Abstract
Obesity is a risk factor for the development of many severe human diseases such as cardiovascular disorders, diabetes, and cancer, which are tightly linked to angiogenesis. The adipose tissue produces several growth factors/hormones including leptin, tumor necrosis factor α, and adiponectin. It has been found that adiponectin levels are reduced in obesity. Here, we report a unique function of adiponectin as a negative regulator of angiogenesis. In vitro, adiponectin potently inhibits endothelial cell proliferation and migration. In the chick chorioallantoic membrane and the mouse corneal angiogenesis assays, adiponectin remarkably prevents new blood vessel growth. Further, we demonstrate that the antiendothelial mechanisms involve activation of caspase-mediated endothelial cell apoptosis. Adiponectin induces a cascade activation of caspases-8, -9, and -3, which leads to cell death. In a mouse tumor model, adiponectin significantly inhibits primary tumor growth. Impaired tumor growth is associated with decreased neovascularization, leading to significantly increased tumor cell apoptosis. These data demonstrate induction of endothelial apoptosis as an unique mechanism of adiponectin-induced antiangiogenesis. Adiponectin, as a direct endogenous angiogenesis inhibitor, may have therapeutic implications in the treatment of angiogenesis-dependent diseases.
Keywords: neovascularization, cancer, adipocyte, endothelium, Acrp30
Obesity has become a global health problem, and it is linked to the development of many angiogenesis-related diseases such as diabetes, cardiovascular disorders, and cancer (1, 2). Several angiogenic growth factors and hormones are produced by the adipose tissue (3), including vascular endothelial growth factor (VEGF), tumor necrosis factor α, and leptin (4–8). Thus adipocyte-derived factors may play critical roles in regulation of global as well as local tissue and organ function by control of angiogenesis. Like any other tissue growth in the body, the expansion of fat mass depends on angiogenesis (9). Thus, the adipose tissue must be able to switch on an angiogenic phenotype to grow.
Switching on angiogenesis usually requires both up-regulation of angiogenic stimulators and down-regulation of angiogenesis inhibitors (10). It is known that actively growing healthy or pathological tissues express high levels of angiogenic factors. However, overexpression of angiogenic factors may not be sufficient to induce angiogenesis. For example, VEGF is expressed at high levels in several quiescent adult tissues that lack active angiogenesis. This observation indicates that up-regulation of angiogenic factor and down-regulation of angiogenesis inhibitors are both necessary to induce angiogenesis. In the adipose tissue, the balance between angiogenic factors and inhibitors has not been studied. Adiponectin is a unique adipocyte-derived hormone, and its functional targets are not only limited to adipose tissues. Indeed, adiponectin accumulates to very high levels in the circulation. It has recently been found that adiponectin may protect against diabetes and arteriosclerosis (11–14). In obese individuals, adiponectin levels are decreased, while active angiogenesis occurs in the adipose tissue (15). Thus we hypothesize that adiponectin might be a negative regulator of angiogenesis. Here, we provide evidence that adiponectin is a direct angiogenesis inhibitor that induces apoptosis in activated endothelial cells. Further, because angiogenesis is critical for tumor growth and metastasis, we investigate the antitumor activity of adiponectin.
Methods
Reagents, Cells, and Animals. Recombinant full-length human adiponectin or mouse adiponectin (Acrp30) was produced and purified as described (15). Recombinant human fibroblast growth factor 2 (FGF-2) was obtained from Amersham Pharmacia and UpJohn, and the VEGF165 was provided by R & D Systems. Primary bovine capillary endothelial (BCE) cells were obtained from Judah Folkman's laboratory; human dermal microvascular endothelial (HDME) cells were obtained from PromoCell (Heidelberg); porcine aortic endothelial (PAE) cells were obtained from Lena Claesson-Welsh's laboratory; and rat vascular smooth muscle (rVSM) cells were provided by Johan Thyberg (Karolinska Institutet). Female or male 5- to 6-wk-old C57BL/6 mice were acclimated and caged in groups of six or fewer. Mice were anaesthetized by injection of a mixture of dormicum/hypnorm (1:1) before all procedures and killed with a lethal dose of CO2. All animal studies were reviewed and approved by the animal care and use committee of the Stockholm Animal Board.
Proliferation Assay. A standard 72-h BCE cell proliferation assay was performed as described (7). PAE/FGFR-1 cells or rVSM cells were grown in 10% heat-inactivated FCS-F12-Ham's medium. Approximately 10,000 cells per well were seeded in 24-well plates. Samples were assayed in 5% FCS-Ham's medium containing 10–15 ng/ml FGF-2. Murine T241 fibrosarcoma cells were assayed (10,000 cells per well in 24-well plates) in 5% FCS-DMEM containing various concentrations of adiponectin. After 72-h incubation, cells were trypsinized, resuspended in Isoton II solution (Kebo Lab, Stockholm), and counted with a Coulter counter. Results are presented as mean cell number per well (+SEM).
Cell Migration Assay. The motility response of PAE/VEGFR-2 cells to VEGF was assayed by using a modified Boyden chamber technique as described (16). rVSM cells at the density of 25,000 cells per well were seeded in the upper chambers in serum-free F12-Ham's medium containing 0.25% BSA and adiponectin samples. F12-Ham's medium containing 2% FCS was added to the lower chambers. After a 6- to 9-h incubation, the medium was removed and cells attached to the filter were fixed in 99% methanol and stained with a Giemsa solution. All experiments were performed in six replicates. The number of cells migrating through the filter was counted and plotted as mean number of migrating cells per optic field (×20 magnification) (+SEM).
Chick Chorioallantoic Membrane (CAM) Assay. After 3-d incubation at 37°C, fertilized white Leghorn eggs (OVA Production, Sorgarden, Sweden) were cracked, and chick embryos with intact yolks were carefully placed in 20 × 100-mm plastic Petri dishes. After 6 days of incubation in 4% CO2 at 37°C, methylcellulose disks containing 5, 10, or 20 μg of adiponectin or PBS were implanted on the CAM of individual embryos. After 2–8 days of incubation, CAMs were examined for the formation of avascular zones around the field of the implanted disks by using a stereoscope (n = 5 embryos per group). Photographs (×20 magnification) were taken on day 8 after implantation.
Mouse Corneal Micropocket Assay. The mouse corneal assay was performed as described (7). Micropellets containing 30 ng of FGF-2, 360 ng of adiponectin, or both were surgically implanted. Eyes were examined on day 5 after pellet implantation. Vessel lengths and clock hours of circumferential neovascularization were measured under a stereoscope (n = 10–11 eyes per group). Areas of maximal neovascularization were calculated.
Morphological Detection of Cellular Apoptosis. BCE or HDME cells grown to 60–70% confluency in 12-well plates were incubated for 6–48 h with various concentrations of human adiponectin in 5% bovine calf serum-DMEM or 1% FCS in endothelial cell growth medium MV (PromoCell), respectively. Cells were harvested and resuspended in PBS containing 30 mM glycerol and 0.1 M NaCl; the cells were dried onto slides and fixed with acetone/methanol (1:1). The cells were stained with Hoecsht dye 33258 (500 ng/ml). Apoptotic cells were counted in random fields under a microscope (×60 magnification, at least 10 fields per sample).
Caspase Activity Assay. BCE cells grown to 70–80% confluency in 12-well plates were incubated for 3–24 h with various concentrations of human adiponectin in 5% BCS-DMEM. The activities of caspase-3-, -8, and -9 were determined fluorometrically by cleavage of substrates: DEVD-7-amino-4-methylcoumarin (AMC), IETD-AMC, or LEHD-AMC (Peptide Institute, Osaka), respectively, according to described methods (17). Approximately 3–5 × 105 cells were used for each sample. To inhibit caspase activity, some samples were preincubated for 1 h with z-DEVD-fmk, z-IEDT-fmk, or z-LEHD-fmk (20 μM) (Enzyme Systems Products, Livermore, CA). Cleavage of the fluorogenic peptide substrates was monitored by AMC release in a Fluoroscan II plate reader (Labsystems, Chicago). Fluorescence units were converted to picomole of AMC by using a standard curve generated with free AMC. Data were analyzed by linear regression. Quantification of caspase activity was calculated as fold increase over control samples.
Tumor Experiments. Female 6- to 7-wk-old C57BL/6 mice were used for tumor studies. WT or GFP-expressing murine T241 fibrosarcoma cells growing in log phase were harvested and resuspended in PBS, and 1 × 106 cells in 100 μl were implanted s.c. in the middle dorsum of each animal as described (18). Mice were treated with murine adiponectin (Acrp30; 50 μg per mouse) by daily intralesional injections throughout the experiment (n = 6 mice per group). Control animals were injected with PBS. Visible tumors were present after 72 h and measured by using digital calipers on the indicated time points. Tumor volumes were calculated as reported (18). After a 2-wk treatment, the control tumors approached to the size of the Swedish ethical upper limit (1.5 cm3), and the experiment was terminated at that point. Mice were killed, and the tumor tissues were removed and weighed.
Immunohistochemistry. Growth factor-implanted corneas were snap-frozen in TissueTek (HistoLab, Gothenburg, Sweden) on dry ice and stored at -80°C. Histological sections (12 μm) were stained for CD31 as described (7). Tumors were dissected and fixed in 3% paraformaldehyde, dehydrated, and embedded in paraffin. Thin sections (5 μm) were deparaffinized, blocked, and incubated overnight at 4°C with a biotinylated rat anti-mouse CD31 Ab (1:100; Pharmingen), followed by amplification of the signal by using NEN TSA amplification (Perkin–Elmer) protocol for biotinylated primary Abs. The reaction was developed by addition of diaminobenzidine substrate. Microphotographs were taken under a microscope (×20 magnification) and analyzed by using photoshop 7.0 (Adobe Systems, Mountain View, CA). Vessel numbers per field (×10 magnification) were quantified in five to eight random fields per group (four tumors per group, two fields per tumor). The terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) staining was performed according to a standard but modified fluorescein in situ death detection protocol (Amersham Biosciences). In brief, deparaffinized thin tumor sections (5 μm) were blocked by using 3% H2O2 in methanol for 15 min; antigenic epitopes were retrieved, and the TUNEL reaction mixture was added. The sections were photographed and signals were quantified under fluorescent microscope (×60 magnification) in 5–10 random fields per group.
Whole-Mount Staining. Adiponectin-treated or control GFP-expressing T241 tumors were removed on day 8 after implantation for whole-mount immunohistochemical analysis. Tumors were dissected into thin slices and fixed with 3% paraformaldehyde overnight. Ab epitopes were exposed by proteinase K, and the tissues were permeabilized. Blood vessel endothelial cells were detected by using a rat anti-mouse CD31 (1:300; Pharmingen) primary Ab followed by a secondary goat anti-rat-IgG Ab labeled with Cy5 (Zymed). Apoptotic cells were visualized by TUNEL in situ death detection kit as above. Samples were examined by confocal microscopy (×20 magnification; LSM510 confocal microscope, Zeiss). The 3D images were assembled by scanning 10 layers (2–3 μm apart) of each sample by using lsm510 software (Zeiss) and photoshop 7.0.
Statistics. Results are presented as mean (+SEM). Statistical evaluation of the results was made by using standard two-tailed Student's t test with excel 5 (Microsoft). Statistical significance was defined as P < 0.05.
Results
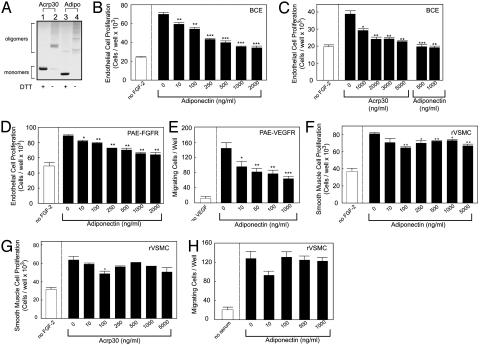
Inhibition of Endothelial Cell Growth. To study whether adiponectin could inhibit endothelial cell growth, the effect of full-length purified recombinant human and mouse adiponectin (15) were determined by using endothelial cell proliferation and migration assays. Under nonreducing conditions, both human and mouse adiponectin form trimers and large oligomeric complexes (Fig. 1A). These large complexes are biologically active (19). Addition of physiologically relevant concentrations of human adiponectin to BCE cells resulted in a dose-dependent inhibition of FGF-2-stimulated endothelial proliferation, with an IC50 of 250 ng/ml (Fig. 1B). Similarly, mouse adiponectin also inhibited BCE proliferation (Fig. 1C). Because endothelial cell growth and angiogenesis can be stimulated by several angiogenic factors, we next determined whether adiponectin could block individual angiogenic factor-induced endothelial cell growth. In FGF receptor-1-overexpressing PAE cells/FGFR, human adiponectin significantly inhibited FGF-2-induced proliferation in a dose-dependent manner, with an IC50 of 250 ng/ml (Fig. 1D). To study VEGF-induced endothelial activity, we performed endothelial cell migration assay because VEGF preferentially induced endothelial cell migration rather than proliferation. VEGF receptor-2-overexpressing PAE (PAE/VEGFR-2) cells exhibited dramatically increased cell motility upon stimulation with VEGF. Human adiponectin significantly inhibited VEGF-induced PAE/VEGFR-2 cell migration (Fig. 1E). These data suggest that adiponectin blocks a common pathway for endothelial cell proliferation and migration. To study whether adiponectin also inhibited nonendothelial cell growth, we determined its inhibitory activity on rVSM cells. Similar to previous findings (20), adiponectin significantly inhibited rVSM cell proliferation (Fig. 1 F and G). However, the concentrations required to reach a maximal inhibitory effect on rVSM cells were higher than for endothelial cells. Interestingly, adiponectin showed no inhibitory effect on rVSM cell migration (Fig. 1H) or FGF-2-stimulated fibroblast proliferation (data not shown). These findings indicate that adiponectin selectively inhibits endothelial cell growth at low concentrations. Consistent with our data, it has been reported that adiponectin exhibits specific binding to endothelial cells in vitro and that it accumulates in the vascular wall in vivo, suggesting the existence of a cell surface receptor for adiponectin (21–23). Indeed, adiponectin receptors (AdipoR1 and AdipoR2) have recently been detected on endothelial cells (24).
Fig. 1.
Adiponectin inhibits endothelial growth. (A) Recombinant mouse (Acrp30) or human adiponectin (Adipo) proteins (1 μg) were analyzed on a polyacrylamide gel followed by staining with Coomassie brilliant blue. Trimeric/oligomeric (lanes 2 and 4) and monomeric (lanes 1 and 3) forms of adiponectin were detected under nonreducing and reducing/alkylating conditions, respectively. (B and C) FGF-2-stimulated BCE cells were incubated with various concentrations of human or mouse adiponectin. (D) Human adiponectin was incubated with PAE/FGFR-1 cells stimulated with FGF-2. (E) The motility response of PAE/VEGFR-2 cells to VEGF with or without human adiponectin was assayed. FGF-2-stimulated rVSM cells were incubated with human (F) or mouse (G) adiponectin. (H) The motility response of rVSM cells to serum was assayed with or without human adiponectin. Values represent mean number of cells per well (+SEM). *, P < 0.05; **, P < 0.01; ***, P < 0.001.
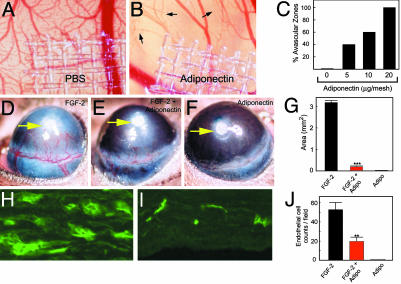
Inhibition of Angiogenesis in Vivo. To investigate its antiangiogenic activity in vivo, adiponectin was tested in the CAM and the mouse corneal angiogenesis assays. In the CAM model, adiponectin induced avascular zone formation in the developing embryos. Notably, newly formed microvessels were regressed around the area of adiponectin-implanted disks (Fig. 2B, arrows). This antiangiogenic activity appeared to be dose-dependent (Fig. 2C). In contrast, control CAMs, implanted with disks without adiponectin, had no avascular zones (Fig. 2A). In the mouse corneas, adiponectin significantly inhibited FGF-2-induced neovascularization (Fig. 2E) as compared to FGF-2 alone (Fig. 2D). Adiponectin alone did not induce angiogenesis in this model (Fig. 2F). Quantification analysis revealed the antineovascularization effect of adiponectin to be significant, with 94% reduction of vascularization area (P < 0.001) (Fig. 2G). Immunohistological examination of the corneas revealed that the number of newly formed microvessels was significantly reduced in the FGF-2/adiponectin-coimplanted corneas as compared with the FGF-2-implanted eyes (P < 0.01; Fig. 2 H–J). These findings demonstrate that adiponectin is a potent inhibitor of angiogenesis in vivo.
Fig. 2.
Antiangiogenic activity of adiponectin. Disks containing human adiponectin were implanted on the developing CAMs. Microphotographs (×20) on day 8 of a control CAM (A) and an adiponectin-implanted CAM (B;20 μg per disk) are shown. Arrows point to regressing blood vessels. (C) The number of CAMs with avascular zones was quantified at 48 h after implantation. Pellets containing FGF-2 (D), FGF-2/adiponectin (E), or adiponectin alone (F) were implanted into mouse corneal micropockets. Photographs represent ×20 magnification of the mouse eye, and positions of implanted pellets are indicated by arrows. (G) Corneal neovascularization was quantified on day 5 as mean maximal area (+SEM). (H and I) Immunohistochemical labeling of blood vessels in sections of FGF-2-(H) or FGF-2/adiponectin-(I) implanted corneas (×20). (J) The number of microvessels per microscopic field was quantified. **, P < 0.01; ***, P < 0.001.
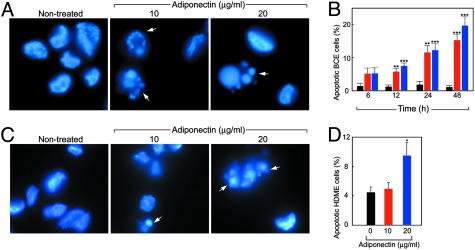
Induction of Endothelial Cell Apoptosis and Activation of Caspases. To elucidate the possible mechanisms of adiponectin-mediated antiangiogenesis, we investigated the effect of adiponectin on endothelial cell apoptosis. We found that addition of adiponectin to BCE cells induced a significant proportion of cells to undergo apoptosis, as determined by the appearance of apoptotic bodies (Fig. 3A). The induction of endothelial apoptosis became more pronounced after prolonged incubation, with ≈20% of the total cell population showing clear signs of apoptosis by 48 h (Fig. 3B). Similar findings were also observed by using HDME cells (Fig. 3 C and D).
Fig. 3.
Adiponectin induces endothelial apoptosis. (A and B) Apoptotic bodies of adiponectin-treated BCE cells were detected at 24 h by fluorescent microscopy and quantified at different time points. Bars: black, control; red, 10 μg/ml; blue, 20 μg/ml adiponectin. (C and D) Apoptosis of HDME cells after 24-h incubation with adiponectin. Values represent mean percentage of apoptotic cells per total number of cells per field. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
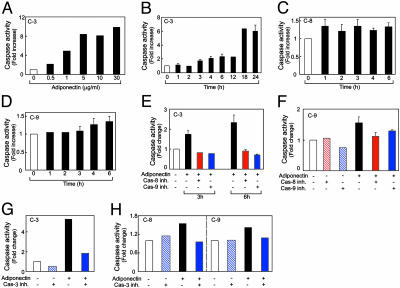
To investigate apoptotic pathways, activation of endothelial caspases was analyzed. First, we determined the involvement of caspase-3, an apoptotic effector, in BCE cells. Activation of procaspase-3 by adiponectin was found to be dose and time dependent. A maximal activation was observed at ≈5 μg/ml adiponectin (Fig. 4A). Notably, the physiological levels of adiponectin in both human and mouse serum have been reported to range from 2 to 17 μg/ml (15, 25). Thus, the concentrations of adiponectin used in our system to sufficiently induce apoptosis of activated endothelial cells are physiologically relevant. To standardize our assay systems and compare efficacies of activation of different caspases, we used 1 μg/ml adiponectin in subsequent studies. We found that caspase-3 activity was first elevated at 3–4 h and reached a maximal level (≈6-fold over control) after 18–24 h of incubation (Fig. 4B). Activation of pro-caspase-8 was detected already 1 h after addition of adiponectin, and this level of activation persisted during prolonged incubations (Fig. 4C). In contrast, activation of procaspase-9 seemed to closely mirror the activation pattern of caspase-3 (Fig. 4D). These findings suggest that adiponectin initially activates caspase-8, which subsequently leads to activation of caspases-3 or -9. Pro-caspase-8 is an intracellular component that directly communicates with the death domain of cell membrane receptors, whereas pro-caspase-9 is activated by apoptotic signals released by the mitochondria.
Fig. 4.
Activation of endothelial caspase pathways. (A) Dose-dependent activation of caspase-3 in adiponectin-treated BCE cells at 24 h. (B–D) Time course determination of activation of caspase-3-like (B), caspase-8-like (C), and caspase-9-like (D) enzymes in BCE cells treated with 1 μg/ml human adiponectin. Activation of caspase-3 at 3 or 6 h (E and G), caspase-9 at 6 h (F and H), and caspase-8 at 6 h (H) was determined in the presence or absence of specific caspase inhibitors.
Consistent with these data, addition of cell permeable-specific, irreversible inhibitors of caspase-8 (z-IETD-fmk), caspase-9 (z-LEHD-fmk), or caspase-3-like (z-DEVD-fmk) enzymes to BCE cells revealed that both z-IETD-fmk and z-LEHD-fmk completely prevented adiponectin-induced activation of caspase-3 (Fig. 4E). In addition, z-IETD-fmk blocked activation of caspase-9 (Fig. 4F). Thus, it is plausible that activation of caspase-8 is the immediate early event of adiponectin-triggered endothelial apoptosis. Furthermore, we found that preincubation of BCE cells with a specific caspase-3 inhibitor repressed not only the activation of caspase-3 (Fig. 4G) but also the activation of caspases-8 and -9 after 6 h of incubation (Fig. 4H), indicating the existence of an caspase-3-mediated amplification loop in the adiponectin-trigged caspase pathway (26).
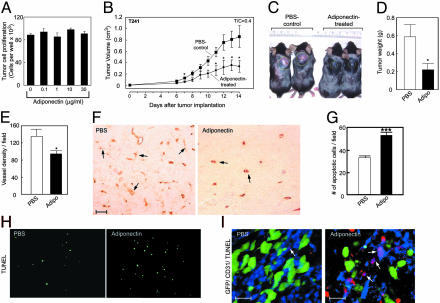
Suppression of Tumor Growth and Neovascularization. The antiangiogenic activity of adiponectin encouraged us to determine whether adiponectin could suppress pathological angiogenesis. Tumor growth is angiogenesis-dependent, and suppression of angiogenesis has been shown to inhibit tumor growth (27). To study the possible antitumor activity of adiponectin, we chose a hypervascularized murine T241 fibrosarcoma model. Incubation of T241 tumor cells with adiponectin, even at high concentrations, did not affect tumor cell growth in vitro (Fig. 5A), suggesting that adiponectin had no direct effect on tumor cells per se. In vivo, intralesional administration of murine adiponectin resulted in significant suppression of tumor growth. After a 2-wk treatment, ≈60% reduction of tumor volumes and weights was observed (Fig. 5 B–D). Neovascularization of adiponectin-treated tumors was significantly reduced as compared with control tumors (Fig. 5 E and F). Interestingly, reduction of tumor neovascularization led to a remarkable increase of tumor apoptosis (Fig. 5 G and H). The increase of tumor cell apoptosis could be caused by deprivation of survival factors supplied by blood vessels.
Fig. 5.
Suppression of tumor growth and induction of apoptosis. (A) T241 tumor cell growth rates in vitro in the presence and absence of mouse adiponectin. (B) Tumor volumes represent mean determinants of treated and control groups (+SEM). (C) Typical examples of tumor-bearing mice of the control or adiponectin-treated groups on day 14. (D) Tumor weights at necropsy. (E) Tumor vascular density was quantified as numbers of vessels per field (×10). (F) Tumor neovascularization was detected by using an anti-CD31 Ab in the adiponectin-treated and control tumors (×20). (Scale bar, 50 μm.) (G) Quantification of TUNEL-positive, apoptotic tumor cells. *, P <0.05; ***, P <0.001. (H) TUNEL staining (green) for visualization of apoptotic cells in tumor sections. (I) Whole-mount staining of tumor blood vessels [×20; green, GFP-T241 tumor cells; blue, CD31-positive tumor vessels; red, TUNEL-positive apoptotic cells (scale bar, 25 μm)]. Arrows indicate CD31/TUNEL double-positive structures.
To further study whether tumor vessel endothelial cells became apoptotic, we colocalized the CD31-positive signals with TUNEL-positive cells. Interestingly, a remarkable overlap between CD31- and TUNEL-positive signals was found in adiponectin-treated tumors (Fig. 5I). In contrast, only a small number of CD31/TUNEL double positive structures was found in the PBS-treated control tumors (Fig. 5I). These data demonstrate that adiponection induces tumor vessel apoptosis, which is consistent with our in vitro data that adiponectin selectively induces endothelial cell apoptosis.
Discussion
Adiponectin is produced by adipocytes and thought to be an important regulator of lipid and glucose metabolism (13, 14). Our present data demonstrate that adiponectin is a negative regulator of angiogenesis. This finding provides important clues to understanding the biological functions of this protein as a master regulator of many systems. Indeed, the adipose tissue is an important and perhaps the largest endocrine organ in the body. In addition to adiponectin, leptin, resistin, and adipsin are examples of other adipose-derived endocrine hormones or adipokines (3, 28). Although their main targets are located in distal sites, the local functions have not been fully investigated. Recently, leptin has been found to stimulate angiogenesis (6–8). Thus, adiponectin and leptin have opposite effects in regulation of angiogenesis. Like most tissues in the adult body, the quiescent vasculature in the adipose tissue may represent a net balance between the production levels of angiogenic factors and inhibitors. Overproduction of angiogenic factors or decreased levels of inhibitors would result in adipose tissue neovascularization and expansion. Recently, it has been shown that expansion of fat mass in the body is angiogenesis dependent (9). To switch on an angiogenic phenotype, the adipose tissue may have to down-regulate the expression of angiogenesis inhibitors. Our data provide an example of an endogenous adipose-derived angiogenesis inhibitor. Indeed, the levels of adiponectin are decreased in obese animals and humans (13, 15). In contrast, production of leptin is usually elevated in the obese state (29, 30), and it is known that dysregulation of leptin in mice and humans lead to obesity (31, 32). Surprisingly, deletion of adiponectin in mice does not result in any differences in body weight, suggesting that the adiponectin system might be redundant under physiological conditions (33). Similarly, overexpression of adiponectin in mice did not result in a significant difference of body weight or adiposity (34). Indeed, our present data show that treatment of mice with recombinant adiponectin does not affect adipogenesis (unpublished data), a process that probably depends on angiogenesis. The fact that adiponectin preferentially targets tumor blood vessels reflects that the rate of angiogenesis in the tumor vasculature is higher than that in the slowly expanding adipose tissue. Thus, it appears the antiangiogenic activity of adiponectin is more pronounced under pathological conditions. Another possibility is that tumors produce additional molecules that facilitate the antiangiogenic activity of adiponectin. However, this hypothesis remains to be further investigated.
Very recently, adiponectin has been reported to stimulate endothelial cell growth and angiogenesis (35). Most of these experimental data were obtained by in vitro assays using endothelial cells derived from large vessels. The discrepancy of these findings from our present data could be because different systems were used. In those studies, human umbilical vein endothelial cells were used for the in vitro studies, whereas we use capillary endothelial cells in our assays. It is believed that capillary endothelial cells are most relevant for studying angiogenic processes in vitro because capillaries usually sprout from microvessels but not large vessels. In addition, the effect of adiponectin on endothelial cells in vitro cannot be directly translated into its in vivo angiogenic activity. For example, transforming growth factor type β is a potent inhibitor for endothelial cell growth in vitro but stimulates angiogenesis in vivo (36, 37). Our in vivo results are consistent with the in vitro finding obtained from capillary endothelial cells, suggesting that adiponectin is a potent angiogenesis inhibitor.
There is a correlation between obesity and high incidences of certain cancers, including breast, colon/rectal, and prostate cancer (38, 39). Our findings that adiponectin inhibits tumor neovascularization may imply that obese individuals with low levels of adiponectin are more susceptible to develop various forms of tumors. It would be interesting to investigate whether the reported increased cancer incidences in the obese population (38, 40) correlate with significantly decreased levels of adiponectin. In this study, we also provide molecular mechanisms of the antiangiogenic activity of adiponectin. The finding that adiponectin induces endothelial apoptosis indicates that this protein acts as a direct endogenous inhibitor for blood vessel growth. Indeed, administration of adiponectin to tumor-bearing animals resulted in apoptosis of a significant proportion of tumor blood vessels. Adiponectin may have several therapeutic advantages as compared to angiogenic factor antagonists. As the genome of tumor cells is unstable, the production of multiple angiogenic factors is switched on during tumor progression (41). Thus, antiangiogenic therapy by using, e.g., an anti-VEGF agent may encounter drug resistance problems. In contrast, induction of endothelial apoptosis by adiponectin seems to target a common angiogenic pathway. In this regard, adiponectin may be an effective novel anticancer agent.
In addition to suppression of tumor growth, adiponectin has recently been reported to protect apolipoprotein E-deficient mice from development of atherosclerotic plaques (12). Although the underlying mechanisms need to be further investigated, inhibition of angiogenesis could be involved in the antiatherogenic effect of adiponectin, as suppression of angiogenesis can prevent atherosclerotic plaque growth (42). In type II diabetic individuals, decreased levels of adiponectin may contribute to several severe complications. For example, diabetic retinopathy represents a switch of pathological angiogenesis in the retina. Could the pathological neovascularization in the eye and other tissues be caused by decreased levels of adiponectin in diabetic patients? Our finding that adiponectin acts as a potent angiogenesis inhibitor suggests this possibility.
Acknowledgments
We thank Meit Björndahl and Maya Nisancioglu for critical reading of the manuscript. This work is supported by the Human Frontier Science Program, the Swedish Cancer Foundation, the Stockholm Cancer Foundation, the European Community FP5 program, the Karolinska Institute Foundation, the Åke Wibergs Foundation, the Swedish Research Council, and the Heart and Lung Foundation. Y.C. is supported by the Swedish Research Council.
Abbreviations: VEGF, vascular endothelial growth factor; FGF, fibroblast growth factor; BCE, bovine capillary endothelial; HDME, human dermal microvascular endothelial; PAE, porcine aortic endothelial; rVSM, rat vascular smooth muscle; CAM, chick chorioallantoic membrane; TUNEL, terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling; AMC, 7-amino-4-methylcoumarin.
References
- 1.Friedman, J. M. (2000) Nature 404, 632-634. [DOI] [PubMed] [Google Scholar]
- 2.Kopelman, P. G. (2000) Nature 404, 635-643. [DOI] [PubMed] [Google Scholar]
- 3.Guerre-Millo, M. (2002) J. Endocrinol. Invest. 25, 855-861. [DOI] [PubMed] [Google Scholar]
- 4.Frater-Schroder, M., Risau, W., Hallmann, R., Gautschi, P. & Bohlen, P. (1987) Proc. Natl. Acad. Sci. USA 84, 5277-5281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Claffey, K. P., Wilkison, W. O. & Spiegelman, B. M. (1992) J. Biol. Chem. 267, 16317-16322. [PubMed] [Google Scholar]
- 6.Bouloumie, A., Drexler, H. C., Lafontan, M. & Busse, R. (1998) Circ. Res. 83, 1059-1066. [DOI] [PubMed] [Google Scholar]
- 7.Cao, R., Brakenhielm, E., Wahlestedt, C., Thyberg, J. & Cao, Y. (2001) Proc. Natl. Acad. Sci. USA 98, 6390-6395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sierra-Honigmann, M. R., Nath, A. K., Murakami, C., Garcia-Cardena, G., Papapetropoulos, A., Sessa, W. C., Madge, L. A., Schechner, J. S., Schwabb, M. B., Polverini, P. J., et al. (1998) Science 281, 1683-1686. [DOI] [PubMed] [Google Scholar]
- 9.Rupnick, M. A., Panigrahy, D., Zhang, C. Y., Dallabrida, S. M., Lowell, B. B., Langer, R. & Folkman, M. J. (2002) Proc. Natl. Acad. Sci. USA 99, 10730-10735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Folkman, J. (2002) Semin. Oncol. 29, 15-18. [DOI] [PubMed] [Google Scholar]
- 11.Matsuda, M., Shimomura, I., Sata, M., Arita, Y., Nishida, M., Maeda, N., Kumada, M., Okamoto, Y., Nagaretani, H., Nishizawa, H., et al. (2002) J. Biol. Chem. 277, 37487-37491. [DOI] [PubMed] [Google Scholar]
- 12.Okamoto, Y., Kihara, S., Ouchi, N., Nishida, M., Arita, Y., Kumada, M., Ohashi, K., Sakai, N., Shimomura, I., Kobayashi, H., et al. (2002) Circulation 106, 2767-2770. [DOI] [PubMed] [Google Scholar]
- 13.Ukkola, O. & Santaniemi, M. (2002) J. Mol. Med. 80, 696-702. [DOI] [PubMed] [Google Scholar]
- 14.Yamauchi, T., Kamon, J., Waki, H., Terauchi, Y., Kubota, N., Hara, K., Mori, Y., Ide, T., Murakami, K., Tsuboyama-Kasaoka, N., et al. (2001) Nat. Med. 7, 941-946. [DOI] [PubMed] [Google Scholar]
- 15.Arita, Y., Kihara, S., Ouchi, N., Takahashi, M., Maeda, K., Miyagawa, J., Hotta, K., Shimomura, I., Nakamura, T., Miyaoka, K., et al. (1999) Biochem. Biophys. Res. Commun. 257, 79-83. [DOI] [PubMed] [Google Scholar]
- 16.Brakenhielm, E., Cao, R. & Cao, Y. (2001) FASEB J. 15, 1798-1800. [DOI] [PubMed] [Google Scholar]
- 17.Kohler, C., Orrenius, S. & Zhivotovsky, B. (2002) J. Immunol. Methods 265, 97-110. [DOI] [PubMed] [Google Scholar]
- 18.Eriksson, A., Cao, R., Pawliuk, R., Berg, S. M., Tsang, M., Zhou, D., Fleet, C., Tritsaris, K., Dissing, S., Leboulch, P. & Cao, Y. (2002) Cancer Cell 1, 99-108. [DOI] [PubMed] [Google Scholar]
- 19.Pajvani, U. B., Du, X., Combs, T. P., Berg, A. H., Rajala, M. W., Schulthess, T., Engel, J., Brownlee, M. & Scherer, P. E. (2003) J. Biol. Chem. 278, 9073-9085. [DOI] [PubMed] [Google Scholar]
- 20.Arita, Y., Kihara, S., Ouchi, N., Maeda, K., Kuriyama, H., Okamoto, Y., Kumada, M., Hotta, K., Nishida, M., Takahashi, M., et al. (2002) Circulation 105, 2893-2898. [DOI] [PubMed] [Google Scholar]
- 21.Okamoto, Y., Arita, Y., Nishida, M., Muraguchi, M., Ouchi, N., Takahashi, M., Igura, T., Inui, Y., Kihara, S., Nakamura, T., et al. (2000) Horm. Metab. Res. 32, 47-50. [DOI] [PubMed] [Google Scholar]
- 22.Ouchi, N., Kihara, S., Arita, Y., Maeda, K., Kuriyama, H., Okamoto, Y., Hotta, K., Nishida, M., Takahashi, M., Nakamura, T., et al. (1999) Circulation 100, 2473-2476. [DOI] [PubMed] [Google Scholar]
- 23.Ouchi, N., Kihara, S., Arita, Y., Okamoto, Y., Maeda, K., Kuriyama, H., Hotta, K., Nishida, M., Takahashi, M., Muraguchi, M., et al. (2000) Circulation 102, 1296-1301. [DOI] [PubMed] [Google Scholar]
- 24.Yamauchi, T., Kamon, J., Ito, Y., Tsuchida, A., Yokomizo, T., Kita, S., Sugiyama, T., Miyagishi, M., Hara, K., Tsunoda, M., et al. (2003) Nature 423, 762-769. [DOI] [PubMed] [Google Scholar]
- 25.Berg, A. H., Combs, T. P., Du, X., Brownlee, M. & Scherer, P. E. (2001) Nat. Med. 7, 947-953. [DOI] [PubMed] [Google Scholar]
- 26.Kulik, G., Carson, J. P., Vomastek, T., Overman, K., Gooch, B. D., Srinivasula, S., Alnemri, E., Nunez, G. & Weber, M. J. (2001) Cancer Res. 61, 2713-2719. [PubMed] [Google Scholar]
- 27.Cao, R., Wu, H. L., Veitonmaki, N., Linden, P., Farnebo, J., Shi, G. Y. & Cao, Y. (1999) Proc. Natl. Acad. Sci. USA 96, 5728-5733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mora, S. & Pessin, J. E. (2002) Diabetes Metab. Res. Rev. 18, 345-356. [DOI] [PubMed] [Google Scholar]
- 29.Frederich, R. C., Hamann, A., Anderson, S., Lollmann, B., Lowell, B. B. & Flier, J. S. (1995) Nat. Med. 1, 1311-1314. [DOI] [PubMed] [Google Scholar]
- 30.Caprio, S., Tamborlane, W. V., Silver, D., Robinson, C., Leibel, R., McCarthy, S., Grozman, A., Belous, A., Maggs, D. & Sherwin, R. S. (1996) Am. J. Physiol. 271, E626-E630. [DOI] [PubMed] [Google Scholar]
- 31.Pelleymounter, M. A., Cullen, M. J., Baker, M. B., Hecht, R., Winters, D., Boone, T. & Collins, F. (1995) Science 269, 540-543. [DOI] [PubMed] [Google Scholar]
- 32.Clement, K., Vaisse, C., Lahlou, N., Cabrol, S., Pelloux, V., Cassuto, D., Gourmelen, M., Dina, C., Chambaz, J., Lacorte, J. M., et al. (1998) Nature 392, 398-401. [DOI] [PubMed] [Google Scholar]
- 33.Maeda, N., Shimomura, I., Kishida, K., Nishizawa, H., Matsuda, M., Nagaretani, H., Furuyama, N., Kondo, H., Takahashi, M., Arita, Y., et al. (2002) Nat. Med. 8, 731-737. [DOI] [PubMed] [Google Scholar]
- 34.Yamauchi, T., Kamon, J., Waki, H., Imai, Y., Shimozawa, N., Hioki, K., Uchida, S., Ito, Y., Takakuwa, K., Matsui, J., et al. (2003) J. Biol. Chem. 278, 2461-2468. [DOI] [PubMed] [Google Scholar]
- 35.Ouchi, N., Kobayashi, H., Kihara, S., Kumada, M., Sato, K., Inoue, T., Funahashi, T. & Walsh, K. (2004) J. Biol. Chem. 279, 1304-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muller, G., Behrens, J., Nussbaumer, U., Bohlen, P. & Birchmeier, W. (1987) Proc. Natl. Acad. Sci. USA 84, 5600-5604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang, E. Y. & Moses, H. L. (1990) J. Cell Biol. 111, 731-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Calle, E. E., Rodriguez, C., Walker-Thurmond, K. & Thun, M. J. (2003) N. Engl. J. Med. 348, 1625-1638. [DOI] [PubMed] [Google Scholar]
- 39.Bergstrom, A., Pisani, P., Tenet, V., Wolk, A. & Adami, H. O. (2001) Int. J. Cancer 91, 421-430. [DOI] [PubMed] [Google Scholar]
- 40.Okasha, M., McCarron, P., McEwen, J. & Smith, G. D. (2002) J. Epidemiol. Community Health 56, 780-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Folkman, J. (2002) Cancer Cell 1, 113-115. [DOI] [PubMed] [Google Scholar]
- 42.Moulton, K. S., Heller, E., Konerding, M. A., Flynn, E., Palinski, W. & Folkman, J. (1999) Circulation 99, 1726-1732. [DOI] [PubMed] [Google Scholar]