Abstract
In the title compound, C26H22N2O4, the pyrrolidine ring adopts a twisted conformation and the other five-membered rings adopt envelope conformations with the spiro C atoms as the flap atoms. The naphthalene ring system of the dihydroacenaphthylene group forms dihedral angles of 89.2 (9) and 75.5 (6)° with the pyrrolidine and indole rings, respectively. The pyrrolidine ring makes a dihedral angle of 80.1 (9)° with the indole ring. In the crystal, molecules are linked by weak C—H⋯O hydrogen bonds, forming chains along the b-axis direction.
Related literature
For the biological activity of naphthalene derivatives, see: Wiltz et al. (1998 ▶); Wright et al. (2000 ▶); Varma et al. (1994 ▶). For a related structure, see: Wei et al. (2012 ▶). For ring conformations, see: Cremer & Pople (1975 ▶).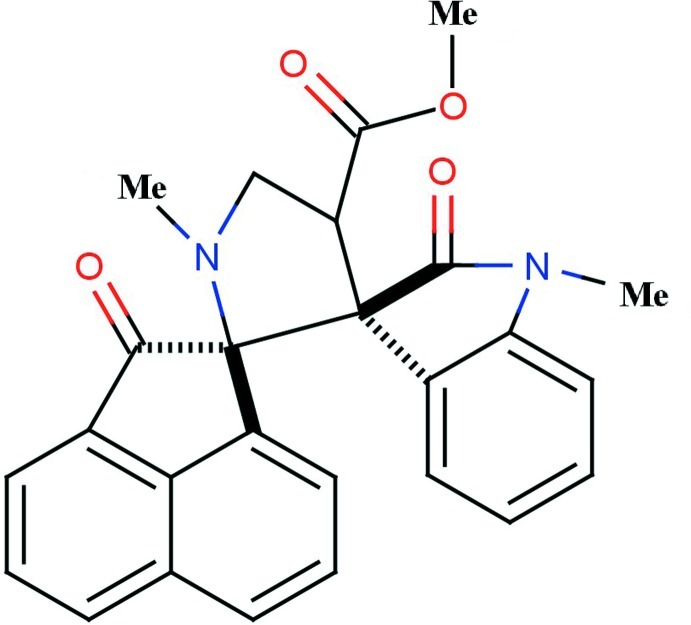
Experimental
Crystal data
C26H22N2O4
M r = 426.46
Monoclinic,

a = 15.4839 (4) Å
b = 9.5832 (2) Å
c = 15.6375 (4) Å
β = 115.184 (1)°
V = 2099.81 (9) Å3
Z = 4
Mo Kα radiation
μ = 0.09 mm−1
T = 293 K
0.25 × 0.22 × 0.19 mm
Data collection
Bruker APEXII CCD area-detector diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 2008 ▶) T min = 0.977, T max = 0.983
19264 measured reflections
4057 independent reflections
3017 reflections with I > 2σ(I)
R int = 0.031
Refinement
R[F 2 > 2σ(F 2)] = 0.038
wR(F 2) = 0.103
S = 1.03
4057 reflections
292 parameters
H-atom parameters constrained
Δρmax = 0.17 e Å−3
Δρmin = −0.17 e Å−3
Data collection: APEX2 (Bruker, 2008 ▶); cell refinement: SAINT (Bruker, 2008 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 (Farrugia, 2012 ▶); software used to prepare material for publication: SHELXL97 and PLATON (Spek, 2009 ▶).
Supplementary Material
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536813000470/lx2278sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536813000470/lx2278Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C10—H10⋯O2i | 0.93 | 2.60 | 3.268 (2) | 130 |
Symmetry code: (i)  .
.
Acknowledgments
ASP thanks the University Grants Commission, India, for a Minor Research Project.
supplementary crystallographic information
Comment
Naphthalene derivatives have manifested applications in many fields, for example, as a colorant, explosive, disinfectant, insecticide and plant hormone auxin. Naphthalene is believed to play a role in the chemical defence against biological enemies (Wiltz et al., 1998; Wright et al., 2000). It may be produced by metabolic processes in termites or by associated microorganisms which inhabit, e.g., the termite guts (Varma et al., 1994). In view of these importance and continuation of our work on the crystal structure analyis of napthalene derivatives, the crystal structure of the title compound has been carried out and the results are presented here.
X–Ray analysis confirms the molecular structure and atom connectivity as illustrated in Fig. 1. The geometry of acenaphthylene and pyrrolidine ring systems are comparable with the related structure [Wei et al. (2012)]. The sum of the angles at N1 [338.2 (1)°] and N2 [359.4 (1)°] of the pyrrolidine rings are in accordance with sp3 and sp2 hybridizations. The naphthalene ring system of the dihydroacenaphthylene group [C7–C16] forms dihedral angles of 89.2 (8) and 75.5 (5)° with the central pyrrolidine ring [N1/C2–C5] and the indole ring [N2/C4/C17–C23], respectively. It clearly shows that the naphthalene ring system of the dihydroacenaphthylene group attached to the central pyrrolidine ring are almost perpendicular to each other. Also the dihedral angle between the central pyrrolidine and the indole ring forms a a dihedral angle of 80.1 (8)°.
The central pyrrolidine ring adopts twisted conformations on N1 and C2 atoms with the pukering parameter of q2 = 0.3942 (2) Å, φ = 13.72 (3)° (Cremer & Pople, 1975). The pyrrolidine ring [N2/C4/C17–C19] in the indole group adopts envelope conformations, q2 = 0.0889 (2) Å and φ = 219.39 (1)°, and with atom C17 deviating -0.0565 (2) Å from the least–squares plane passing through the remaining four atoms (N2/C19/C18/C4) of that ring. In the crystal the molecules are linked by weak intermolecular C—H···O hydrogen bonds (Table 1), forming one-dimensional chains along the b–axis.
Experimental
To a mixture of 1eq of (E)–methyl 2–(1–methyl–2–oxoindolin –3–ylidene) acetate, 1eq of isatin and 1.5eq of acenaphthylene–1,2 –dione were dissolved in acetonitrile. This reaction mixture refluxed at 80°C for 8 hours. The reaction mixture was monitored for completion by thin layar chromatography. Upon completion, the reaction mixture was extracted with ethyl acetate and water. The product was dried and purified by coloumn chromatography using ethyl acetate and hexane (1:9) as an elutent to affored pure Dispiro oxindole. Yield (78%). Single crystals suitable for X–ray diffraction were obtained by slow evaporation of a solution of the title compound in ethyl acetate at room temperature.
Refinement
All H atoms were fixed geometrically and allowed to ride on their parent C atoms, with C—H distances fixed in the range 0.93–0.97 Å with Uiso(H) = 1.5Ueq(C) for methyl H 1.2Ueq(C) for other H atoms. The positions of methyl hydrogens were optimized rotationally.
Figures
Fig. 1.
The molecular structure of the title compound with the atom numbering scheme. Displacement ellipsoids are drawn at the 30% probability level. H atoms are presented as small spheres of arbitrary radius.
Crystal data
| C26H22N2O4 | F(000) = 896 |
| Mr = 426.46 | Dx = 1.349 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2ybc | Cell parameters from 4057 reflections |
| a = 15.4839 (4) Å | θ = 1.5–25.8° |
| b = 9.5832 (2) Å | µ = 0.09 mm−1 |
| c = 15.6375 (4) Å | T = 293 K |
| β = 115.184 (1)° | Block, colourless |
| V = 2099.81 (9) Å3 | 0.25 × 0.22 × 0.19 mm |
| Z = 4 |
Data collection
| Bruker APEXII CCD area-detector diffractometer | 4057 independent reflections |
| Radiation source: fine-focus sealed tube | 3017 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.031 |
| ω and φ scans | θmax = 25.8°, θmin = 2.6° |
| Absorption correction: multi-scan (SADABS; Bruker, 2008) | h = −18→18 |
| Tmin = 0.977, Tmax = 0.983 | k = −11→11 |
| 19264 measured reflections | l = −19→18 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.038 | Hydrogen site location: difference Fourier map |
| wR(F2) = 0.103 | H-atom parameters constrained |
| S = 1.03 | w = 1/[σ2(Fo2) + (0.0432P)2 + 0.5222P] where P = (Fo2 + 2Fc2)/3 |
| 4057 reflections | (Δ/σ)max < 0.001 |
| 292 parameters | Δρmax = 0.17 e Å−3 |
| 0 restraints | Δρmin = −0.17 e Å−3 |
Special details
| Geometry. All esds (except the esd in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell esds are taken into account individually in the estimation of esds in distances, angles and torsion angles; correlations between esds in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell esds is used for estimating esds involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > 2sigma(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1 | 0.69067 (8) | 0.37535 (12) | 0.28269 (9) | 0.0565 (3) | |
| O2 | 0.87808 (9) | 0.87118 (12) | 0.39458 (9) | 0.0552 (3) | |
| O3 | 0.64333 (11) | 0.58931 (15) | 0.48841 (11) | 0.0762 (4) | |
| O4 | 0.65285 (10) | 0.81185 (13) | 0.45236 (10) | 0.0648 (4) | |
| N1 | 0.87057 (9) | 0.51097 (14) | 0.41944 (9) | 0.0456 (3) | |
| N2 | 0.73670 (10) | 0.90053 (13) | 0.26438 (10) | 0.0490 (3) | |
| C1 | 0.91612 (13) | 0.37648 (19) | 0.42302 (14) | 0.0590 (5) | |
| H1A | 0.8683 | 0.3050 | 0.3999 | 0.088* | |
| H1B | 0.9596 | 0.3560 | 0.4871 | 0.088* | |
| H1C | 0.9505 | 0.3800 | 0.3844 | 0.088* | |
| C2 | 0.81537 (13) | 0.51929 (18) | 0.47465 (12) | 0.0537 (4) | |
| H2A | 0.8562 | 0.5123 | 0.5418 | 0.064* | |
| H2B | 0.7673 | 0.4465 | 0.4568 | 0.064* | |
| C3 | 0.77008 (12) | 0.66228 (17) | 0.44928 (11) | 0.0468 (4) | |
| H3 | 0.8174 | 0.7303 | 0.4890 | 0.056* | |
| C4 | 0.75428 (11) | 0.68832 (15) | 0.34492 (11) | 0.0385 (3) | |
| C5 | 0.81077 (10) | 0.56359 (15) | 0.32472 (10) | 0.0377 (3) | |
| C6 | 0.74144 (11) | 0.44844 (15) | 0.25997 (12) | 0.0410 (4) | |
| C7 | 0.75797 (10) | 0.43438 (15) | 0.17441 (11) | 0.0399 (4) | |
| C8 | 0.71903 (12) | 0.34921 (17) | 0.09679 (12) | 0.0503 (4) | |
| H8 | 0.6677 | 0.2913 | 0.0878 | 0.060* | |
| C9 | 0.75897 (13) | 0.35191 (18) | 0.03135 (12) | 0.0566 (5) | |
| H9 | 0.7321 | 0.2965 | −0.0225 | 0.068* | |
| C10 | 0.83614 (14) | 0.43324 (18) | 0.04395 (12) | 0.0544 (4) | |
| H10 | 0.8613 | 0.4304 | −0.0004 | 0.065* | |
| C11 | 0.87793 (12) | 0.52114 (16) | 0.12320 (11) | 0.0437 (4) | |
| C12 | 0.83529 (10) | 0.52056 (15) | 0.18635 (10) | 0.0375 (3) | |
| C13 | 0.86999 (10) | 0.59774 (15) | 0.27095 (11) | 0.0377 (3) | |
| C14 | 0.95079 (11) | 0.67546 (17) | 0.29344 (12) | 0.0470 (4) | |
| H14 | 0.9769 | 0.7255 | 0.3497 | 0.056* | |
| C15 | 0.99391 (13) | 0.67866 (19) | 0.23017 (13) | 0.0550 (5) | |
| H15 | 1.0482 | 0.7331 | 0.2454 | 0.066* | |
| C16 | 0.95951 (13) | 0.60548 (19) | 0.14783 (13) | 0.0545 (4) | |
| H16 | 0.9898 | 0.6111 | 0.1078 | 0.065* | |
| C17 | 0.79970 (12) | 0.82915 (16) | 0.33990 (12) | 0.0430 (4) | |
| C18 | 0.65287 (11) | 0.70826 (15) | 0.27105 (11) | 0.0394 (4) | |
| C19 | 0.64718 (11) | 0.83487 (16) | 0.22577 (11) | 0.0429 (4) | |
| C20 | 0.56296 (13) | 0.88569 (19) | 0.15782 (13) | 0.0555 (5) | |
| H20 | 0.5609 | 0.9701 | 0.1276 | 0.067* | |
| C21 | 0.48165 (13) | 0.8071 (2) | 0.13602 (14) | 0.0601 (5) | |
| H21 | 0.4237 | 0.8390 | 0.0902 | 0.072* | |
| C22 | 0.48497 (12) | 0.6824 (2) | 0.18089 (14) | 0.0584 (5) | |
| H22 | 0.4292 | 0.6317 | 0.1657 | 0.070* | |
| C23 | 0.57069 (11) | 0.63162 (17) | 0.24860 (12) | 0.0489 (4) | |
| H23 | 0.5727 | 0.5470 | 0.2785 | 0.059* | |
| C24 | 0.75568 (16) | 1.03779 (19) | 0.23647 (17) | 0.0747 (6) | |
| H24A | 0.7189 | 1.1066 | 0.2511 | 0.112* | |
| H24B | 0.7383 | 1.0385 | 0.1698 | 0.112* | |
| H24C | 0.8224 | 1.0589 | 0.2701 | 0.112* | |
| C25 | 0.68278 (13) | 0.68033 (18) | 0.46619 (12) | 0.0511 (4) | |
| C26 | 0.56590 (17) | 0.8428 (3) | 0.4604 (2) | 0.0891 (8) | |
| H26A | 0.5144 | 0.7906 | 0.4139 | 0.134* | |
| H26B | 0.5525 | 0.9408 | 0.4503 | 0.134* | |
| H26C | 0.5727 | 0.8178 | 0.5224 | 0.134* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.0570 (7) | 0.0447 (7) | 0.0803 (9) | −0.0125 (6) | 0.0413 (7) | −0.0052 (6) |
| O2 | 0.0555 (7) | 0.0507 (7) | 0.0624 (8) | −0.0152 (6) | 0.0281 (6) | −0.0144 (6) |
| O3 | 0.0945 (10) | 0.0629 (8) | 0.1036 (11) | −0.0088 (8) | 0.0734 (10) | 0.0024 (8) |
| O4 | 0.0775 (9) | 0.0521 (7) | 0.0881 (10) | 0.0029 (6) | 0.0577 (8) | −0.0005 (7) |
| N1 | 0.0475 (8) | 0.0464 (8) | 0.0437 (8) | 0.0061 (6) | 0.0202 (6) | 0.0065 (6) |
| N2 | 0.0579 (9) | 0.0333 (7) | 0.0603 (9) | −0.0026 (6) | 0.0295 (7) | 0.0028 (6) |
| C1 | 0.0604 (11) | 0.0532 (11) | 0.0598 (11) | 0.0149 (9) | 0.0223 (9) | 0.0104 (9) |
| C2 | 0.0652 (11) | 0.0552 (10) | 0.0481 (10) | 0.0046 (9) | 0.0313 (9) | 0.0099 (8) |
| C3 | 0.0543 (10) | 0.0472 (9) | 0.0452 (9) | −0.0055 (8) | 0.0272 (8) | −0.0025 (7) |
| C4 | 0.0422 (8) | 0.0358 (8) | 0.0422 (8) | −0.0021 (6) | 0.0225 (7) | −0.0013 (7) |
| C5 | 0.0387 (8) | 0.0352 (8) | 0.0410 (8) | −0.0005 (6) | 0.0188 (7) | 0.0005 (6) |
| C6 | 0.0364 (8) | 0.0335 (8) | 0.0546 (10) | 0.0022 (6) | 0.0207 (7) | 0.0012 (7) |
| C7 | 0.0392 (8) | 0.0320 (8) | 0.0448 (9) | 0.0037 (6) | 0.0144 (7) | 0.0016 (7) |
| C8 | 0.0502 (10) | 0.0387 (9) | 0.0521 (10) | −0.0008 (7) | 0.0121 (8) | −0.0043 (8) |
| C9 | 0.0714 (12) | 0.0467 (10) | 0.0434 (10) | 0.0045 (9) | 0.0164 (9) | −0.0074 (8) |
| C10 | 0.0735 (12) | 0.0481 (10) | 0.0468 (10) | 0.0071 (9) | 0.0308 (9) | 0.0015 (8) |
| C11 | 0.0523 (10) | 0.0399 (8) | 0.0421 (9) | 0.0067 (7) | 0.0231 (8) | 0.0047 (7) |
| C12 | 0.0379 (8) | 0.0331 (7) | 0.0397 (8) | 0.0048 (6) | 0.0148 (7) | 0.0037 (6) |
| C13 | 0.0362 (8) | 0.0369 (8) | 0.0411 (8) | 0.0011 (6) | 0.0176 (7) | 0.0010 (7) |
| C14 | 0.0439 (9) | 0.0511 (9) | 0.0485 (9) | −0.0084 (7) | 0.0219 (8) | −0.0058 (8) |
| C15 | 0.0484 (10) | 0.0592 (11) | 0.0664 (12) | −0.0132 (8) | 0.0332 (9) | −0.0058 (9) |
| C16 | 0.0595 (11) | 0.0573 (11) | 0.0617 (11) | −0.0001 (9) | 0.0402 (10) | 0.0039 (9) |
| C17 | 0.0493 (9) | 0.0376 (8) | 0.0502 (9) | −0.0058 (7) | 0.0290 (8) | −0.0091 (7) |
| C18 | 0.0411 (8) | 0.0375 (8) | 0.0454 (9) | 0.0009 (6) | 0.0241 (7) | −0.0023 (7) |
| C19 | 0.0502 (9) | 0.0367 (8) | 0.0483 (9) | 0.0029 (7) | 0.0273 (8) | −0.0026 (7) |
| C20 | 0.0653 (12) | 0.0455 (10) | 0.0572 (11) | 0.0148 (9) | 0.0274 (10) | 0.0066 (8) |
| C21 | 0.0496 (10) | 0.0622 (12) | 0.0623 (12) | 0.0147 (9) | 0.0177 (9) | −0.0045 (10) |
| C22 | 0.0416 (10) | 0.0616 (11) | 0.0709 (12) | 0.0012 (8) | 0.0230 (9) | −0.0092 (10) |
| C23 | 0.0457 (9) | 0.0454 (9) | 0.0608 (11) | −0.0004 (7) | 0.0277 (8) | −0.0006 (8) |
| C24 | 0.0924 (16) | 0.0405 (10) | 0.0951 (16) | −0.0103 (10) | 0.0438 (13) | 0.0119 (10) |
| C25 | 0.0649 (11) | 0.0489 (10) | 0.0522 (10) | −0.0087 (8) | 0.0371 (9) | −0.0056 (8) |
| C26 | 0.0934 (17) | 0.0816 (16) | 0.127 (2) | 0.0184 (13) | 0.0803 (17) | 0.0055 (14) |
Geometric parameters (Å, º)
| O1—C6 | 1.2135 (18) | C9—C10 | 1.369 (3) |
| O2—C17 | 1.2178 (19) | C9—H9 | 0.9300 |
| O3—C25 | 1.198 (2) | C10—C11 | 1.408 (2) |
| O4—C25 | 1.328 (2) | C10—H10 | 0.9300 |
| O4—C26 | 1.436 (2) | C11—C12 | 1.402 (2) |
| N1—C2 | 1.453 (2) | C11—C16 | 1.408 (2) |
| N1—C1 | 1.459 (2) | C12—C13 | 1.407 (2) |
| N1—C5 | 1.4622 (19) | C13—C14 | 1.367 (2) |
| N2—C17 | 1.354 (2) | C14—C15 | 1.410 (2) |
| N2—C19 | 1.403 (2) | C14—H14 | 0.9300 |
| N2—C24 | 1.455 (2) | C15—C16 | 1.360 (3) |
| C1—H1A | 0.9600 | C15—H15 | 0.9300 |
| C1—H1B | 0.9600 | C16—H16 | 0.9300 |
| C1—H1C | 0.9600 | C18—C23 | 1.379 (2) |
| C2—C3 | 1.513 (2) | C18—C19 | 1.389 (2) |
| C2—H2A | 0.9700 | C19—C20 | 1.374 (2) |
| C2—H2B | 0.9700 | C20—C21 | 1.379 (3) |
| C3—C25 | 1.494 (2) | C20—H20 | 0.9300 |
| C3—C4 | 1.564 (2) | C21—C22 | 1.376 (3) |
| C3—H3 | 0.9800 | C21—H21 | 0.9300 |
| C4—C18 | 1.514 (2) | C22—C23 | 1.387 (2) |
| C4—C17 | 1.539 (2) | C22—H22 | 0.9300 |
| C4—C5 | 1.590 (2) | C23—H23 | 0.9300 |
| C5—C13 | 1.520 (2) | C24—H24A | 0.9600 |
| C5—C6 | 1.572 (2) | C24—H24B | 0.9600 |
| C6—C7 | 1.471 (2) | C24—H24C | 0.9600 |
| C7—C8 | 1.371 (2) | C26—H26A | 0.9600 |
| C7—C12 | 1.400 (2) | C26—H26B | 0.9600 |
| C8—C9 | 1.403 (2) | C26—H26C | 0.9600 |
| C8—H8 | 0.9300 | ||
| C25—O4—C26 | 117.20 (15) | C12—C11—C16 | 116.49 (15) |
| C2—N1—C1 | 115.01 (14) | C10—C11—C16 | 127.37 (16) |
| C2—N1—C5 | 107.70 (12) | C7—C12—C11 | 122.73 (14) |
| C1—N1—C5 | 115.53 (13) | C7—C12—C13 | 113.46 (13) |
| C17—N2—C19 | 111.21 (13) | C11—C12—C13 | 123.65 (14) |
| C17—N2—C24 | 123.77 (15) | C14—C13—C12 | 118.01 (14) |
| C19—N2—C24 | 124.40 (15) | C14—C13—C5 | 132.55 (14) |
| N1—C1—H1A | 109.5 | C12—C13—C5 | 109.14 (12) |
| N1—C1—H1B | 109.5 | C13—C14—C15 | 119.08 (15) |
| H1A—C1—H1B | 109.5 | C13—C14—H14 | 120.5 |
| N1—C1—H1C | 109.5 | C15—C14—H14 | 120.5 |
| H1A—C1—H1C | 109.5 | C16—C15—C14 | 122.79 (16) |
| H1B—C1—H1C | 109.5 | C16—C15—H15 | 118.6 |
| N1—C2—C3 | 102.59 (13) | C14—C15—H15 | 118.6 |
| N1—C2—H2A | 111.2 | C15—C16—C11 | 119.94 (15) |
| C3—C2—H2A | 111.2 | C15—C16—H16 | 120.0 |
| N1—C2—H2B | 111.2 | C11—C16—H16 | 120.0 |
| C3—C2—H2B | 111.2 | O2—C17—N2 | 125.31 (15) |
| H2A—C2—H2B | 109.2 | O2—C17—C4 | 126.49 (15) |
| C25—C3—C2 | 114.31 (14) | N2—C17—C4 | 108.19 (13) |
| C25—C3—C4 | 114.51 (14) | C23—C18—C19 | 118.95 (15) |
| C2—C3—C4 | 105.51 (13) | C23—C18—C4 | 132.22 (14) |
| C25—C3—H3 | 107.4 | C19—C18—C4 | 108.63 (13) |
| C2—C3—H3 | 107.4 | C20—C19—C18 | 122.52 (16) |
| C4—C3—H3 | 107.4 | C20—C19—N2 | 127.77 (16) |
| C18—C4—C17 | 101.45 (12) | C18—C19—N2 | 109.61 (14) |
| C18—C4—C3 | 117.85 (12) | C19—C20—C21 | 117.63 (17) |
| C17—C4—C3 | 108.74 (12) | C19—C20—H20 | 121.2 |
| C18—C4—C5 | 115.01 (12) | C21—C20—H20 | 121.2 |
| C17—C4—C5 | 110.31 (12) | C22—C21—C20 | 121.07 (17) |
| C3—C4—C5 | 103.43 (12) | C22—C21—H21 | 119.5 |
| N1—C5—C13 | 111.32 (12) | C20—C21—H21 | 119.5 |
| N1—C5—C6 | 112.02 (12) | C21—C22—C23 | 120.68 (17) |
| C13—C5—C6 | 101.67 (12) | C21—C22—H22 | 119.7 |
| N1—C5—C4 | 102.87 (11) | C23—C22—H22 | 119.7 |
| C13—C5—C4 | 117.40 (12) | C18—C23—C22 | 119.14 (16) |
| C6—C5—C4 | 111.90 (11) | C18—C23—H23 | 120.4 |
| O1—C6—C7 | 126.75 (15) | C22—C23—H23 | 120.4 |
| O1—C6—C5 | 124.52 (14) | N2—C24—H24A | 109.5 |
| C7—C6—C5 | 108.34 (12) | N2—C24—H24B | 109.5 |
| C8—C7—C12 | 119.87 (15) | H24A—C24—H24B | 109.5 |
| C8—C7—C6 | 132.72 (15) | N2—C24—H24C | 109.5 |
| C12—C7—C6 | 107.13 (13) | H24A—C24—H24C | 109.5 |
| C7—C8—C9 | 118.06 (16) | H24B—C24—H24C | 109.5 |
| C7—C8—H8 | 121.0 | O3—C25—O4 | 123.53 (17) |
| C9—C8—H8 | 121.0 | O3—C25—C3 | 125.34 (17) |
| C10—C9—C8 | 122.29 (16) | O4—C25—C3 | 111.12 (14) |
| C10—C9—H9 | 118.9 | O4—C26—H26A | 109.5 |
| C8—C9—H9 | 118.9 | O4—C26—H26B | 109.5 |
| C9—C10—C11 | 120.90 (16) | H26A—C26—H26B | 109.5 |
| C9—C10—H10 | 119.5 | O4—C26—H26C | 109.5 |
| C11—C10—H10 | 119.5 | H26A—C26—H26C | 109.5 |
| C12—C11—C10 | 116.09 (15) | H26B—C26—H26C | 109.5 |
| C1—N1—C2—C3 | −174.54 (14) | C7—C12—C13—C5 | −0.36 (17) |
| C5—N1—C2—C3 | −44.14 (16) | C11—C12—C13—C5 | −175.79 (13) |
| N1—C2—C3—C25 | 158.48 (14) | N1—C5—C13—C14 | −50.7 (2) |
| N1—C2—C3—C4 | 31.78 (17) | C6—C5—C13—C14 | −170.18 (17) |
| C25—C3—C4—C18 | −8.3 (2) | C4—C5—C13—C14 | 67.4 (2) |
| C2—C3—C4—C18 | 118.29 (15) | N1—C5—C13—C12 | 122.71 (13) |
| C25—C3—C4—C17 | 106.29 (16) | C6—C5—C13—C12 | 3.27 (15) |
| C2—C3—C4—C17 | −127.12 (14) | C4—C5—C13—C12 | −119.15 (14) |
| C25—C3—C4—C5 | −136.46 (14) | C12—C13—C14—C15 | 2.0 (2) |
| C2—C3—C4—C5 | −9.87 (16) | C5—C13—C14—C15 | 174.98 (16) |
| C2—N1—C5—C13 | 163.81 (13) | C13—C14—C15—C16 | −1.2 (3) |
| C1—N1—C5—C13 | −66.09 (17) | C14—C15—C16—C11 | −0.5 (3) |
| C2—N1—C5—C6 | −83.11 (15) | C12—C11—C16—C15 | 1.3 (2) |
| C1—N1—C5—C6 | 47.00 (18) | C10—C11—C16—C15 | −176.25 (17) |
| C2—N1—C5—C4 | 37.23 (15) | C19—N2—C17—O2 | −169.50 (15) |
| C1—N1—C5—C4 | 167.34 (13) | C24—N2—C17—O2 | 1.8 (3) |
| C18—C4—C5—N1 | −145.28 (12) | C19—N2—C17—C4 | 9.62 (17) |
| C17—C4—C5—N1 | 100.76 (14) | C24—N2—C17—C4 | −179.04 (16) |
| C3—C4—C5—N1 | −15.38 (14) | C18—C4—C17—O2 | 169.98 (15) |
| C18—C4—C5—C13 | 92.14 (15) | C3—C4—C17—O2 | 45.1 (2) |
| C17—C4—C5—C13 | −21.82 (18) | C5—C4—C17—O2 | −67.68 (19) |
| C3—C4—C5—C13 | −137.96 (13) | C18—C4—C17—N2 | −9.13 (15) |
| C18—C4—C5—C6 | −24.86 (17) | C3—C4—C17—N2 | −134.01 (13) |
| C17—C4—C5—C6 | −138.82 (13) | C5—C4—C17—N2 | 113.21 (14) |
| C3—C4—C5—C6 | 105.04 (14) | C17—C4—C18—C23 | −169.12 (16) |
| N1—C5—C6—O1 | 49.29 (19) | C3—C4—C18—C23 | −50.6 (2) |
| C13—C5—C6—O1 | 168.23 (14) | C5—C4—C18—C23 | 71.8 (2) |
| C4—C5—C6—O1 | −65.65 (19) | C17—C4—C18—C19 | 5.61 (15) |
| N1—C5—C6—C7 | −123.94 (13) | C3—C4—C18—C19 | 124.14 (14) |
| C13—C5—C6—C7 | −5.00 (15) | C5—C4—C18—C19 | −113.43 (14) |
| C4—C5—C6—C7 | 121.13 (13) | C23—C18—C19—C20 | −1.4 (2) |
| O1—C6—C7—C8 | 5.6 (3) | C4—C18—C19—C20 | −176.94 (15) |
| C5—C6—C7—C8 | 178.65 (16) | C23—C18—C19—N2 | 175.13 (14) |
| O1—C6—C7—C12 | −168.02 (15) | C4—C18—C19—N2 | −0.41 (17) |
| C5—C6—C7—C12 | 5.01 (16) | C17—N2—C19—C20 | 170.31 (16) |
| C12—C7—C8—C9 | 0.0 (2) | C24—N2—C19—C20 | −1.0 (3) |
| C6—C7—C8—C9 | −172.97 (16) | C17—N2—C19—C18 | −5.99 (18) |
| C7—C8—C9—C10 | 1.7 (3) | C24—N2—C19—C18 | −177.26 (16) |
| C8—C9—C10—C11 | −1.4 (3) | C18—C19—C20—C21 | 1.0 (2) |
| C9—C10—C11—C12 | −0.6 (2) | N2—C19—C20—C21 | −174.81 (16) |
| C9—C10—C11—C16 | 176.92 (17) | C19—C20—C21—C22 | 0.1 (3) |
| C8—C7—C12—C11 | −2.1 (2) | C20—C21—C22—C23 | −0.8 (3) |
| C6—C7—C12—C11 | 172.48 (13) | C19—C18—C23—C22 | 0.6 (2) |
| C8—C7—C12—C13 | −177.61 (13) | C4—C18—C23—C22 | 174.91 (16) |
| C6—C7—C12—C13 | −3.00 (17) | C21—C22—C23—C18 | 0.4 (3) |
| C10—C11—C12—C7 | 2.4 (2) | C26—O4—C25—O3 | −3.1 (3) |
| C16—C11—C12—C7 | −175.42 (14) | C26—O4—C25—C3 | 176.22 (17) |
| C10—C11—C12—C13 | 177.41 (14) | C2—C3—C25—O3 | −7.5 (3) |
| C16—C11—C12—C13 | −0.4 (2) | C4—C3—C25—O3 | 114.4 (2) |
| C7—C12—C13—C14 | 174.18 (14) | C2—C3—C25—O4 | 173.17 (15) |
| C11—C12—C13—C14 | −1.3 (2) | C4—C3—C25—O4 | −64.93 (19) |
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C10—H10···O2i | 0.93 | 2.60 | 3.268 (2) | 130 |
Symmetry code: (i) x, −y+3/2, z−1/2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: LX2278).
References
- Bruker (2008). APEX2, SADABS and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- Cremer, D. & Pople, J. A. (1975). J. Am. Chem. Soc. 97, 1354–1358.
- Farrugia, L. J. (2012). J. Appl. Cryst. 45, 849–854.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Spek, A. L. (2009). Acta Cryst. D65, 148–155. [DOI] [PMC free article] [PubMed]
- Varma, A., Kolli, B. K., Paul, J., Saxena, S. & Konig, H. (1994). FEMS Microbiol. Rev. 15, 9–28.
- Wei, A. C., Ali, M. A., Choon, T. S., Arshad, S. & Razak, I. A. (2012). Acta Cryst. E68, o1340–o1341. [DOI] [PMC free article] [PubMed]
- Wiltz, B. A., Henderson, G. & Chen, J. (1998). Environ. Entomol. 27, 936–940.
- Wright, M. S., Lax, A. R., Henderson, G. & Chen, J. A. (2000). Mycologia, 92, 42–45.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536813000470/lx2278sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536813000470/lx2278Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report



