Abstract
X-rays have been used for non-invasive high-resolution imaging of thick biological specimens since their discovery in 1895. They are widely used for structural imaging of bone, metal implants, and cavities in soft tissue. Recently, a number of new contrast methodologies have emerged which are expanding X-ray’s biomedical applications to functional as well as structural imaging. These techniques are promising to dramatically improve our ability to study in situ biochemistry and disease pathology. In this review, we discuss how X-ray absorption, X-ray fluorescence, and X-ray excited optical luminescence can be used for physiological, elemental, and molecular imaging of vasculature, tumours, pharmaceutical distribution, and the surface of implants. Imaging of endogenous elements, exogenous labels, and analytes detected with optical indicators will be discussed.
1. Introduction
There are two broad categories for medical imaging by X-rays: structural imaging, which reveals anatomical structure, and functional imaging measuring changes in biological function including metabolism, blood flow, regional chemical composition and biochemical processes. X-rays are widely used in structural imaging of bone, teeth, microcalcinations, lungs, and orthopedic devices. However, endogenous soft tissue types are difficult to distinguish using conventional X-ray projection imaging. Distinguishing tissue types for functional imaging requires either exogenous contrast agents (e.g. radiopaque agents to view vasculature and flow in angiography), or technique that are more sensitive to tissue differences (or both). This review describes the opportunities for functional imaging based upon X-ray attenuation, X-ray fluorescence (XRF), and X-ray excited optical luminescence (XEOL) for non-invasive biochemical imaging. In addition, different types of contrast agents and their contrast mechanisms are discussed in order to increase the contrast to noise ratio and reduce the X-ray dose. These functional imaging techniques promise to dramatically improve our ability to study in situ biochemistry and disease pathology.
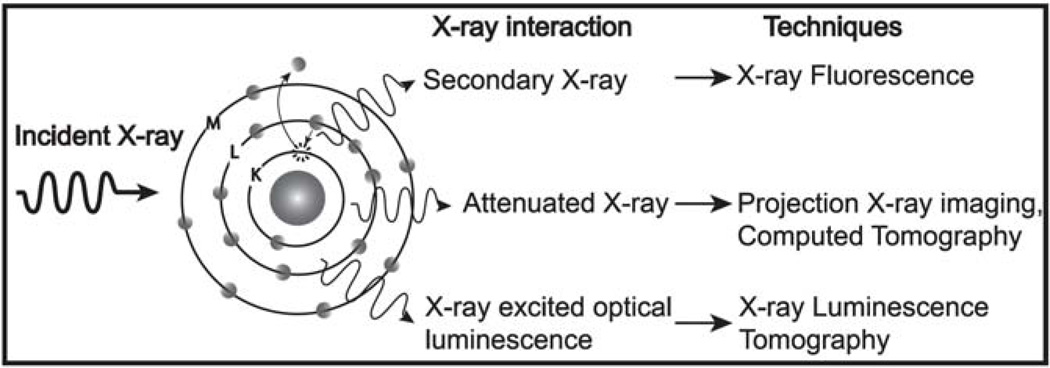
These various X-ray methodologies utilize several different types of interactions between X-rays and matter that may be employed for imaging and analysis (see Fig. 1). First, X-rays can be absorbed or scattered by the tissue thereby attenuating the transmitted X-ray intensity. This is the most widely used technique for structural, vasculature, and gastrointestinal tract imaging, however, it is not very sensitive to small amounts of X-ray absorption because noise on the transmitted X-ray signal can obscure small decreases due to attenuation. Second, when atoms in a tissue sample absorb X-rays, some of the energy is released via secondary X-ray emission (i.e. X-ray fluorescence, XRF). Each element has a unique XRF spectrum providing a robust fingerprint for elemental analysis. Third, the absorbed X-ray energy can also generate optical luminescence in scintillators such as rare-earth doped phosphors. Optical luminescence has been used to detect X-rays since Röntgen’s original discovery in 1895. In these studies, the scintillators are placed outside the tissue and used to detect X-ray attenuation and fluorescence. Recently, these nanophosphors have been injected into tissues as a contrast agent. The optical luminescence can be combined with colorimetric indicator dyes for high resolution chemical imaging in tissue. Each technique has advantages and limitations for various applications, and they can also be used together to provide complimentary structural and functional information.
Figure 1.
Diagram depicting the possible interactions between X-rays and a sample for various different X-ray techniques.
In addition to external X-ray sources, radioisotope-labeled analytes are widely used as in vivo molecular imaging contrast agents. They have been applied to many research and diagnostic applications including: the study of the biodistribution of pharmaceuticals and nanoparticles, glucose metabolism tracking for cancer identification, and imaging of beta-amyloid plaques in Alzheimer’s patients.1 Two principal imaging techniques are available, single photon emission computed tomography (SPECT), and positron emission tomography (PET). SPECT radioisotopes generate γ-rays directly or through bremsstrahlung radiation from high energy beta emitters, while PET radioisotopes emit positrons which generate a pair of anti-parallel 511 keV γ-rays upon annihilation with electrons in the sample. SPECT imaging requires collimation optics to localize the radio-emission source and most of the γ-rays are absorbed during this collimation. In contrast, physical collimators are not needed for PET imaging because the emission angle is known to lie along the line connecting the pair of simultaneously detected γ-rays. Consequently, PET provides two to three orders of magnitude more sensitivity than SPECT.2 PET also provides higher spatial resolution for large objects, ~1–3 mm for μ-PET and 5–10 mm for clinical-PET depending upon the sample size, imaging geometry and radioisotope,3–4 compared to ~15–20 mm for SPECT of a human brain,5 although high resolution SPECT is possible at the expense of sensitivity using pinhole apertures.2, 6 However, PET radioisotope contrast agents have several limitations. A cyclotron and on-site chemical synthesis facilities are necessary for generating radioisotopes and producing the radiopharmaceuticals.7 Furthermore, the short radioisotope half-life of most positron emitters (e.g. 20 minutes for 11C and 2 hours for 18F) makes it difficult to prepare a sufficient amount of radiopharmaceuticals for clinical studies.8 The short decay time also necessitates frequent recalibration of the remaining dose during the course of a day. Positron emitters with longer half-lives (e.g. 8.3 h for 52Fe and 4.2 days for 124I) are less commonly used and require a higher energy synchrotron to produce.
Another approach to imaging radioisotopes in tissue is to measure visible light generated during decay events. This light is generated either through Čerenkov radiation by emission of high energy charged particles (e.g. α, β−, or β+ emission) moving faster than the speed of light in the tissue,9–10 or by absorption of radiation by scintillators in the tissue (scintillation proximity assays).11 The advantage of these optical luminescence techniques is that they can be performed rapidly in a small animal multimodal bioluminescence imaging system and the luminescence image can be superimposed upon fluorescent, X-ray, and white light images acquired in the same system. However, the image spatial resolution is poor because the visible light scatters as it propagates through the tissue, similar to bioluminescence.12
In the next three sections, we describe the principles, instrumentation, and biomedical applications used for three different X-ray imaging techniques: X-ray attenuation, X-ray fluorescence (XRF), and X-ray excited optical luminescence (XEOL) to emphasize the potential for chemical specificity and the detection of various exogenous labels and endogenous analytes. These techniques can generally provide high resolution images because the external source can be focused or collimated. The final section is a discussion of the challenges and opportunities for elemental, molecular, and perfusion imaging for intrinsic and contrast-enhanced imaging with these techniques. We will not emphasize radioimaging based upon γ-emission from radioactive analytes within the sample (e.g. PET and SPECT) which are reviewed in detail elsewhere.2, 13
2. X-ray imaging based on X-ray attenuation
2.1 Projection X-ray imaging and Computed tomography (CT)
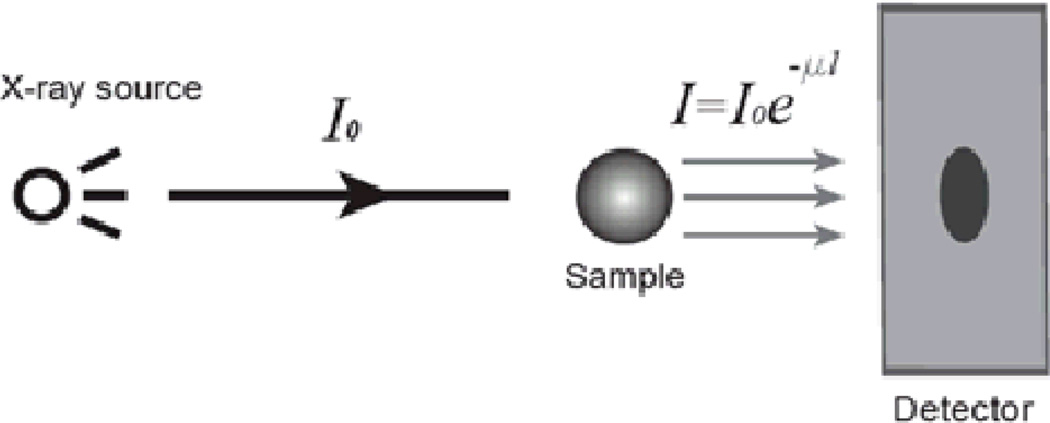
X-ray projection imaging and computed tomography (CT) are techniques which detect X-ray attenuation in a sample (see Fig. 2). The image contrast depends upon the relative attenuation of the objects in the sample. For monochromatic X-ray radiation, the intensity decreases as the X-rays propagate through the tissue according to the Lambert-Beer law.
| (1) |
where I0 is the incident intensity, l is the path length, and μ is the sample’s linear attenuation coefficient. This coefficient depends upon the elemental composition of the sample and is larger for electron-dense materials.
Figure 2.
Schematic showing projection X-ray imaging.
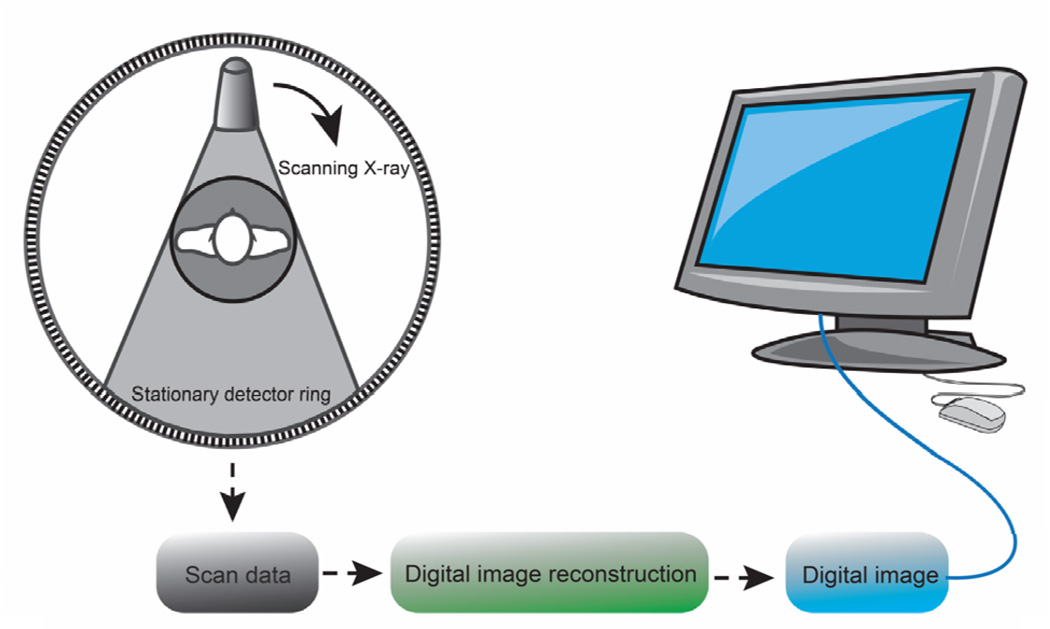
Projection X-ray imaging generates two-dimensional images that highlight changes in electron density of the tissue. Though materials such as bone, kidney stones, and metal implants have high contrast against soft tissue, it is impossible to determine the attenuation coefficient independent of the path length from a single image, and overlapping layers of soft tissue or complex bone can make images difficult to interpret. Hounsfield addressed this problem in 1973 by developing X-ray computed tomography (CT) to determine the three-dimensional spatial distribution of attenuation within a sample using multiple X-ray images acquired at different angles.11 A back projection algorithm such as the Radon transform is used to reconstruct the three dimensional image from each two dimensional projection (Figure 3).2, 13–14
Figure 3.
Schematic showing the three phase process of the formation of a CT image.
The spatial resolution of CT images is determined by the X-ray beam focal spot size and energy, the detectors, and the distance between the source, object, and imaging optics. High spatial resolution requires collimation of the X-ray source and collected X-rays to remove scattered X-ray photons. However, collimation reduces the intensity of the collected signal. There is also an inherent tradeoff between minimum resolution and dose because one needs a certain number of X-rays absorbed per pixel for good contrast between adjacent voxels with similar absorption. In general, the X-ray dose must increases by a factor of 1/r4 to maintain a constant signal/noise ratio, thus imaging at 50 µm resolution requires 104 higher dose than at 500 µm resolution.15
X-ray dose should be limited in vivo because high local doses >100 mGy can cause acute symptoms such as radiation burns, radiation sickness, and hair loss,16–17 while effective whole-body doses are associated with increased cancer risks. The local absorbed dose is reported in Gray (Gy)= 1 J/kg, while the effective whole body dose, reported in Seivert (Sv), is the average dose over the whole body weighted by mass, radiation type, and tissue type. Typical local doses in CT range from 0.01 to 40 mGy, while typical effective whole body dose for CT ranges from 0.01 to 20 mSv depending upon the required image quality (e.g. resolution/contrast) and organs in question.18–20 At a high effective whole body X-ray dose the incidence of cancer is proportional to dose, with a 5.5% chance of developing cancer per Sv.21 It is controversial whether doses of <100 mSv correlate with increased or even decreased cancer risk. However, the goal for imaging is to use the minimum dose necessary for good imagery with no “safe upper limit.” Relatively higher doses are permissible in certain applications such as cancer treatment, especially in conjunction with radiation therapy. For small animals, weekly irradiation with 1.5 Gy, 2.2 Gy, and 3 Gy resulted in tumors in 0 %, 35%, and 100% of the mice respectively.22 Ex vivo samples disintegrate at doses of >109~1010 Gy, although 1015 Gy synchrotron pulses may be used if the pulse is short compared to the ablation process.23
Synchrotron radiation is well-suited for high resolution imaging especially ex vivo because the X-ray beam is intense, monochromatic, and highly collimated. The expected spatial resolution is on the order of 0.2 mm to 0.5 mm for medical CT systems and less than submicrometer for synchrotron based CT systems by using 30~90kV X-rays.24–26
2.2 X-ray imaging contrast agents
The ability to discriminate between two materials depends on the accuracy with which their linear X-ray attenuation coefficient, μ can be determined. X-ray contrast is often reported in Hounsfield units (HU) which are defined as the relative difference in linear attenuation coefficient between the sample and water:
| (2) |
This ranges from μ=−1000 for air to 0 for water and 600–3000 for different types of bone at different X-ray energies.27 The linear attenuation coefficient depends on the photon energy of the X-ray beam (E), the electron density of the material (ρe), and the effective atomic number of the material (Z) and can be approximated as the sum of Compton scatter and photoelectric contributions:
| (3) |
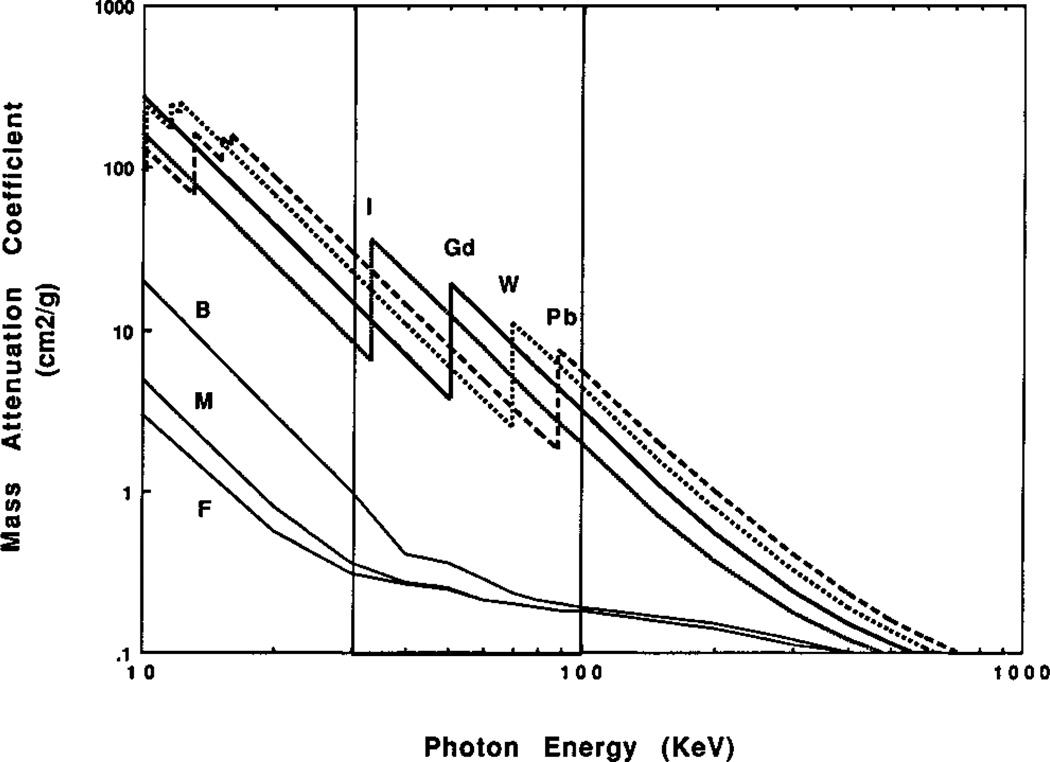
where a associated with scattering and is only weakly dependent on energy level, while b is a constant related to absorption.26, 28 Thus X-ray attenuation inreases with increasing atomic number and decreases with increasing incident X-ray energy. In addition, inner orbital transitions create sharp step-functions in the absorption and scattering coefficients as shown in Figure 4. Composite materials such as tissues and bones have mass attenuation coefficients (μ/material density) that are equal to a mass weighted sum of the composite elements. The mass attenuation coefficients of bone, muscle, and fat become silmilar at high X-ray photon energies around 100 keV and greater.
Figure 4.
Mass attenuation coefficients for various elements and tissues as a function of photon energy (on a log-log scale). (B: bone, M: muscle, F: fat). The vertical lines show the typical region used for X-ray projection imaging and CT. Note the mass weighed attenuation coefficient is equal to the linear coeffficient divided by the density. Reproduced with permission from Ref. 32.
Conventional projection X-ray imaging employs a polychromatic X-ray source, and measures the average transmittance of all X-ray energies. Recently energy disperse cameras have been developed to measure the energy of the transmitted X-rays reaching each pixel.29–31 These hyperspectral images can be analyzed to determine the elemental distribution of concentrated heavy element contrast agents based upon the K-edge absorption of high Z elements (see Fig. 4).32 As an alternative to polychromatic spectral imaging, monochromatic synchrotron radiation can be used to collect a series of images at different X-ray energies.33
In addition to differentiating tissues based upon elemental composition, contrast agents may be used to highlight vasculature before and after intravenous injection of contrast agents. Contrast agents can also be chemically functionalized with antibodies, aptamers, and other molecular recognition elements to target molecules expressed on the surface of specific cells and tissues. An important goal is to develop contrast agents to label tumors via leaky vasculature and molecular targeting. Properties including mass attenuation coefficient, density, and typical dose for a variety of X-ray contrast agents are summarized in Table 1. When choosing the proper contrast agent for a given application, toxicity and circulation time must also be considered.
Table 1.
Examples of X-ray contrast agents
| Contrast agent (or tissue) | μ (cm2/g)* | Density (g/cm3) | Application | Dose (/Kg body weight) |
Reference |
|---|---|---|---|---|---|
| Iohexol | 1.093 | 0.924 | Cardiac angiography, liver imaging | ~600 mg I | 51 |
| Iodixanol | 1.156 | 2.295 | Cardiac angiography | ~600 mg I | 52–53 |
| Diatrizoate | 1.454 | 1.66 | Cardiac angiography | 600 mg I | 54–58 |
| Ioxaglate | 1.408 | Cardiac angiography | 600 mg I/m | 58–60 | |
| Silver iodide | 2.130 | 5.675 | Retrograde pyelography, liver imaging | 400 mg | 61–63 |
| Cesium chloride | 1.969 | 3.99 | Bronchography | ~400 mg | 61, 64 |
| Barium sulfate | 1.616 | 4.5 | Gastrointestinal tract | 100 mg | 65–66 |
| Bismuth subnitrate | 1.437 | 4.93 | Gastrointestinal tract | 67–68 | |
| Bismuth sulfide | 1.813 | 6.78 | Gastrointestinal tract | 120 mg | 69–71 |
| Gold nanoparticle | 1.72 | 19.32 | Vascular casting, tumor | 1000~2700 mg | 36, 72 |
| Silver nanoparticle | 1.907 | 10.49 | Vascular casting | 27 mg | 37 |
| Gadolinium oxide | 2.511 | 7.4 | Hepatobiliary | 200~400 mg | 38, 42, 73–75 |
| Gadolinium-DTPA** | 0.855 | Angiography | 0.3 mmol | 76 | |
| Ytterbium-DTPA** | 0.935 | Angiography | 45, 77–80 | ||
| Stannic oxide | 1.660 | 6.95 | Liver imaging | 350 mg Sn | 81–86 |
| Calcium tungstate | 1.871 | 6.06 | Bronchography | 87–89 | |
| Thorium oxide | 2.223 | 10.00 | Cerebral arteriography, liver imaging | 230 mg | 90–91 |
| Tantalum oxide | 2.399 | 8.2 | Gastrointestinal tract, hypervascular tumors | 92–93 |
:The mass-weighted attenuation coefficient is for 80 KeV X-rays. The mass-weighted attenuation coefficient for soft tissue and cortical bone are 0.182 and 0.223 cm2/g, respectively. Note: the linear attenuation coefficient, μ (cm−1) is the product of the mass-weighted coeffeficient times the density.
:DTPA is gadopentetic acid
Contrast agents containing iodine and barium are comercially available for X-ray examinations due to their high contrast with respect to soft tissue. High contrast is observed due to the electron density and atomic number of these elements (Z = 53 for iodine, Z = 56 for barium) and the favorable location of the K-absorption edge relative to the typical X-ray source energy spectrum. Though iodine-based compounds are often used as intravenous contrast agents due to their high solubility, they have the limitations of short imaging times due to rapid renal clearance, renal toxicity, and vascular permeation.34–35
To address renal toxicity and increase circulation time especially in tumor labeling applications, researchers are increasingly turning to nanoparticles in place of molecular contrast agents. Nanoparticles with diameters larger than 5 nm are not cleared by the kidneys and can have much longer blood circulation times. Several nanoparticles have been investigated for gastrointestinal imaging, imaging of vasculature, and tumor labeling. Though barium sulfate is used as a contrast agent to improve the visualization of the gastrointestinal tract, it is insoluble in water. If the barium sulfate settles during an X-ray exam, the images will be compromised. In addition, accumulated barium sulfate can potentially block constrictions in the gastrointestinal tract. Though bismuth based compounds are another such long lived contrast agent (e.g. 5% suspension of bismuth subnitrate),36 the toxicity of bismuth subnitrate at high doses has obviated their use in gastrointestinal tract imaging. Although Rabin used bismuth sulfide as a contrast agent to image vasculature of a live Balb/C mouse,37 the LD50 of bismuth sulfide nanoparticles showed a similar profile to that of clinically used iopromide in hepatocyte cultures. They also found that lower volumes of bismuth sulfide nanoparticles can be used with a longer vascular half-life (>2 h) without confronting viscosity problems of iodine based agents.
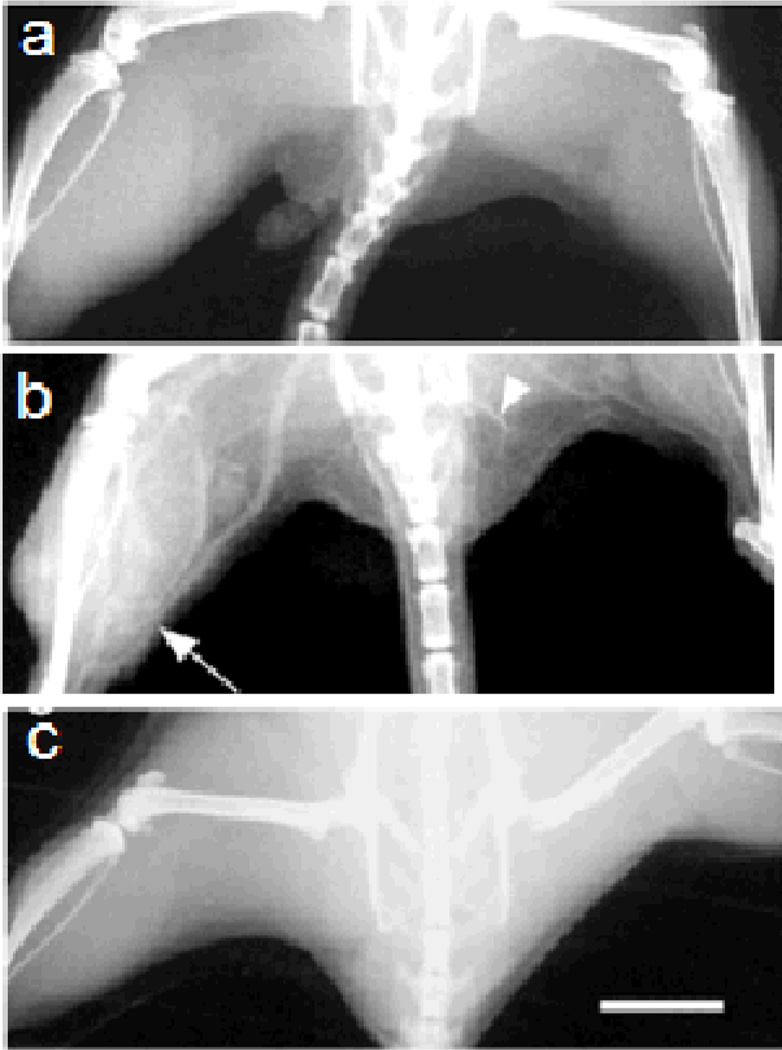
Gold nanoparticles are also promising X-ray contrast agents because of their high density and X-ray attenuation,38 low toxicity,39 and ease of functionalization with thiol-based compounds.40–41 For example, Hainfeld et al. have imaged a 0.1 mm blood vessel injected with 1.9 nm gold nanoparticles in Balb/C mice with a clinical mammography X-ray unit (Figure 5).38 In the image, a 5 mm tumor growing in one thigh can be distinguished by its increased vascularity and consequently higher gold content.
Figure 5.
X-ray images of mouse hind legs in vivo. (a) Before injection; (b) 2 min. post tail vein injection of gold nanoparticles; (c) 2 min. after equal weight of iodine contrast agent (Omnipaque). Arrow points to leg with tumor and increased vascularity. Arrowhead points to 0.1 mm diameter vessel. Scale bar represents 5 mm. Reproduced with permission from Ref. 38.
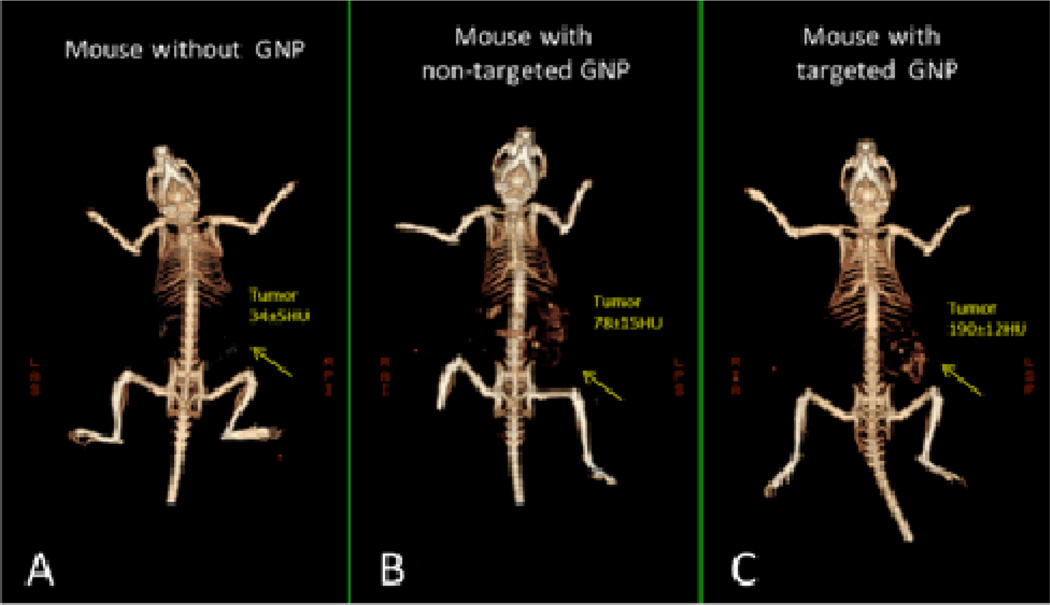
Recent progress in the use of gold nanoparticles as a CT contrast agent has been made by Popovtzer and co-workers.42 They demonstrated the feasibility of functionalized gold nanoparticles as a contrast agent which selectively and sensitively targets tumour antigens. Compared with unfunctionalized gold nanoparticles, these immuno-targeted gold nanoprobes bind to surface receptors on the cancer cells which provides distinct features for identifying and localizing the targeted cancer. Popovtzer and coworkers recently have demonstrated that a small tumor, which is currently undetectable through anatomical computed tomography, can be enhanced and becomes clearly visible via the use of anti-epidermal growth factor receptor conjugated gold nanoparticles (30 nm).43 These molecularly-targeted gold nanoparticles were intravenously injected into nude mice implanted with human squamous cell carcinoma head and neck cancer. Their results also show that active tumour targeting is more efficient and specific than passive targeting (see Figure. 6).
Figure 6.
In vivo X-ray CT volume-rendered images of (A) a mouse before GNP injection, (B) a mouse 6 hours post injection of non-specific IgG GNP as a passive targeting experiment, and (C) a mouse 6 hours post injection of anti EGFR coated GNP that specifically targeted the SCC head and neck tumor. The anti-EGFR targeted GNP shows clear contrast enhancement of the tumor (C, yellow arrow), which was undetectable without the GNP contrast agents (A, yellow arrow). CT numbers represents the average HU of the whole tumor area. All scans were performed using a clinical CT at 80 kVp, 500 mAs, collimation 0.625×64 mm and 0.521 pitch size (A 64-detector CT scanner, LightSpeed VCT, GE Medical Systems). Reproduced with permission from Ref. 43.
Gadolinium oxide nanoparticles have also been investigated for CT contrast imaging following intravenous injection.44–45 Their relatively high atomic number (Z), low toxicity, and long circulation time in the blood pool permit their use as an excellent contrast agent. Meanwhile, these nanoparticles show excellent T1-weighted magnetic resonance imaging (MRI) which is used in dual mode imaging.46–47 Recently, some rare earth (Tb, Eu) doped gadolinium oxide or oxysulfide nanoparticles have been developed for multimodal imaging with MRI and X-ray luminescence tomography.48–50
3. X-ray fluorescence (XRF)
Hyperspectral computed tomography can determine elemental composition from the K-edge absorption of concentrated high Z elements in a sample.29–30 However, for low element concentrations, few photons are absorbed by the sample compared to the number of photons transmitted, and signal from X-ray absorption can be obscured by the noise in the background of transmitted photons. XRF, a positive contrast technique, has a much lower background and can be more sensitive to low elemental concentrations than CT. Bazalova and coworkers have simulated the contrast-to-noise ratio (CNR) for phantoms containing gold nanoparticles and cisplatin by K-edge transmission CT and X-ray fluorescence. From their simulations they concluded that with large XRF detectors (a 10 cm diameter ring, 2 cm tall). At concentration of gold nanoparticles and cisplatin of 0.4% or less, X-ray fluorescence computed tomography provides a higher contrast-to-noise ratio than K-edge CT. For example, the CNR of a 2 mm object with a gold or platinum concentration of 0.4% was calculated to be approximately 5 for gold and ~ 6 for platinum at a dose of 2 mGy. Meanwhile, for the same size object and concetration, the CNR for CT was approximately 2 for both gold and platinum.94 The tradeoff will depend upon sample concentration, voxel size, and the XRF photon-collection efficiency, with smaller voxels, lower concentrations, and higher collection efficiencies favoring XRF.
3.1 Principles of X-ray Fluorescence
When an X-ray photon is absorbed by an atom, some of its energy can be reemitted as secondary X-ray fluorescence. Each element has a unique X-ray fluorescence spectrum enabling quantitative elemental analysis. XRF is widely applied in the fields of geology, archaeology, cosmochemistry, and materials and environmental sciences.95–97 This technique is also applicable to the study of a variety of biological samples95–109 to investigate metal toxicity95–96, 109, the uptake and distribution of metallopharmacueticals99, and intracellullar elemental distributions.95–99, 106–109 In addition, in vivo studies have been performed to non-invasively determine the lead concentration in the bones of children and young adults,100–101 arsenic in human skin,110–111 and iodine in the thyroid112.
X-ray fluorescence utilizes the unique nuclear charge of elements in order to determine sample composition.95–96 Excitation of a core-shell electron of an atom via absorption of an X-ray photon (or other particle such as a proton or neutron) results in the ejection of an excited photoelectron.95, 97 The ionization process for inner shell electrons was determined to be independent of the ionization source by both Meitner and Robinson in the early 1920’s.113 The transition that occurs creates a vacancy within the core-shell leaving the atom in a highly energetic state. The vacancy is then filled by a neighboring higher-shell electron and upon relaxation secondary X-rays are emitted. The energy of the secondary X-rays is equal to the binding energy difference between the inner and higher energy shell and is proportional to the squared nuclear charge of the element, allowing for identification, while the number of X-rays emitted is directly proportional to atomic abundance.95–96
The energy of the emitted secondary X-rays can be calculated by Moseley’s law:
| (4) |
where E is the energy of the spectral line of interest, Z is atomic number, b is a charge screening constant dependent on the line series (e.g. K or L lines), n1 and n2 are the energy levels involved in the transition, and Ry is the Rydberg energy (Ry = mec2α2/2 ≈ 13.606 eV, where me is the mass of the electron, c is the speed of light, and α is the fine structure constant). For the Kα line, b is equal to 1 and n1 and n2 are equal to 1 and 2, respectively.114
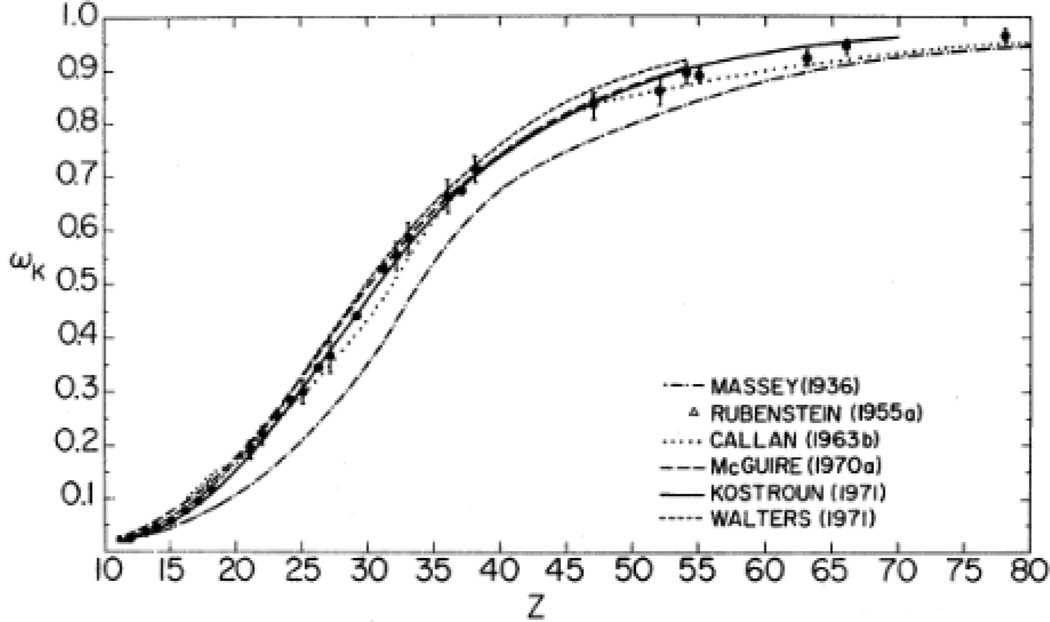
X-ray fluoresence experiments commonly probe excitation of an electron in the K shell (n1=1) or L-shell (n1=2). The experimental and theoretical fluorescence yields of both the K and L-shells have been studied extensively and have been reported in the literature as a function of the atomic number. The fluorescence yield is the probability that an electron vacancy generates a secondary X-ray photon upon filling of the vacancy in a radiative transition. Theoretical calculations show that as the atomic number increases, the fluorescence yield increases for the total emission from the K- shell. Low atomic number elements, Z=5 to Z=17 (boron to chlorine) have fluorescent yields less than 0.1 according to calculations reported by McGuire, Kostroun, and Walters in the late 1960’s to early 1970’s. The results of the theoretical calculations performed by these scientists as well as others can be seen in Figure 7. Experimental results by Konstantinov, Bailey, and Pahor confirm low fluorescent yields for elements with Z = 13~17 however, the fluorescent yields of elements with Z<13 were not reported due to dependence on chemical state.113
Figure 7.
K-shell fluorescence yield for all K-shell transitions determined by theoretical calculations of Massey and Burhop, Rubenstein, Callan, McGuire, Kostroun, Chen and Craseman, and Walters and Bhalla. Reproduced with permission from Ref. 113.
Low atomic number elements generate low energy secondary X-rays. These X-rays are readily absorbed and exponentially attenuated by a tissue, according to equations (1) and (3). The secondary Kα X-rays generated from elements with Z<14 have a mean free path equal to a micron or less in tissue samples.107 Therefore imaging in tissue samples necessitates that the elements have a high atomic number and the X-ray detector has a high collection efficiency of photons in order to achieve sensitive measurements.
The number of secondary X-ray photons that can be detected per voxel from a sample with an elemental concentration c (ppm), is given by equation (5):
| (5) |
Where D is the X-ray dose applied to the sample (Grays), Γ is the product of the X-ray fluorescence quantum yield and a conversion factor between the dose in grays and the number of photons absorbed per volume, c is the concentration, V is the voxel volume, and L is the X-ray collection efficiency including transmission through the tissue and photodetector yield. This equation was modified from Carpenter et al.,115 who use it to determine number of visible photons collected after irradiation of a sample containing X-ray scintillators. The equation assumes a relatively low concentration so that self-absorption effects are negligible. According to the equation, the number of collected X-rays per voxel increases for increasing element concentrations, larger imaging voxels, and higher Z elements with larger quantum effieiciencies as well as better collection optics.
The XRF examples provided in this review utilize energy dispersive detection of secondary fluorescent X-rays. Energy dispersive detectors efficiently collect incident X-rays and detect the energy of each X-ray based upon the electric charge generated in the photodetector. The energy resolution depends upon the X-ray energy and detector bandgap as well as heterogeneity in detector efficiency and is ~ 120 eV at an X-ray energy of 6 keV for HPGe and Si(Li) detectors.116 This resolution is far too poor to observe spectral shifts due chemical binding, but the high collection efficiency makes it preferable for in vivo imaging. Alternatively, wavelength dispersive detectors operate by diffracting a collimated X-ray fluorescence beam according to Bragg’s Law and are capable of achieving a higher resolution.117–118 However, these detectors cannot measure more than one X-ray energy simultaneously, and require collimation of the X-ray beam, both of which result in poor X-ray collection efficiency.118 In order to compensate for a lower X-ray collection volume than with an energy dispersive detector, a very high X-ray flux would be necessary to achieve adequate resolution for use for in vivo studies.
X-ray fluorescence research now includes both conventional and microXRF with smaller beam sizes. Three variations of XRF, X-ray fluorescence computed tomography (XRCT), Synchrotron (SXRF), and Confocal XRF (CXRF) techniques and their applications are discussed below. In addition to these variations, total reflection (TXRF), grazing exit (GEXRF), grazing incidence (GIXRF) and portable XRF techniques have been developed. West et al. provide a thorough review of advancements in these techniques as well as in X-ray optics and detectors.117
3.2 X-ray Fluorescence Computed Tomography
X-ray fluorescence computed tomography (XRCT) can be used to image the 3-D elemental composition in a sample. This tomography technique combines principles of CT imaging of X-ray attenuation with the measurement of secondary X-ray fluorescence from elements within the sample. XRCT produces three-dimensional elemental maps by reconstructing 2D distributions including corrections for the attenuation of the excitation and fluorescence X-rays. XRCT is conducted by irradiating a sample with an external X-ray beam and collecting the X-ray fluorescence with an energy dispersive detector. This detector measures the energy of each X-ray fluorescence photon by counting the electron/hole pairs produced upon absorption in the detector. In order to minimze the detection of elastic and Compton scattered photons and improve the signal-to-background ratio, the fluorescence detector is positioned 90° from the incident beam.102, 107–108 X-ray transmittance is simultaenously acquired using a conventional CT detector in order to provide co-registered XRF and CT images. Samples are scanned and rotated through a series of angles and the emitted fluorescence intensity is collected at each orientation.119 Mathematical back projection algorithms are then used on the measurements acquired to turn sinogram plots into tomograms.102, 107–108, 119 Several image reconstruction and attenuation correction methods for the absorption of X-rays by a sample have been developed for use in XRCT.107–108
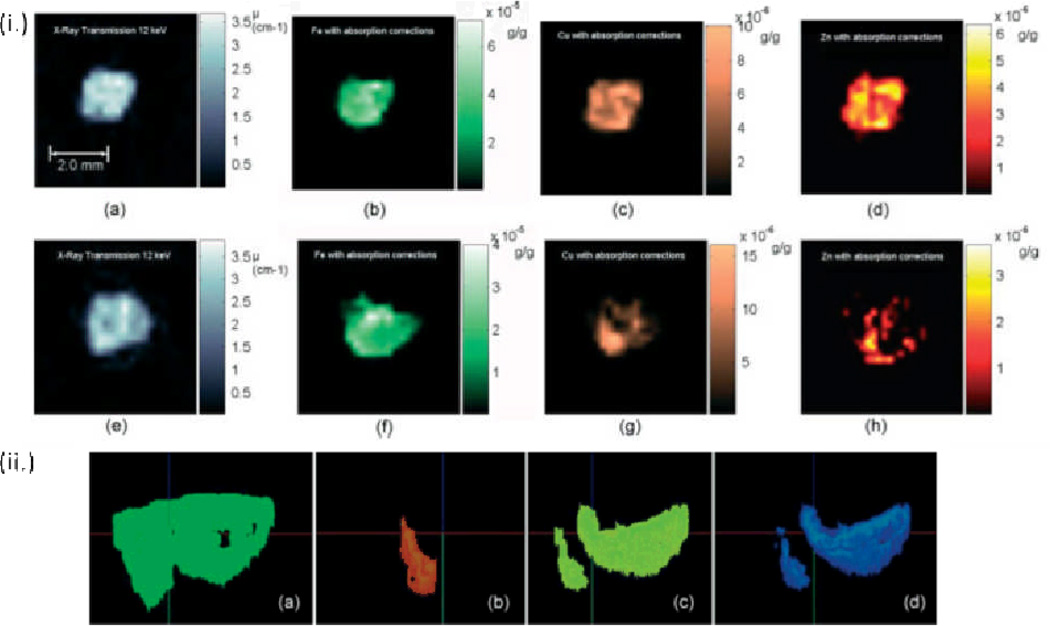
To study the putitive link between carcinogenesis and metal ion content in tissue, Pereira et al. used XRF μCT in the analysis of iron, copper, and zinc in breast, prostate, and lung biopsy tissues at the Brazilian Synchrotron Light Laboratory (LNLS).102 Prior to analysis, samples 1.5~2.0 mm thick and 4–5 mm high were frozen and dried to reduce the attenuation of fluorescent X-rays. Using a filtered back-projection algorithm with absorption corrections, they acheived a spatial resolution of 200 µm. Both healthy and cancerous breast tissue were analyzed for comparison. XRF studies revealed an iron content of approximately 50 µg/g in healthy tissue and an increase of 40% for cancerous tissue as shown in Figure 8. A doubling in the zinc concentration from approximately 7 µg/g for healthy tissue was observed in the cancerous sample. In addition to the varying metal concentrations, a heterogenoeus metal distribution was observed in the healthy tissue.102 In earlier work by the same authors, XRF μCT was performed on intenstine samples.119
Figure 8.
(i.) X-ray transmission microtomographies of (a) cancerous and (e) healthy breast tissue in the same patient. (1) (b)– (d) and (f) – (h) show X-ray fluorescence microtomographies of cancerous and healthy breast tissue respectively. The scale bar is 2 mm. (ii.) 3-Dimensional XRF μCT images of (a) healthy breast tissue, (b) iron (c) copper and (d) zinc showing the heterogeneous distribution of metal ion content. Reproduced with permission from Ref. 102.
3.3 Synchrotron X-ray Fluorescence Microscopy
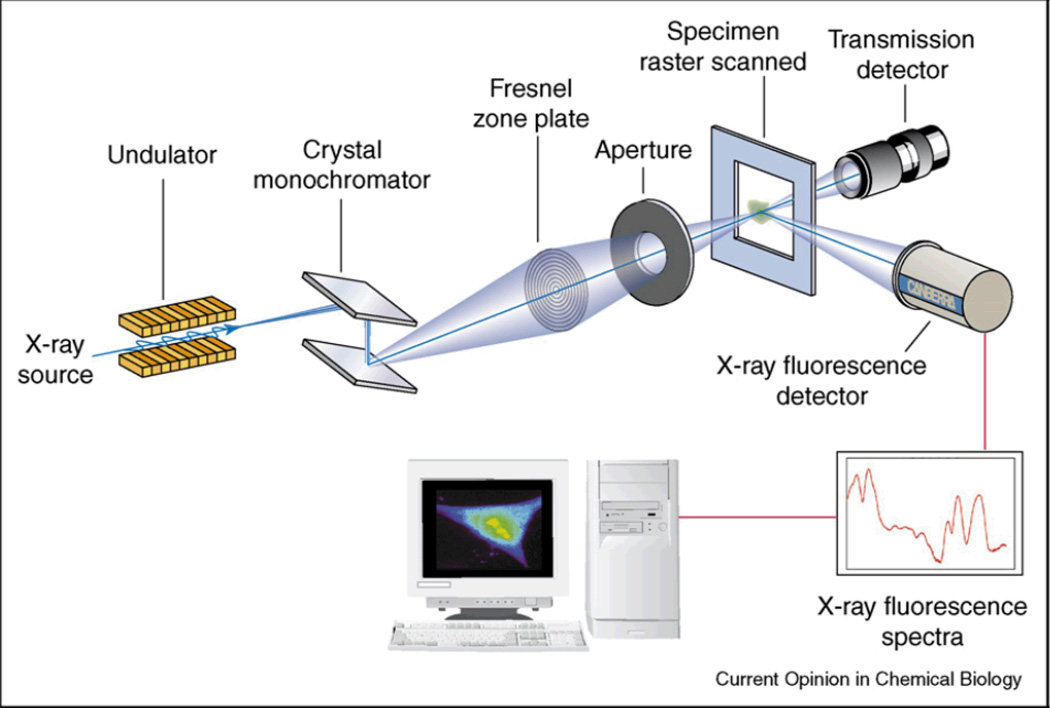
Synchrotron radiation sources provide a highly coherent bright beam with a high flux for XRF analysis.109 On average, third generation synchrotron sources provide a brilliance on the order of 1017 to 1020 ph/s/mm2/mmrad2/0.1%BW.120 Synchrotron energy can be tuned by changing the cycle frequency over a broad range of energies ranging from infrared to X-ray radiation.120 Imaging with synchrotron based micro X-ray fluorescence has improved detection limits and spatial resolution compared to conventional μ-XRF instruments with X-ray tubes used in laboratory settings. In combination with X-ray focusing optics, monochromatic X-ray beams can be produced by synchrotron sources with submicron diameter beam sizes.121–123 The high resolution images that can be achieved with XRF are excellent for biopsies and cell studies.124 However, the X-ray dose required to achieve a significant signal per voxel for small voxels, limits the use of these techniques for in vivo studies (see equation (5)). Figure 9 shows a schematic of a typical SXRF experimental configuration.
Figure 9.
Schematic of an X-ray fluorescence microscope with a Fresnel zone plate for beam focusing, and energy dispersive detector for multi-element analysis. Reproduced with permission from Ref. 121.
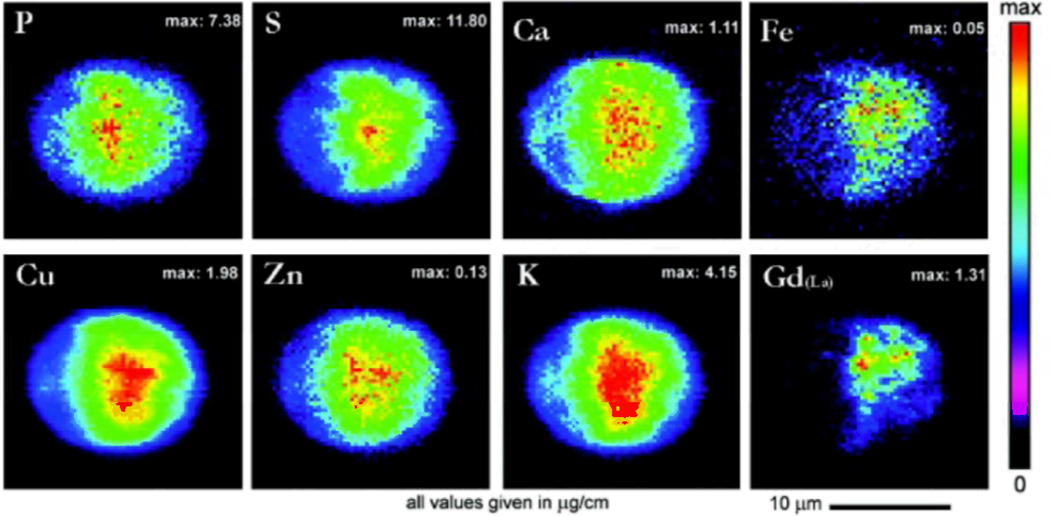
The deep penetration depth of hard X-rays in Synchrotron X-ray fluorescence experiments, makes the technique highly applicable for cell study. Cells can be analyzed in their hydrated states.109, 121 SXRF is capable of achieving single cell and subcellular resolution.125 A variety of cellular studies utilizing SXRF have been conducted, including the determination of the distribution of cis-diamminedichloro-platinum (II) (CDDP) in cis-platin sensitive and resistant cells by Shimura et al..99, 121 The distribution of MRI contrast agents in cells was also studied independently by both Endres and Marmorato.98, 106 A study of the distribution of Gadolinium containing contrast agents capable of crossing cell membranes in NIH/3T3, MDCK, and RAW 264.7 cell cultures was conducted by Endres et al. at the the 2-ID-E beamline at Argonne National Laboratory.95, 106 Figure 10 shows the distribution of several elements in addition to Gadolinium mapped in MDCK cells.106 Marmorato et al. also studied the distribution of a potential MRI contrast agent, colbalt ferrite nanoparticles, in Balb/3T3 mouse fibroblasts.98
Figure 10.
Intensity-weighted elemental maps of phosphorus, sulfur, calcium, iron, copper, zinc, and potassium in an MDCK cell incubated with a Gadolinium (III) contrast agent (Gadolinium (III) (4,7,1-Triscarboxymethyl-6-[4-(3-{4-[2-(4-Dimethylaminophenyl)vinyl] phenyl)-thioureido)benzyl]-1,4,7,10-Tetraazacyclododec-1-yl}-acetic acid). Reproduced with permission from Ref. 106.
Qin et. al. have used SXRF at the 2-ID-D beamline of the Advanced Photon Source at Argonne National laboratory to determine the topographic distribution of phosphorus, sulfur, and zinc in 4 µm thick sections of thoracic aorta of Sprague Dawley rats to elucidate copper distribution in tissues pertaining to collagen and elastin formation and for determining the role of metals in cardiovascular diseases. Qin et al. discovered that the majority of copper present was located in the elastic laminae in aortic walls and phosphorus, sulfur, and zinc in the vascular smooth cells.104 The technique has also been applied by Leskovjan et al. for the study of iron, copper, and zinc content in brain tissue and amyloid plaques from cortex and hippocampi samples, thought to be linked to Alzheimer’s disease. The study was conducted using 30 µm thick whole brain coronal cryosections from transgenic mice with a similar gene for the development of amyloid plaques to those associated with Alzheimer’s disease in humans.103
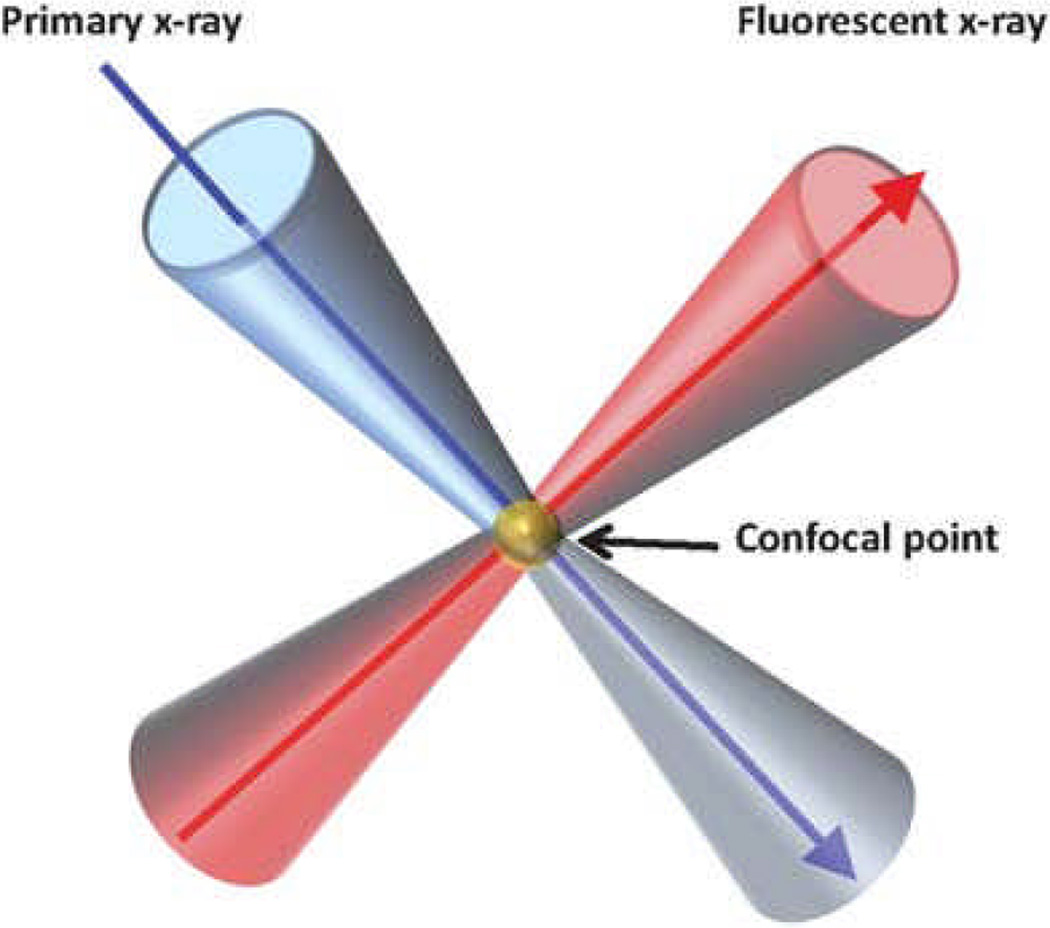
3.4 Confocal X-ray Fluorescence Microscopy
Confocal X-ray fluorescence microscopy (CXRF) is used to acquire 3D depth profiling of the elemental composition of a sample.97, 126–129 CXRF defines a microscopic sample volume through which the sample is scanned utilizing polycapillary lenses composed of a bundle of hollow glass capillary tubes operating under total external reflection to focus the X-ray beam.97, 127–130 The axial resolution of CXRF is determined by the resolution of the focusing lens and becomes poorer when imaging elements with low atomic numbers due to lens wavelength dependence. The current limit for axial resolution for confocal tomography is 5 µm,97 although there is a tradeoff between focal distance, focal spot size, and X-ray energy.130 The confocal arrangement reduces background intensity.96, 129 However, self-absorption of X-ray fluorescence within a sample limits the imaging depth for low energy X-ray fluorescence.127
Gibson and Kumakhov were the first to propose the use of a polycapillary lens for XRF in 1992.127, 129 The use of polycapillary lenses in XRF has since evolved. In 2006, a confocal X-ray fluorescence microscope with a single-bounce monocapillary lens for the excitation optic and a polycapillary lens for X-ray collection was constructed at the Cornell High Energy Synchrotron Source (CHESS) for the analysis of historic paintings. Depth resolution of 35 µm was achieved with this instrument at an excitation energy of 8 keV and greater. Depth resolution studies were conducted on titanium, copper, and gold metal films.128 Prior to 2006, a confocal instrument was constructed at the Hamburg Synchrotron Radiation Laboratory (HASYLAB) in Germany capable of an axial resolution of 20~40 µm for fluorescent radiation energies ranging from 4 to 20 keV and a limit of detection on the sub-femtogram level using a second generation synchrotron source.126
Although the use of synchrotron radiation in conjuction with confocal XRF has shown great promise, limited availability of synchrotron instrumentation has stimulated research in alternate confocal configurations with alternative radiation sources. Nakano et al. have developed a laboratory confocal XRF set-up utilizing a 30 Watt metal-ceramic X-ray tube with a Molybdenum target as an excitation source attached to a full lens polycapillary. Using this excitation source, a depth resolution of 45 µm for the Au Lβ line was observed.129 Subsequent improvements yielded a depth resolution of 13.7 µm for the same analytical line using a confocal arrangement with a fine focus metal-ceramic X-ray tube with a molybdenum target, improved optics, and an increased sensitive area for the detector.123
3.5 Multiplexed X-ray Fluorescence
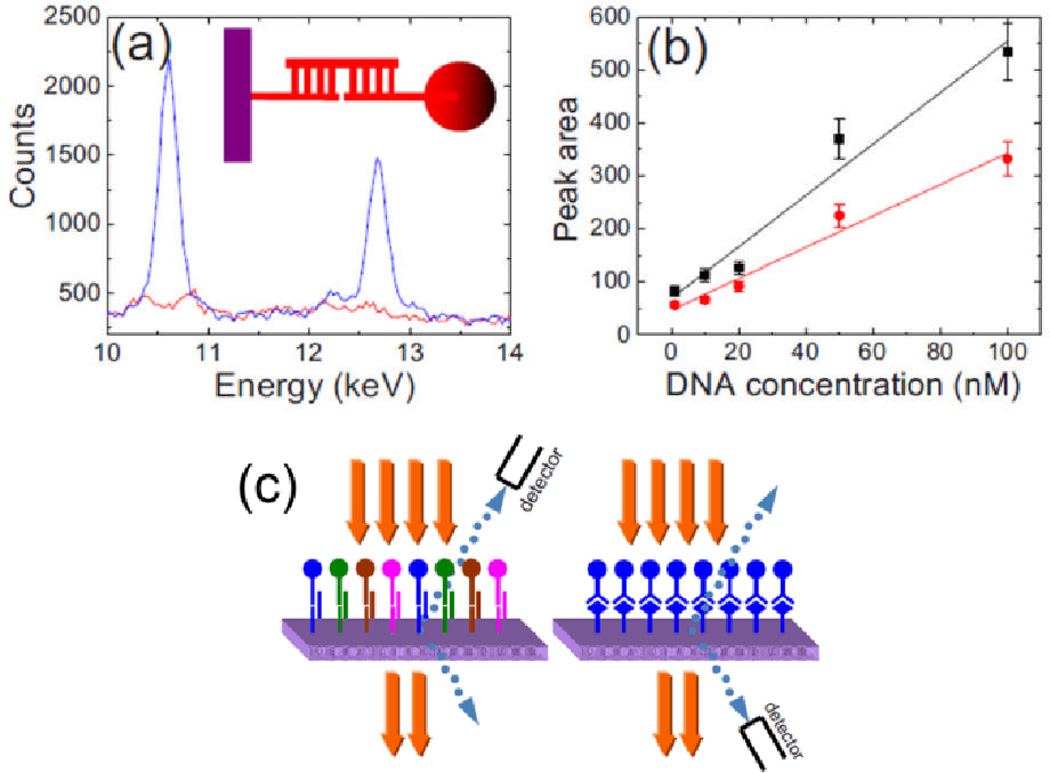
In addition to studying the elemental composition of cells and tissues, XRF has been extended for the analysis of multiplexed biomarker assays using nanoparticles with distinct elemental composition as chemical labels. To detect single strand DNA (ssDNA) Hossain et al. synthesized a series of indium, bismuth, tin, and lead-tin alloy nanoparticles. The synthesized nanoparticles were modified with thiolated probe single strand DNA and immobilized on ssDNA modified aluminum plates. XRF spectra were obtained for the detection of 100 nM DNA using a mini X-ray tube operating at 40 kV. The peak area for L-band X-ray fluorescence lines of the spectra were analyzed as a function of ssDNA concentration (Figure 12). They also observed a higher sensitivity for DNA detection measurements with lead-tin and bismuth nanoparticles than with indium and tin particles, due to the greater X-ray absoption and fluorescence efficiency exhibited by these particles. The study was also extended to the detection of four different types of single strand DNA using lead, bismuth, indium, and tin nanoparticles, showing the ability of the technique for multiplexed analysis with different element labels. It is believed that approximately 50 different peaks can be detected by this method, based upon the availability of elements from which nanoparticles can be synthesized.131
Figure 12.
(a) X-ray fluorescence spectrum and schematic of experimental set-up for the detection of 100 nM ssDNA with synthesized lead-tin alloy nanoparticles, (b) Plot of peak area vs. concentration for the Lα1 represented in black with a higher sensitivity than the Lβ1 line of lead represented in red. (c) Schematic of experimental set-up. Reproduced with permission from Ref. 131.
To represent detection in tissue, Hossain et al. also performed studies of prostate specific membrane antigens (PSMAs) on polymethyl methacrylate coated plates.131 It is expected that this technique can be expanded for in situ imaging within tissue. Their method provides a novel means for high resolution labeling using X-ray fluorescence analysis.
Cheong et al. have also demonstrated the use of nanoparticles in XRF imaging using a polymethyl methacrylate phantom. X-ray fluorescence computed tomography was performed at an X-ray energy of 110 kVp. Saline solutions containing 1~2% gold nanoparticles inside a PMMA phantom were imaged and the gold concentration in the sample was quantified. Although, this study is not currently practical for in vivo measurements due to long scanning time on the order of 30 hours, in vivo imaging may be feasible with improvements in acquisition geometry.132 Takeda et al. imaged cerebral uptake of I in living mice in 1.5 hours (0.36 Gy dose), and proposed different illumination and detector geometries to reduce acquisition time by a factor of 190.133 The group previously used XRF for simulatenous imaging of 0.25 mg/L iodine contrast agents and 30% Xe gas in phantoms demonstrating proof-of-principle for multi-analyte tomographic imaging.134
4. X-ray luminescence imaging
XRF is an excellent technique for non-invasive elemental analysis of endogenous tissue and exogenous pharmaceutical or contrast agents. However, the number of fluorescent X-rays collected limits the sensitivity, which in turn limits the resolution at a given X-ray dose. In addition, although XRF is well suited for elemental analysis, it provides no molecular information unless nanoparticles are used as molecular labels as proposed by Hossain et al.131 In order to detect a wider variety of analytes with molecular information, X-ray excited optical luminescence (XEOL) based techniques were recently developed with lower backgrounds and lower X-ray doses. The number of visible photons detected is given by equation (5), although Γ is much higher than for XRF because one generates ~60,000 visible photons/MeV X-ray (or 4,800 for a 40 keV X-ray) as oppose to ~1 XRF photon for a high Z element. The collection efficiency, L, depends upon both the collection optics and the attenuation of light as it passes through the tissue.115
4.1 X-ray luminescence computed tomography (XLCT)
The ability to specifically target biological processes in vivo makes nanoparticles promising molecular imaging agents for X-ray excited optical techniques. Though optical microscopy (e.g. scanning confocal microscopy) is an excellent technique for biomedical imaging, it has limited utility in tissues deeper than 1 mm because almost all light is scattered, resulting in poor resolution.135–137
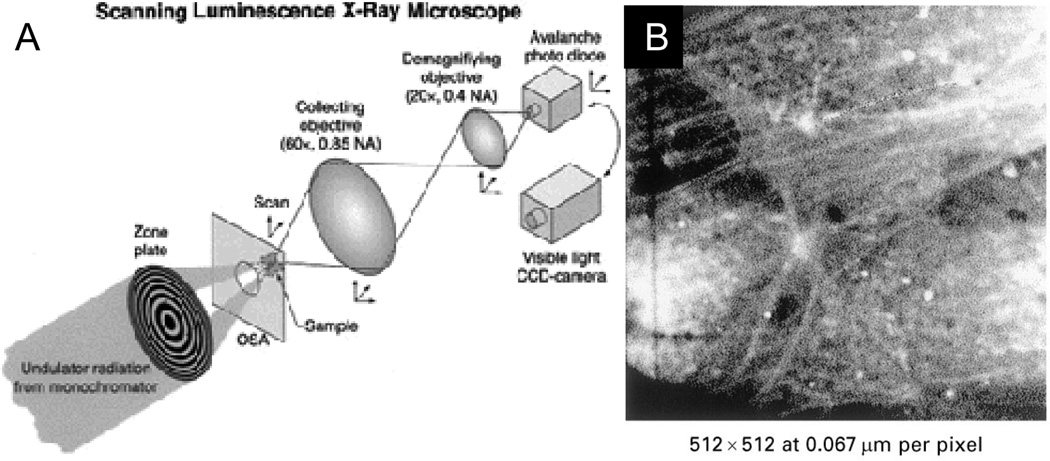
Through the use of a scanning luminescence X-ray microscope, Morrone imaged the optical emission of terbium labeled actin in a single cell to obtain ~50 nm resolution based on the selective excitation of lanthanide organo-polychelate complexes and optical detection of their luminescence (see Fig. 13).138 The high resolution of this technique lies in the fact that the X-ray excited luminescence is only produced in the path of the narrow scanning X-ray beam. However, this small voxel size and weak scintillation yield necessitated the use of a very intense X-ray dose exceeding 108 Gy. To increase the scintillation efficiency, Adam and coworkers proposed using quantum dots for multiplexed cell labeling.139 Alternative optical microscopy techniques have been developed for near-field imaging in the absence of light scattering.140–142 However, the principle of scanning X-ray excitation can also be applied to imaging in tissues thicker than 1 mm where optical scattering prevents high-resolution optical imaging. In accordance with equation (5), increasing the voxel volume and the scintillation efficiency dramatically reduces the necessary dose to levels acceptable for in vivo imaging.
Figure 13.
(A) Schematic of a scanning luminescence X-ray microscope (SLXM) configured for luminescence detection using an avalanche photodiode. (B) SLXM image of actin filmaents in a 3T3 fiberblast labelled with a Tb-polychelated secondary antibody bound to anti-actin. Reproduced with permission from Ref. 138.
With a 1 mm X-ray beam and 1~100 cGy radiation dose, Pratx et al. developed X-ray luminescence computed tomography (XLCT) and achieved a spatial resolution of 1 mm through tissue-mimicking material.143 X-ray scintillators are a series of materials that convert X-ray photons to visible photons when excited by an X-ray source. The basic principle of XLCT is based on luminescence measurements from selectively excited nanoscintillators (Figure 14). XLCT is similar to XFCT in its sample irradiation method, however, XLCT uses photodetectors to capture the optical photons emitted from the nanoscintillators rather than an X-ray spectrometer for secondary fluorescent X-ray detection. Because the X-ray does not scatter much in tissue and the X-ray luminescence is only generated on the path of the narrow X-ray beam, the optical detector need not spatially resolve the source of luminescence. As long as the luminesence can be detected, the spatial resolution is defined by the narrow X-ray beam. The advantages of this technique are its combination of high sensitivity of radioluminescent nanoparticles and the high spatial localization of collimated X-ray beams. Pratx and coworkers recently used XLCT to image the cross sectional distribution of microsize phosphor particles in 4.5 cm of an agar tissue phantom.144 Carpenter recently completed a numerical phantom experiment to simulate the X-ray dose (Gy) required to achieve a signal-to-noise ratio of 10 for varying concentrations. They found that picomolar (ng/ml) concentrations of 10 nm X-ray phosphor are detectable for a mammographic-like dose.115 They have also shown that X-ray luminescence can be observed in small animal models.115, 143
Figure 14.
Schematic showing the principle of XLCT. A computer-controlled collimated X-ray beam selectively excites the sample while photo-detectors measure the light coming out. Inset figure shows the X-ray excited luminescence of X-ray phosphor (Gd2O2S:Eu). Reproduced with permission from Ref. 144.
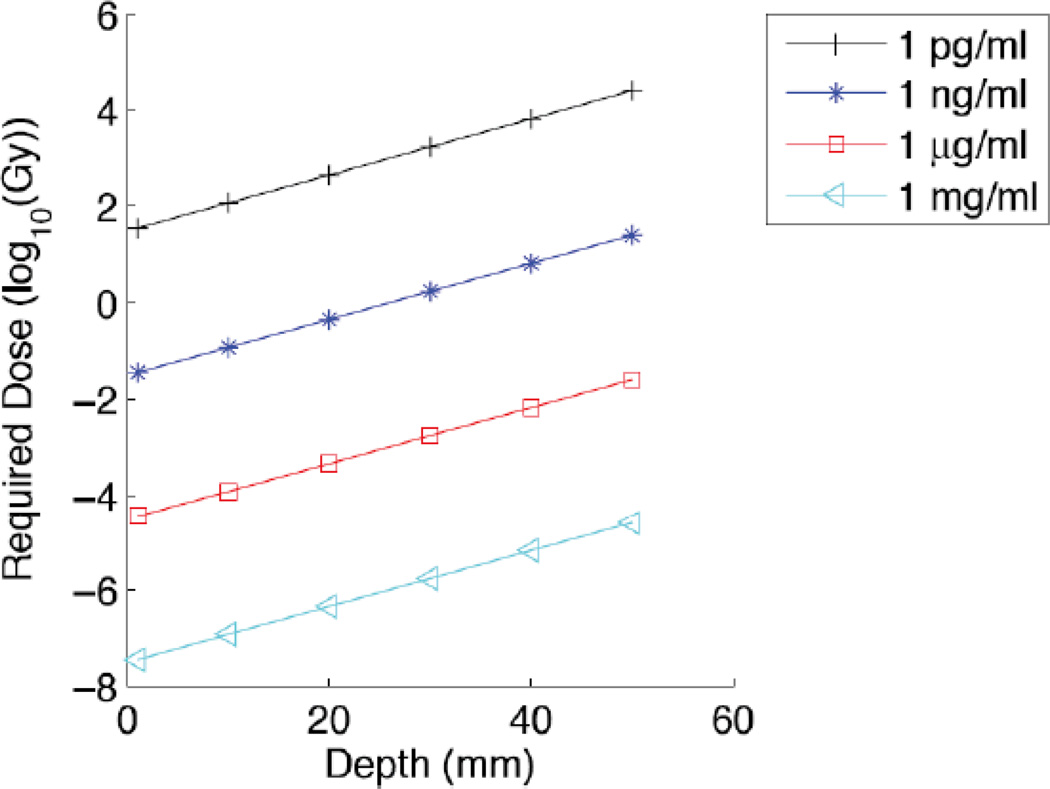
Although high resolution was demonstrated using XLCT, the image is acquired slowly by scanning the X-ray beam through the sample and rotating the sample through 180°. In addition, there are applications such as tumor-resection where the X-ray excitation angle is limited by geometry, and where increased rapid acquisition is critical. Limited-angle X-ray luminescence tomography was deveoped to address these applications based on a hybrid X-ray/optical reconstruction, which allows XLCT spatial encoding in a limited-angle geometry, and diffuse optical spatial discrimination for the remaining dimensions. Figure 15 shows a simulation of the required dose to image a 6 mm object through varying tissue depths and particle concentrations. According to the model, µg·mL−1 particle concentrations may be observed through 5 cm of tissue with ~10 mGy doses. The limited angle technique is expected to be especially useful in surgical applicaions such as breast or brain excision due to rapid acquisition speed, convenient geometric configuration, high depth resolution, and low X-ray dose.145
Figure 15.
Numerical simulation of a limited angle XLT detection of a 6 mm diameter object buried at varying depths beneath the tissue surface. The object is labeled with varying scintillator concentrations as indicated. The y-axis shows the required X-ray dose to achieve a signal/noise ratio of 10. Reproduced with permission from Ref. 145.
4.2 X-ray excited optical luminescence indicators
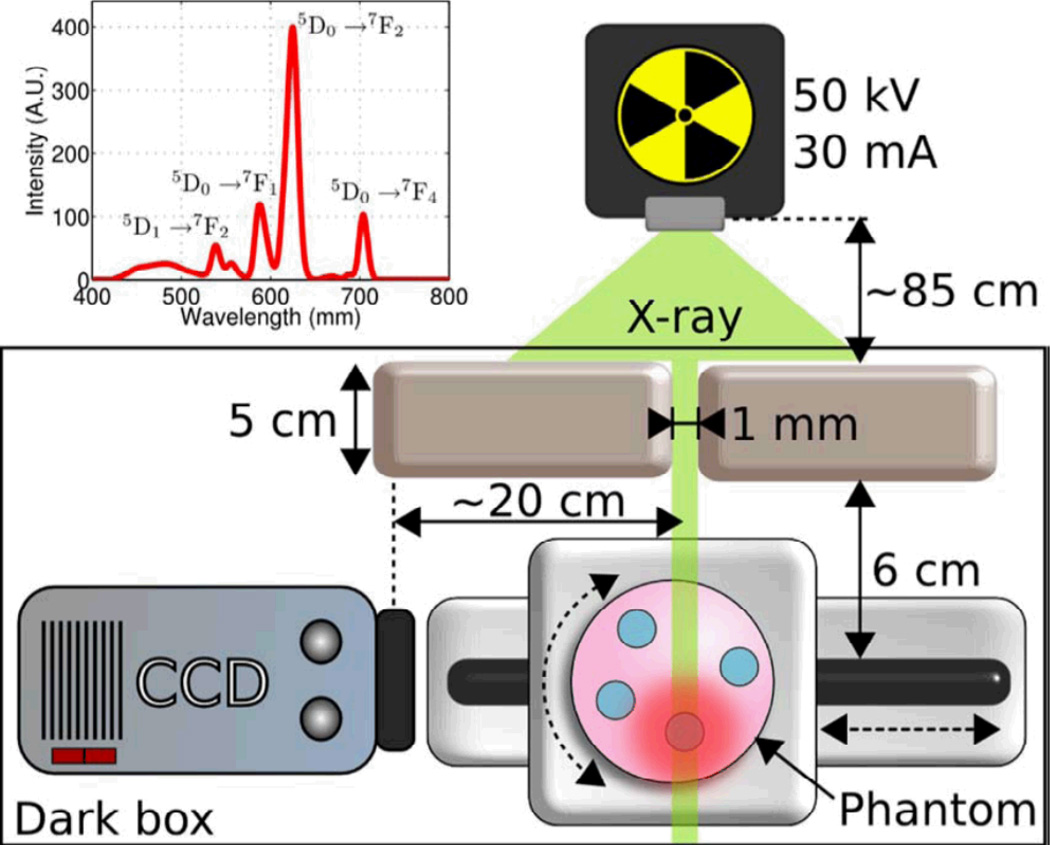
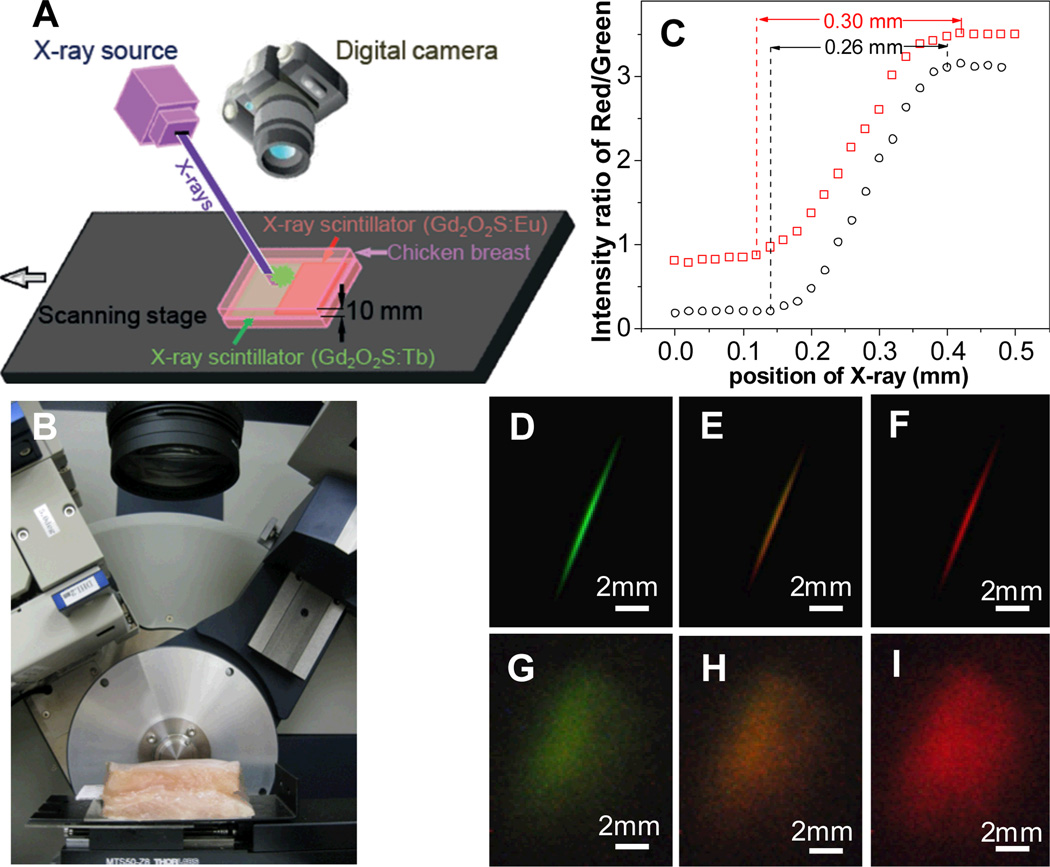
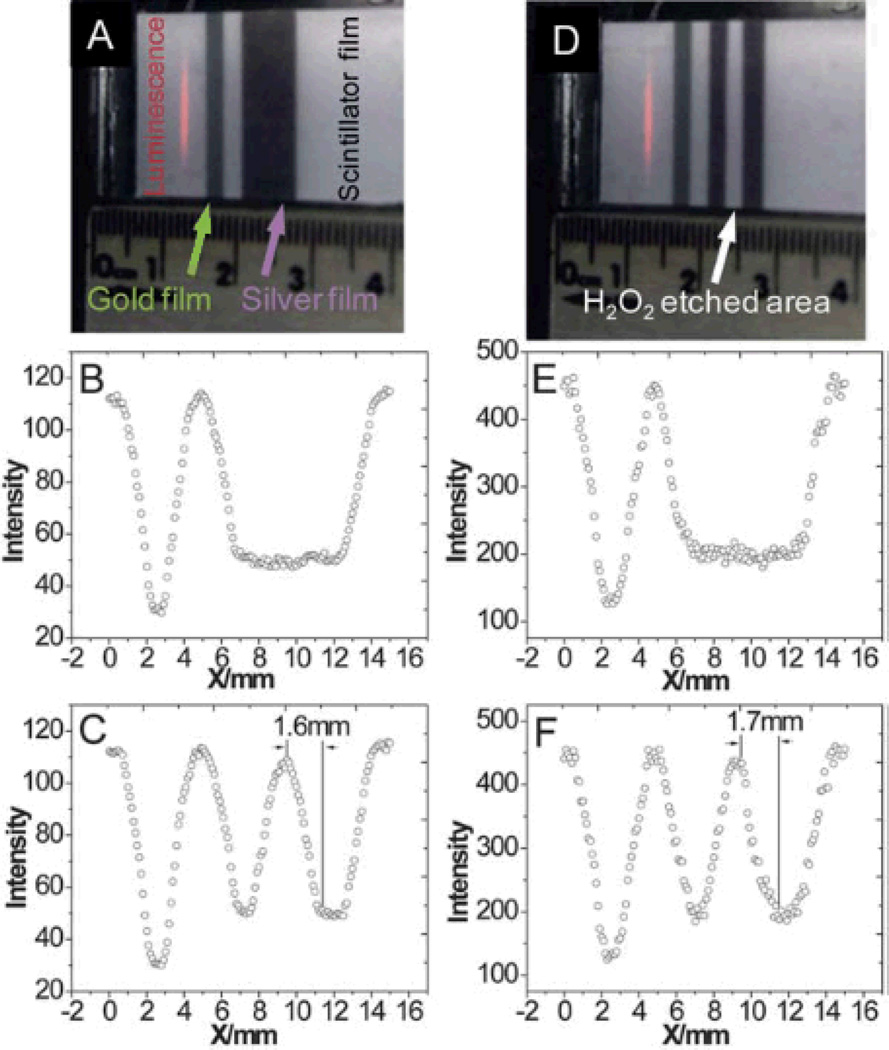
X-ray excited optical luminescence (XEOL) can be used not only to locate X-ray scintillators in tisssue, but also serve as a local light source for fluorescent and colorimetric chemical indicators. Our group has developed a scanning XEOL technique to detect chemical concentrations on the surface of a sensor film through tissue with high spatial resolution.48–49 The technique uses the X-rays to locally excite one small region of a scintillator film at a time. The visible luminescence generated by the scintillators then passes through a thin sensor layer containing indicator dyes that alter the luminescence spectrum. A high-resolution image is formed by scanning the X-ray excitation spot across the sample and measuring the luminescence spectrum at each point. To demonstrate the feasiblity of this high-resolution imaging, we imaged the red and green luminescence from a scintillator film with a red emitting Gd2O2S:Eu region and a green emitting Gd2O2S:Tb region (Fig. 16). The film was imaged first without tissue (Fig. 16D, E, F), and then with the film inserted between two 10 mm thick slices of chicken breast tissue. Even though the luminescent images (Fig. 16G, H, I) were blurred by the thick tissue, the location of the luminescence source could be resolved by the narrow X-ray beam. Figure 16C plots the ratio of red/green intensity ratio versus the sample position in millimeters. The one dimensional beam profile is approximately square with a small tail. The 90%/10% knife-edge resolution was 0.16 mm without tissue, and 0.17 mm with tissue; the full knife-edge resolution including the tails was approximately 0.26 mm without tissue and 0.30 mm with tissue. The small resolution increase in the tissue was likely due to imperfect sample alignment and scattering of the incident X-rays in the tissue. Even higher resolution is possible with narrower X-ray beams.
Figure 16.
High-resolution XEOL imaging through tissue. Green X-ray scintillators (Gd2O2S:Tb) and red scintillators (Gd2O2S:Eu) were deposited as a film with a sharp interface between the red and green sections. The film was irradiated by a narrow rectangular X-ray beam, and a photograph of the luminescence was acquired at a series of sample positions (20 µm steps). (A) Setup schematic, (B) setup photograph, (C) Ratio of red to green light intensity scanned at different positions (20 µm step size) with/without 10 mm of tissue. (D, E, F) photos of luminescence (with room light blocked) as the sample was moved across the red/green (Tb/Eu) phosphor interface, at displacements of 0.12, 0.22, and 0.42 mm, respectively. (G, H, I) correspond to the same position of D, E, and F, respectively, but with the film inserted into chicken breast. The luminescent image is blurred by the scattering in the tissue from 0.26 to~8.5 mm. Reproduced with permission from Ref. 48.
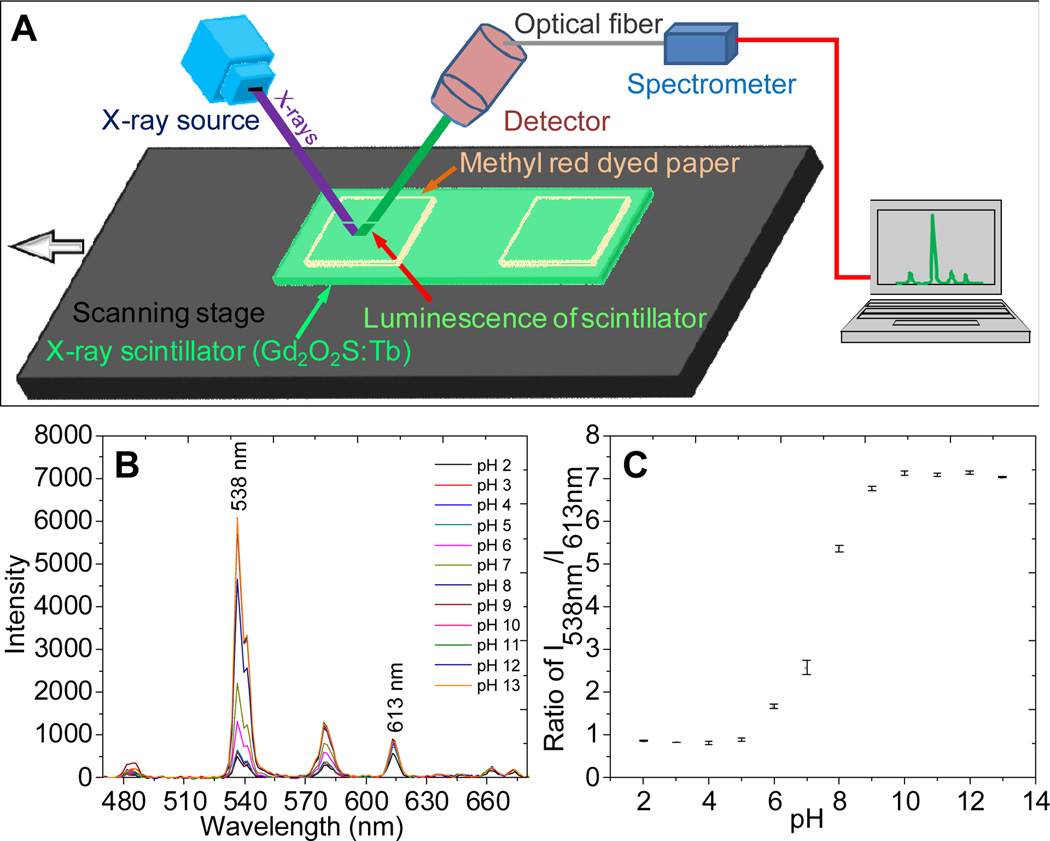
In order to demonstate the chemical sensitivity of the technique, we analyzed luminesence spectra of the scintillators passing through methyl red dyed pH paper. (Figure 17B). The ratio of the peaks at 538 nm and 613 nm was used to identify the pH with a dynamic range between pH 6~9 and noise level of 0.05 pH units. The availability of a wide range of nanoscintillators and organic sensing dyes allows XEOL to provide high chemical sensitivity and selectivity.
Figure 17.
An X-ray excited pH sensor, formed by measuring X-ray excited luminescence spectra through methyl red-dyed paper. (A) Schematic showing the pH sensor and (B) spectra of Gd2O2S:Tb through methyl red paper at different pH values. (C) Calibration curve: peak ratio as a function of pH. Error bars represent the standard deviation of five replicable trials. Reproduced with permission from Ref. 48.
This imaging technique is unique in measuring local chemical concentrations on the surface of implanted medical devices. The surface of implants are susceptible to bacterial infection and the formation of biofilms which are resistant to antibiotic treatments in part due to heterogeneous pH and oxygen concentration.146–147 We expect that the ability to study local chemical concentrations such as pH or released antimicrobial agents will be useful in early detction of biofilms and studying the effectiveness of antimicrobial surface coatings. For example, silver nanoparticles are commonly utilized as antimicrobial agents, however their in vivo effectiveness is controversial because compounds such as albumin chelate with silver ions and reduce free ion conentrations.148–151 To demonstrate that we are able to sensitively detect silver nanoparticles through tissue we depositecd a 5 nm thick film of silver nanoparticles on a scintillator film. Through XEOL, patterns of silver deposition and dissolution on a scintillator (Gd2O2S:Eu) film were imaged through 1 cm of pork tissue (Figure 18).49 We expect that this in situ high resolution surface imaging will be useful for studying localized infection and developing antimicrobial surfaces.
Figure 18.
(A), (D) Images of a gold and silver coated scintillator film before and after H2O2 etching. (B), (E) Intensity of red light scanned at different positions with/without 10 mm of tissue before H2O2 etching. (C), (F) Intensity of red light scanned at different positions with/without 10 mm of tissue after H2O2 etching. The resolution through 10 mm tissue is 1.7 mm. Reproduced with permission from Ref. 49.
4.3 X-ray scintillators
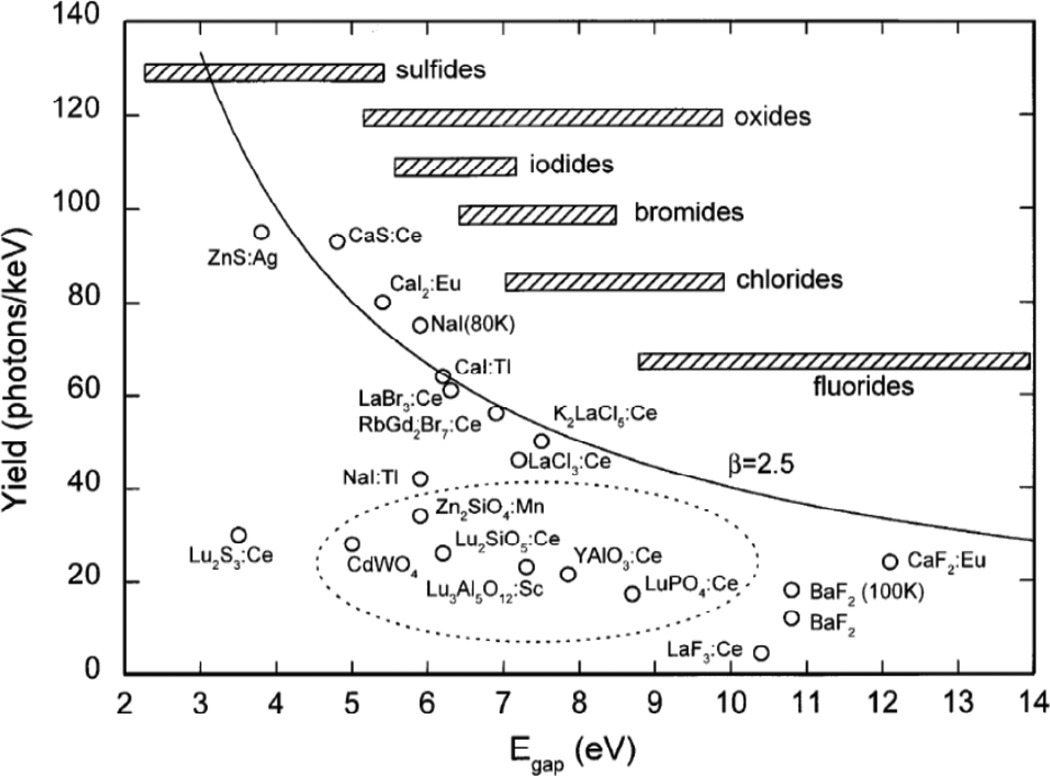
X-ray scintillators, materials which emit visible light upon irradiation by X-rays, are often used in X-ray imaging screens and films for projection imaging, fluoroscopy, and CT. The visible light emitted by the scintillators is then collected by a photodetector. The fundamental limit of the light yield (Y) of an activated scintillator (photons emitted/MeV) is determined by the band gap Egap (eV) of the host material and is approximately given by equation (6) with a maximum of close to 40% energy efficiency.152
| (6) |
Figure 19 shows the fundamental limit on light output for various phosphors. The hatched bars indicate the typical range of band gap values associated with the specific type of compound indicated. The fundamental limit is smallest for fluorides with the largest band gap and the limit is largest for sulfides with small band gaps.
Figure 19.
Light yield of scintillators and cathode ray tube phosphors. Reproduced with permission from Ref. 152.
Discovered in 1896, CaWO4, was used for the next 80 years in X-ray scintillator screens due to its reasonable X-ray absorption in the 20–100 keV range and blue emission.153 However, CaWO4 suffers from low X-ray to light conversion efficiency (only 15,000 optical photons /Mev). Currently, alkali metal halides containing small amounts of activator such as NaI:Tl and CsI:Tl are being widely used because of their superior conversion efficiency (64,000 optical photons/MeV for CsI:Tl). However, most alkali metal halides are hygroscopic requiring that the scintillators be kept moisture-free. Recently, optical ceramic scintillators have become popular due to their light conversion efficiency and chemical stability. Bismuth germanate oxide (BGO), a ceramic scinitillator, is often used due to its rapid response and little or no afterglow. The speed of response and high density make the scintillator ideal for high energy and high acquisition rate X-ray measurements. BGO is not hygroscopic, however, its low conversion efficiency (only 9,000 optical photon/MeV) results in poorer energy resolution than detectors based on alkali metal halides. Rare earth oxysulfide scintillators (M2O2S:Ln, M= Gd or Y. Ln= Ce, Pr, Eu, Tm, or Tb) are a promising class of ceramic scinitillators with improved absorption, greater density and higher X-ray to light conversion efficiency (60,000 optical photons/Mev).
Though the aforementioned scintillators discussed are typically used in projection X-ray imaging and CT, scintillator particles with sizes ranging from nanometers to micrometers are used as XLCT contrast agents.144, 154 Recently, rare-earth doped oxide and oxysulfide nanoparticles have been investigated as labels for low-background imaging using up-conversion,155 radioluminescence,153, 156 long-lifetime phosphorescence,157–158 and proximity scintillation assays. These nanoparticles are attractive imaging agents due to their chemical stability, photostability, large X-ray absorption cross-section and scintillation efficiency, and relatively low toxicity, especially after silica encapsulation.159 X-ray scintillators may also be coupled to photodynamic nanoparticles to enhance reactive oxygen production,160 although their effectiveness depends greatly upon their ability to target the reactive oxygen damage to the nucleus or other sensitive sites.137
5. Discussion and Outlook
Recent developments in X-ray imaging and contrast agents are opening up new opportunities for high-resolution functional imaging. Three general categories of analytes can be detected: endogenous elements within a sample detected via X-ray attenuation and XRF, analytes that are labeled with exogenous contrast agents and detected via attenuation, XRF, and XLT, and analytes that are detected with optical indicators via functional XLT. In all cases, a key advantage of X-ray imaging is that high resolution images can be generated because X-rays can penetrate deeply through tissue and have relatively low scattering coefficients. The images can also be co-registered with structural information from X-ray attenuation imaging and integrated with other imaging techniques such as diffuse optical fluorescence tomography and MRI. The main disadvantage of X-ray imaging, however, is that large X-ray doses cause short term tissue damage/radiation sickness, and increase the long term risk of developing cancer.160, 197–198 Consequently, the challenge will be to maximize the analytical sensitivity, specificity, image resolution, and acquisition rate while minimizing the X-ray dose, contrast concentration, and contrast toxicity. Each application has its own considerations, and we will separately discuss imaging of native analytes, contrast labeled molecules, and analytes detected with functional XLT.
Endogenous Analytes
Contrast-free X-ray attenuation imaging of endogenous tissue is widely used in medical diagnosis. Different types of tissue are distinguished by their elemental composition and X-ray attenuation coefficient. For example, bone has more calcium and phosphorous than fat, muscle, or blood and thus attenuates X-rays more strongly at all energies used in projection imaging (see Fig. 4). Conventional imaging measures the average attenuation of polychromatic X-rays. Contrast can therefore be significantly improved by hyperspectral imaging at a number of different X-ray energies in order to differentiate tissues by their different elemental compositions and X-ray attenuation spectra.199 However, the most distinct spectral features are from K-edge absorbance, and unfortunately the most abundant endogenous elements in the human body (O, C, H, N, Ca, P, K, S, Na, and Cl) do not have sharp K-edge features in the 20~100 keV energy range used for imaging. Detection of less abundant elements is possible, but generally requires more X-ray intensity because of the large background.
XRF it is a positive contrast technique with little background and is therefore more sensitive than X-ray attenuation imaging for detecting low elemental concentrations. This high sensitivity allows for submicron analysis of trace amounts (ng/mL) of metal ions and other elements (see Fig. 10). However, ultra-high resolution imaging is only practical in cells and tissue sections, not in vivo, because very intense X-rays are needed in order to collect a sufficient number of fluorescent photons per pixel. Lower doses can be used in vivo imaging but at lower spatial resolution (equation (5)). For example XRF has been used to image native iodine in thyroid biopsies,200 image iodine uptake in the brains of living mice133 and measure lead concentration in vivo in the bones of children.100–101 The major limitation to XRF sensitivity is the collection angle efficiency of the detector. For example, in Takeda’s study of cerebral iodine uptake in mice, the detector captured only a 0.02% of the radiation solid angle.133 Larger spectroscopic detectors and improved geometries are expected to enhance the sensitivity and reduce acquisition rate especially for tomographic images. Collimators may also increase resolution and acquisition rate, at the expense of sensitivity.201
Exogenous Labels
Conventional images often miss important features, which could be imaged using the appropriate contrast agent. For example, fat and fibroglandular tissue are easily distinguished in mammograms because fat is comprised of large carbon-hydrogen chains and is less radiopaque than firbroglandular tissue containing heavier elements including oxygen (especially in water), phosphorous (as phosphate ions and in nucleic acids), and ions such sodium, potassium, calcium, and chloride. Breast tumors display a similarly large contrast compared to fat, and on average are slightly more opaque than fibroglandular tissue, but the latter contrast is difficult to discern.202 This difficulty of differentiating normal fibroglandular tissue from tumors severely limits the utility of mammograms for patients with large percentages of fibrous breast tissue, especially premenopausal women.203–205 Methods to increase contrast are therefore urgently needed.
Several types of molecular and nanoparticle contrast agents are being developed for projection and CT imaging (see Table. 1). For example, iodine based agents are commonly used for imaging vasculature (e.g. Fig 5). This contrast can be dramatically improved with hyperspectral imaging and spectral analysis near the K-edge absorption band of the contrast agent.29–31, 199 For example, the linear attenuation coefficient for iodine increases by a factor of 1.6 immediately above the K-absorption edge at 33.17 keV providing a distinct feature for detecting iodine concentrations. Synchrotrons are ideal sources for intense wavelength-tunable monochromatic X-ray but are prohibitively large and costly for most medical applications. Less expensive and more compact sources are being developed using free electron lasers (FEL) and electron beams interacting with ultrafast infrared laser pulses,206–207 or conventional X-ray sources with quasi-monochromatic Bragg filters.208 An alternative hyperspectral imaging approach is to use conventional polychromatic X-ray sources and measure the energy of each collected X-ray photon using an energy dispersive camera.29–31 These energy dispersive cameras are easily interfaced with existing X-ray sources, but have a lower energy resolution and thus lower sensitivity than monochromatic synchrotron and FEL sources.
XRF is more sensitive than attenuation imaging because the background is lower and there is consequently less background interference and less shot noise on the background. The XRF signal is usually weaker, however, for three reasons. First not all absorbed photons result in X-ray fluorescence, and the quantum efficiency is low for small element numbers. Second, the fluorescent X-rays are absorbed by the tissue especially for low energy X-ray fluorescent photons in deep tissue. Third, the detector is often small or placed distantly from the sample which limits the collected solid angle. New developments in spectral imaging cameras are expected to greatly improve sensitivity and acquisition speed. 133, 201, 209
For analysis of low Z elements, it is possible to shift XRF photons to higher energies by replacing electrons with more massive negative subatomic particles (i.e. pions, with a mass 273 times greater than an electron, and muons, with a mass aproximately 207 times the mass of the electron).210–211 The emission energy is proportional to the mass through the Rydberg energy in equation (4). Reidy et al. report the detection of elements with atomic numbers 6 to 20 by the generation of muonic X-rays.211 Pions and muons beams can penetrate deeply into tissue, but have short lifetimes in the rest frame (2.55×10−8 s and 2.23×10−6 s, respectively), and require a synchrotron source.210–212
Nanoparticles are also attractive as contrast agents and molecular labels for CT and XRF because they have longer circulation time than many molecular contrast agents. They can be functionalized with peptide, aptamer, or antibody molecular targeting agents, and provide a large signal per binding event. Popovtzer showed that gold nanoparticles can be selectively targeted to tumors using antibodies and can be observed in CT (Fig. 6). The contrast is expected to be much higher using K-edge imaging of the gold. For example, X-ray attenuation from gold increases by a factor of 2.5 at the L3 absorption edge (11.92 keV) and a factor of 4.16 at the K-edge (80.72 keV). Many different types of nanoparticles can be fabricated with different elemental composition and surface chemistry in order to simultaneously image multiple molecular and physiological markers (see Fig. 12), as well as blood and gas pools.134 Nanoparticles also have room on their surface and within the particle for additional functional “cargo” such as fluorescent dyes, MRI contrast molecules, and encapsulated drugs. The challenge will be to maximize specific molecular labeling, while improving biocompatibility and clearance or elimination.
X-ray excited luminescent nanoparticles are also very promising as contrast agents. Typically a few thousand visible photons are generated per absorbed X-ray photon (depending upon the incident X-ray energy) compared to at most one fluorescent X-ray for XRF. Some light is lost during propagation through the tissue, but a reasonable flux of near-infrared light penetrates through several centimeters of tissue (Fig. 15). In principle very high optical collection efficiencies are possible using large area photodetectors and potentially integrating sphere optics. In limited angle configurations the XLT images can be acquired rapidly, although with some loss of resolution along one dimension.145 A wide variety of contrast agents with unique spectra have been developed for scintillator film applications and these can be used for XLT contrast (see Table. 2). We expect that continuing development of scintillator nanomaterials, targeting moieties, and instrumentation will greatly expand the utility of XLT. Many of the scintillator materials also have large magnetic permeability and serve as effective MRI contrast agents.50
Table 2.
Examples of X-ray scintillators
| Scintillator | Decay time (ns) |
Maximum Emission Wavelength (nm) |
Conversion efficiency (visible photons/MeV) |
Hygroscopicity | Density g/cm3 | Reference |
|---|---|---|---|---|---|---|
| CsI:Tl | 800 | 550 | 66,000 | Yes | 4.51 | 161–163 |
| CsI:Na | 630 | 425 | 49,000 | Yes | 4.51 | 164–165 |
| NaI:Tl | 230 | 415 | 44,000 | Yes | 3.67 | 161–162, 165 |
| LiI:Eu | 1,200 | 475 | 15,000 | Yes | 4.08 | 166–167 |
| LaBr3:Ce | 35 | 358 | 61,000 | Yes | 5.3 | 168 |
| K2LaI5: Ce | 24 | 420 | 55,000 | Yes | 4.4 | 169 |
| CaF2:Eu | ~1,000 | 435 | 24,000 | No | 3.18 | 161, 170 |
| SrI2:Ce,Na | 27 (25%), 450 (75%) | 404 | 16,000 | Yes | 4.59 | 171 |
| SrI2:Eu | 1,200 | 435 | 120,000 | Yes | 4.59 | 171 |
| BaFBr:Eu | 800 | 390 | 60,000 | Yes | 4.56 | 172–173 |
| LaOBr:Tb | ~1000,000 | 425 | 67,000 | Yes | 6.3 | 174–175 |
| LaOBr:Tm | ~1000,000 | 374, 472 | 6,0000 | Yes | 6.1 | 156 |
| ZnS:Ag | 1,200 | 450 | 49,000 | No | 3.9 | 176 |
| M’-YTaO4 | 3,000 | 337 | 40,000 | No | 7.5 | 156, 173 |
| M’-YTaO4:Nb | ~2,000 | 410 | 40,000 | No | 7.5 | 177–178 |
| BaHfO3:Ce | 25 | 400 | 40,000 | No | 8.5 | 175, 179 |
| Bi4Ge3O12 (BGO) | 300 | 480 | 9,000 | No | 7.1 | 161, 180 |
| CaWO4 | 8,000 | 425 | 15,800 | No | 6.1 | 181–182 |
| CdWO4 | 5,000 | 495 | 20,000 | No | 7.9 | 183 |
| YAlO3:Ce | 24 | 360 | 20,100 | No | 5.35 | 184–185 |
| Y3Al5O12:Ce | 90~120 | 550 | 16,700 | No | 4.55 | 161–162, 186 |
| LuAlO3:Ce | 18 | 365 | 12,000 | No | 8.34 | 175, 187 |
| Lu3Al5O12:Ce | 55 | 530 | 12,500 | No | 6.7 | 188–189 |
| Lu2SiO5:Ce | 30 | 425 | 33,000 | No | 7.4 | 190 |
| Lu2O3:Tb,Eu | ~1,000 | 612 | 30,000 | No | 9.4 | 191 |
| Gd2O3:Eu | ~1,000 | 612 | 40,000 | No | 5.91 | 172, 192 |
| Gd2O2S:Eu | ~1000,000 | 623 | 60,000 | No | 7.3 | 193 |
| Gd2O2S:Tb | ~1000,000 | 545 | 60,000 | No | 7.3 | 179 |
| Gd2O2S:Pr,Ce,F | 3,000 | 510 | 48,000 | No | 7.3 | 179 |
| Gd3Ga5O12:Cr,Ce | 140,000 | 730 | 40,000 | No | 7.1 | 178, 194 |
| Gd2SiO5:Ce | 60 | 430 | 8,000 | No | 6.7 | 195–196 |
X-ray excited optical luminescence indicators
Fluorescent and colorimetric indicators and stains are widely used for histology and biomedical research because of their high sensitivity and specificity for a wide variety of analytes. XEOL indicators combine the advantages of optical indicators with high resolution, low background imaging from scanning X-ray excitation. A particularly attractive application is imaging chemical and mechanical changes on implanted medical devices, which can become infected. Many analytes can be measured (e.g. pH, silver metal dissolution, oxygen, and protease activity). Relatively thick scintillator films may be used to improve X-ray capture, and signal strength especially at high energies. The two dimensional nature of the surfaces simplifies and speeds up imaging. An important challenge will be to minimize the spectral distortion caused by wavelength dependent tissue scattering and absorption, especially for deeply implanted devices. Several techniques are possible, including calculation of spectral ratios using closely spaced spectral peaks, use of spectral reference regions near to the sensor region, and lifetime-based imaging. Another technical challenge will be integrating the sensor film onto the implanted device while minimizing delamination, abrasion, and reducing bacterial adhesion. After sensor development, the next step will be integration of sensing with active components such as combining magnetic or photothermal hyperthermia and drug release with local pH sensors to monitor the effect of therapies on local biofilm chemistry.
In addition to chemical analysis on surfaces, XEOL can be used for three dimensional tomography similar to XLT and limited-angle XLT. For example, our group has developed hollow nanoparticles with encapsulated magnetic iron oxide nanoparticles. The luminescence is quenched by iron oxide in the core and increases as the iron oxide is etched.50 We have also encapsulated indicator dyes and are studying leaching rate as a function of pH. In future we expect that these types of sensors will be useful for local drug delivery while measuring the amount of drug delivered in time. Additional sensors with unique optical emission spectra can be used for simultaneously studying changes in cellular chemistry such as endosomal pH changes upon apoptosis. Such in situ sensors will be useful for optimizing therapeutic agents and monitoring effectiveness.
Overall, functional X-ray imaging techniques are rapidly advancing. Development of monochromatic X-ray sources, energy sensitive detectors, and hyperspectral image analysis algorithms are improving contrast-free images. New contrast agents for projection imaging and XRF are dramatically improving specificity. XLT-based labels and indicators are also highly promising for rapid and sensitive chemical analysis in tissue on the surface of implanted medical devices. With further improvements in instrumentation and contrast agents, we expect a bright future for high resolution molecular imaging with X-rays.
Figure 11.
Schematic of a confocal 3D XRF configuration. Reproduced with permission from Ref. 129.
Acknowledgements
This work was supported in part through a SC Space Grants Consortium grant under award number NNG05GI68G and NIH Center of Biomaterials for Tissue Regeneration (CBTR) grants 5P20RR021949 and 8P20GM103444..
Notes and references
- 1.Ono M. Chemical and Pharmaceutical Bulletin. 2009;57:1029–1039. doi: 10.1248/cpb.57.1029. [DOI] [PubMed] [Google Scholar]
- 2.Rahmim A, Zaidi H. Nuclear medicine communications. 2008;29:193. doi: 10.1097/MNM.0b013e3282f3a515. [DOI] [PubMed] [Google Scholar]
- 3.Levin CS, Hoffman EJ. Phys. Med. Bio. 1999;44:781. doi: 10.1088/0031-9155/44/3/019. [DOI] [PubMed] [Google Scholar]
- 4.Derenzo SE, Moses WW, Huesman RH, Budinger TF. Annals of nuclear medicine. 1993;7:3–3. [Google Scholar]
- 5.Kojima A, Matsumoto M, Takahashi M, Hirota Y, Yoshida H. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 1989;30:508. [PubMed] [Google Scholar]
- 6.Beekman FJ, van der Have F, Vastenhouw B, van der Linden AJA, van Rijk PP, Burbach JPH, Smidt MP. Journal of Nuclear Medicine. 2005;46:1194–1200. [PubMed] [Google Scholar]
- 7.Stocklin G, Pike V. Radiopharmaceuticals for positron emission tomography: methodological aspects. Springer Netherlands: 1993. [Google Scholar]
- 8.Philip H E. Methods. 2002;27:208–217. [Google Scholar]
- 9.Lucignani G. European Journal of Nuclear Medicine and Molecular Imaging. 2011;38:592–595. doi: 10.1007/s00259-010-1708-6. [DOI] [PubMed] [Google Scholar]
- 10.Robertson R, Germanos M, Li C, Mitchell G, Cherry S, Silva M. Phys. Med. Bio. 2009;54:N355–N365. doi: 10.1088/0031-9155/54/16/N01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glickman J, Schmid A, Ferrand S. Assay Drug Dev. Technol. 2008;6:433–455. doi: 10.1089/adt.2008.135. [DOI] [PubMed] [Google Scholar]
- 12.Troy T, Jekic-McMullen D, Sambucetti L, Rice B. Molecular Imaging. 2004;3:9–23. doi: 10.1162/15353500200403196. [DOI] [PubMed] [Google Scholar]
- 13.Rahmim A. Iranian Journal of Nuclear Medicine. 2006;14:1–20. [Google Scholar]
- 14.Muehllehner G, Karp JS. Physics in Medicine and Biology. 2006;51:R117. doi: 10.1088/0031-9155/51/13/R08. [DOI] [PubMed] [Google Scholar]
- 15.Badea CT, Drangova M, Holdsworth DW, Johnson GA. Physics in Medicine and Biology. 2008;53:R319. doi: 10.1088/0031-9155/53/19/R01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nussbaum RH, Kohnlein W. Occupational, industrial and environmental toxicology. Mosby Publishing: St. Louis. 1997:517–530. [Google Scholar]
- 17.Cohen BL. Cancer risk from low-level radiation. American Journal of Roentgenology. 2002;179:1137–1143. doi: 10.2214/ajr.179.5.1791137. [DOI] [PubMed] [Google Scholar]
- 18.Furlow B. Radiologic Technology. 2010;81:437–450. [PubMed] [Google Scholar]
- 19.Frush DP, Donnelly LF, Rosen NS. Pediatrics. 2003;112:951–957. doi: 10.1542/peds.112.4.951. [DOI] [PubMed] [Google Scholar]
- 20.Brenner DJ, Hall EJ. New England Journal of Medicine. 2007;357:2277–2284. doi: 10.1056/NEJMra072149. [DOI] [PubMed] [Google Scholar]
- 21.ICRP. Recommendations of the International Commission on Radiological Protection. Ann. ICRP; 2007. pp. 2–4. ICRP Publication 103. [Google Scholar]
- 22.Tanooka H. International Journal of Radiation Biology. 2001;77:541–551. doi: 10.1080/09553000110034612. [DOI] [PubMed] [Google Scholar]
- 23.Marchesini S, Chapman H, Hau-Riege S, London R, Szoke A, He H, Howells M, Padmore H, Rosen R, Spence J, Weierstall U. Opt. Express. 2003;11:2344–2353. doi: 10.1364/oe.11.002344. [DOI] [PubMed] [Google Scholar]
- 24.Paulus MJ, Gleason SS, Kennel SJ, Hunsicker PR, Johnson DK. Neoplasia (New York, NY) 2000;2:62. doi: 10.1038/sj.neo.7900069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burstein P, Bjorkholm PJ, Chase RC, Seguin FH. Nuclear Instruments and Methods in Physics Research. 1984;221:207–212. [Google Scholar]
- 26.Wildenschild D, Vaz CMP, Rivers ML, Rikard D, Christensen BSB. Journal of Hydrology. 2002;267:285–297. [Google Scholar]
- 27.Haidekker MA, Xplore I. Advanced biomedical image analysis. Wiley Online Library; 2011. [Google Scholar]
- 28.Denison C, Carlson WD, Ketcham RA. Journal of Metamorphic Geology. 1997;15:29–44. [Google Scholar]
- 29.Pan D, Roessl E, Schlomka J-P, Caruthers SD, Senpan A, Scott MJ, Allen JS, Zhang H, Hu G, Gaffney PJ, Choi ET, Rasche V, Wickline SA, Proksa R, Lanza GM. Angewandte Chemie. 2010;122:9829–9833. doi: 10.1002/anie.201005657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schlomka JP, Roessl E, Dorscheid R, Dill S, Martens G, Istel T, Bäumer C, Herrmann C, Steadman R, Zeitler G, Livne A, Proksa R. Physics in Medicine and Biology. 2008;53:4031. doi: 10.1088/0031-9155/53/15/002. [DOI] [PubMed] [Google Scholar]
- 31.Bornefalk H. Physics in Medicine and Biology. 2012;57:N83. doi: 10.1088/0031-9155/57/7/N83. [DOI] [PubMed] [Google Scholar]
- 32.Yu S, Watson AD. Chemical reviews. 1999;99:2353–2378. doi: 10.1021/cr980441p. [DOI] [PubMed] [Google Scholar]
- 33.Lewis R. Physics in Medicine and Biology. 1997;42:1213. doi: 10.1088/0031-9155/42/7/001. [DOI] [PubMed] [Google Scholar]
- 34.Singh J, Daftary A. Journal of Nuclear Medicine Technology. 2008;36:69–74. doi: 10.2967/jnmt.107.047621. [DOI] [PubMed] [Google Scholar]
- 35.Mcclennan BL. Investigative Radiology. 1994;29:S46. doi: 10.1097/00004424-199405001-00008. [DOI] [PubMed] [Google Scholar]
- 36.Rumpel T. Muenchen Med Wschr. 1897;44:420–421. [Google Scholar]
- 37.Rabin O, Perez JM, Grimm J, Wojtkiewicz G, Weissleder R. Nature Materials. 2006;5:118–122. doi: 10.1038/nmat1571. [DOI] [PubMed] [Google Scholar]
- 38.Hainfeld J, Slatkin D, Focella T, Smilowitz H. British journal of radiology. 2006;79:248. doi: 10.1259/bjr/13169882. [DOI] [PubMed] [Google Scholar]
- 39.Zhang XD, Wu HY, Wu D, Wang YY, Chang JH, Zhai ZB, Meng AM, Liu PX, Zhang LA, Fan FY. International journal of nanomedicine. 2010;5:771. doi: 10.2147/IJN.S8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Woehrle GH, Brown LO, Hutchison JE. Journal of the American Chemical Society. 2005;127:2172–2183. doi: 10.1021/ja0457718. [DOI] [PubMed] [Google Scholar]
- 41.DeLong RK, Reynolds CM, Malcolm Y, Schaeffer A, Severs T, Wanekaya A. Nanotechnology, Science and Applications. 2010;3:53–63. doi: 10.2147/NSA.S8984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Popovtzer R, Agrawal A, Kotov NA, Popovtzer A, Balter J, Carey TE, Kopelman R. Nano Letters. 2008;8:4593–4596. doi: 10.1021/nl8029114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reuveni T, Motiei M, Romman Z, Popovtzer A, Popovtzer R. International journal of nanomedicine. 2011;6:2859. doi: 10.2147/IJN.S25446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Atkins HL, Fairchild RG, Robertson JS, Greenberg D. Radiology. 1975;115:431–437. doi: 10.1148/115.2.431. [DOI] [PubMed] [Google Scholar]
- 45.Seltzer S. Contrast Media in Computed Tomography. 1981;76 [Google Scholar]
- 46.Ahrén M, Selegård La, Klasson A, Söderlind F, Abrikossova N, Skoglund C, Bengtsson Tr, Engström M, Käll P-O, Uvdal K. Langmuir. 2010;26:5753–5762. doi: 10.1021/la903566y. [DOI] [PubMed] [Google Scholar]
- 47.Li IF, Su C-H, Sheu H-S, Chiu H-C, Lo Y-W, Lin W-T, Chen J-H, Yeh C-S. Advanced Functional Materials. 2008;18:766–776. [Google Scholar]
- 48.Chen H, Patrick AL, Yang Z, VanDerveer DG, Anker JN. Analytical Chemistry. 2011;83:5045–5049. doi: 10.1021/ac200054v. [DOI] [PubMed] [Google Scholar]
- 49.Chen H, Longfield DE, Varahagiri VS, Nguyen KT, Patrick AL, Qian H, VanDerveer DG, Anker JN. Analyst. 2011;136:3438–3445. doi: 10.1039/c0an00931h. [DOI] [PubMed] [Google Scholar]
- 50.Chen H, Colvin DC, Qi B, Moore T, He J, Mefford OT, Alexis F, Gore JC, Anker JN. Journal of Materials Chemistry. 2012;22:12802–12809. doi: 10.1039/C2JM15444G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hounsfield GN. British journal of radiology. 1973;46:1016. doi: 10.1259/0007-1285-46-552-1016. [DOI] [PubMed] [Google Scholar]
- 52.Herman GT. Real-Time Imaging. 1995;1:3–18. [Google Scholar]
- 53.Natterer F, Wübbeling F. Mathematical methods in image reconstruction. Society for Industrial Mathematics; 2001. [Google Scholar]
- 54.Olivecrona H. Neuroradiology. 1977;14:175–181. doi: 10.1007/BF00496981. [DOI] [PubMed] [Google Scholar]
- 55.Bettmann M, Bourdillon P, Barry W, Brush K, Levin D. Radiology. 1984;153:583–587. doi: 10.1148/radiology.153.3.6387783. [DOI] [PubMed] [Google Scholar]
- 56.Nelson R, Chezmar J, Peterson J, Bernardino M. American Journal of Roentgenology. 1989;153:973–976. doi: 10.2214/ajr.153.5.973. [DOI] [PubMed] [Google Scholar]
- 57.Leander P, Golman K, Strandede P, Klaveness JO, Besjakov J, Falt K. Investigative Radiology. 1993;28:513. doi: 10.1097/00004424-199306000-00009. [DOI] [PubMed] [Google Scholar]
- 58.Blum AG, Simon J, Cotten A, Quirin-Cosmidis I, Boyer B, Boutry N, Antonini JP. Investigative Radiology. 2000;35:304. doi: 10.1097/00004424-200005000-00004. [DOI] [PubMed] [Google Scholar]
- 59.Dawson P, Howell M. British journal of radiology. 1986;59:987. doi: 10.1259/0007-1285-59-706-987. [DOI] [PubMed] [Google Scholar]
- 60.Hill JA, Cohen MB. The American journal of cardiology. 1994;74:57–63. doi: 10.1016/0002-9149(94)90492-8. [DOI] [PubMed] [Google Scholar]
- 61.Katzberg RW. Radiology. 1997;204:297. doi: 10.1148/radiology.204.2.9240511. [DOI] [PubMed] [Google Scholar]
- 62.Hlatky MA, Morris KG, Pieper KS, Davidson CJ, Schwab SJ, Bashore TM. Journal of the American College of Cardiology. 1990;16:871–877. doi: 10.1016/s0735-1097(10)80335-3. [DOI] [PubMed] [Google Scholar]
- 63.Grainger RG. The British journal of radiology. 1982;55:1. doi: 10.1259/0007-1285-55-649-1. [DOI] [PubMed] [Google Scholar]
- 64.Bettmann M. American Journal of Roentgenology. 1982;139:787–794. doi: 10.2214/ajr.139.4.787. [DOI] [PubMed] [Google Scholar]
- 65.Havron A, Davis MA, Seltzer SE, Paskins-Hurlburt AJ, Hessel SJ. Journal of Computer Assisted Tomography. 1980;4:642. doi: 10.1097/00004728-198010000-00014. [DOI] [PubMed] [Google Scholar]
- 66.Uhle AA, Pfahler GE, Mackinney WH, Miller AG. Annals of Surgery. 1910;51:546. doi: 10.1097/00000658-191004000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shapiro R, Papa D. Annals of the New York Academy of Sciences. 1959;78:756–763. doi: 10.1111/j.1749-6632.1959.tb56061.x. [DOI] [PubMed] [Google Scholar]
- 68.Shapiro R. Acta radiologica. 1956;46:635. doi: 10.3109/00016925609171456. [DOI] [PubMed] [Google Scholar]
- 69.Bachem C, Gunther HZ. Fhr Rontgenkunde. 1910;12:369. [Google Scholar]
- 70.POV Reports The Diagostic Imaging Marketplace in the US. Cedar Grove, NJ: POV Inc; 1998. [Google Scholar]
- 71.Owens JM, Biery DN. Radiographic interpretation for the small animal clinician. Wiley-Blackwell; 1999. [Google Scholar]
- 72.Cannon WB. Journal of the Boston Society of Medical Sciences. 1898;2:59. [Google Scholar]
- 73.Xiao M, Nyagilo J, Arora V, Kulkarni P, Xu D, Sun X, Davé DP. Nanotechnology. 2010;21:035101. doi: 10.1088/0957-4484/21/3/035101. [DOI] [PubMed] [Google Scholar]
- 74.Cai QY, Kim SH, Choi KS, Kim SY, Byun SJ, Kim KW, Park SH, Juhng SK, Yoon KH. Investigative Radiology. 2007;42:797. doi: 10.1097/RLI.0b013e31811ecdcd. [DOI] [PubMed] [Google Scholar]
- 75.Kim D, Park S, Lee JH, Jeong YY, Jon S. Journal of the American Chemical Society. 2007;129:7661–7665. doi: 10.1021/ja071471p. [DOI] [PubMed] [Google Scholar]
- 76.Liu H, Wang H, Guo R, Cao X, Zhao J, Luo Y, Shen M, Zhang G, Shi X. Polym. Chem. 2010;1:1677–1683. [Google Scholar]
- 77.McDonald MA, Watkin KL. Investigative Radiology. 2003;38:305. [PubMed] [Google Scholar]
- 78.Seltzer S, Davis M, Judy P, Havron A. 1979:400. [Google Scholar]
- 79.Watkin KL, McDonald MA. Academic radiology. 2002;9:S285. doi: 10.1016/s1076-6332(03)80205-2. [DOI] [PubMed] [Google Scholar]
- 80.Sarkar SK, Rycyna RE, Lenkinski RE, Solleveld HA, Kinter LB. Magnetic Resonance in Medicine. 1991;17:328–335. doi: 10.1002/mrm.1910170205. [DOI] [PubMed] [Google Scholar]
- 81.Thomsen H, Almen T, Morcos S. Journal belge de radiologie. 2004;87:88–92. [Google Scholar]
- 82.Strunk HM, Schild H. European radiology. 2004;14:1055–1062. doi: 10.1007/s00330-004-2260-1. [DOI] [PubMed] [Google Scholar]
- 83.Gupta AK, Alberico RA, Litwin A, Kanter P, Grossman ZD. Journal of Computer Assisted Tomography. 2002;26:869. doi: 10.1097/00004728-200211000-00001. [DOI] [PubMed] [Google Scholar]
- 84.Albrecht T, Dawson P. British journal of radiology. 2000;73:878. doi: 10.1259/bjr.73.872.11026864. [DOI] [PubMed] [Google Scholar]
- 85.Vehmas T, Markkola A. Acta radiologica. 1998;39:223–226. doi: 10.1080/02841859809172184. [DOI] [PubMed] [Google Scholar]
- 86.Janon E. American Journal of Roentgenology. 1989;152:1348–1348. doi: 10.2214/ajr.152.6.1348-a. [DOI] [PubMed] [Google Scholar]
- 87.Unger E, Gutierrez F. Investigative Radiology. 1986;21:802–807. doi: 10.1097/00004424-198610000-00007. [DOI] [PubMed] [Google Scholar]
- 88.Zeman HD, Siddons DP. Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment. 1990;291:67–73. doi: 10.1016/j.nima.2014.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zwicker C, Langer M, Urich V, Felix R. Contrast opacification for CT from iodine, gadolinium and ytterbium: In vitro and animal experiments. New York: Georg Thieme Verlag Stuttgart; 1993. pp. 255–259. [DOI] [PubMed] [Google Scholar]
- 90.Fischer HW. Radiology. 1957;68:488–498. doi: 10.1148/68.4.488. [DOI] [PubMed] [Google Scholar]
- 91.Fischer H. The American journal of roentgenology, radium therapy, and nuclear medicine. 1957;77:64. [PubMed] [Google Scholar]
- 92.Sailer R, Kissler B, Stauch G, Franken T, Huth F. Fortschritte auf dem Gebiete der Röntgenstrahlen und der Nuklearmedizin. 1973;119:727. [PubMed] [Google Scholar]
- 93.Sargent EN, Sherwin R. American Journal of Roentgenology. 1971;113:660–679. doi: 10.2214/ajr.113.4.660. [DOI] [PubMed] [Google Scholar]
- 94.Bazalova M, Kuang Y, Pratx G, Xing L. Medical Imaging, IEEE Transactions on. 2012;31:1620–1627. doi: 10.1109/TMI.2012.2201165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Christoph J F. Current Opinion in Chemical Biology. 2007;11:121–127. doi: 10.1016/j.cbpa.2007.02.039. [DOI] [PubMed] [Google Scholar]
- 96.McRae R, Bagchi P, Sumalekshmy S, Fahrni CJ. Chemical Reviews. 2009;109:4780–4827. doi: 10.1021/cr900223a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.de Jonge MD, Vogt S. Current Opinion in Structural Biology. 2010;20:606–614. doi: 10.1016/j.sbi.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 98.Marmorato P, Ceccone G, Gianoncelli A, Pascolo L, Ponti J, Rossi F, Salomé M, Kaulich B, Kiskinova M. Toxicology Letters. 2011;207:128–136. doi: 10.1016/j.toxlet.2011.08.026. [DOI] [PubMed] [Google Scholar]
- 99.Shimura M, Saito A, Matsuyama S, Sakuma T, Terui Y, Ueno K, Yumoto H, Yamauchi K, Yamamura K, Mimura H, Sano Y, Yabashi M, Tamasaku K, Nishio K, Nishino Y, Endo K, Hatake K, Mori Y, Ishizaka Y, Ishikawa T. Cancer Research. 2005;65:4998–5002. doi: 10.1158/0008-5472.CAN-05-0373. [DOI] [PubMed] [Google Scholar]
- 100.Todd AC, Chettle DR. Environmental health perspectives. 1994;102:172. doi: 10.1289/ehp.94102172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hoppin JA, Aro A, Williams PL, Hu H, Ryan PB. Environmental health perspectives. 1995;103:78. doi: 10.1289/ehp.9510378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pereira GR, Rocha HS, Calza C, Anjos MJ, Lima I, Pérez CA, Lopes RT. X-Ray Spectrometry. 2011;40:260–264. [Google Scholar]
- 103.Leskovjan AC, Kretlow A, Lanzirotti A, Barrea R, Vogt S, Miller LM. NeuroImage. 2011;55:32–38. doi: 10.1016/j.neuroimage.2010.11.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Qin Z, Toursarkissian B, Lai B. Metallomics. 2011;3:823–828. doi: 10.1039/c1mt00033k. [DOI] [PubMed] [Google Scholar]
- 105.Núñez-Milland DR, Baines SB, Vogt S, Twining BS. Journal of Synchrotron Radiation. 2010;17:560–566. doi: 10.1107/S0909049510014020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Endres PJ, MacRenaris KW, Vogt S, Allen MJ, Meade TJ. Molecular Imaging. 2006;5:485–497. [PubMed] [Google Scholar]
- 107.Rivière PJL. Physics in Medicine and Biology. 2004;49:2391. doi: 10.1088/0031-9155/49/11/019. [DOI] [PubMed] [Google Scholar]
- 108.Miqueles EX, De Pierro AR. Physics in Medicine and Biology. 2010;55:1007. doi: 10.1088/0031-9155/55/4/007. [DOI] [PubMed] [Google Scholar]
- 109.Ortega R, Devès G, Carmona A. Journal of The Royal Society Interface. 2009;6:S649–S658. doi: 10.1098/rsif.2009.0166.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Studinski R, O'Meara J, McNeill F. X-Ray Spectrometry. 2008;37:51–57. [Google Scholar]
- 111.Studinski RCN, McNeill FE, Chettle DR, O'Meara JM. Physics in Medicine and Biology. 2005;50:521. doi: 10.1088/0031-9155/50/3/009. [DOI] [PubMed] [Google Scholar]
- 112.Milakovic M, Berg G, Eggertsen R, NystrÖM E, Olsson A, Larsson A, Hansson M. Journal of Internal Medicine. 2006;260:69–75. doi: 10.1111/j.1365-2796.2006.01660.x. [DOI] [PubMed] [Google Scholar]
- 113.Bambynek W, Crasemann B, Fink RW, Freund HU, Mark H, Swift CD, Price RE, Rao PV. Reviews of Modern Physics. 1972;44:716–813. [Google Scholar]
- 114.Moseley HGJ. Philosophical Magazine. 1914;27:703–714. [Google Scholar]
- 115.Carpenter C, Sun C, Pratx G, Rao R, Xing L. Medical physics. 2010;37:4011. doi: 10.1118/1.3457332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Matthias F, Stephan F, Jens H, Josef J. Journal of X-Ray Science and Technology. 2003;11:83–112. [Google Scholar]
- 117.West M, Ellis AT, Potts PJ, Streli C, Vanhoof C, Wegrzynek D, Wobrauschek P. Journal of Analytical Atomic Spectrometry. 2011;26:1919–1963. [Google Scholar]
- 118.Frankel RS, Aitken DW. Appl. Spectrosc. 1970;24:557–566. [Google Scholar]
- 119.Pereira GR, Anjos MJ, Rocha HS, Faria P, Pérez CA, Lopes RT. Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment. 2007;580:951–954. doi: 10.1016/j.nima.2007.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Couprie ME, Filhol JM. Comptes Rendus Physique. 2008;9:487–506. [Google Scholar]
- 121.Fahrni CJ. Current Opinion in Chemical Biology. 2007;11:121–127. doi: 10.1016/j.cbpa.2007.02.039. [DOI] [PubMed] [Google Scholar]
- 122.Janssens K, Proost K, Falkenberg G. Spectrochimica Acta Part B: Atomic Spectroscopy. 2004;59:1637–1645. [Google Scholar]
- 123.Tsuji K, Nakano K. Journal of Analytical Atomic Spectrometry. 2011;26:305–309. [Google Scholar]
- 124.Takeda T, Zeniya T, Wu J, Yu Q, Lwin TT, Tsuchiya Y, Rao DV, Yuasa T, Yashiro T, Dilmanian FA, Itai Y, Akatsuka T. Proc. SPIE. 2002:300–309. [Google Scholar]
- 125.Punshon T, Guerinot ML, Lanzirotti A. Annals of Botany. 2009 doi: 10.1093/aob/mcn264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Janssens K, Proost K, Falkenberg G. Spectrochimica Acta Part B: Atomic Spectroscopy. 2004;59:1637–1645. [Google Scholar]
- 127.Vincze L, Vekemans B, Brenker FE, Falkenberg G, Rickers K, Somogyi A, Kersten M, Adams F. Analytical Chemistry. 2004;76:6786–6791. doi: 10.1021/ac049274l. [DOI] [PubMed] [Google Scholar]
- 128.Woll AR, Mass J, Bisulca C, Huang R, Bilderback DH, Gruner S, Gao N. Applied Physics A: Materials Science & Processing. 2006;83:235–238. [Google Scholar]
- 129.Nakano K, Tsuji K. Journal of Analytical Atomic Spectrometry. 2010;25:562–569. [Google Scholar]
- 130.Schields PJ, Gibson DM, Gibson WM, Gao N, Huang H, Ponomarev IY. Powder Diffraction. 2002;17:70–80. [Google Scholar]
- 131.Hossain M, Wang C, Su M. Applied Physics Letters. 2010;97:263704. [Google Scholar]
- 132.Cheong SK, Jones BL, Siddiqi AK, Liu F, Manohar N, Cho SH. Phys. Med. Bio. 2010;55:647. doi: 10.1088/0031-9155/55/3/007. [DOI] [PubMed] [Google Scholar]
- 133.Takeda T, Wu J, Huo Q, Yuasa T, Hyodo K, Dilmanian FA, Akatsuka T. Journal of Synchrotron Radiation. 2008;16:57–62. doi: 10.1107/S0909049508031853. [DOI] [PubMed] [Google Scholar]
- 134.Yu Q, Takeda T, Yuasa T, Hasegawa Y, Wu J, Thet-Thet-Lwin T, Hyodo K, Dilmanian F, Itai Y, Akatsuka T. Journal of Synchrotron Radiation. 2001;8:1030–1034. doi: 10.1107/s0909049500020483. [DOI] [PubMed] [Google Scholar]
- 135.Liu Y, Chen W, Wang S, Joly AG. Applied Physics Letters. 2008;92:043901. [Google Scholar]
- 136.Wang G, Cong W, Durairaj K, Qian X, Shen H, Sinn P, Hoffman E, McLennan G, Henry M. Opt. Express. 2006;14:7801–7809. doi: 10.1364/oe.14.007801. [DOI] [PubMed] [Google Scholar]
- 137.Chen W, Zhang J. Journal of Nanoscience and Nanotechnology. 2006;6:1159–1166. doi: 10.1166/jnn.2006.327. [DOI] [PubMed] [Google Scholar]
- 138.Moronne MM. Ultramicroscopy. 1999;77:23–36. doi: 10.1016/s0304-3991(99)00002-9. [DOI] [PubMed] [Google Scholar]
- 139.Adam J-F, Moy J-F, Susini J. Review of Scientific Instruments. 2005;76 091301-091301-091315. [Google Scholar]
- 140.Shroff H, Galbraith CG, Galbraith JA, Betzig E. Nat Meth. 2008;5:417–423. doi: 10.1038/nmeth.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hein B, Willig KI, Hell SW. Proceedings of the National Academy of Sciences. 2008;105:14271. doi: 10.1073/pnas.0807705105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hecht B, Sick B, Wild UP, Deckert V, Zenobi R, Martin OJF, Pohl DW. The Journal of Chemical Physics. 2000;112:7761–7774. [Google Scholar]
- 143.Pratx G, Carpenter CM, Sun C, Lei X. Medical Imaging, IEEE Transactions on. 2010;29:1992–1999. doi: 10.1109/TMI.2010.2055883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pratx G, Carpenter CM, Sun C, Rao RP, Xing L. Opt. Lett. 2010;35:3345–3347. doi: 10.1364/OL.35.003345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Carpenter C, Pratx G, Sun C, Xing L. Phys. Med. Bio. 2011;56:3487. doi: 10.1088/0031-9155/56/12/003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wolcott RD, Ehrlich GD. JAMA: The Journal of the American Medical Association. 2008;299:2682–2684. doi: 10.1001/jama.299.22.2682. [DOI] [PubMed] [Google Scholar]
- 147.Bryers JD. Biotechnology and Bioengineering. 2008;100:1–18. doi: 10.1002/bit.21838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Schierholz J, Lucas L, Rump A, Pulverer G. Journal of Hospital infection. 1998;40:257–262. doi: 10.1016/s0195-6701(98)90301-2. [DOI] [PubMed] [Google Scholar]
- 149.Ivanova E, Hasan J, Truong V, Wang J, Raveggi M, Fluke C, Crawford R. Applied Microbiology and Biotechnology. 2011:1–9. doi: 10.1007/s00253-011-3195-5. [DOI] [PubMed] [Google Scholar]
- 150.Darouiche RO. Clinical infectious diseases. 1999;29:1371. doi: 10.1086/313561. [DOI] [PubMed] [Google Scholar]
- 151.Wong KKY, Liu X. MedChemComm. 2010;1:125–131. [Google Scholar]
- 152.Dorenbos P. Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment. 2002;486:208–213. doi: 10.1016/s0168-9002(01)01386-9. [DOI] [PubMed] [Google Scholar]
- 153.Feldmann C, Jüstel T, Ronda CR, Schmidt PJ. Advanced Functional Materials. 2003;13:511–516. [Google Scholar]
- 154.Pratx G, Carpenter CM, Sun C, Xing L. Medical Imaging, IEEE Transactions on. 2010;29:1992–1999. doi: 10.1109/TMI.2010.2055883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Luo X, Cao W. Science in China Series B: Chemistry. 2007;50:505–513. doi: 10.1007/s11426-007-0047-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Issler SL, Torardi CC. Journal of Alloys and Compounds. 1995;229:54–65. [Google Scholar]
- 157.Hu L, Yan B, Zhang J, Wang X. Journal of Physics D: Applied Physics. 2007;40:7519. [Google Scholar]
- 158.Mao S, Liu Q, Gu M, Mao D, Chang C. Journal of Alloys and Compounds. 2008;465:367–374. [Google Scholar]
- 159.Osseni SA, Lechevallier S, Verelst M, Dujardin C, Dexpert-Ghys J, Neumeyer D, Leclercq M, Baaziz H, Cussac D, Santran V, Mauricot R. J. Mater. Chem. 2011;21:18365–18372. [Google Scholar]
- 160.Morgan NY, Kramer-Marek G, Smith PD, Camphausen K, Capala J. Radiation Research. 2009;171:236–244. doi: 10.1667/RR1470.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Holl I, Lorenz E, Mageras G. Nuclear Science, IEEE Transactions on. 1988;35:105–109. [Google Scholar]
- 162.Sakai E. Nuclear Science, IEEE Transactions on. 1987;34:418–422. [Google Scholar]
- 163.Valentine JD, Moses WW, Derenzo SE, Wehe DK, Knoll GF. Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment. 1993;325:147–157. [Google Scholar]
- 164.De Haas JTM, Dorenbos P, Van Eijk C. Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment. 2005;537:97–100. [Google Scholar]
- 165.De Haas JTM, Dorenbos P. Nuclear Science, IEEE Transactions on. 2008;55:1086–1092. [Google Scholar]
- 166.Syntfeld A, Moszynski M, Arlt R, Balcerzyk M, Kapusta M, Majorov M, Marcinkowski R, Schotanus P, Swoboda M, Wolski D. Nuclear Science Symposium Conference Record, 2004 IEEE. 2004;Vol. 1543:1545–1550. [Google Scholar]
- 167.Murray R. Nuclear Instruments. 1958;2:237–248. [Google Scholar]
- 168.van Loef EVD, Dorenbos P, van Eijk CWE, Krämer KW, Güdel HU. Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment. 2002;486:254–258. [Google Scholar]
- 169.van Loef EVD, Dorenbos P, van Eijk CWE, Krämer KW, Güdel HU. Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment. 2005;537:232–236. [Google Scholar]
- 170.Menefee J, Swinehart C, O'Dell E. Nuclear Science, IEEE Transactions on. 1966;13:720–724. [Google Scholar]
- 171.van Loef EV, Wilson CM, Cherepy NJ, Hull G, Payne SA, Choong WS, Moses WW, Shah KS. Nuclear Science, IEEE Transactions on. 2009;56:869–872. [Google Scholar]
- 172.Ronda CR. Luminescence: from theory to applications. LibreDigital; 2008. [Google Scholar]
- 173.Brixner LH. Materials Chemistry and Physics. 1987;16:253–281. [Google Scholar]
- 174.Marvin J W. Journal of Luminescence. 2002;100:35–45. [Google Scholar]
- 175.Nikl M. Measurement Science and Technology. 2006;17:R37. [Google Scholar]
- 176.Derenzo SE, Moses WW. Heavy scintillators for scientific and industrial applications. 1993:125–135. [Google Scholar]
- 177.van Loef EV, Higgins WM, Glodo J, Brecher C, Lempicki A, Venkataramani V, Moses WW, Derenzo SE, Shah KS. Nuclear Science, IEEE Transactions on. 2007;54:741–743. [Google Scholar]
- 178.Greskovich C, Duclos S. Annual review of materials science. 1997;27:69–88. [Google Scholar]
- 179.Eijk CWEv. Physics in Medicine and Biology. 2002;47:R85. doi: 10.1088/0031-9155/47/8/201. [DOI] [PubMed] [Google Scholar]
- 180.Weber MJ, Monchamp RR. Journal of Applied Physics. 1973;44:5495–5499. [Google Scholar]
- 181.Zdesenko YG, Avignone Iii F, Brudanin V, Danevich F, Nagorny S, Solsky I, Tretyak V. Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment. 2005;538:657–667. [Google Scholar]
- 182.Moszyński M, Balcerzyk M, Czarnacki W, Nassalski A, Szczęśniak T, Kraus H, Mikhailik V, Solskii I. Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment. 2005;553:578–591. [Google Scholar]
- 183.Lempicki A, Randles MH, Wisniewski D, Balcerzyk M, Brecher C, Wojtowicz AJ. Nuclear Science, IEEE Transactions on. 1995;42:280–284. [Google Scholar]
- 184.Moszynski M, Kapusta M, Mayhugh M, Wolski D, Flyckt SO. Nuclear Science, IEEE Transactions on. 1997;44:1052–1061. [Google Scholar]
- 185.Mihóková E, Nikl M, Mareš JA, Beitlerová A, Vedda A, Nejezchleb K, Blažek K, D’Ambrosio C. Journal of Luminescence. 2007;126:77–80. [Google Scholar]
- 186.Melcher C, Manente R, Schweitzer J. Nuclear Science, IEEE Transactions on. 1989;36:1188–1192. [Google Scholar]
- 187.Mares JA, Beitlerova A, Nikl M, Solovieva N, D'Ambrosio C, Blazek K, Maly P, Nejezchleb K, de Notaristefani F. Radiation Measurements. 2004;38:353–357. [Google Scholar]
- 188.Mares JA, Nikl M, Mihokova E, Kvapil J, Giba J, Blazek K. Journal of Luminescence. 1997;72–74:737–739. [Google Scholar]
- 189.Dujardin C, Pedrini C, Blanc W, Gacon J, Spijker JC, Frijns O, Eijk CWE, Dorenbos P, Chen R, Fremout A. Journal of Physics: Condensed Matter. 1998;10:3061. [Google Scholar]
- 190.Melcher C, Schweitzer J. 1991;vol. 221:228–231. [Google Scholar]
- 191.van Eijk CWE. Nuclear Instruments and Methods in Physics Research Section A: Accelerators, Spectrometers, Detectors and Associated Equipment. 2003;509:17–25. [Google Scholar]
- 192.Kang Y, Park S, Lenggoro I, Okuyama K. Journal of Physics and Chemistry of Solids. 1999;60:379–384. [Google Scholar]
- 193.Okumura M, Tamatani M, Matsuda N, Takahara T, Fukuta Y. Ceramic scintillator, method for producing same, and X-ray detector and X-ray CT imaging equipment using same. U.S. Patent 6 384 417. 2002
- 194.Deych R, Dolazza E. Sensors Applications Symposium, 2007. SAS '07. IEEE; 2007. pp. 1–6. [Google Scholar]
- 195.Takagi K, Fukazawa T. Applied Physics Letters. 1983;42:43–45. [Google Scholar]
- 196.Ishibashi H, Shimizu K, Susa K, Kubota S. Nuclear Science, IEEE Transactions on. 1989;36:170–172. [Google Scholar]
- 197.Cohen B. Radiation Dose from Adult and Pediatric Multidetector Computed Tomography. 2007:33–49. [Google Scholar]
- 198.Badea C, Drangova M, Holdsworth D, Johnson G. Phys. Med. Bio. 2008;53:R319. doi: 10.1088/0031-9155/53/19/R01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kang DG, Sung Y, Kim SS, Lee SD, Kim CY. 2009:4173–4176. [Google Scholar]
- 200.Takeda T, Zeniya T, Wu J, Yu Q, Lwin TT, Tsuchiya Y, Rao DV, Yuasa T, Yashiro T, Dilmanian FA, Itai Y, Akatsuka T. Proceedings of SPIE. 2002:300–309. [Google Scholar]
- 201.Meng LJ, Li N, La Riviere PJ. Nuclear Science, IEEE Transactions on. 2011:1–1. doi: 10.1109/TNS.2011.2167632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Saunders R, Samei E, Badea C, Yuan H, Ghaghada K, Qi Y, Hedlund L, Mukundan S. 2008:69130Y. [PMC free article] [PubMed] [Google Scholar]
- 203.Yaffe M. Breast Cancer Research. 2008;10:209. doi: 10.1186/bcr2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Johns PC, Yaffe MJ. Phys. Med. Bio. 1987;32:675. doi: 10.1088/0031-9155/32/6/002. [DOI] [PubMed] [Google Scholar]
- 205.Carroll F. Journal of cellular biochemistry. 2003;90:502–508. doi: 10.1002/jcb.10632. [DOI] [PubMed] [Google Scholar]
- 206.Carroll FE, Mendenhall MH, Traeger RH, Brau C, Waters JW. American Journal of Roentgenology. 2003;181:1197–1202. doi: 10.2214/ajr.181.5.1811197. [DOI] [PubMed] [Google Scholar]
- 207.Edwards G, Austin R, Carroll F, Copeland M, Couprie M, Gabella W, Haglund R, Hooper B, Hutson M, Jansen E. Review of Scientific Instruments. 2003;74:3207. [Google Scholar]
- 208.Pole DJ, Popovic K, Williams MB. Proc. SPIE Medical Imaging 2008. 2008;6913:691344–691341. [Google Scholar]
- 209.Huo Q, Sato H, Yuasa T, Akatsuka T, Wu J, Lwin TT, Takeda T, Hyodo K. X-Ray Spectrometry. 2009;38:439–445. [Google Scholar]
- 210.Lewis CA, O'Leary K, Jackson DF, Lam GKY. Physics in Medicine and Biology. 1982;27:683. doi: 10.1088/0031-9155/27/5/003. [DOI] [PubMed] [Google Scholar]
- 211.Reidy JJ, Hutson RL, Daniel H, Springer K. Analytical Chemistry. 1978;50:40–44. [Google Scholar]
- 212.Jackson DF, Lewis CA, O'Leary K. Physical Review A. 1982;25:3262–3276. [Google Scholar]