Abstract
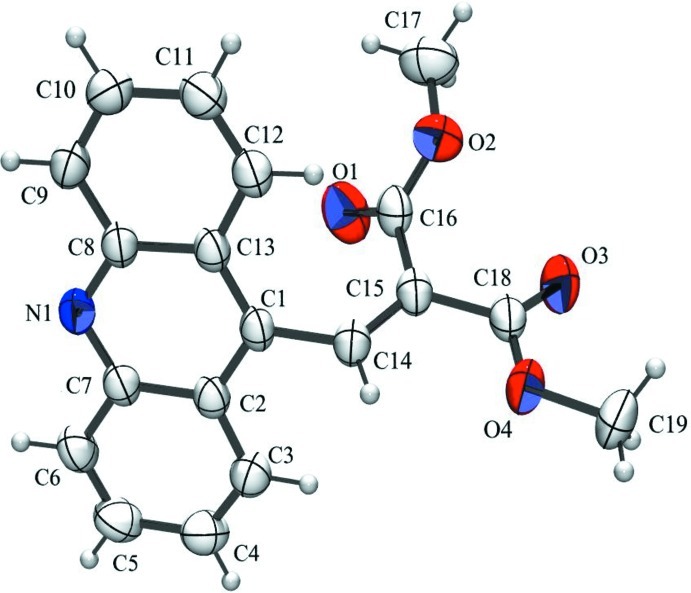
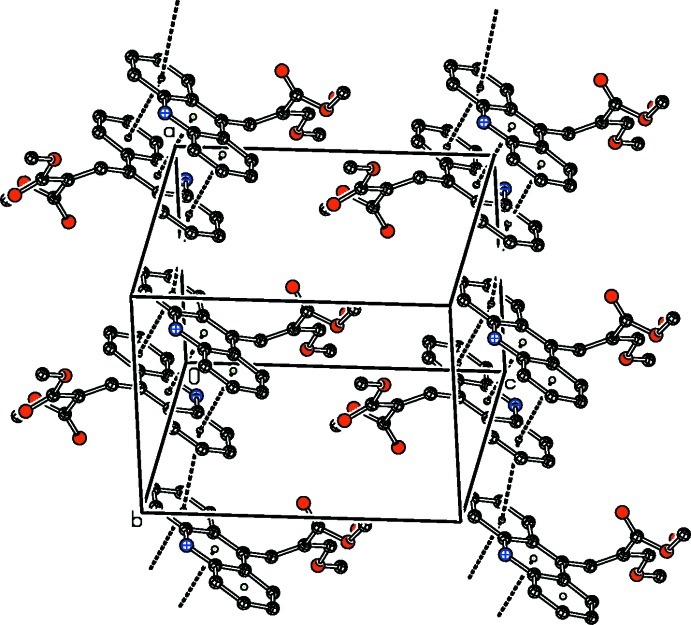
In the title compound, C19H15NO4, the acridine system is essentially planar (r.m.s. deviation = 0.015 Å). The crystal packing exhibits π–π interactions between pairs of centrosymmetric molecules, one of them between the central heterocyclic rings and others between the outer benzene rings of the acridine systems, with centroid–centroid distances of 3.692 (1) and 3.754 (1) Å, respectively. These pairs are further linked by additional π–π interactions along the a-axis direction through one of the two outer benzene ring of neighboring molecules, with a centroid–centroid distance of 3.642 (2) Å.
Related literature
For background to acridines, see: Kumar et al. (2012 ▶). For the biological activity of acridine derivatives, see: Pigatto et al. (2011 ▶); Das et al. (2011 ▶); Kumar et al. (2012 ▶). For the synthesis of acridines, see: Tomar et al. (2010 ▶). For related structures, see: Buckleton & Waters (1984 ▶).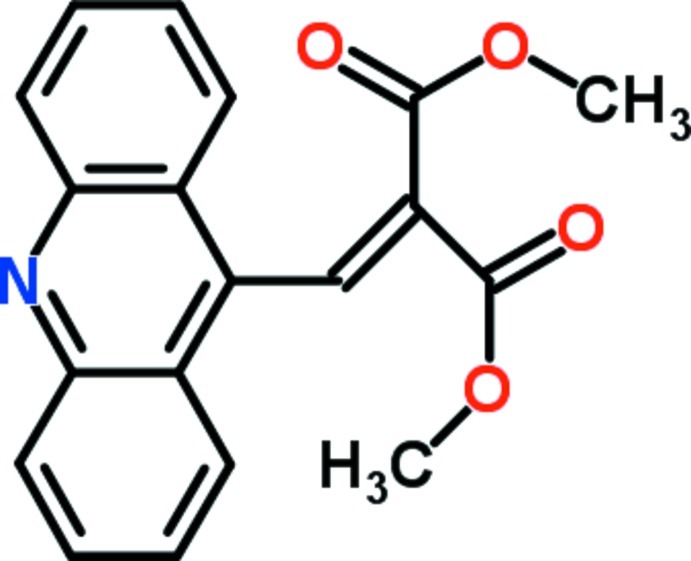
Experimental
Crystal data
C19H15NO4
M r = 321.32
Triclinic,
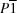
a = 8.3022 (2) Å
b = 9.0208 (3) Å
c = 12.0334 (4) Å
α = 96.468 (2)°
β = 93.652 (2)°
γ = 117.422 (2)°
V = 787.98 (4) Å3
Z = 2
Mo Kα radiation
μ = 0.10 mm−1
T = 295 K
0.32 × 0.28 × 0.22 mm
Data collection
Nonius KappaCCD diffractometer
10674 measured reflections
3626 independent reflections
2805 reflections with I > 2σ(I)
R int = 0.050
Refinement
R[F 2 > 2σ(F 2)] = 0.052
wR(F 2) = 0.148
S = 1.05
3626 reflections
218 parameters
H-atom parameters constrained
Δρmax = 0.26 e Å−3
Δρmin = −0.24 e Å−3
Data collection: COLLECT (Nonius, 1997 ▶); cell refinement: SCALEPACK (Otwinowski & Minor, 1997 ▶); data reduction: DENZO (Otwinowski & Minor, 1997 ▶) and SCALEPACK; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 for Windows (Farrugia, 2012 ▶); software used to prepare material for publication: WinGX (Farrugia, 2012 ▶).
Supplementary Material
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536813000500/lr2094sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536813000500/lr2094Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536813000500/lr2094Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Acknowledgments
This work received partial support from CNPq and PROPESQ/UEPB. The authors thank the Instituto de Física de São Carlos – USP for allowing the use of the KappaCCD diffractometer.
supplementary crystallographic information
Comment
The pharmacological uses of acridine derivatives have been well documented (Kumar et al. 2012). Amongst these applications can be highlighted: Proflavin an antibacterial agent against many Gram positive bacteria; Bucricaine which is used topically for surface anesthesia (Kumar et al. 2012); Quinacrine and 9-aminoacridine that act as antimalarial and disinfectant agents respectively (Das et al. 2011); and several antitumor agents as nitracrina, amsacrine and 9-arylacridines which are potent topoisomerase inhibitors (Pigatto et al. 2011).
In this work, we report the structure of the title compound synthesized by the reaction between acridine-9-carbaldehyde and dimethyl malonate. The mean plane analysis of molecule shows that the acridine ring is essentially planar. The deviation observed is maximum for the C5 [(0.0311 (2) Å].The crystal packing exhibits π- π interactions between pairs of centrosymmetric molecules, one of them between the central heterocyclic rings and others between the side benzene rings of the acridine moieties, with centroid-centroid distances of 3.692 (1) and 3.754 (1) Årespectively. These pairs are further linked by additional π- π interactions along the a axis through one of the two side benzene ring of neighboring molecules, with centroid-centroid distance of 3.642 (2) Å.
Experimental
In a Dean-Stark apparatus, acridine-9-carbaldehyde (0.1 mmol), dimethyl malonate (0.1 mmol) and morpholine (0.01 mmol) were refluxed in toluene (10 ml). The reaction mixture was refluxed at 383 K for 24 h, and the solvent was evaporated under reduced pressure. The title compound was purified by flash chromatography on silica gel (230–400 mesh) Merck (Germany), eluting with n-hexane/ethyl acetate (9.5:0.5) to give analytically pure yellow crystals of 2-acridin-9-yl-methylene-malonic acid dimethyl ester; yield 33%, M.p. 405–407 K. Crystals suitable for suitable for single-crystal X-ray diffraction were grown by slow evaporation at 289 K of a solution of the pure title compound in absolute ethanol.
FTIR (KBr, cm-1) Umax: 2954, 2924, 1730, 1628, 1435, 1266, 1232, 1076, 785, 749. NMR 1H (300 MHz, DMSO-d6) δ 3.15 (s, 3H), 3.92 (s, 3H), 7.65 (m, 2H), 7.89 (m, 2H), 7.99 (d, 2H,J = 8.6 Hz), 8.20 (d, 2H, J = 8.6 Hz), 8.70 (s, 1H). HRMS calcd for C19H15NO4 = 321.1001, found = 322.0617.
Refinement
All H-atoms were included in the refinement at calculated positions [C—H = 0.93 Å (aromatic) and 0.96 Å (methyl), with Uiso(H) = 1.2Ueq(aromatic C) or 1.5Ueq(methyl C)],also using a riding-model approximation. The maximum and minimum residual electron density peaks were located 0.16 and 0.77 Å, from the C9 and H14 atoms respectively.
Figures
Fig. 1.
The molecular structure of the title compound. with the ellipsoids drawn at the 50% probability level.
Fig. 2.
A partial view of the packing showing the π - π interactions.
Crystal data
| C19H15NO4 | Z = 2 |
| Mr = 321.32 | F(000) = 336 |
| Triclinic, P1 | Dx = 1.354 Mg m−3 |
| Hall symbol: -P 1 | Mo Kα radiation, λ = 0.71073 Å |
| a = 8.3022 (2) Å | Cell parameters from 6418 reflections |
| b = 9.0208 (3) Å | θ = 2.5–27.5° |
| c = 12.0334 (4) Å | µ = 0.10 mm−1 |
| α = 96.468 (2)° | T = 295 K |
| β = 93.652 (2)° | Prism, yellow |
| γ = 117.422 (2)° | 0.32 × 0.28 × 0.22 mm |
| V = 787.98 (4) Å3 |
Data collection
| Nonius KappaCCD diffractometer | 2805 reflections with I > 2σ(I) |
| Radiation source: Enraf Nonius FR590 | Rint = 0.050 |
| Horizonally mounted graphite crystal monochromator | θmax = 27.5°, θmin = 2.6° |
| Detector resolution: 9 pixels mm-1 | h = −10→10 |
| CCD rotation images,thick slices scans | k = −11→10 |
| 10674 measured reflections | l = −15→15 |
| 3626 independent reflections |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.052 | H-atom parameters constrained |
| wR(F2) = 0.148 | w = 1/[σ2(Fo2) + (0.0817P)2 + 0.1056P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.05 | (Δ/σ)max < 0.001 |
| 3626 reflections | Δρmax = 0.26 e Å−3 |
| 218 parameters | Δρmin = −0.24 e Å−3 |
| 0 restraints | Extinction correction: SHELXL97 (Sheldrick, 2008), Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| Primary atom site location: structure-invariant direct methods | Extinction coefficient: 0.168 (19) |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| C2 | 0.18856 (17) | −0.04107 (16) | 0.05238 (10) | 0.0416 (3) | |
| C8 | 0.27051 (18) | 0.29435 (17) | 0.10755 (11) | 0.0439 (3) | |
| C1 | 0.12960 (17) | 0.00751 (16) | 0.15015 (10) | 0.0409 (3) | |
| C16 | 0.25132 (19) | −0.00447 (18) | 0.38809 (12) | 0.0475 (3) | |
| N1 | 0.32779 (16) | 0.24918 (14) | 0.01345 (9) | 0.0477 (3) | |
| C14 | 0.02296 (18) | −0.12607 (17) | 0.21649 (11) | 0.0455 (3) | |
| H14 | −0.0906 | −0.2111 | 0.1815 | 0.055* | |
| O4 | −0.19557 (15) | −0.38893 (13) | 0.31911 (9) | 0.0630 (3) | |
| C13 | 0.16978 (17) | 0.17719 (16) | 0.17992 (10) | 0.0412 (3) | |
| C12 | 0.11370 (19) | 0.24024 (18) | 0.27615 (11) | 0.0484 (3) | |
| H12 | 0.0464 | 0.1665 | 0.3236 | 0.058* | |
| C11 | 0.1572 (2) | 0.4058 (2) | 0.29913 (12) | 0.0544 (4) | |
| H11 | 0.1202 | 0.4443 | 0.3625 | 0.065* | |
| C6 | 0.3548 (2) | 0.0411 (2) | −0.11100 (12) | 0.0549 (4) | |
| H6 | 0.4194 | 0.1228 | −0.1546 | 0.066* | |
| C9 | 0.3139 (2) | 0.46746 (18) | 0.13512 (13) | 0.0538 (4) | |
| H9 | 0.3808 | 0.5443 | 0.0892 | 0.065* | |
| O2 | 0.22592 (15) | 0.05138 (14) | 0.48843 (9) | 0.0593 (3) | |
| O1 | 0.39648 (15) | 0.04600 (18) | 0.35414 (10) | 0.0745 (4) | |
| O3 | 0.02856 (18) | −0.31220 (15) | 0.46082 (10) | 0.0716 (4) | |
| C7 | 0.28933 (18) | 0.08723 (17) | −0.01311 (11) | 0.0445 (3) | |
| C18 | −0.0314 (2) | −0.28576 (17) | 0.37642 (11) | 0.0488 (3) | |
| C5 | 0.3247 (2) | −0.1193 (2) | −0.14145 (14) | 0.0618 (4) | |
| H5 | 0.3696 | −0.1466 | −0.2051 | 0.074* | |
| C3 | 0.1592 (2) | −0.20857 (18) | 0.01646 (12) | 0.0508 (3) | |
| H3 | 0.0939 | −0.2935 | 0.0577 | 0.061* | |
| C15 | 0.07425 (18) | −0.13590 (17) | 0.32134 (11) | 0.0452 (3) | |
| C10 | 0.2585 (2) | 0.52130 (19) | 0.22792 (13) | 0.0578 (4) | |
| H10 | 0.2871 | 0.6345 | 0.2449 | 0.069* | |
| C4 | 0.2252 (2) | −0.2462 (2) | −0.07701 (14) | 0.0588 (4) | |
| H4 | 0.2048 | −0.3563 | −0.0989 | 0.071* | |
| C19 | −0.3052 (3) | −0.5404 (2) | 0.36533 (18) | 0.0765 (5) | |
| H19A | −0.4202 | −0.6062 | 0.3179 | 0.115* | |
| H19B | −0.3267 | −0.5095 | 0.4398 | 0.115* | |
| H19C | −0.2415 | −0.6059 | 0.3690 | 0.115* | |
| C17 | 0.3884 (3) | 0.1685 (3) | 0.56385 (16) | 0.0767 (5) | |
| H17A | 0.3545 | 0.2006 | 0.6338 | 0.115* | |
| H17B | 0.4557 | 0.2672 | 0.5302 | 0.115* | |
| H17C | 0.4634 | 0.1156 | 0.5779 | 0.115* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C2 | 0.0385 (6) | 0.0446 (7) | 0.0367 (6) | 0.0149 (5) | 0.0014 (5) | 0.0111 (5) |
| C8 | 0.0422 (7) | 0.0438 (7) | 0.0379 (6) | 0.0126 (5) | 0.0030 (5) | 0.0124 (5) |
| C1 | 0.0387 (6) | 0.0446 (7) | 0.0350 (6) | 0.0143 (5) | 0.0027 (5) | 0.0143 (5) |
| C16 | 0.0493 (7) | 0.0517 (7) | 0.0466 (7) | 0.0249 (6) | 0.0081 (6) | 0.0219 (6) |
| N1 | 0.0484 (6) | 0.0451 (6) | 0.0397 (6) | 0.0120 (5) | 0.0080 (5) | 0.0136 (5) |
| C14 | 0.0465 (7) | 0.0429 (7) | 0.0428 (7) | 0.0157 (5) | 0.0075 (5) | 0.0136 (5) |
| O4 | 0.0636 (7) | 0.0495 (6) | 0.0614 (7) | 0.0110 (5) | 0.0127 (5) | 0.0236 (5) |
| C13 | 0.0395 (6) | 0.0448 (7) | 0.0348 (6) | 0.0153 (5) | 0.0023 (5) | 0.0117 (5) |
| C12 | 0.0506 (7) | 0.0535 (8) | 0.0411 (7) | 0.0230 (6) | 0.0081 (6) | 0.0134 (6) |
| C11 | 0.0619 (9) | 0.0575 (9) | 0.0440 (7) | 0.0291 (7) | 0.0051 (6) | 0.0058 (6) |
| C6 | 0.0510 (8) | 0.0602 (9) | 0.0419 (7) | 0.0153 (6) | 0.0115 (6) | 0.0105 (6) |
| C9 | 0.0574 (8) | 0.0425 (7) | 0.0497 (8) | 0.0127 (6) | 0.0043 (6) | 0.0139 (6) |
| O2 | 0.0593 (6) | 0.0619 (7) | 0.0536 (6) | 0.0279 (5) | 0.0027 (5) | 0.0030 (5) |
| O1 | 0.0492 (6) | 0.1023 (10) | 0.0627 (7) | 0.0255 (6) | 0.0115 (5) | 0.0222 (6) |
| O3 | 0.0931 (9) | 0.0618 (7) | 0.0579 (7) | 0.0302 (6) | 0.0085 (6) | 0.0315 (5) |
| C7 | 0.0399 (6) | 0.0491 (7) | 0.0366 (6) | 0.0137 (5) | 0.0038 (5) | 0.0109 (5) |
| C18 | 0.0622 (8) | 0.0441 (7) | 0.0440 (7) | 0.0252 (6) | 0.0168 (6) | 0.0155 (6) |
| C5 | 0.0587 (9) | 0.0693 (10) | 0.0482 (8) | 0.0241 (8) | 0.0113 (7) | −0.0008 (7) |
| C3 | 0.0500 (8) | 0.0477 (7) | 0.0493 (8) | 0.0180 (6) | 0.0048 (6) | 0.0107 (6) |
| C15 | 0.0502 (7) | 0.0455 (7) | 0.0425 (7) | 0.0220 (6) | 0.0119 (6) | 0.0165 (5) |
| C10 | 0.0661 (9) | 0.0442 (8) | 0.0545 (8) | 0.0204 (7) | 0.0005 (7) | 0.0048 (6) |
| C4 | 0.0603 (9) | 0.0542 (8) | 0.0561 (9) | 0.0246 (7) | 0.0041 (7) | 0.0001 (7) |
| C19 | 0.0826 (12) | 0.0459 (9) | 0.0854 (13) | 0.0120 (8) | 0.0267 (10) | 0.0254 (8) |
| C17 | 0.0776 (12) | 0.0741 (11) | 0.0667 (11) | 0.0323 (9) | −0.0107 (9) | −0.0063 (9) |
Geometric parameters (Å, º)
| C2—C1 | 1.4037 (18) | C6—C5 | 1.351 (2) |
| C2—C3 | 1.425 (2) | C6—C7 | 1.427 (2) |
| C2—C7 | 1.4345 (17) | C6—H6 | 0.9300 |
| C8—N1 | 1.3482 (18) | C9—C10 | 1.358 (2) |
| C8—C9 | 1.424 (2) | C9—H9 | 0.9300 |
| C8—C13 | 1.4356 (17) | O2—C17 | 1.441 (2) |
| C1—C13 | 1.4049 (19) | O3—C18 | 1.1981 (17) |
| C1—C14 | 1.4823 (16) | C18—C15 | 1.4899 (18) |
| C16—O1 | 1.1940 (17) | C5—C4 | 1.415 (2) |
| C16—O2 | 1.3226 (18) | C5—H5 | 0.9300 |
| C16—C15 | 1.497 (2) | C3—C4 | 1.358 (2) |
| N1—C7 | 1.3381 (18) | C3—H3 | 0.9300 |
| C14—C15 | 1.3315 (18) | C10—H10 | 0.9300 |
| C14—H14 | 0.9300 | C4—H4 | 0.9300 |
| O4—C18 | 1.3293 (19) | C19—H19A | 0.9600 |
| O4—C19 | 1.4474 (18) | C19—H19B | 0.9600 |
| C13—C12 | 1.4301 (19) | C19—H19C | 0.9600 |
| C12—C11 | 1.355 (2) | C17—H17A | 0.9600 |
| C12—H12 | 0.9300 | C17—H17B | 0.9600 |
| C11—C10 | 1.419 (2) | C17—H17C | 0.9600 |
| C11—H11 | 0.9300 | ||
| C1—C2—C3 | 123.94 (12) | N1—C7—C6 | 117.91 (12) |
| C1—C2—C7 | 117.80 (12) | N1—C7—C2 | 123.52 (12) |
| C3—C2—C7 | 118.24 (12) | C6—C7—C2 | 118.55 (13) |
| N1—C8—C9 | 117.44 (12) | O3—C18—O4 | 124.20 (13) |
| N1—C8—C13 | 123.21 (12) | O3—C18—C15 | 123.33 (14) |
| C9—C8—C13 | 119.35 (12) | O4—C18—C15 | 112.42 (12) |
| C2—C1—C13 | 119.54 (11) | C6—C5—C4 | 120.40 (14) |
| C2—C1—C14 | 117.57 (12) | C6—C5—H5 | 119.8 |
| C13—C1—C14 | 122.87 (12) | C4—C5—H5 | 119.8 |
| O1—C16—O2 | 124.32 (15) | C4—C3—C2 | 121.06 (14) |
| O1—C16—C15 | 124.28 (14) | C4—C3—H3 | 119.5 |
| O2—C16—C15 | 111.38 (11) | C2—C3—H3 | 119.5 |
| C7—N1—C8 | 118.15 (11) | C14—C15—C18 | 122.41 (13) |
| C15—C14—C1 | 126.22 (12) | C14—C15—C16 | 122.17 (11) |
| C15—C14—H14 | 116.9 | C18—C15—C16 | 115.18 (11) |
| C1—C14—H14 | 116.9 | C9—C10—C11 | 120.38 (14) |
| C18—O4—C19 | 115.93 (13) | C9—C10—H10 | 119.8 |
| C1—C13—C12 | 124.38 (12) | C11—C10—H10 | 119.8 |
| C1—C13—C8 | 117.77 (12) | C3—C4—C5 | 120.61 (15) |
| C12—C13—C8 | 117.83 (12) | C3—C4—H4 | 119.7 |
| C11—C12—C13 | 120.94 (13) | C5—C4—H4 | 119.7 |
| C11—C12—H12 | 119.5 | O4—C19—H19A | 109.5 |
| C13—C12—H12 | 119.5 | O4—C19—H19B | 109.5 |
| C12—C11—C10 | 120.94 (14) | H19A—C19—H19B | 109.5 |
| C12—C11—H11 | 119.5 | O4—C19—H19C | 109.5 |
| C10—C11—H11 | 119.5 | H19A—C19—H19C | 109.5 |
| C5—C6—C7 | 121.14 (14) | H19B—C19—H19C | 109.5 |
| C5—C6—H6 | 119.4 | O2—C17—H17A | 109.5 |
| C7—C6—H6 | 119.4 | O2—C17—H17B | 109.5 |
| C10—C9—C8 | 120.55 (13) | H17A—C17—H17B | 109.5 |
| C10—C9—H9 | 119.7 | O2—C17—H17C | 109.5 |
| C8—C9—H9 | 119.7 | H17A—C17—H17C | 109.5 |
| C16—O2—C17 | 116.36 (13) | H17B—C17—H17C | 109.5 |
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: LR2094).
References
- Buckleton, J. S. & Waters, T. N. (1984). Acta Cryst. C40, 1587–1589.
- Das, S., Kundu, S. & Suresh, K. G. (2011). DNA Cell Biol. 30, 525–535. [DOI] [PubMed]
- Farrugia, L. J. (2012). J. Appl. Cryst. 45, 849–854.
- Kumar, R., Kaur, M. & Kumari, M. (2012). Acta Pol. Pharm. 69, 3–9. [PubMed]
- Nonius (1997). COLLECT. Nonius BV, Delft, The Netherlands.
- Otwinowski, Z. & Minor, W. (1997). Methods in Enzymology, Vol. 276, Macromolecular Crystallography, Part A, edited by C. W. Carter Jr & R. M. Sweet, pp. 307–326. New York: Academic Press.
- Pigatto, M. C., Lima, M. C. A., Galdino, S. L., Pitta, I. R., Vessecchi, R., Assis, M. D., Santos, J. S., Costa, T. C. T. D. & Lopes, P. N. (2011). Eur. J. Med. Chem. 1, 4245–4251. [DOI] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Tomar, V., Bhattacharjee, G., Uddin, K., Rajakumar, S., Srivastava, K. & Puri, S. K. (2010). Eur. J. Med. Chem. 45, 745–751. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) I, global. DOI: 10.1107/S1600536813000500/lr2094sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536813000500/lr2094Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536813000500/lr2094Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report