Summary
Purpose
To investigate brain electrical activity in Q54 mice that display spontaneous seizures because of a gain-of-function mutation of the Scn2a sodium channel gene, and to evaluate the efficacy of low frequency deep brain stimulation (DBS) for seizure frequency reduction.
Methods
EEG, EMG, and hippocampal deep electrodes were implanted into Q54 mice expressing an epileptic phenotype (n = 6). Chronic six channel recordings (wideband, 0.1–300 Hz) were stored 24 hours a day for more than 12 days. Low Frequency stimulation (LFS) (3Hz, square wave, biphasic, 100μs, 400μA) was applied to the ventral hippocampal commisure (VHC) in alternating five minute cycles (on or off) 24 hours a day for a period of four days.
Results
LFS (3Hz) resulted in a significant reduction in seizure frequency and duration (21% and 35%, p<0.05), when applied to the VHC of epileptic Q54 mice (n = 6). Seizure frequency was not directly affected by stimulation state (“on” versus “off”).
Conclusion
LFS applied at a frequency of 3Hz significantly reduced seizure frequency and duration in the Q54 model. Furthermore, the reduction of seizure frequency and duration by LFS was not immediate but had a delayed and lasting effect, supporting complex, indirect mechanisms of action.
Keywords: Epilepsy, DBS, LFS, Sodium Channel, Scn2a
INTRODUCTION
Epilepsy, one of the most prevalent neurologic disorders today, is characterized by the presence of recurrent, spontaneous seizures, resulting from the uncontrolled, abnormal excitation of neuronal populations within the brain. Because neuronal excitability is highly regulated by voltage-gated sodium channels, they play an essential role in both the development and treatment of patients with epilepsy. Moreover, the genetic component of inherited epilepsies in humans has been linked to mutations in several genes coding for the sodium channel protein, including: Scn1a, Scn2a, and Scn1b (Meisler and Kearney, 2005). In fact, two of the most commonly prescribed antiepileptic drugs (AEDs) are known sodium channel inhibitors: phenytoin (Dilantin) and carbamazepine (Tegretol).
Although numerous AEDs are readily available, more than 25% of patients do not respond well or become resistant to them over time (Enna and Coyle, 1998). Unfortunately, only about half of these patients are then considered good candidates for remaining neurosurgical treatment, typically involving the surgical resection of seizure foci. One potential alternative therapy for medically intractable epilepsies is deep brain stimulation (DBS). DBS is an alternative surgical treatment involving the implantation of one or more electrodes into the central nervous system. Implanted electrodes deliver electrical impulses to specific brain regions enabling direct and controlled changes in brain activity. DBS is a recognized and approved therapy by the Food and Drug Administration (FDA) for the treatment of several neurological diseases including Parkinson’s, essential tremor, and dystonia (Halpern et al., 2007; Yu and Neimat, 2008). Presently, DBS is being investigated as a potential therapy for other neurological disorders including depression, obsessive-compulsive disorder, and epilepsy.
The application of DBS therapies to a variety of neurological disorders is possible due to the inherent flexibility of stimulation parameters including location, timing, and intensity. Although high frequency stimulation (HFS) is traditionally used in DBS therapies, low frequency stimulation (LFS), in the range of 0 – 10 Hz, is also a strong candidate for epilepsy therapy. Not only has LFS been shown experimentally to reduce seizure generation and frequency both in vivo and in vitro, but LFS inherently requires less current injection, minimizing the potential for stimulus induced tissue or electrode damage.
To date, LFS has shown an antiepileptic effect in several animal models of epilepsy. Low frequency stimulations (1 and 1.3 Hz) were able to suppress high extracellular potassium and bicuculine induced seizures in the rat hippocampus in vitro (Jerger and Schiff, 1995; Albensi et al., 2004). Similarly, multiple studies have shown a suppressive effect of preemptive 1Hz stimulation on amygdala kindled afterdischarges in the in vivo rat model (Velisek et al., 2002; Goodman et al., 2005). An earlier study also demonstrated a significant reduction in amygdala-kindled seizure frequency when 3Hz stimulation was applied after kindling (Gaito et al, 1980). In contrast, two prior amygdala-kindling studies have argued a proconvuslive effect of 3 Hz stimulation (Cain and Corcorain, 1981; Minabe et al, 1986). However, in these studies the stimulation was applied at a substantial increase in stimulus amplitude (1000–1500 μA) and/or pulse width (≥ 1ms) and in one case also combined with a known convulsive frequency of 60Hz. Suppression of seizure activity by LFS has also been seen in a limited number of human studies. For example, a 0.5 Hz stimulus applied to ictal zones resulted in a reduction of seizure initiation in 4 of the 5 identified seizure onset zones (Schrader et al., 2006). In fact, the majority of uncontrolled human studies have yielded exceptional seizure control (Lüders, 2004). One of the reasons for the inability of this success to translate to controlled studies is likely due to the fact that ideal parameters have yet to be identified and customized specifically for seizure suppression. Previous studies have targeted the subthalamic nucleus (STN) based primarily on its success in the treatment of Parkinson’s disease and the convenience it provided for approving experimental protocols. However, when treating seizures that involve a variety of brain regions, a more diverse stimulation may be required to affect multiple epileptic foci. For example, stimulation of white matter tracts could serve to distribute the effects of stimulation from a single electrode contact to multiple epileptic zones and/or to a large region of the brain thereby preventing the seizure from propagating outside the region of influence of the electrode.
The aim of this study is to evaluate the suppression of spontaneous seizures via stimulation of white matter tracts connecting bilateral hippocampi, the ventral hippocampal commissure (VHC) (Figure 1, A). We hypothesize that the stimulation of white matter tracts directly connected to epileptic zones may prevent seizure activity and suppress epileptic foci. In addition, by stimulating these axonal-rich white matter tracks we can maximize any therapeutic effect by targeting multiple downstream epileptic foci with a single stimulation target.
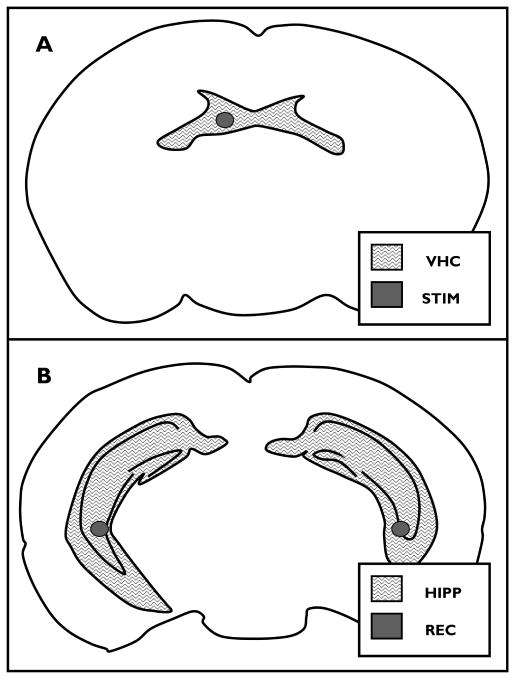
Figure 1. Stimulation and recording electrodes.
A) stimulation electrode target (STIM) within the ventral hippocampal commissure(VHC) in a coronal section of the mouse brain (0.82 mm posterior to Bregma), and B) bilateral hippocampal recording electrode targets (REC) within bilateral hippocampi (HIPP) in a coronal section of themouse brain (2.8 mm posterior to Bregma) (images modified from (Paxinos and Franklin, 2001)).
The specific hypothesis to be tested is that low frequency stimulation of 3Hz applied to the ventral hippocampal commissural (VHC) fibers will reduce seizure frequency in Q54 mice. A gain-of-function voltage-gated sodium channel mutation of the Scn2a gene in the Q54 line of mice developed by Kearney et. al., results in the development of spontaneous, generalized seizures (Kearney et al., 2001). Because the Q54 Scn2a mutation affects pyramidal cells of the hippocampus and seizures dominate this area in Q54 mice, this model was chosen to study the effects of VHC stimulation for the purpose of seizure reduction. To test our hypothesis, stimulation electrodes were implanted into the VHC of seizure-prone Q54 transgenic mice and seizure frequency and duration were evaluated with both depth and surface electrodes before, during, and following applied LFS.
METHODS
Animals
Experiments were performed on the Q54 strain of transgenic mice expressing the GAL879–881QQQ mutation of Scn2a gene (Kearney et al., 2001). Q54 transgenic mice were bred and genotyped as previously described (Kile et al., 2008). Experiments were conducted on heterozygous female Q54 transgenics (n = 6), between two and six months, ensuring the onset of generalized seizures while minimizing the possibility for seizure induced damage. Animals were housed in microisolator cages and maintained in a veterinarian monitored animal care facility with a 12hr:12hr light:dark cycle at room temperature (20–25°C). All animal protocols were reviewed and approved by the Institutional Animal Care and Use Committee of Case Western Reserve University.
Electrode implantation
Adult Q54 transgenic mice (n = 6) were anesthetized with isoflurane (5% induction, 2% maintenance) inhalation, and mounted into a stereotaxic frame. Polyimide-insulated stainless steel monopolar microelectrodes (0.1mm diameter) (Plastics One Inc. Roanoke, VA) were implanted bilaterally into the pyramidal cell layer of the posterior hippocampus (AP, −2.8; ML, ±3; DV, −3.5 [mm from Bregma]). A twisted-pair polyimide-coated stainless steel bipolar electrode (0.25mm diameter) (Plastics One Inc. Roanoke, VA) was implanted into the ventral hippocampal commissural (VHC) fibers (AP, −0.8; ML, −0.5; DV, −2.2). In addition, four 1/16″ stainless steel EEG screws (A-M Systems, Carlsberg WA) were implanted into the lateral frontal and lateral parietal (AP, 1.5; ML, ±1.5, or AP, −2.5; ML, ±1.0) regions of the skull for differential EEG recordings. EMG signals were recorded from fine polyimide insulated stainless steel wire electrodes sewn into the neck muscles (Cooner Wire, Chatsworth, CA). Chronic recording and stimulation targets are shown in Figure 1, and stereotaxic implantation sites are shown in relation to the mouse skull in Supplementary Figure 1. Following electrophysiological experiments, mice were anesthetized and decapitated using a small animal guillotine. The brain was rapidly removed and fixed in 10% formalin. Nissl stained coronal sections (60 μm thick) were viewed with a light microscope to verify electrode placement.
Stimulation and recording
Ten days following implantation, six-channel (4 EEG, 1 EMG, 1 Deep Hippocampal Electrode) wideband (0.3 – 300 Hz) recordings of electrical activity were acquired at a rate of 2KHz 24 hours per day for a minimum overall period of 12 days consisting of the following consecutive 4 day periods: baseline (pre-LFS), stimulation (LFS), recovery (post-LFS). During LFS, animals were stimulated with 3Hz charge-balanced biphasic, square wave pulses (100us, ≤400μA) delivered to the VHC. Stimulation was continuously delivered with a 50% power cycle (5 min on, 5 min off). Simultaneous video monitoring and six-channel electrical recordings were maintained throughout.
Seizure classification was determined by inspection of EEG recordings and observed video behavior (Figure 2) according to an adjusted version of the Racine scale (Racine, 1972): stage 1: EEG seizures without detectable motor manifestation, 2: mouth and facial clonus, head nodding, 3: bilateral forelimb clonus, 4: forelimb clonus and rearing, 5: forelimb clonus, rearing, and falling. Further description of EEG differential signal calculation is provided in supplemental material.
Figure 2. Q54 model seizures.
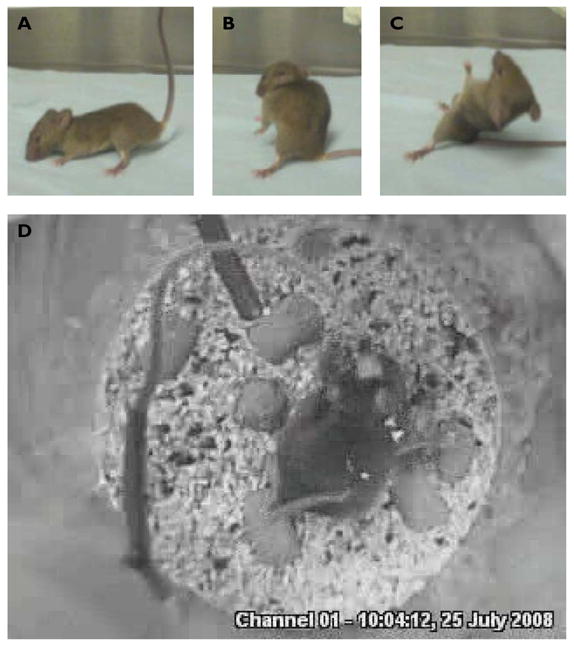
A–C) Q54 mice exhibited motor seizures as previously described (Kearney et al., 2001). D) During chronic electrical recordings, electrode implanted mice were video monitored from above to confirm occurrence of motor seizures.
All signals were amplified (5000 gain) with a GRASS EEG Amplifier (Model 7P511J/K, Grass Technologies, West Warwick, RI), and recorded onto a personal computer using a National Instruments Data Acquisition System and Labview software (National Instruments, Austin, TX). Stimulation current was applied with a digital stimulus isolator (Model DS8000/DLS100, World Precision Instruments, Sarasota, FL). Off-line data analyses were performed in Polyman (v1.2.6.603, Kemp & Roessen, Den Haag, Netherlands), Spike2 (v5.02, Cambridge Electronic Design Limited, Cambridge, England), and Matlab (v7.6, Mathworks, Natick, MA) Software. Video monitoring was conducted with small USB digital video camera (Creative Labs, Singapore) and video monitoring freeware.
Data analysis
Measurements throughout this text are expressed as mean ± standard deviation (SD) unless otherwise noted, with n, h, d, s, and m, indicating the number of animals, hours, days, seizures, and total measures evaluated per group per test. Seizure frequency data was found to be normally distributed as confirmed by both Kolmogorov-Smirnov and Ryan-Joiner goodness-of-fit tests (p-values >0.15 and >0.1 respectively). Unless otherwise noted, select results obtained were evaluated for statistical significance using an independent/unpaired two-tailed Student’s t-test for two samples of equal size and equal variance with a significance level, α = 0.05. For evaluation of statistical significance of seizure frequency changes during differing recording periods (pre-LFS, LFS, and post-LFS) t-values were calculated from dependent/paired data and a one-tailed assumption.
RESULTS
Baseline seizure activity
In order to evaluate the effect of LFS on seizure activity, chronically recorded seizures in Q54 mice were characterized and quantified. After a minimum of six days of recovery from surgical implant, baseline activity was recorded for a period of at least four days. Baseline data were analyzed for the presence of high frequency, high amplitude activity characteristic of seizure in EEG channels and matched with video recordings (Figure 3). Seizures were evaluated according to modified Racine scale (Racine, 1972), and only seizures greater than or equal to stage four were counted in seizure frequency measurements. A typical seizure recorded from the chronic preparation is shown in Fig 3. Seizure activity was primarily identified by the presence of high frequency activity across all channels including EEG, EMG, and deep electrodes (DPE). Seizures were confirmed in video footage by the presence of bilateral forelimb clonus, rearing, and falling behaviors (Figure 2). Two complete days of baseline data, immediately preceding stimulation, were analyzed for each animal, and seizure frequency noted as the number of seizures per hour. The average seizure frequency was 14.6±6 seizures/hr (n = 6, d = 2, h = 24, m = 288) (Table 1). Seizure frequency was reasonably stable across all hours of the day with a mean seizure frequency range from 12.4 to 16.9 s/hr over the entire 48 hour period. Seizure frequency was found to be slightly greater during more active hours (6pm-6am), 14.9±1 seizures/hr (n = 6, d = 2, h = 12, m = 144) versus 13.8±1 seizures/hr (n = 6, d = 2, h = 12, m = 144), although this result was not found to be of statistical significance. From these data, the four hour period from 9am – 1pm was selected for seizure frequency analysis for all animals (n = 6) for all recorded days over all periods: pre-LFS, LFS, and post-LFS. This period of time was selected because the mean seizure frequency was comparable to overall average with a decreased variability, as well as the fact that the animals were generally less active, minimizing EMG interference. During this time period the average seizure frequency during pre-LFS was 14.8±5 seizures/hr (n = 6, d = 4, h = 4, m = 96).
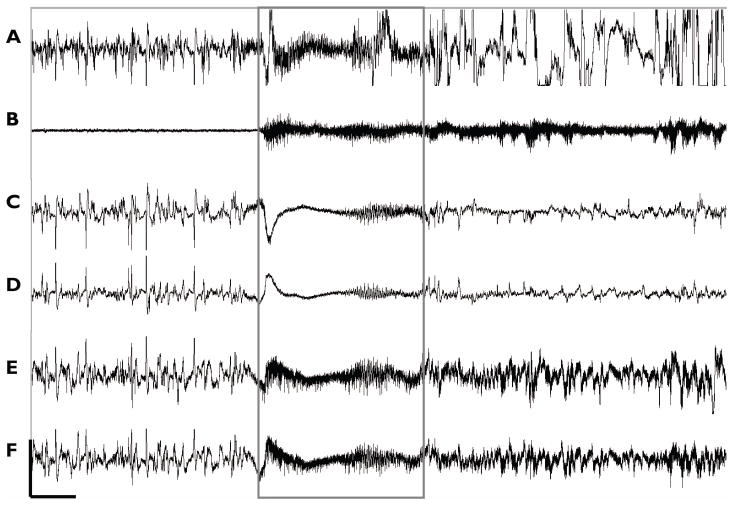
Figure 3. Chronic recording.
Six-channel recordings were maintained for 24 hours a day over entire experimental protocol. Typical recording including A) Hippocampus, B) EMG, C) Right EEG, D) Left EEG, E) Frontal EEG, and F) Parietal EEG, is shown, with seizure (boxed) as identified by high frequency components across all channels as well as by motor seizure activity seen in video monitoring (Scale: 50μV, 2.5s).
Table 1.
Effects of Low Frequency Stimulation on Seizure Frequency and Duration
| Pre-LFS | LFS | Post-LFS | All | ||
|---|---|---|---|---|---|
| Frequency (sz/hr) | 14.8±5 | 11.7±5* | 10.3±5** | 14.6±6 | |
| Duration (s) | Clonic | 11.7±6 | 11.3±5 | 9.5±2 | 10.9±5 |
| Hipp | 8.7±4 | 6.9±2 | 5.7±2* | 7.1±3 | |
Mean seizure duration and frequency ± SEM over four day pre-LFS, LFS, and post-LFS periods is shown for all animals (n = 6, d = 4, h = 4, m = 96). Overall, animals experienced a decrease in average seizure frequency during the period of applied LFS, which persisted during recovery, as well as a significant decrease in seizure duration during post-LFS
= significant difference from pre-LFS, p<0.05;
= significant difference from pre-LFS and LFS, p<0.05.
During all periods, clonic seizures were found to be significantly longer than seizures recorded by hippocampal electrodes (p<0.02).
Effects of Low Frequency Stimulation (LFS) on seizure activity
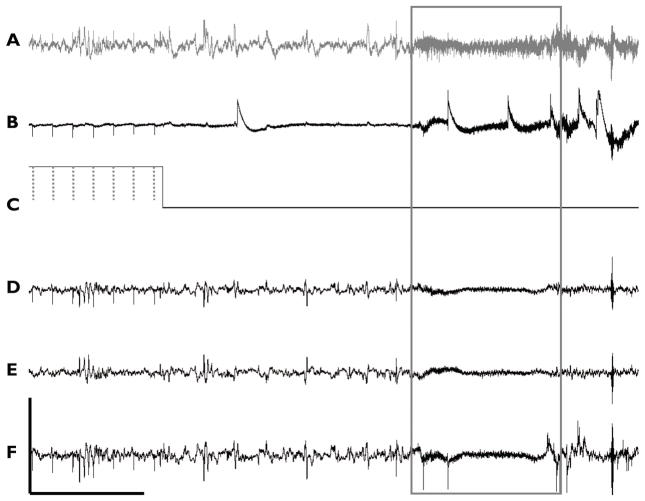
LFS was applied to the VHC in transgenic mice and control animals in order to determine the effect of the stimulation on seizure activity. Biphasic (cathodic-anodic) square wave pulses with a 200μs pulse width (100μs per phase) and ±400μA amplitude were applied at a frequency of 3 Hz cycling between on and off periods every 5 min. This cycling paradigm was applied continuously throughout each day (24 hrs) for a period of four days (3Hz1 – 3Hz4). At the end of four days, stimulation was turned off and followed by another four day recording segment without stimulation, recovery period (R1–R4). An example of chronic recordings during periods of 3 Hz stimulation is shown in Figure 5. This figure also shows a transition in stimulation state from “on” to “off”. Stimulation “on” is labeled with dotted lines which represent the occurrence of a stimulus pulse, visible as stimulation artifacts within the recorded neurological activity. A short time after a switch to an “off” period, a seizure occurs associated with a high frequency signal as indicated by the boxed data.
Figure 5. Stimulation during chronic recording.
Six-channel recordings during periods of cycling stimulation. Channels of A) hippocampus DPE, B) EMG, C) stimulation indicator, D) Left EEG, E) Right EEG, and F) Parietal EEG, are shown, with seizure (boxed) as identified by high frequency components across all channels as well as by motor seizure activity seen in video monitoring. Stimulus artifacts from square wave pulses (dotted line) are easily identifiable over recordings, with the exception of right EEG (Scale: 50μV, 5s).
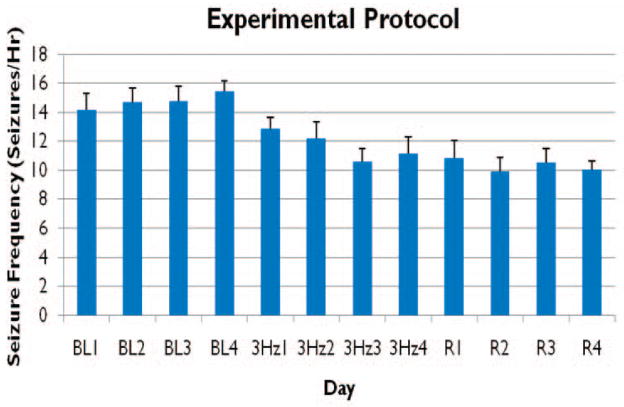
Animals (n = 6) were evaluated both individually and together during three, four day long experimental periods (pre-LFS, LFS, and post-LFS), for a total of 12 consecutive days (Figure 6). Figure 6 shows the mean seizure frequency for all animals during the evaluated hours (9–12), throughout the timeline of this experimental protocol. Individually, five of six animals showed a decrease in mean seizure frequency during 3Hz LFS from pre-LFS, as well as during post-LFS, four of which animals were statistically significant reductions (p-value < 0.05, d = 4, h = 4, m = 16). One animal that did not experience a decrease during pre-LFS or post-LFS periods, experienced an increase in mean seizure frequency by 0.88 seizures/hr during LFS and a further increase of 2.31 seizures/hr during post-LFS. Average mean seizure frequency for all animals decreased from 14.8±5 seizures/hr pre-LFS to 11.7±5 during LFS and finally, 10.3±5 seizures/hr post-LFS (n = 6, d = 4, h = 4, m = 96). Overall, animals exhibited a significant (21%) average decrease in mean seizure frequency from pre-LFS during both LFS and post-LFS (p-value <0.05, Table 1). This decrease in seizure frequency did not recover and was maintained during the post-stimulation period.
Figure 6. Low Frequency Stimulation (LFS) protocol.
LFS (3 Hz) was applied for a period of four days immediately following a four day period without stimulation, pre-LFS (BL 1–4), and preceding an additional four days of recording without stimulation, post-LFS (R 1–4).. Seizures were counted per hour over a period of four hours, 9am – 12pm, each day. Mean seizure frequency (seizures/hour) ± SEM is shown over all experimental days (n=6).
In addition to seizure frequency, seizure duration was evaluated during all three experimental periods (Table 1). Seizure duration was evaluated as the time during motor seizures as reported by video recordings (Clonic) and by the presence of an elevated high frequency component to the deep electrode recording within the hippocampus (Hipp). For all periods, clonic manifestation of seizure activity was found to be significantly longer than hippocampal activity. In addition, hippocampal seizure duration was significantly (35%) reduced following LFS stimulation (n = 6, d = 4, s = 10, m = 40).
To evaluate the effect of stimulation state, “on” versus “off”, on seizure frequency, seizures occurring on stimulation days were noted as occurring during five minute intervals when 3Hz stimulation was applied, “on”, or during the alternating five minute intervals when stimulation was not applied, “off”. The percent of total seizures per hour occurring in either “on” or “off” intervals was calculated for each hour and then averaged over all hours evaluated. During 3 Hz stimulation the mean percent of total seizures during the “on” state was 52.2±17.2%. Of the 96 measures evaluated per state (n = 6, d = 4, h = 4, m = 96), t-tests revealed no significant difference between mean seizure frequency during “on” versus “off” states throughout the LFS period (Figure 7).
Figure 7. Effect of stimulation state on seizure frequency.
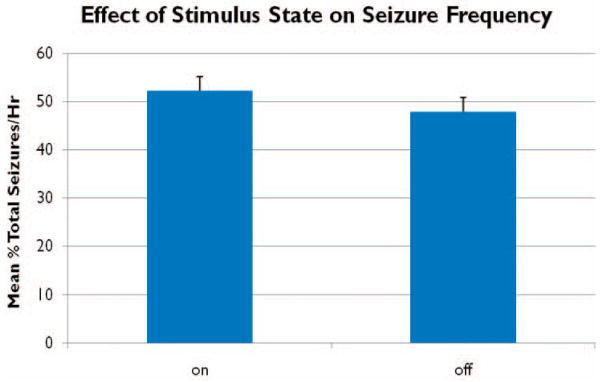
No significant difference in the mean percent of total seizures per hour (n=6, h=4, d=4) was found between on and off states over four days of 3Hz low frequency stimulation.
DISCUSSION
Deep brain stimulation offers a promising and reversible therapeutic alternative for patients with medically intractable seizures. However, before deep brain stimulation (DBS) can be administered as a successful seizure therapy, proposed stimulation targets and waveform parameters need to be identified and evaluated within a realistic model of human epilepsy. Low frequency stimulation (LFS) for example, has benefits over high frequency stimulation (HFS) in that it requires inherently fewer pulses per second, lowering the required current injection and minimizing the potential for stimulation induced damage to the electrode and its surrounding tissue. This study investigated a potential DBS target with a LFS paradigm for the treatment of temporal lobe epilepsy in a genetic model and generated three key findings. First, analysis of seizure activity in Q54 mice reveals a robust and well maintained seizure frequency over several weeks. Second, LFS at 3Hz significantly reduces seizure frequency in a transgenic model of inherited epilepsy. Lastly, the presence of LFS does not seem to have a direct and immediate effect on seizure frequency.
Several animal studies have been conducted on numerous epilepsy models, using an assortment of stimulation parameters and applied to a variety CNS targets. A number of kindling studies have shown that LFS can have an inhibitory effect on seizure generation and propagation, and the results presented here are consistent with these studies. For example, LFS (0.1 – 10 Hz) was able to suppress high extracellular potassium and bicuculine induced seizures in the rat hippocampus in vitro (Jerger and Schiff, 1995; Albensi et al., 2004), and LFS (1 – 3 Hz) had a suppressive effect on afterdischarges elicited by kindling in the amygdala of the rat in vivo (Gaito, 1980; Gaito et al., 1980; Weiss et al., 1995; Velisek et al., 2002; Goodman et al., 2005; Carrington et al., 2007; Zhu-Ge et al., 2007). The kindling model of epilepsy elicits electrographic and behavioral seizures in response to repeated application of intermittent trains of electrical stimulation (Goddard et al., 1969). Because this study indicated a reduced probability of eliciting seizure activity at frequencies outside of 60Hz, Gaito evaluated the effect of several frequencies on the kindling effect in vivo, and determined that 1Hz or 3Hz stimulation was unable to elicit seizure behaviors (Gaito, 1980). Furthermore, 3Hz stimulation applied before and after 60Hz kindling trains was found to have a suppressive effect on kindling due to what was suggested to be an “interference effect” (Gaito et al., 1980). Clinically, LFS (0.5–1 Hz) applied to epileptic zones in humans was shown to have an inhibitory affect (Schrader et al., 2006).
HFS is has been shown to inhibit pathological neuronal activity through direct disruption of local network activity, while LFS is thought to inhibit activity by increasing the threshold for the firing of neuronal action potentials through more complex mechanisms such as long term depression (LTD) (Albensi et al., 2004; Schrader et al., 2006). LTD is characterized by a long-lasting decrease in synaptic efficacy which can be induced by 1Hz or 3Hz LFS applied to the Schaffer collaterals in the hippocampal slice preparation (Christie et al., 1994; Albensi et al., 2007). While the specific role of LTD was not evaluated in this study, the results are consistent with this mechanism. In particular, the effects of LTD following a LFS train can last several hours and a significant reduction in seizure frequency was seen throughout the recovery period following the application of LFS in this study. Moreover, it is possible that CA3 cells were activated antidromically first and then received synaptic input later as is required for LTD (Markram et al. 1997; Kennedy 2005).
Low frequency stimulation of white matter tracts could result in an overall inhibitory effect on downstream neuronal populations through a number of mechanisms either in addition to or as an alternate of LTD. Synaptic depression of excitatory neurotransmission has been suggested as a mechanisms for two in vitro studies: LFS (1Hz) applied to the Schaeffer collaterals in rat hippocampal slices suppressed bicuculline induced seizure activity (Albensi et al, 2004), and LFS (0.5–5Hz) applied directly suppressed seizure-like activity through depression of excitatory neurotransmission (Schiller and Bankirer, 2007). Two additional studies have suggested increased GABA-mediated inhibitory activity as a mechanism of LFS induced seizure suppression (Chkhenkeli et al, 2004, Kinoshita et al, 2005). While the mechanism of LFS seizure reduction does seem to implicate changes in synaptic weights, further research is necessary to determine the specific role of inhibitory and/or excitatory synaptic plasticity.
Data presented here support the hypothesis that LFS can decrease the incidence of seizure activity as demonstrated by a statistically significant reduction of mean seizure frequency during periods of LFS. However, the inhibitory effect results in a modest 20 percent decrease in seizure frequency, suggesting that LFS applied to the VHC is unable to block seizures completely. One reason for this could be due to the fact that the animals in this study have a uniquely high seizure frequency during baseline recordings stemming from foci outside of the stimulation targeted regions. The Q54 model of epilepsy, while based on a sodium channel mutation similar to those found in inherited human epilepsy syndromes it is not limited to the temporal lobe and seizures are distributed throughout the brain involving several brain nuclei and subsequently, a greater number of seizure foci. The VHC pathway in the rat innervates directly the CA3 and CA1 regions of a portion of both hippocampi (Gottlieb and Cowan, 1973). Therefore, LFS stimulation could be effective only on the hippocampl epileptogenic zones directly innervated by the VHC. In support of this, while the duration of clonic seizure activity was not affected by VHC LFS, hippocampal seizure duration recorded by deep electrodes was significantly reduced following LFS periods.
Although the anatomy of the mouse brain is similar in many ways to the human brain, it should be noted that murine hippocampal commissures have differing anatomical characteristics and connectivity in comparison to those in humans. Although the VHC is reduced in humans, the dorsal hippocampal commissure (DHC) is well developed and represents a substantial fiber tract connecting the right and left hippocampi via fornix attachments (Gloor et al., 1993). Therefore this target could also be tested in patients with epilepsy though the DHC. Preliminary cortical evoked potentials studies suggest that this fiber track connects and activates the hippocampus directly in patients with depth electrodes implanted for seizure monitoring (data not shown). Also, the stimulation target within the Q54 mouse model is extremely small resulting in a challenging surgery for electrode implantation. While brain histology was conducted to confirm stimulating and recording locations, even slight error in stimulating electrode placement could have had a significant effect on the activation of VHC by LFS.
In addition, due to the selection of all female mice for this study, catamenial epilepsy, or the modification of seizure frequency or intensity with hormonal changes, should be addressed. While catamenial epilepsy is understood to occur in approximately 10–70% of epileptic women (Case and Reid, 2001), we did not observe this in our mouse model. Q54 mice were observed hourly and/or daily for seizure activity over their lifespan in this and prior research experiments (Kile et al 2008). While seizure frequency did escalate with age, cycling of seizure frequency or severity in female mice during estrous cycles was not observed, nor was a significant difference in seizure susceptibility found between sexes. In addition, the estrous cycle in mice is approximately 4–6 days, and each phase of the experiment was conducted for four days.
In conclusion, the Q54 model of epilepsy exhibits chronic spontaneous seizures at a reliable and sustainable high incidence that is maintained over several days. 3 Hz LFS significantly reduces seizure frequency and duration, but the effect is not immediate, supporting that underlying mechanisms of action may be complex and long lasting. The goal of this study was to evaluate the effect of low frequency deep brain stimulation applied to the VHC fibers on seizure activity. This study provides evidence to support the hypothesis that stimulation of a fiber tract at low frequency can reduce seizure frequency and duration but its effect may be limited to epileptogenic zones innervated by the stimulated fiber track. Furthermore, these data suggest that LFS might be a viable alternative to high frequency stimulation currently used in deep brain stimulation for the treatment of epileptic seizures. Future studies into the mechanisms of action of LFS are underway in order to guide the selection of optimal LFS parameters for the successful suppression of epileptic seizures in humans.
Supplementary Material
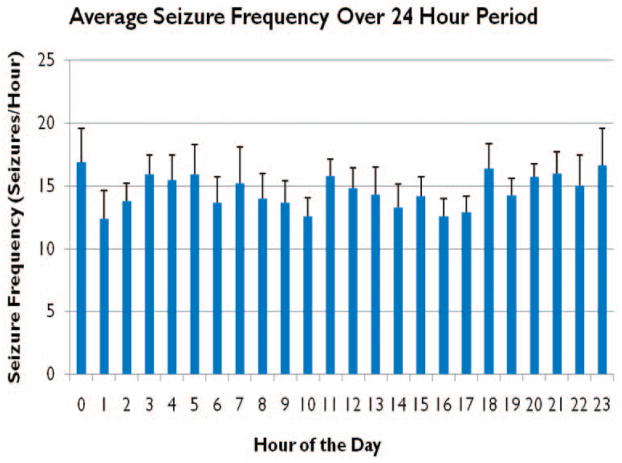
Figure 4. Daily Seizure Frequency.
Average seizure frequency over all animals (n=6) during baseline data was found to be 14.6±1 seizures/hr. Seizure frequency was slightly greater during the daytime hours (6am-6pm), 14.9±1 seizures/hr, versus 13.8±1 seizures/hr during the nighttime hours (6pm-6am), although this result was not found to be statistically significant (data is presented here as mean seizure frequency ± SEM).
Acknowledgments
This work is supported by a Grant from the Walter H Coulter Foundation, a Department of Education GAANN Neural Engineering Training Grant, and an Ohio Board of Reagents Innovative Incentive Fellowship. This work could not have been completed without the Q54 strain colony founders donated by Dr. Miriam Meisler and Dr. Jennifer Kearney, as well as the generous time and assistance provided by colleagues: Deborah Sim Barkauskas, Alicia Jensen, Andrew Kibler, David Tang, and Sheela Toprani. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Footnotes
None of the authors has any conflict of interest to disclose.
References
- Albensi BC, Oliver DR, Toupin J, Odero G. Electrical stimulation protocols for hippocampal synaptic plasticity and neuronal hyper-excitability: are they effective or relevant? Exp Neurol. 2007;204:1–13. doi: 10.1016/j.expneurol.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Albensi BC, Ata G, Schmidt E, Waterman JD, Janigro D. Activation of long-term synaptic plasticity causes suppression of epileptiform activity in rat hippocampal slices. Brain Res. 2004;998:56–64. doi: 10.1016/j.brainres.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Bazil CW, Pedley TA. Advances in the medical treatment of epilepsy. Annu Rev Med. 1998;49:135–162. doi: 10.1146/annurev.med.49.1.135. [DOI] [PubMed] [Google Scholar]
- Cain DP, Corcoran ME. Kindling with low-frequency stimulation: Generality, transfer, and recruiting effects. Exp Neurol. 1981;73(1):219–232. doi: 10.1016/0014-4886(81)90057-1. [DOI] [PubMed] [Google Scholar]
- Carrington CA, Gilby KL, McIntyre DC. Effect of focal low-frequency stimulation on amygdala-kindled afterdischarge thresholds and seizure profiles in fast- and slow-kindling rat strains. Epilepsia. 2007;48:1604–1613. doi: 10.1111/j.1528-1167.2007.01077.x. [DOI] [PubMed] [Google Scholar]
- Case AM, Reid RL. Menstrual cycle effects on common medical conditions. Comp Ther. 2001;27(1):65–71. doi: 10.1007/s12019-001-0010-8. [DOI] [PubMed] [Google Scholar]
- Chkhenkeli SA, Sramka M, Lortkipandize GS, Rakviashvili TN, Bregvadze ES, Magalashvili GE, Gagoshidze TS, Chkhenkeli IS. Electrophysiological effects and clinical results of direct brain stimulation for intractable epilepsy. Clin Neurol Neurosurg. 2004;106:318–329. doi: 10.1016/j.clineuro.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Christie BR, Kerr DS, Abraham WC. Flip side of synaptic plasticity: long-term depression mechanisms in the hippocampus. Hippocampus. 1994;4:127–135. doi: 10.1002/hipo.450040203. [DOI] [PubMed] [Google Scholar]
- Durand DM, Bikson M. Suppression and Control of Epileptiform Activity by Electrical Stimulation: A Review. Proceedings of the IEEE. 2001;89:1065–1082. [Google Scholar]
- Durand DM, Jensen A, Bikson M. Suppression of neural activity with high frequency stimulation. Conf Proc IEEE Eng Med Biol Soc. 2006;1:1624–1625. doi: 10.1109/IEMBS.2006.259396. [DOI] [PubMed] [Google Scholar]
- Enna SJ, Coyle JT. Pharmacological management of neurological and psychiatric disorders. New York: McGraw-Hill Health Professions Division; 1998. [Google Scholar]
- Gaito J. Gradient of interference by various frequencies on 60 Hz kindling behavior. Can J Neurol Sci. 1980;7:223–226. doi: 10.1017/s0317167100023246. [DOI] [PubMed] [Google Scholar]
- Gaito J, Nobrega JN, Gaito ST. Interference effect of 3 Hz brain stimulation on kindling behavior induced by 60 Hz stimulation. Epilepsia. 1980;21:73–84. doi: 10.1111/j.1528-1157.1980.tb04046.x. [DOI] [PubMed] [Google Scholar]
- Gleissner U, Helmstaedter C, Schramm J, Elger C. Memory outcome after selective amygdalophippocampaectomy: a study in 140 patients with temporal lobe epilepsy. Epilepsia. 2002;43:87–95. doi: 10.1046/j.1528-1157.2002.24101.x. [DOI] [PubMed] [Google Scholar]
- Gloor P, Salanova V, Olivier A, Quesney LF. The human dorsal hippocampal commissure. An anatomically identifiable and functional pathway. Brain. 1993;116 (Pt 5):1249–1273. doi: 10.1093/brain/116.5.1249. [DOI] [PubMed] [Google Scholar]
- Goddard GV, McIntyre DC, Leech CK. A permanent change in brain function resulting from daily electrical stimulation. Exp Neurol. 1969;25:295–330. doi: 10.1016/0014-4886(69)90128-9. [DOI] [PubMed] [Google Scholar]
- Goodman JH, Berger RE, Tcheng TK. Preemptive low-frequency stimulation decreases the incidence of amygdala-kindled seizures. Epilepsia. 2005;46:1–7. doi: 10.1111/j.0013-9580.2005.03804.x. [DOI] [PubMed] [Google Scholar]
- Gottlieb D, Cowan W. Autoradiographic studies of the commissural and ipsilateral association connections of the hippocampus and dentate gyrus of the rat. J Comp Neurol. 1973;149:393–422. doi: 10.1002/cne.901490402. [DOI] [PubMed] [Google Scholar]
- Halpern C, Hurtig H, Jaggi J, Grossman M, Won M, Baltuch G. Deep brain stimulation in neurologic disorders. Parkinsonism Relat Disord. 2007;13:1–16. doi: 10.1016/j.parkreldis.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Hauser WA, Annegers JF, Kurland LT. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935–1984. Epilepsia. 1993;34:453–468. doi: 10.1111/j.1528-1157.1993.tb02586.x. [DOI] [PubMed] [Google Scholar]
- Jerger K, Schiff SJ. Periodic pacing an in vitro epileptic focus. J Neurophysiol. 1995;73:876–879. doi: 10.1152/jn.1995.73.2.876. [DOI] [PubMed] [Google Scholar]
- Kearney JA, Plummer NW, Smith MR, Kapur J, Cummins TR, Waxman SG, Goldin AL, Meisler MH. A gain-of-function mutation in the sodium channel gene Scn2a results in seizures and behavioral abnormalities. Neuroscience. 2001;102:307–317. doi: 10.1016/s0306-4522(00)00479-6. [DOI] [PubMed] [Google Scholar]
- Kile KB, Tian N, Durand DM. Scn2a sodium channel mutation results in hyperexcitability in the hippocampus in vitro. Epilepsia. 2008;49:488–499. doi: 10.1111/j.1528-1167.2007.01413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita M, Ikeda A, Begum T, Yamamoto J, Hitomi T, Shibasaki H. Low-frequency repetitive transcranial magnetic stimulation for seizure suppression in patients with extratemporal lobe epilepsy – a pilot study. Seizure. 2005;14:387–392. doi: 10.1016/j.seizure.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Lüders HO. Deep brain stimulation and epilepsy. New York: Martin Dunitz; 2004. [Google Scholar]
- McIntyre CC, Savasta M, Kerkerian-Le Goff L, Vitek JL. Uncovering the mechanism(s) of action of deep brain stimulation: activation, inhibition, or both. Clin Neurophysiol. 2004;115:1239–48. doi: 10.1016/j.clinph.2003.12.024. [DOI] [PubMed] [Google Scholar]
- Markram H, Lubke J, Frotscher M, Sakmann B. Regulation of synaptic efficacy by coincidence of postynaptic APs and EPSPs. Science. 1997;275(5297):213–5. doi: 10.1126/science.275.5297.213. [DOI] [PubMed] [Google Scholar]
- Meisler MH, Kearney JA. Sodium channel mutations in epilepsy and other neurological disorders. J Clin Invest. 2005;115(8):2010–7. doi: 10.1172/JCI25466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minabe Y, Tanii Y, Kadono Y, Tsutsumi M, Nakaura I. Low-frequency kindling as a new model of epilepsy. Exp Neurol. 1986;94(3):317–323. doi: 10.1016/0014-4886(86)90105-6. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. San Diego, Calif. London: Academic; 2001. [Google Scholar]
- Penfield W, Baldwin M. Temporal lobe seizures and the technique of subtotal temporal lobectomy. Ann Surg. 1952;136:625–34. [PMC free article] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Schiller Y, Bankirer Y. Cellular mechanisms underlying antiepileptuic effects of low- and high- frequency electrical stimulation in acute epilepsy in neorcortical brain slices in vitro. J Neurophysiol. 2007;97:1887–1902. doi: 10.1152/jn.00514.2006. [DOI] [PubMed] [Google Scholar]
- Schrader LM, Stern JM, Wilson CL, Fields TA, Salamon N, Nuwer MR, Vespa PM, Fried I. Low frequency electrical stimulation through subdural electrodes in a case of refractory status epilepticus. Clin Neurophysiol. 2006;117:781–788. doi: 10.1016/j.clinph.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Velisek L, Veliskova J, Stanton PK. Low-frequency stimulation of the kindling focus delays basolateral amygdala kindling in immature rats. Neurosci Lett. 2002;326:61–63. doi: 10.1016/s0304-3940(02)00294-x. [DOI] [PubMed] [Google Scholar]
- Weiss SR, Li XL, Rosen JB, Li H, Heynen T, Post RM. Quenching: inhibition of development and expression of amygdala kindled seizures with low frequency stimulation. Neuroreport. 1995;6:2171–2176. [PubMed] [Google Scholar]
- Yu H, Neimat JS. The treatment of movement disorders by deep brain stimulation. Neurotherapeutics. 2008;5:26–36. doi: 10.1016/j.nurt.2007.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu-Ge ZB, Zhu YY, Wu DC, Wang S, Liu LY, Hu WW, Chen Z. Unilateral low-frequency stimulation of central piriform cortex inhibits amygdaloid-kindled seizures in Sprague-Dawley rats. Neuroscience. 2007;146:901–906. doi: 10.1016/j.neuroscience.2007.02.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.