Abstract
CD8+ T cells are critical for effective host defenses against viral infections. Studies addressing HIV-induced immune responses in infected individuals have suggested that CD8+ T cells play an important role in controlling viral replication. However, despite an abundance of HIV-specific CD8+ T cells, HIV is not contained in many untreated patients. Active HIV replication is associated with numerous immunologic changes, the most notable and consistent of which is an increase in CD8+ T cells expressing CD38. Previous studies have demonstrated that the expression of CD38 on CD8+ T cells is associated with poor prognostic outcome in infected individuals with detectable plasma viremia; however, the relationship between the expression of CD38 and the frequency of HIV-specific CD8+ T cells is unclear. We demonstrate a correlation between levels of HIV-specific CD8+ T cells and levels of CD8+ T cells expressing CD38 in untreated patients. The distribution of HIV-specific CD8+ T cells was heavily skewed toward CD38+CD8+ T cells in patients with a high percentage of CD38+CD8+ T cells. Spontaneous/Fas-mediated apoptosis in CD38+CD8+ T cells was significantly higher in patients with high percentages of CD38+CD8+ T cells. Our data suggest that a substantial proportion of the HIV-specific CD8+ T cells present in CD38+CD8+ T cells in patients with active viral replication arise by HIV-driven aberrant immune activation and may not manifest effective cytolytic activity against infected targets due to a high degree of susceptibility to apoptosis, thus providing an explanation of why HIV is not successfully contained by CD8+ T cells in such individuals.
CD8+ T cell-mediated immune responses play an important role in host defenses against viral infections. Previous studies of immune responses in HIV-infected individuals have suggested that CD8+ T cells play an important role in controlling viral replication, as evidenced by the emergence of HIV-specific CD8+ cytotoxic T lymphocyte (CTL) activity coinciding with a decline in plasma viremia during primary infection (1, 2) and a diminution of CTL responses preceding disease progression in infected individuals (3, 4). With the recent advent of highly sensitive immunologic assays (5–8), significant progress has been made in understanding the role of HIV-specific CD8+ T cells in the pathogenesis of HIV infection. However, recent studies addressing the relationship between the frequency of HIV-specific CD8+ T cells and the levels of plasma viremia have yielded conflicting results (8–10). One study that used HLA–tetrameric complexes that recognize CD8+ T cells bearing certain T cell receptors has shown an inverse relationship between the frequency of tetramer-positive CD8+ T cells and the level of plasma viremia in infected individuals (9). However, subsequent studies that have examined the capacity of CD8+ T cells to express intracellular IFN-γ in response to a broad range of HIV antigens have shown that a large proportion of infected individuals harbor significant numbers of HIV-specific CD8+ T cells and yet fail to effectively control viral replication, as manifested by high levels of plasma viremia (8, 10).
The deleterious effects of active viral replication on immunologic functions of various subsets of lymphocytes in HIV-infected individuals have been well documented (11–16). Among those, expression of CD38, a surface antigen with multiple functions on CD8+ T cells (17), has been well accepted as a prognostic marker for disease progression in infected individuals who do not control viral replication (18, 19). In addition, it has been speculated that an increase of CD8+ T cells expressing CD38 is a reflection of HIV-mediated immune activation and that these cells are ultimately unable to control viral replication or prevent disease progression (18). The present study was conducted to gain further insight into the pathophysiologic relationship between HIV-specific CD8+ T cells and CD38 expression that results from HIV-driven aberrant immune activation in patients who are not receiving antiviral therapy and to provide a feasible explanation of why HIV is not successfully contained by HIV-specific CD8+ T cells in such individuals.
Materials and Methods
Study Subjects. Forty-two HIV-1-infected individuals who were not receiving antiviral therapy were studied. The mean CD4+ and CD8+ T cell counts and median plasma viremia were 502 cells per mm3 (range, 140–1,190), 1,096 cells per mm3 (range, 390–2,630), and 11,815 copies of HIV RNA per ml of plasma (range, from <50 to 750,000), respectively. Plasma viremia was determined by either RT-PCR (Roche) or ultrasensitive bDNA assay (Chiron) with the lower limit of detection of 50 copies of HIV RNA per ml of plasma. Leukapheresis was conducted in accordance with protocols approved by the Institutional Review Board of the University of Toronto and the National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Stimulation of CD8+ T Cells and Flow Cytometric Analysis of HIV-Specific CD8+ T Cells. Peripheral blood mononuclear cells (PBMC) were obtained from leukapheresis by Ficoll–Hypaque density gradient centrifugation. The frequency of CD8+ T cells specific for HIV was determined by analysis of intracellular IFN-γ-positive cells after stimulation with HIV-1-specific peptides (15-mers overlapping 10 aa). Pools of overlapping peptides spanning HIV-1 Gag, Pol, Env, Nef, Tat, Rev, Vpr, Vif, and Vpu proteins (1 μg each, National Institutes of Health AIDS Reagent Program) and cytomegalovirus (CMV) pp65 protein (BD Pharmingen) were initially incubated with 3 × 106 PBMCs for 10 min in round-bottom 5-ml tubes (BD Biosciences) in the presence of 1 μg of anti-CD28 and CD49d antibody in 0.1 ml of complete medium in a 37°CCO2 incubator. Subsequently, 0.4 ml of complete medium was added to the tube, and the contents were incubated for 2 hours. After the 2-hour incubation, brefeldin A (Sigma) was added to the medium at a final concentration of 10 μg/ml to inhibit secretion of IFN-γ. After 6 h of incubation, the cells were washed twice and fixed with 1× fixing solution (BD Biosciences) for 10 min at room temperature followed by another washing. Cells were permeabilized with 1× Permeabilization Solution 2 (BD Biosciences) and further incubated at room temperature for 10 min. After washing, cells were stained with the following antibodies: FITC-conjugated anti-CD8 (BD Biosciences), phycoerythrin-conjugated anti-IFN-γ (BD Pharmingen), Cy5-conjugated anti-CD38 (Beckman Coulter), and allophycocyanin-conjugated anti-CD3 antibodies (BD Biosciences). With a gate set on CD3+CD8+ lymphocytes, ≈100,000 events were collected by using FACSCalibur (BD Biosciences), and the frequency of IFN-γ+ cells within the CD3+/CD8+/CD38- and CD3+/CD8+/CD38+ population was determined by using cellquest software (BD Biosciences).
Apoptosis Assays. Freshly isolated CD8+ T cells were incubated at 37°C overnight with complete medium in the presence or absence of FLAG-tagged Fas ligand (Kamiya Biomedical, Thousand Oaks, CA). Cells were collected, washed, and stained with phycoerythrin-conjugated anti-CD95 (BD Biosciences) and Cy5-conjugated anti-CD38 (Beckman Coulter) antibodies. After incubation and washing, the cells were resuspended in annexin V binding buffer (BD Pharmingen) and stained with FITC-labeled annexin V according to manufacturer's specifications (BD Pharmingen). Stained cells were analyzed by using FACSCalibur (BD Biosciences), and the frequency of annexin V-positive cells within the CD38-CD8+ and CD38+CD8+ population (in some experiments, CD95 was included in fluorescence-activated cell sorter analysis) was determined by using cellquest (BD Biosciences) and flowjo (Tree Star, Ashland, OR) software.
Statistical Analysis. The correlation between variables was determined by Spearman rank correlation. Medians and distributions of the two independent groups defined by levels of CD38+CD8+ T cells and plasma viremia, levels of CD38+CD8+ T cells and degrees of apoptosis, and levels of CD38+CD8+ T cells and CD95 expression were compared by the Wilcoxon two-sample test. The Bonferroni method was used to adjust P values for multiple testing.
Results
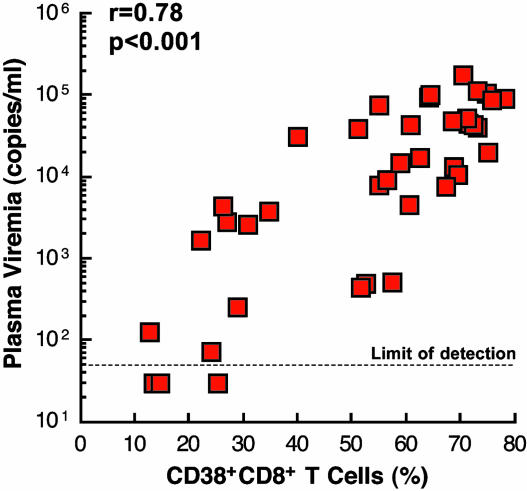
Relationship Between CD38 Expression on CD8+ T Cells and Plasma Viremia. We first sought to establish the relationship between the proportion of CD8+ T cells expressing CD38 and the level of plasma viremia in 42 HIV-1-infected individuals (including a number of long-term non-progressors) who were not receiving any antiretroviral therapy at the time of study (Table 1). A strong positive correlation was observed between these two parameters (Fig. 1; P < 0.001), suggesting that active HIV replication has a profound impact on the expression of CD38 on CD8+ T cells.
Table 1. Profiles of HIV-1-infected patients.
| Patient | Plasma viremia* (copies/ml) | CD4+ T cell count per μl | CD8+ T cell count per μl | CD38+ CD8+ T cells, % |
|---|---|---|---|---|
| 1 | 125 | 1,190 | 1,163 | 12.6 |
| 2 | <50 | 640 | 740 | 13.3 |
| 3 | <50 | 140 | 1,080 | 14.7 |
| 4 | <50 | 780 | 390 | 15.4 |
| 5 | 1,673 | 484 | 1,169 | 22.2 |
| 6 | 72 | 740 | 620 | 24.1 |
| 7 | <50 | 840 | 610 | 25.3 |
| 8 | 4,336 | 440 | 610 | 26.3 |
| 9 | 2,778 | 1,000 | 1,580 | 26.9 |
| 10 | 253 | 474 | 428 | 29.0 |
| 11 | 2,635 | 509 | 1,066 | 30.9 |
| 12 | 3,771 | 587 | 747 | 34.8 |
| 13 | 30,707 | 517 | 749 | 40.1 |
| 14 | 39,595 | 280 | 1,190 | 51.0 |
| 15 | 437 | 704 | 737 | 51.5 |
| 16 | 495 | 1,140 | 670 | 52.5 |
| 17 | 74,477 | 586 | 422 | 55.0 |
| 18 | 8,045 | 548 | 890 | 55.1 |
| 19 | 9,282 | 640 | 2,120 | 56.4 |
| 20 | 750,000 | 214 | 1,242 | 57.4 |
| 21 | 523 | 449 | 881 | 57.5 |
| 22 | 15,049 | 746 | 2,344 | 58.9 |
| 23 | 4,495 | 918 | 1,232 | 60.6 |
| 24 | 5,875 | 480 | 2,020 | 60.6 |
| 25 | 44,122 | 460 | 740 | 60.8 |
| 26 | 17,361 | 340 | 2,630 | 62.5 |
| 27 | 94,973 | 214 | 1,960 | 64.2 |
| 28 | 100,000 | 230 | 960 | 64.4 |
| 29 | 7,731 | 507 | 1,213 | 67.3 |
| 30 | 47,719 | 290 | 1,480 | 68.5 |
| 31 | 13,130 | 210 | 740 | 68.7 |
| 32 | 10,500 | 571 | 710 | 69.4 |
| 33 | 175,956 | 280 | 1,110 | 70.4 |
| 34 | 52,178 | 500 | 790 | 71.1 |
| 35 | 44,846 | 280 | 500 | 71.5 |
| 36 | 42,812 | 541 | 1,271 | 72.5 |
| 37 | 113,067 | 571 | 2,319 | 73.0 |
| 38 | 40,260 | 295 | 1,198 | 73.2 |
| 39 | 105,497 | 180 | 1,290 | 74.9 |
| 40 | 20,220 | 210 | 840 | 75.0 |
| 41 | 88,592 | 200 | 1,110 | 75.8 |
| 42 | 91,561 | 149 | 456 | 78.1 |
Measured by Amplicor or ultracensitive bDNA assay with a detection limit of 50 copies per ml of plasma
Fig. 1.
Correlation between plasma viremia and levels of CD8+ T cells expressing CD38. The level of CD38 expression was determined by flow cytometry by measuring the expression of CD38 on CD3+CD8+ T lymphocytes. The P value was determined by the Spearman rank correlation.
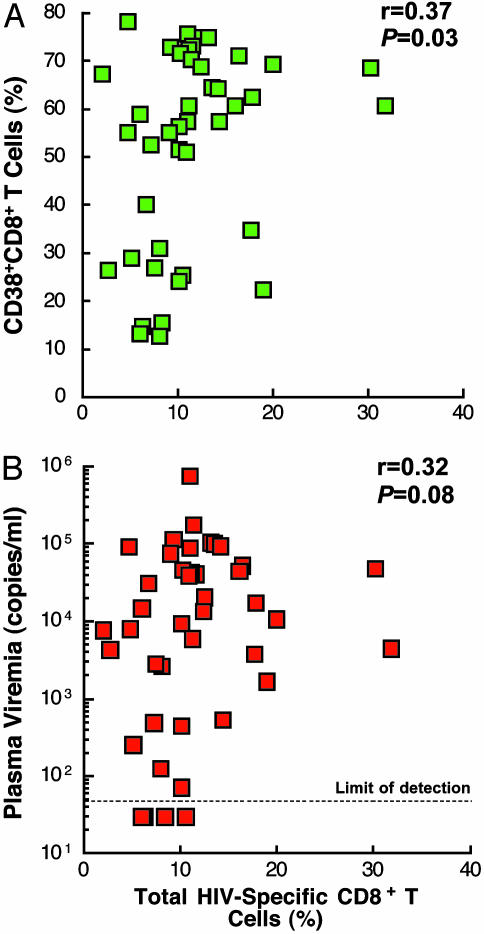
Relationship Between the Frequency of HIV-Specific CD8+ T Cells and Plasma Viremia and the Frequency of HIV-Specific CD8+ T Cells and Levels of CD38. To determine the relationship between HIV-specific CD8+ T cells and CD8+ T cells expressing CD38 in infected individuals, we determined the proportion of cells expressing intracellular IFN-γ using 15-mer overlapping peptides spanning the entire HIV genome and, as a control, pp65 protein from CMV strain AD169 (BD Pharmingen). Upon antigenic stimulation of peripheral blood mononuclear cells for a short (6-h) incubation, the frequency of CD8+ T cells expressing intracellular IFN-γ (referred to as HIV-specific CD8+ T cells hereafter) was determined by four-color flow cytometric analysis. Two separate gates were used to determine the frequency of HIV-specific CD8+ T cells within the CD3+/CD8+/CD38- and CD3+/CD8+/CD38+ populations of cells. Previous studies examining the role of CD8+ T cell-mediated antiviral immunity in HIV infection have mainly focused on the relationship between the frequency of HIV-specific CD8+ T cells and plasma viremia (8, 9). To investigate whether the level of CD38+CD8+ T cells can be correlated with the level of HIV-specific CD8+ T cells in infected patients with high plasma viremia, the relationship between total HIV-specific CD8+ T cells and the level of CD38+CD8+ T cells and between total HIV-specific CD8+ T cells and plasma viremia were determined. As shown in Fig. 2, a more significant correlation was found between total HIV-specific CD8+ T cells and the level of CD38+CD8+ T cells (Fig. 2 A) than between total HIV-specific CD8+ T cells and plasma viremia (Fig. 2B), suggesting that the percentage of CD8+ T cells expressing CD38, along with plasma viremia, can be used as a marker for predicting the level of HIV-specific CD8+ T cells in infected patients in the absence of antiviral therapy.
Fig. 2.
Correlation between the frequency of HIV-specific CD8+ T cells and the level of CD8+ T cells expressing CD38 (A) and the frequency of HIV-specific CD8+ T cells and plasma viremia (B). The total frequency of HIV-specific CD8+ T cells was determined by combining the percentage of CD8+ T cells expressing intracellular IFN-γ in both CD38-CD8+ and CD38+CD8+ T cells upon antigen stimulation. The P values were determined by the Spearman rank correlation.
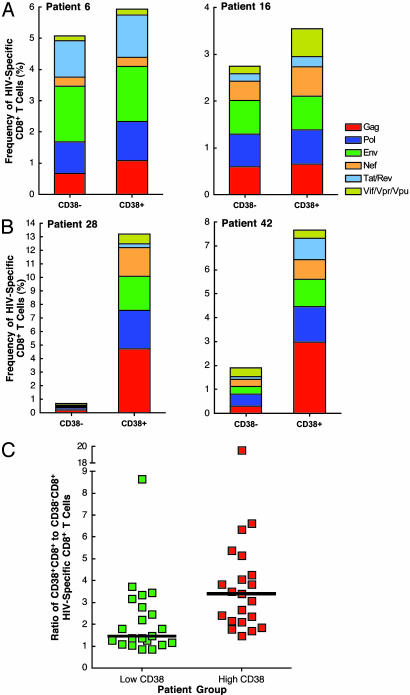
Distribution of HIV-Specific CD8+ T Cells in CD38-CD8+ and CD38+CD8+ T Cells. To examine the distribution of HIV-specific CD8+ T cells within CD38-CD8+ and CD38+CD8+ T cells, we first divided the study subjects into two groups, a low-CD38 group (median, 30.9%; range, 12.6–57.5) and a high-CD38 grou[(median, 69.4%; range, 58.9–78.1), based on the median value of CD8+ T cells expressing CD38 in all patients (58.2%). Fig. 3 shows representative data from two patients in the low-CD38 group (Fig. 3A) and two patients in the high-CD38 group (Fig. 3B). HIV-specific CD8+ T cells were found to be distributed relatively evenly in both populations of CD8+ T cells in the low-CD38 group. In contrast, the distribution of HIV-specific CD8+ T cells was heavily skewed toward the CD38+CD8+ T cells in the high-CD38 group. When the ratios of CD38+CD8+ to CD38-CD8+ HIV-specific CD8+ T cells of all study subjects were stratified based on the low- and high-CD38 groups, a significantly skewed distribution of HIV-specific CD8+ T cells in the CD38+CD8+ T cell population was observed in the high-CD38 group (Fig. 3C; P = 0.002). These results suggest that the majority of HIV-specific CD8+ T cells localize within the CD38+CD8+ T cell population in infected individuals with active viral replication and suggest that HIV-specific CD8+ T cells expressing CD38 may be poor antiviral effectors. In addition, the presence of HIV-specific CD8+ T cells within the CD38-CD8+ T cell population may play a key role in containment of viral replication in infected individuals with low levels of CD38+CD8+ T cells.
Fig. 3.
Distribution of HIV-specific CD8+ T cells in subsets of CD38-CD8+ and CD38+CD8+ T cells. The frequency of HIV-specific CD8+ T cells was determined in the subsets of CD38-CD8+ and CD38+CD8+ T cells. The data from two representative patients in the low-CD38 group (A) and two representative patients in the high-CD38 group (B) are shown. The ratios of CD38+CD8+ to CD38-CD8+ HIV-specific CD8+ T cells were stratified by the low-CD38 and high-CD38 groups of patients (C). The median of HIV-specific CD8+ T cells is shown as a black bar in each patient group.
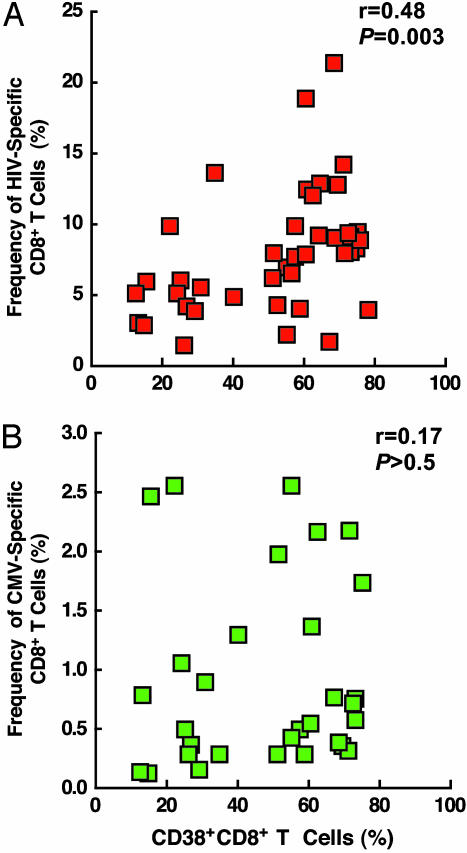
Relationship Between HIV- and CMV-Specific CD8+ T Cells and CD38 Expression. To further delineate the relationship between HIV-specific CD8+ T cells and CD38 expression, the correlation between the frequency of HIV-specific CD8+ T cells within the subset of CD38+CD8+ T cells and the levels of CD8+ T cells expressing CD38 was determined. In addition, similar experiments were conducted by using CMV peptides to address whether HIV-induced aberrant immune activation has any impact on antigen-specific CD8+ T cells other than HIV-specific CD8+ T cells. As shown in Fig. 4A, a statistically significant correlation was observed between the frequency of HIV-specific CD8+ T cells within the CD38+CD8+ T cell subset and the level of CD38+CD8+ T cells (r = 0.48 and P = 0.003), but not between the frequency of CMV-specific CD8+ T cells within the CD38+CD8+ T cell subset and the level of CD38+CD8+ T cells (r = 0.17 and P > 0.5). These results demonstrate that the high levels of HIV-specific CD8+ T cells in infected individuals with active viral replication are found in a functionally abnormal subpopulation of CD8+ T cells, i.e., CD38+CD8+ T cells. In addition, the lack of correlation between CMV-specific CD8+ T cells and the level of CD38+CD8+ T cells suggest that the above observation is due mainly to direct and/or indirect consequences of HIV-mediated aberrant immune activation in infected individuals.
Fig. 4.
Correlation between the frequency of HIV- and CMV-specific CD8+ T cells within the population of CD38+CD8+ T cells and the level of CD8+ T cells expressing CD38. (A) The correlation between the frequency of HIV-specific CD8+ T cells within CD38+CD8+ T cells and the level of CD38 expression on CD8+ T cells were determined. (B) The correlation between the frequency of CMV-specific CD8+ T cells within CD38+CD8+ T cells and the level of CD38 expression on CD8+ T cells were determined. The P values were determined by the Spearman rank correlation.
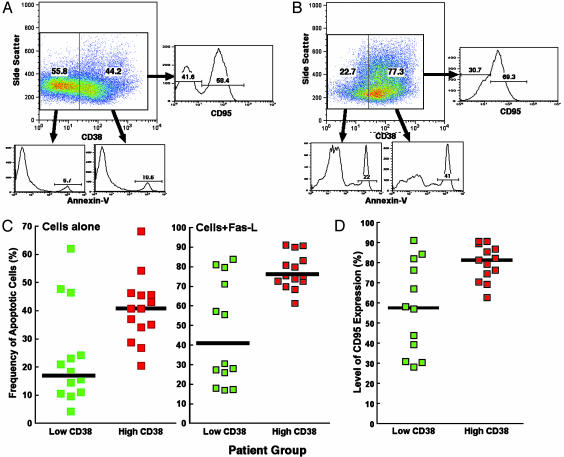
Relationship Between Levels of Spontaneous and Fas-Mediated Apoptosis and Levels of CD38 Expression on CD8+ T Cells. To delineate possible explanations for the lack of containment of viral replication despite an abundance of HIV-specific CD8+ T cells in the CD38+CD8+ T cell compartment of infected individuals whose CD8+ T cells expressed a high level of CD38, we determined the levels of spontaneous and Fas-mediated apoptosis in CD38-CD8+ and CD38+CD8+ T cells using additional patients. Freshly isolated CD8+ T cells were incubated overnight in the presence or absence of Fas ligand, and the levels of apoptosis in CD38-CD8+ and CD38+CD8+ T cells were determined (Fig. 5 A and B). The levels of spontaneous apoptosis in the CD38-CD8+ T cell population were not significantly different between the low-CD38 group (median, 11.0%; range, 0.9–43.2) and the high-CD38 group (median, 22.5%; range, 7.7–44.0) (P = 0.15; data not shown). However, the levels of spontaneous apoptosis were significantly higher in CD38+CD8+ T cells of the high-CD38 group (median, 40.8%; range, 20.5–68.2) relative to those of the low-CD38 group (median, 17.0%; range, 4.2–62.1) of infected individuals (P = 0.02) (Fig. 5C). When Fas ligand was added to the CD8+ T cell culture, no statistical difference was observed in the CD38-CD8+ T cell population between two patient groups (P > 0.5; data not shown); however, the levels of Fas-mediated apoptosis were significantly higher in CD38+CD8+ T cells of the high-CD38 group (median, 75.3%; range, 61.2–91.0) compared with those of the low-CD38 group (median, 40.1%; range, 17.0–83.9) (P < 0.01) of patients (Fig. 5C). The enhanced levels of apoptosis were associated with upregulation of CD95 on CD38+CD8+ T cells in the high-CD38 group (median, 81.5%; range, 62.8–90.8) relative to those of the low-CD38 group (median, 57.6%; range, 28.2–91.2) of patients (P = 0.03). Indeed, the highest levels of apoptosis were observed in the subset of CD38+CD95+CD8+ T cells (data not shown). A significant correlation was observed between the levels of CD95 expression and the degrees of apoptosis in the CD38+CD8+ T compartment of both the low-CD38 group (r = 0.86 and P < 0.001) and the high-CD38 group (r = 0.74 and P < 0.01). These results suggest that the substantial proportion of CD8+ T cells expressing CD38 is highly susceptible to spontaneous and Fas-mediated (as a consequence of up-regulation of CD95) apoptosis, resulting in a population of cells that has a poor antiviral effector function despite the presence of high levels of HIV-specific CD8+ T cells.
Fig. 5.
Relationship between degrees of spontaneous and Fas-mediated apoptosis and levels of CD38 expression in CD8+ T cells. The degree of apoptosis in CD38-CD8+ and CD38+CD8+ T cells from representative patients from the low-CD38 group (A) and the high-CD38 group (B). The frequency of cells undergoing spontaneous and Fas-mediated apoptosis was determined in the CD38+CD8+ T cell compartment in each group of patients (C). The levels of CD38+CD8+ T cells expressing CD95 were also determined (D).
Discussion
Recent developments in immunologic assays that accurately and specifically measure T cell responses against various antigens (5–8) have significantly advanced our understanding of the pathogenesis of HIV, particularly the role of CD8+ T cells in viral infection. Using an assay that measures CD8+ T cell responses against a broad range of HIV-derived peptides that eliminates the HLA-haplotype requirement of an antigen-presenting cell, we have examined the relationship between HIV-specific CD8+ T cell responses and the level of CD8+ T cells that express CD38. One of the most prominent features of HIV infection is chronic elevation of the level of CD8+ T cells in infected individuals with high levels of viremia (12). Among the many immunologic surface markers expressed on CD8+ T cells, the presence of CD38 has consistently been shown to be associated with disease progression in HIV infection, and its expression has been speculated to be directly associated with HIV-mediated immune activation that renders such CD8+ T cells unable to control viral replication or prevent disease progression (18, 19). Given recent controversies regarding the association between the frequency of HIV-specific CD8+ T cells and plasma viremia (8–10), we addressed the above issue in 42 HIV-infected individuals, who were not receiving antiviral therapy, by incorporating the level of CD38 expression on CD8+ T cells in our analyses. We found a significant correlation between the frequency of HIV-specific CD8+ T cells and the level of CD38+CD8+ T cells in infected individuals we examined. We observed a significantly skewed distribution of HIV-specific CD8+ T cells in the population of CD38+CD8+ T cells in those individuals harboring a high frequency of CD8+ T cells expressing CD38. The strongest correlation was found between the level of HIV-specific CD8+ T cells within the CD38+CD8+ T cell population and the proportion of CD8+ T cells expressing CD38; this correlation was not observed in the case of CMV-specific CD8+ T cells.
In an effort to delineate the possible defect in CD38+CD8+ T cells of the high-CD38 group of patients, we investigated the role of spontaneous and Fas-mediated apoptosis in this population of cells. It has been proposed that apoptosis plays an important role in the pathogenesis of HIV infection and is a possible cause of the loss of CD4+ T cells in infected individuals (20). Several studies have demonstrated enhanced levels of spontaneous and/or Fas-mediated cell death in a number of subsets of lymphocytes (21–25), including HIV-specific CD8+ T cells of HIV-infected individuals (26). In particular, one study has reported that HIV-specific CD8+ T cells are more prone to CD95/Fas-induced apoptosis compared with CMV-specific CD8+ T cells in some infected individuals (26). Our present study provides a mechanistic explanation for this observation by demonstrating that a substantial proportion of CD8+ T cells expressing CD38 in infected individuals with active viral replication are susceptible to spontaneous and Fas-mediated cell death. Considering that the bulk of HIV-specific CD8+ T cells resides within the CD38+CD8+ T cells of infected individuals and that these cells are prone to apoptosis (26), our findings add yet another possible explanation for the apparent failure of containment of HIV in certain infected individuals. In this regard, increased levels of CD38 expression and susceptibility to spontaneous and CD95/Fas-mediated apoptosis in CD8+ T cells of infected individuals with active viral replication may seriously compromise the effectiveness of HIV-specific CD8+ T cells in eliminating productively infected target cells. Future studies addressing possible causes of spontaneous apoptosis and the mechanism by which CD38+CD8+ T cells up-regulate CD95 expression along with strategies aimed at preventing HIV-induced immune activation should improve our understanding of the dynamic relationship between the physiologic state of HIV-specific cytotoxic T lymphocytes and containment of viral replication in HIV-infected patients.
Acknowledgments
We thank the patients for their participation in this study. We also thank Paul Parks, Rowena Dimayuga, and the staff of the National Institute of Allergy and Infectious Diseases HIV Clinic for their invaluable assistance with the execution of this study.
Abbreviation: CMV, cytomegalovirus.
References
- 1.Borrow, P., Lewicki, H., Hahn, B. H., Shaw, G. M. & Oldstone, M. B. (1994) J. Virol. 68, 6103-6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koup, R. A., Safrit, J. T., Cao, Y., Andrews, C. A., McLeod, G., Borkowsky, W., Farthing, C. & Ho, D. D. (1994) J. Virol. 68, 4650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carmichael, A., Jin, X., Sissons, P. & Borysiewicz, L. (1993) J. Exp. Med. 177, 249-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klein, M. R., van Baalen, C. A., Holwerda, A. M., Kerkhof Garde, S. R., Bende, R. J., Keet, I. P., Eeftinck-Schattenkerk, J. K., Osterhaus, A. D., Schuitemaker, H. & Miedema, F. (1995) J. Exp. Med. 181, 1365-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Altman, J. D., Moss, P. A., Goulder, P. J., Barouch, D. H., McHeyzer-Williams, M. G., Bell, J. I., McMichael, A. J. & Davis, M. M. (1996) Science 274, 94-96. [DOI] [PubMed] [Google Scholar]
- 6.Kern, F., Surel, I. P., Brock, C., Freistedt, B., Radtke, H., Scheffold, A., Blasczyk, R., Reinke, P., Schneider-Mergener, J., Radbruch, A., et al. D. (1998) Nat. Med. 4, 975-978. [DOI] [PubMed] [Google Scholar]
- 7.Appay, V., Nixon, D. F., Donahoe, S. M., Gillespie, G. M., Dong, T., King, A., Ogg, G. S., Spiegel, H. M., Conlon, C., Spina, C. A., et al. (2000) J. Exp. Med. 192, 63-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Betts, M. R., Ambrozak, D. R., Douek, D. C., Bonhoeffer, S., Brenchley, J. M., Casazza, J. P., Koup, R. A. & Picker, L. J. (2001) J. Virol. 75, 11983-11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ogg, G. S., Jin, X., Bonhoeffer, S., Dunbar, P. R., Nowak, M. A., Monard, S., Segal, J. P., Cao, Y., Rowland-Jones, S. L., Cerundolo, V., et al. (1998) Science 279, 2103-2106. [DOI] [PubMed] [Google Scholar]
- 10.Gea-Banacloche, J. C., Migueles, S. A., Martino, L., Shupert, W. L., McNeil, A. C., Sabbaghian, M. S., Ehler, L., Prussin, C., Stevens, R., Lambert, L., et al. (2000) J. Immunol. 165, 1082-1092. [DOI] [PubMed] [Google Scholar]
- 11.Lane, H. C., Masur, H., Edgar, L. C., Whalen, G., Rook, A. H. & Fauci, A. S. (1983) N. Engl. J. Med. 309, 453-458. [DOI] [PubMed] [Google Scholar]
- 12.Pantaleo, G. & Fauci, A. S. (1995) Annu. Rev. Immunol. 13, 487-512. [DOI] [PubMed] [Google Scholar]
- 13.Grossman, Z., Feinberg, M. B. & Paul, W. E. (1998) Proc. Natl. Acad. Sci. USA 95, 6314-6319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hazenberg, M. D., Hamann, D., Schuitemaker, H. & Miedema, F. (2000) Nat. Immunol. 1, 285-289. [DOI] [PubMed] [Google Scholar]
- 15.Moir, S., Malaspina, A., Ogwaro, K. M., Donoghue, E. T., Hallahan, C. W., Ehler, L. A., Liu, S., Adelsberger, J., Lapointe, R., Hwu, P., et al. (2001) Proc. Natl. Acad. Sci. USA 98, 10362-10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kottilil, S., Chun, T. W., Moir, S., Liu, S., McLaughlin, M., Hallahan, C. W., Maldarelli, F., Corey, L. & Fauci, A. S. (2003) J. Infect. Dis. 187, 1038-1045. [DOI] [PubMed] [Google Scholar]
- 17.Savarino, A., Bottarel, F., Malavasi, F. & Dianzani, U. (2000) AIDS 14, 1079-1089. [DOI] [PubMed] [Google Scholar]
- 18.Giorgi, J. V., Liu, Z., Hultin, L. E., Cumberland, W. G., Hennessey, K. & Detels, R. (1993) J. Acquired Immune Defic. Syndr. 6, 904-912. [PubMed] [Google Scholar]
- 19.Liu, Z., Cumberland, W. G., Hultin, L. E., Prince, H. E., Detels, R. & Giorgi, J. V. (1997) J. Acquired Immune Defic. Syndr. Hum. Retrovirol. 16, 83-92. [DOI] [PubMed] [Google Scholar]
- 20.Meyaard, L., Otto, S. A., Jonker, R. R., Mijnster, M. J., Keet, R. P. & Miedema, F. (1992) Science 257, 217-219. [DOI] [PubMed] [Google Scholar]
- 21.Finkel, T. H., Tudor-Williams, G., Banda, N. K., Cotton, M. F., Curiel, T., Monks, C., Baba, T. W., Ruprecht, R. M. & Kupfer, A. (1995) Nat. Med. 1, 129-134. [DOI] [PubMed] [Google Scholar]
- 22.Bofill, M., Gombert, W., Borthwick, N. J., Akbar, A. N., McLaughlin, J. E., Lee, C. A., Johnson, M. A., Pinching, A. J. & Janossy, G. (1995) Am. J. Pathol. 146, 1542-1555. [PMC free article] [PubMed] [Google Scholar]
- 23.Muro-Cacho, C. A., Pantaleo, G. & Fauci, A. S. (1995) J. Immunol. 154, 5555-5566. [PubMed] [Google Scholar]
- 24.Samuelsson, A., Sonnerborg, A., Heuts, N., Coster, J. & Chiodi, F. (1997) AIDS Res. Hum. Retroviruses 13, 1031-1038. [DOI] [PubMed] [Google Scholar]
- 25.Gougeon, M. L. (2003) Nat. Rev. Immunol. 3, 392-404. [DOI] [PubMed] [Google Scholar]
- 26.Mueller, Y. M., De Rosa, S. C., Hutton, J. A., Witek, J., Roederer, M., Altman, J. D. & Katsikis, P. D. (2001) Immunity 15, 871-882. [DOI] [PubMed] [Google Scholar]