale ME, Felson D. Meniscal subluxation: association with osteoarthritis and joint space narrowing. Osteoarthritis Cartilage. 1999;7(6):526–532. doi: 10.1053/joca.1999.0256. [DOI] [PubMed] [Google Scholar]
- 9.Cline GA, Meyer JM, Stevens R, Buckland-Wright C, Peterfy C, Beary JF. Comparison of fixed flexion, fluoroscopic semi-flexed and MTP radiographic methods for obtaining the minimum medial joint space width of the knee in longitudinal osteoarthritis trials. Osteoarthritis Cartilage. 2006;14(Suppl A):A32-6. A32–A36. doi: 10.1016/j.joca.2006.02.023. Epub;%2006 May 8.() [DOI] [PubMed] [Google Scholar]
- 10.Le Graverand MP, Mazzuca S, Lassere M, Guermazi A, Pickering E, Brandt K, et al. Assessment of the radioanatomic positioning of the osteoarthritic knee in serial radiographs: comparison of three acquisition techniques. Osteoarthritis Cartilage. 2006;14(Suppl A):37–43. doi: 10.1016/j.joca.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 11.Guermazi A, Eckstein F, Hellio Le Graverand-Gastineau MP, Conaghan PG, Burstein D, Keen H, et al. Osteoarthritis: current role of imaging. Med Clin North Am. 2009;93(1):101–126. doi: 10.1016/j.mcna.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Hunter DJ, Le Graverand MP, Eckstein F. Radiologic markers of osteoarthritis progression. Curr Opin Rheumatol. 2009;21(2):110–117. doi: 10.1097/BOR.0b013e3283235add. [DOI] [PubMed] [Google Scholar]
- 13.Reichmann WM, Maillefert JF, Hunter DJ, Katz JN, Conaghan PG, Losina E. Responsiveness to change and reliability of measurement of radiographic joint space width in osteoarthritis of the knee: a systematic review. Osteoarthritis Cartilage. 2011;19(5):550–556. doi: 10.1016/j.joca.2011.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Food and Drug Administration. Guidance for industry on clinical development programs for drugs, devices and biological products intended for the treatment of osteoarthritis (OA) 1999 www.fda.gov/downloads/Drugs/GuidanceComlianceRegulatoryInformation/Guidances/ucm071577.pdf.
- 15.Abramson SB, Berenbaum F, Hochberg MC, Moskowitz RW. Introduction to OARSI FDA initiative OAC special edition. Osteoarthritis and Cartilage. 2011;19(5):475–477. doi: 10.1016/j.joca.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 16.Hunter DJ, Zhang W, Conaghan PG, Hirko K, Menashe L, Reichmann WM, et al. Responsiveness and reliability of MRI in knee osteoarthritis: a meta-analysis of published evidence. Osteoarthritis Cartilage. 2011;19(5):589–605. doi: 10.1016/j.joca.2010.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eckstein F, McCulloch CE, Lynch JA, Nevitt M, Kwoh CK, Maschek S, et al. How Do Short-Term Rates of Femorotibial Cartilage Change Compare to Long-term Changes? Four Year Follow-up Data From the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2012 doi: 10.1016/j.joca.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Le Graverand MP, Buck RJ, Wyman BT, Vignon E, Mazzuca SA, Brandt KD, et al. Change in regional cartilage morphology and joint space width in osteoarthritis participants versus healthy controls: a multicentre study using 3. 0 Tesla MRI and Lyon-Schuss radiography. Ann Rheum Dis. 2010;69(1):155–162. doi: 10.1136/ard.2008.099762. [DOI] [PubMed] [Google Scholar]
- 19.Duryea J, Neumann G, Niu J, Totterman S, Tamez J, Dabrowski C, et al. Comparison of radiographic joint space width with magnetic resonance imaging cartilage morphometry: analysis of longitudinal data from the Osteoarthritis Initiative. Arthritis Care Res (Hoboken ) 2010;62(7):932–937. doi: 10.1002/acr.20148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cicuttini F, Hankin J, Jones G, Wluka A. Comparison of conventional standing knee radiographs and magnetic resonance imaging in assessing progression of tibiofemoral joint osteoarthritis. Osteoarthritis Cartilage. 2005;13(8):722–727. doi: 10.1016/j.joca.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 21.Neumann G, Hunter D, Nevitt M, Chibnik LB, Kwoh K, Chen H, et al. Location specific radiographic joint space width for osteoarthritis progression. Osteoarthritis Cartilage. 2009;17(6):761–765. doi: 10.1016/j.joca.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peterfy CG, Schneider E, Nevitt M. The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthritis Cartilage. 2008;16(12):1433–1441. doi: 10.1016/j.joca.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pelletier JP, Raynauld JP, Berthiaume MJ, Abram F, Choquette D, Haraoui B, et al. Risk factors associated with the loss of cartilage volume on weight-bearing areas in knee osteoarthritis patients assessed by quantitative magnetic resonance imaging: a longitudinal study. Arthritis Res Ther. 2007;9(4):R74. doi: 10.1186/ar2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wirth W, Eckstein F. A technique for regional analysis of femorotibial cartilage thickness based on quantitative magnetic resonance imaging. IEEE Trans Med Imaging. 2008;27(6):737–744. doi: 10.1109/TMI.2007.907323. [DOI] [PubMed] [Google Scholar]
- 25.Wirth W, Hellio Le Graverand MP, Wyman BT, Maschek S, Hudelmaier M, Hitzl W, et al. Regional analysis of femorotibial cartilage loss in a subsample from the Osteoarthritis Initiative progression subcohort. Osteoarthritis Cartilage. 2009;17(3):291–297. doi: 10.1016/j.joca.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.KELLGREN JH, LAWRENCE JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eckstein F, Cotofana S, Wirth W, Nevitt M, John MR, Dreher D, et al. Greater rates of cartilage loss in painful knees than in pain-free knees after adjustment for radiographic disease stage: data from the osteoarthritis initiative. Arthritis Rheum. 2011;63(8):2257–2267. doi: 10.1002/art.30414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eckstein F, Nevitt M, Gimona A, Picha K, Lee JH, Davies RY, et al. Rates of Change and Sensitivity to Change in Cartilage Morphology in Healthy Knees and in Knees With Mild, Moderate, and End-Stage Radiographic Osteoarthritis: Results From 831 Participants From the Osteoarthritis Initiative. Arthritis Care & Research. 2011;63(3):311–319. doi: 10.1002/acr.20370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eckstein F, Wirth W, Nevitt MC. Recent advances in osteoarthritis imaging-the Osteoarthritis Initiative. Nat Rev Rheumatol. 2012:10. doi: 10.1038/nrrheum.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Felson DT, Niu J, Guermazi A, Sack B, Aliabadi P. Defining radiographic incidence and progression of knee osteoarthritis: suggested modifications of the Kellgren and Lawrence scale. Ann Rheum Dis. 2011;70(11):1884–1886. doi: 10.1136/ard.2011.155119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Altman RD, Gold GE. Atlas of individual radiographic features in osteoarthritis, revised. Osteoarthritis Cartilage. 2007;15(Suppl A):1–56. doi: 10.1016/j.joca.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 32.Kothari M, Guermazi A, von Ingersleben G, Miaux Y, Sieffert M, Block JE, et al. Fixed-flexion radiography of the knee provides reproducible joint space width measurements in osteoarthritis. Eur Radiol. 2004;14(9):1568–1573. doi: 10.1007/s00330-004-2312-6. [DOI] [PubMed] [Google Scholar]
- 33.Nevitt MC, Peterfy C, Guermazi A, Felson DT, Duryea J, Woodworth T, et al. Longitudinal performance evaluation and validation of fixed-flexion radiography of the knee for detection of joint space loss. Arthritis Rheum. 2007;56(5):1512–1520. doi: 10.1002/art.22557. [DOI] [PubMed] [Google Scholar]
- 34.Peterfy C, Li J, Zaim S, Duryea J, Lynch J, Miaux Y, et al. Comparison of fixed-flexion positioning with fluoroscopic semi-flexed positioning for quantifying radiographic joint-space width in the knee: test-retest reproducibility. Skeletal Radiol. 2003;32(3):128–132. doi: 10.1007/s00256-002-0603-z. [DOI] [PubMed] [Google Scholar]
- 35.Schneider E, NessAiver M, White D, Purdy D, Martin L, Fanella L, et al. The osteoarthritis initiative (OAI) magnetic resonance imaging quality assurance methods and results. Osteoarthritis Cartilage. 2008;16(9):994–1004. doi: 10.1016/j.joca.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duryea J, Li J, Peterfy CG, Gordon C, Genant HK. Trainable rule-based algorithm for the measurement of joint space width in digital radiographic images of the knee. Med Phys. 2000;27(3):580–591. doi: 10.1118/1.598897. [DOI] [PubMed] [Google Scholar]
- 37.Buckland-Wright JC, Macfarlane DG, Williams SA, Ward RJ. Accuracy and precision of joint space width measurements in standard and macroradiographs of osteoarthritic knees. Ann Rheum Dis. 1995;54(11):872–880. doi: 10.1136/ard.54.11.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eckstein F, Ateshian G, Burgkart R, Burstein D, Cicuttini F, Dardzinski B, et al. Proposal for a nomenclature for magnetic resonance imaging based measures of articular cartilage in osteoarthritis. Osteoarthritis Cartilage. 2006;14(10):974–983. doi: 10.1016/j.joca.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 39.Wirth W, Nevitt M, Hellio Le Graverand MP, Benichou O, Dreher D, Davies RY, et al. Sensitivity to change of cartilage morphometry using coronal FLASH, sagittal DESS, and coronal MPR DESS protocols--comparative data from the Osteoarthritis Initiative (OAI) Osteoarthritis Cartilage. 2010;18(4):547–554. doi: 10.1016/j.joca.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Benichou OD, Hunter DJ, Nelson DR, Guermazi A, Eckstein F, Kwoh K, et al. One-year change in radiographic joint space width in patients with unilateral joint space narrowing: data from the Osteoarthritis Initiative. Arthritis Care Res (Hoboken ) 2010;62(7):924–931. doi: 10.1002/acr.20149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raynauld JP, Martel-Pelletier J, Berthiaume MJ, Labonte F, Beaudoin G, de Guise JA, et al. Quantitative magnetic resonance imaging evaluation of knee osteoarthritis progression over two years and correlation with clinical symptoms and radiologic changes. Arthritis Rheum. 2004;50(2):476–487. doi: 10.1002/art.20000. [DOI] [PubMed] [Google Scholar]
- 42.Bruyere O, Genant H, Kothari M, Zaim S, White D, Peterfy C, et al. Longitudinal study of magnetic resonance imaging and standard X-rays to assess disease progression in osteoarthritis. Osteoarthritis Cartilage. 2007;15(1):98–103. doi: 10.1016/j.joca.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 43.Le Graverand MP, Vignon EP, Brandt KD, Mazzuca SA, Piperno M, Buck R, et al. Head-to-head comparison of the Lyon Schuss and fixed flexion radiographic techniques. Long-term reproducibility in normal knees and sensitivity to change in osteoarthritic knees. Ann Rheum Dis. 2008;67(11):1562–1566. doi: 10.1136/ard.2007.077834. [DOI] [PubMed] [Google Scholar]
- 44.Merle-Vincent F, Vignon E, Brandt K, Piperno M, Coury-Lucas F, Conrozier T, et al. Superiority of the Lyon schuss view over the standing anteroposterior view for detecting joint space narrowing, especially in the lateral tibiofemoral compartment, in early knee osteoarthritis. Ann Rheum Dis. 2007;66(6):747–753. doi: 10.1136/ard.2006.056481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mazzuca SA, Hellio Le Graverand MP, Vignon E, Hunter DJ, Jackson CG, Kraus VB, et al. Performance of a non-fluoroscopically assisted substitute for the Lyon schuss knee radiograph: quality and reproducibility of positioning and sensitivity to joint space narrowing in osteoarthritic knees. Osteoarthritis Cartilage. 2008 doi: 10.1016/j.joca.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 46.Hunter DJ, Niu J, Zhang Y, Totterman S, Tamez J, Dabrowski C, et al. Change in cartilage morphometry: a sample of the progression cohort of the Osteoarthritis Initiative. Ann Rheum Dis. 2009;68(3):349–356. doi: 10.1136/ard.2007.082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Eckstein F, Maschek S, Wirth W, Hudelmaier M, Hitzl W, Wyman B, et al. One year change of knee cartilage morphology in the first release of participants from the Osteoarthritis Initiative progression subcohort: association with sex, body mass index, symptoms and radiographic osteoarthritis status. Ann Rheum Dis. 2009;68(5):674–679. doi: 10.1136/ard.2008.089904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eckstein F, Wirth W, Hudelmaier M, Stein V, Lengfelder V, Cahue S, et al. Patterns of femorotibial cartilage loss in knees with neutral, varus, and valgus alignment. Arthritis Rheum. 2008;59(11):1563–1570. doi: 10.1002/art.24208. [DOI] [PubMed] [Google Scholar]
- 49.Wirth W, Buck R, Nevitt M, Le Graverand MP, Benichou O, Dreher D, et al. MRI-based extended ordered values more efficiently differentiate cartilage loss in knees with and without joint space narrowing than region-specific approaches using MRI or radiography - data from the OA initiative. Osteoarthritis Cartilage. 2011;19(6):689–699. doi: 10.1016/j.joca.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moisio K, Chang A, Eckstein F, Chmiel JS, Wirth W, Almagor O, et al. Varus-valgus alignment: Reduced risk for subsequent cartilage loss in the less loaded compartment. Arthritis Rheum. 2011:10. doi: 10.1002/art.30216. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Table 1: Mean change (MC in μm), standard deviation (SD in μm) of the change, and 95% confidence intervals (CI, in μm) of the change in cartilage thickness (ThCtAB) and medial joint space width (JSW) in the coronal FLASH sample (n=445 knees) over 12 months.
Online Table 2: Mean change (MC in μm), standard deviation (SD in μm) of the change, and 95% confidence intervals (CI, in μm) of the change in cartilage thickness (ThCtAB) and medial joint space width (JSW) in the sagittal DESS sample over 12 (n=375) and 24 months (n=522).
Online Table 3: Pearson correlation coefficients between change in joint space width (JSW) and cartilage thickness (ThCtAB) in the medial femorotibial compartment (MFTC) of knees from the coronal FLASH (n=445 knees) and the sagittal DESS (24 months: n=522 knees; 12 months: n=375 knees) sample.


