Abstract
CD4+ CD45RO+ T cells are the major latent viral reservoir in HIV-infected individuals and hence a major obstacle in curing the disease. An anti-CD45RO immunotoxin (IT) can decrease the number of both productively and latently infected CD4+ T cells obtained from HIV-infected individuals with detectable viremia. In this study, we determined whether this IT could also kill latently infected replication-competent CD4+ T cells obtained from infected individuals without detectable plasma viremia. Our results demonstrate that ex vivo treatment with the anti-CD45RO IT significantly reduced the frequency of these cells. In contrast, the IT had only a modest effect on the cytomegalalovirus-specific memory responses of CD8+ T cells. These results suggest that purging latent cells from infected individuals on highly active antiretroviral therapy with the anti-CD45RO IT might reduce the HIV latent reservoir without seriously compromising CD8+ T cell memory responses.
Although the implementation of highly active antiretroviral therapy (HAART) and other advances in our understanding of HIV pathogenesis and therapy have dramatically improved the survival and quality of life of HIV-infected individuals, HIV cannot yet be eradicated from infected individuals. Several studies have demonstrated that in individuals receiving HAART, the frequency of HIV-infected cells is reduced to fewer than one cell per 106 resting CD4+ T cells (1–5). However, even after years with viremia below the limit of quantitation (BLQ), the frequency of these infected cells does not decrease further (1, 4, 6, 7). The therapeutic approaches evaluated to date have failed to demonstrate a significant and persistent decline of this latent viral reservoir (8, 9).
We have previously established a model to characterize the different subsets of T cells infected in vitro with HIV or purified from HIV-infected individuals by using immunotoxins (ITs) directed against different cellular antigens. We have demonstrated that an anti-CD45RO IT can significantly reduce the number of HIV latently infected cells either generated in vitro or purified from HIV-infected individuals with detectable viremia (10, 11). The present study was designed to determine whether the anti-CD45RO IT could reduce the frequency of CD4+ latently infected cells obtained from HIV-infected individuals with viremia BLQ. We also determined whether the IT would eliminate CD8+ T cells that respond against cytomegalovirus (CMV).
Methods
Study Design. Peripheral blood mononuclear cells (PBMCs) from 24 individuals on HAART with viremia BLQ were screened for the presence of replication-competent virus. This was accomplished by two rounds of coculture with activated allogeneic PBMCs followed by p24 assays. Cells were also evaluated by real-time PCR (RT-PCR) for the presence of cDNA for RU5, which was chosen because of the sensitivity of the assay. Apheresis was then performed only on individuals in whom replication-competent virus (p24-secreting cells after coculture) could be detected. CD4+ T cells were isolated to determine the frequency of infected cells cultured either in complete medium (CM) or with the IT. For this purpose, cells were cultured in limiting dilution. Cells were treated for 6 days with either CM or CM plus the anti-CD45RO IT. The remaining cells were then cocultured twice (usually for 12 days) with phytohemagglutinin (PHA)-activated PBMCs to stimulate viral production from surviving latently infected cells. Wells containing 30 pg/ml p24 were considered positive. The frequency of cells with replication competent virus was calculated by multiple linear regression analysis (12–14). The cells remaining were also used to determine the number of HIV cDNA copies of RU5 by using RT-PCR. The same samples of apheresed PBMCs were then analyzed for responses against CMV, which was chosen because virtually all individuals are positive, and it gives a strong CD8+ response, which can be measured in most HIV+ individuals. This was accomplished by measuring levels of intracellular IFN-γ after stimulation with CMV 11-mer peptides. Those cells that were positive were then treated with either CM or IT and reductions in the numbers of CD8+ memory cells, and their CMV-specific memory responses were determined. This analysis was carried out by a combination of flow cytometric analysis for surface markers and by intracellular staining for IFN-γ. The study was approved by the Institutional Review Board of the University of Texas Southwestern Medical School. All patients signed informed consent for both the collection of blood samples and for apheresis.
IT. The IT was prepared by coupling UCHL-1, a murine IgG2a monoclonal antibody directed against human CD45RO (15), to deglycosylatd ricin A chain, as described (16).
p24 Assays. p24 antigen in cell-free supernatants was measured by ELISA (NEN Life Science Products).
HIV+ Individuals. Study individuals were recruited from the Aston and Amelia Court Clinics at the University of Texas Southwestern Medical Center, Dallas. All subjects were on HAART and had viremia of <400 copies per ml for a minimum of 1 month (Table 1). As noted, all patients signed informed consent.
Table 1. Study population.
| Patient | CD4+ cells per μl | %CD4+ cells | Time on HAART, months | Viremia, copies/ml | Time with viremia BLQ, months |
|---|---|---|---|---|---|
| 1* | 457 | 17 | 72 | <50 | 30 |
| 2 | 871 | 50.7 | 36 | <50 | 30 |
| 3† | 1,321 | 40 | 30 | <50 | 28 |
| 4 | 2,026 | 38.8 | 60 | <50 | 46 |
| 5* | 1,013 | 29 | 54 | <50 | 40 |
| 6* | 868 | 24.9 | 69 | <50 | 33 |
| 7† | 677 | 39.5 | 35 | <50 | 34 |
| 8 | 389 | 12.7 | 50 | <50 | 29 |
| 9* | 391 | 19 | 36 | <50 | 34 |
| 10 | 206 | 16 | 5 | <400 | 1 |
| 11† | 592 | 28 | 10 | <400 | 3 |
| 12 | 526 | 15.4 | 27 | <400 | 15 |
| 13 | 641 | 31.2 | 6 | <50 | 1 |
| 14 | 875 | 38.8 | 5 | <400 | 2 |
| 15 | 504 | 8.4 | 25 | <400 | 12 |
| 16 | 335 | 12 | 14 | <50 | 10 |
| 17 | 223 | 18.6 | 7 | <50 | 4 |
| 18 | 354 | 22.3 | 36 | <50 | 33 |
| 19 | 285 | 17 | 18 | <50 | 17 |
| 20 | 585 | 34.6 | 64 | <400 | 60 |
| 21 | 1,233 | 29.3 | 72 | <50 | 60 |
| 22* | 185 | 10.1 | 27 | <50 | 24 |
| 23* | 561 | 19.6 | 41 | <50 | 40 |
| 24 | 682 | 30 | 45 | <50 | 38 |
| Median (range) for all individuals | 573 (185—2,026) | 23.6 (8.4—50.7) | 35.5 (5—72) | 29 (1—60) | |
| Median (range) for apheresis individuals | 561 (185—1,013) | 19.6 (10—39.5) | 41 (27—72) | 34 (24—40) |
Individuals who underwent apheresis
Women
Flow Cytometric Analysis. Cells obtained from HIV-infected individuals with viremia BLQ were frozen at -80°C in 10% DMSO, and cells were stored at -140°C until analysis was performed. Some samples were freshly processed and analyzed. Eight-parameter flow cytometric analysis was performed on a custom LSR-II flow cytometer (Becton Dickinson) by using FITC, Pacific Blue, phycoerythrin (PE), PE-Cy7 (Texas Red), PerCP-Cy5. 5 (True Red), and allophycocyanin (APC) as the six fluorescent parameters. Immunophenotyping was carried out by using FITC-anti-CD45RO, PE-anti-CD28, Pacific Blue-anti-CD3 (custom conjugate, Molecular Probes), True Red-anti-CD4, Texas Red-anti-CD8, and APC-anti-CD95. For cytokine flow cytometry, FITC-anti-IFNγ, PE-anti-CD69, Pacific Blue-anti-CD3, True Red-anti-CD4, Texas Red-anti-CD8, and APC-anti-CD45RO were used. All antibodies were obtained from Becton Dickinson Immunocytometry Systems unless otherwise stated. The criteria for defining and quantifying both memory and naïve T cells have been described (17). Naïve CD8+ cells are CD95lo, CD28hi, and CD45RO-, whereas memory cells are CD95hi, CD28heterogeneous, and CD45ROheterogeneous. Small numbers of CD4+ memory T cells, and up to 50–80% of the CD8+ memory T cells, were CD45RO-/lo. PBMCs were also evaluated for functional activity before and after treatment with CM ± IT. Specific mixtures of CMV peptides, overlapping by 10 aa, and derived from CMV pp65 (UL83, 111 peptides) and IE-1 (UL123, 82 peptides), were used to evaluate the effector potential of these cells as described (18). One million PBMC were incubated with these antigens (2 μg/ml per peptide final concentration) in the presence of antibodies against CD28 and CD49d (1 μg each) for 1 h at 37°C in a 5% CO2 incubator. Brefeldin A (10 μg/ml; Sigma) was added, and cultures were incubated for an additional 5 h. Antigen-specific responses were determined by intracellular staining for IFN-γ as described (18). Antibodies alone were used as negative control, and Staphylococcus enterotoxin B (1 μg/ml final concentration) was used as a positive control. Cells were stored overnight at 4°C after stimulation and stained the next day. Data files were acquired by using the FACS-DiVA digital acquisition platform, and analysis was performed by using the paint-a-gate pro software program (Becton Dickinson Immunocytometry Systems).
Quantitation of HIV-1 RU5 DNA by TaqMan RT-PCR. Approximately 3 × 107 CD4+ T cells per experiment were treated with CM or the IT for 6 days and then cultured with PHA-activated PBMCs for 12 days. Remaining cells were assayed by RT-PCR by using primers for transcripts that corresponded to RU5, as described (19). This assay detects 1–10 copies of proviral RU5 per 106 cells.
Limiting Dilution Cultures and ex Vivo Treatment of Cells with ITs. Mononuclear cell apheresis yielded 2–18 × 108 PBMCs per sample. CD4+ T cells were purified by negative selection and cultured in limiting dilution in three to five replicate wells including three to four different cell concentrations ranging from 5 × 106 to 100 × 106 cells per well in CM ± IT. After 6 days in culture, cells were washed and cocultured twice with PHA-activated PBMCs. Cell frequencies were calculated by using a multiple regression equation (12–14).
Statistical Analysis. For pairwise comparisons, a t test or the nonparametric Mann–Whitney rank sum test was used. Correlations were calculated by using the Spearman correlation coefficient. Data were analyzed by using a sigma stat 2000 software package (Jondel Corporation).
Results
Patient Demographics, Screening, and Apheresis. Twenty-four (21 male and 3 female) HIV-infected adults with viremia <400 copies/ml (BLQ) (18 had <50 copies per ml) were enrolled in the study. At time of enrollment, the median time on HAART was 35.5 months (range, 5–72 months); the median time with viremia BLQ was 29 months (1–60 months). Median CD4 counts and percentages were 573 cells per mm3 (185–2,026 cells per mm3) and 23.6% (8.4–50.7%), respectively. Table 1 summarizes the patient demographics.
All 24 individuals were screened for the recovery of HIV-infected replication-competent CD4+ T cells. Such cells were detected in 7 of these 24 individuals. At time of enrollment, all had viremia <50 copies per ml. The median time these seven individuals had been on HAART was 46 months (27–71 months), the median time with viremia BLQ was 33 months (24–40 months), and the median CD4 counts and percentages of T cells were 561 cells per mm3 (range 185–1,013 cells per mm3) and 19.6% (10.1–39.5%), respectively. These patients were analyzed for anti-CMV responses.
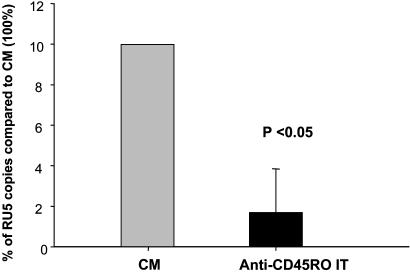
Treatment of CD4+ T Cells Obtained from HIV-Infected Individuals with Viremia BLQ with the Anti-CD45RO IT Significantly Reduced the Number of Copies of RU5 cDNA That Could Be Detected by RT-PCR. RT-PCR was performed on CD4+ T cells isolated from all 24 HIV-infected individuals enrolled in the study to determine the number HIV cDNA RU5 copies after treatment with the IT or CM. In 22 of 23 samples in which RU5 could be detected, treatment with the anti-CD45RO IT reduced the number of copies of RU5 cDNA compared with CM controls by 83% (range 15% to >90%) (Fig. 1).
Fig. 1.
HIV RU5 copy number in CD4+ T cells from HIV-infected individuals with viremia BLQ after ex vivo treatment with CM or IT. Thirty million CD4+ T cells obtained from 23 HIV-infected individuals with viremia BLQ were cultured with either CM or anti-CD45RO IT for 6 days. Cells were then washed and stimulated twice with PHA-activated PBMCs. Finally, DNA was extracted, and the number of copies of HIV RU5 was determined by RT-PCR. The median reduction in the number of HIV RU5 copies after treatment with anti-CD45RO IT compared with the control (CM taken as 100%) was 83%. The RU5 copy numbers declined from a median (range) of 2,669 (17–2,515,904) in cells treated with CM to 160 (5–380,202) in cells treated with the IT (P = 0.012).
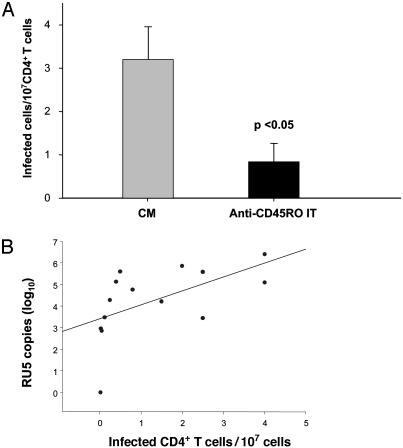
Treatment with the Anti-CD45RO IT Significantly Reduced the Frequency of HIV-Infected Replication-Competent CD4+ T Cells from Individuals with Viremia BLQ. Seven individuals, whose samples yielded HIV-infected replication competent CD4+ T cells, underwent apheresis to obtain sufficient numbers of PBMCs to set up limiting dilution cultures. In five of these seven individuals, we were able to detect and precisely measure the frequency of HIV-infected replication-competent cells. The mean (±SE) frequencies of HIV-infected CD4+ T cells in samples cultured in CM vs. CM plus the anti-CD45RO IT were 3.20 ± 0.80 vs. 0.84 ± 0.43 per 107 CD4+ cells. This result represented a 74% reduction (P = 0.028) (Fig. 2A), which was comparable to the reductions observed by RT-PCR. Indeed, there was a significant correlation between the number of RU5 copies and the frequency of replication competent CD4+ T cells (r = 0.56, P = 0.036) measured in these individuals with viremia BLQ (Fig. 2B).
Fig. 2.
Frequencies of HIV-infected replication-competent CD4+ T cells after ex vivo treatment with CM or IT. CD4+ T cells obtained from seven HIV-infected individuals with both viremia BLQ and replication-competent virus were plated in limiting dilution cultures at different concentrations ranging from 5 × 106 to 100 × 106 cells per well with CM ± the anti-CD45RO IT for 6 days. Cells were then washed and stimulated twice with PHA-activated PBMCs. p24 concentrations were determined in cell supernatants, and the frequencies of HIV-infected cells were calculated as described in Methods. The absolute frequencies of HIV-infected cells are plotted. (A) Mean number of HIV-infected cells. This frequency was reduced from a mean of 3.2 cells/107 CD4+ T cells after culture with CM to 0.84 cells/107 CD4+ T cells after treatment with the anti-CD45RO IT (P < 0.05). (B) Correlation between number of copies of RU5 and the frequency of HIV-infected cells in samples from the patients analyzed. n = 14; r = 0.56; P = 0.036.
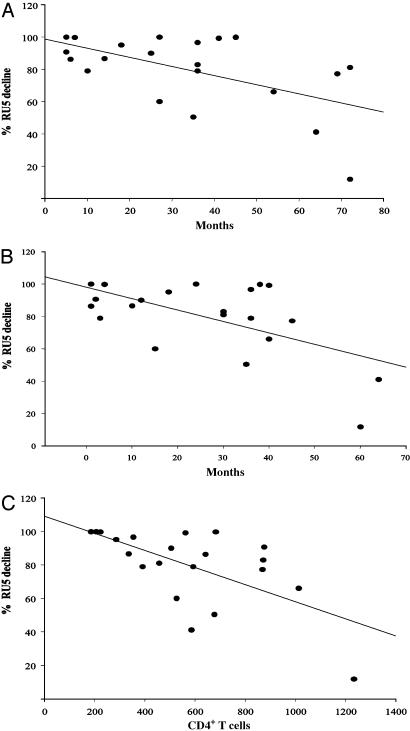
Correlations Between the Depletion of HIV-Latently Infected Cells and Clinical Characteristics of the Study Individuals. We determined whether any clinical or laboratory parameters correlated with variations (15% to >90%) in the efficacy of the IT in the 23 patients. As shown in Fig. 3, there were significant negative correlations between the percentage reduction of RU5 copies and (i) time that the patient had been on HAART; (ii) time with viremia BLQ; and (iii) CD4 count and percentage. In addition, there was a significant positive correlation between the RU5 copy number detected in CM cultures and the percentage reduction observed after treatment with the anti-CD45RO IT. In contrast, there was no correlation between the number of RU5 copies and the duration of either viremia BLQ or of HAART. It is therefore possible that when HIV-infected cells are still in the process of becoming quiescent (5, 7) or resting (20), they are more easily activated after stimulation. When a larger number of cells can become activated, there is a larger pool of cells available to demonstrate the effect of the IT. Regardless, treatment with the anti-CD45RO IT consistently reduced the number of RU5 copies in 22 of 23 samples tested, irrespective of either the number of RU5 copies detected initially or the duration of time during which the individuals had undetectable viremia.
Fig. 3.
Correlations between the effect of the anti-CD45RO IT in decreasing the number of copies of HIV RU5 and clinical parameters. The plots depicted show the negative correlations between the effect of the anti-CD45 RO IT and time on HAART; n = 21; r = -0.48; P = 0.029 (A); time with viremia BLQ; n = 21; r =-0.43; P = 0.049 (B); and the CD4 counts; n = 21; r =-0.58; P = 0.006 (C).
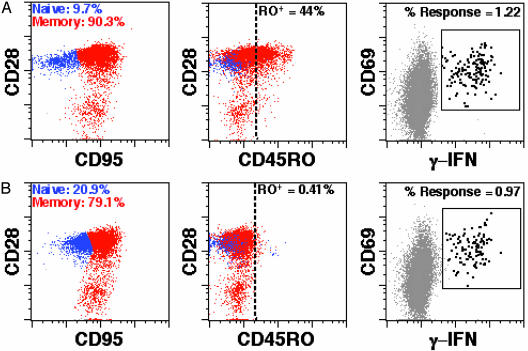
The Effect of the IT on CD8+ T Cell Memory Responses Against human CMV. We measured the CMV-specific responses of CD8+ T cells obtained from the HIV-infected individuals with replication-competent virus and CMV memory responses. Cells collected by apheresis were cultured with CM ± IT for 6 days. Cells were then washed, purified, and cultured with CMV peptides. Six hours later, the presence of intracellular IFN-γ was determined by flow cytometry. Fig. 4 shows the data from one patient, and Table 2 summarizes the results of all three patients. CMV responses of the CD8+ memory T cells were reduced by only 19–23%, which correlated with the fraction of pretreatment CD45R0+ cells specific for these peptide antigens (data not shown). In addition, >95% the CD4+ CD45RO+ memory cells were depleted by IT treatment (data not shown), and little functional CMV memory remained. Therefore, the anti-CD45RO IT was very effective in eliminating the CD4+ cells latently infected with HIV, but it had only a modest effect on the CD8+ memory T cell response to CMV.
Fig. 4.
The effect of the anti-CD45RO IT on naive and memory CD8+ T cells. (A) CM; (B) IT-treated. PBMCs from patient no. 23 were cultured for 6 days with CM ± the anti-CD45RO IT. Cells were stained as described in Methods and gated on CD8+ small lymphocytes. After IT treatment, CD45RO+ cells were depleted with 99.1% efficacy within the total CD8+ pool. After 6 h of coculture with CMV pp65 peptide antigen, 79.5% of the antigen-specific CD8+ T cell responses of IT-treated cells remained as determined by flow cytometry. Similar results were obtained with cells from two other patients (Table 2).
Table 2. Percentage of CD8+ T cell specific for CMV peptides (CD69+ IFN-γ+).
| CMV pp65
|
CMV IE-1
|
|||||
|---|---|---|---|---|---|---|
| Patient no. | Pretreatment | CM treated | IT treated | Pretreatment | CM treated | IT treated |
| 5 | 1.08 | 1.12 | 0.92 | 1.27 | 1.3 | 1.07 |
| 7 | 1.25 | 1.23 | 0.99 | 0.57 | 0.55 | 0.39 |
| 23 | 1.28 | 1.22 | 0.97 | 0.88 | 0.87 | 0.69 |
Three patients with PBMCs that responded to both CMV pp65 and IE-1 antigens were evaluated as described in Methods pretreatment, after 6 days of culture in CM or CM plus CD45RO IT. Controls exhibited only slight deviations from pretreatment samples, and antigen-specific CD8+ T cell responses after IT challenge were reduced by an average of 20%.
Phenotypic analysis of the CD8+ subset revealed that the total memory compartment was reduced only moderately (5–15%, data not shown) as a result of IT treatment. Taken together with the functional data, our results indicate that only CD8+ T cells expressing high levels of CD45RO are targeted, and that the remaining pool of CD45RO-/low CD8+ T cells constitute the majority of the memory/effector cells present before treatment with IT.
Discussion
Despite the striking success of HAART in controlling HIV infection, it does not eliminate the latent viral reservoir, and this reservoir is a major obstacle in curing the disease. Hence, patients require life-long therapy (1, 5, 21–24). The CD45RO+ CD4+ memory T cells appear to harbor the majority of the latent virus. To date, several approaches have been aimed at eliminating these cells (9, 25–29), but none have been highly successful (29–31). Another proposed strategy involves the use of HIV-based vaccines to stimulate anti-HIV cytotoxic T lymphocytes, which might recognize and eliminate the latent reservoir (32–34). More recently, several groups demonstrated that IL-7 and prostatin enhanced viral expression in latent cells. After this stimulation, the cells became susceptible to the effect of an IT directed against viral proteins (30, 31, 35, 36).
We have previously demonstrated that an anti-CD45RO IT can significantly reduce the number of HIV-latently infected cells either generated in vitro or purified from HIV-infected individuals with detectable viremia (10, 11). The present study was designed to determine whether the anti-CD45RO IT could also reduce the frequency of CD4+-latently infected T cells obtained from HIV-infected individuals with viremia BLQ. The major findings to emerge from our study are as follows: (i) Using cells from individuals with undetectable plasma viremia, the anti-CD45RO IT significantly reduced the frequency of latently infected CD4+ T cells. (ii) The IT spared the CD45RA+ cells and some of the CD8+ CD45ROlo memory cells. However, when CMV responses of CD8+ memory T cells could be measured, they were only modestly reduced. (iii) There was a strong correlation in the observed reductions of HIV cDNA RU5 copies and frequencies of HIV-infected CD4+ T cells after ex vivo treatment with the IT. (iv) CD4 counts, time on HAART, and the duration of undetectable viremia showed a negative correlation with the percent reductions in RU5 copies observed after treatment with IT.
These results suggest either that more recently acquired latently infected cells are more susceptible to the effect of the anti-CD45RO IT than long-lived latently infected cells or that the effect of the IT on the latter cannot be detected because the cells cannot be activated. This could be due to the fact that the longer the individuals maintain a state of latency, the more “resting” the cells become, and these cells may be harder to activate. In agreement with this hypothesis, Chun et al. (37) recently demonstrated that resting cells from viremic individuals can produce virus spontaneously, whereas resting cells from nonviremic individuals cannot, despite that fact that they contain similar amounts of HIV proviral DNA. Indeed, long-term memory CD4+ T cells may also down-regulate CD45RO.
To our knowledge, no therapy has been able to decrease the number of HIV-infected cells from HIV-infected individuals with viremia BLQ. We have previously demonstrated that the anti-CD45RO IT decreased the number of latent cells obtained from HIV-infected individuals with detectable viremia (10). We also performed a pilot study with three individuals with viremia BLQ and showed that the IT could significantly decrease these cells (10). In the present study, we have confirmed and extended those studies and have shown that the same IT can spare most of the CMV-specific memory T cells.
Taken together, our results suggest this IT should be tested for its ability to purge one of the critical latent viral reservoirs in patients on HAART with viremia BLQ. Viral latency in lymphocytes is a particularly attractive setting in which to use ITs, because lymphoid cells are accessible to the administered IT, relatively low doses of the IT should be effective, and if the “purge” were to last for 1 week or less, the immunogenicity of the IT would not present a problem. Until such a trial is carried out, we will not know whether a 10-fold depletion of latently infected cells can be achieved in vivo and/or whether this will have any impact on the disease.
Acknowledgments
We thank Ms. Linda Owens for administrative assistance, Dr. Victor Ghetie for preparing the immunotoxin, Dr. Asuncion Mejías for help with regulatory documents, Drs. Philip Keiser and Doug Hardy and the personnel at Aston Center and Amelia Court Clinics for assistance with patient recruitment, and Mr. Fred Scott and Mr. John Fulmer for technical assistance. We thank Drs. Victor Garcia and Jonathan Uhr for reviewing the manuscript. We are especially grateful to the individuals who participated in the study. This work was supported by National Institutes of Health Grant AI-43222, Grant 010019-0100 from the Higher Education Coordinating Board of the State of Texas, and a grant from the Horchow Foundation.
Abbreviations: HAART, highly active antiretroviral therapy; BLQ, below the limit of quantitation; IT, immunotoxin; PBMC, peripheral blood mononuclear cell; RT-PCR, real-time PCR; CM, complete medium; CMV, cytomegalovirus; PHA, phytohemagglutinin.
References
- 1.Blankson, J. N., Persaud, D. & Siliciano, R. F. (2002) Annu. Rev. Med. 53, 557-593. [DOI] [PubMed] [Google Scholar]
- 2.Chun, T. W., Stuyver, L., Mizell, S. B., Ehler, L. A., Mican, J. A., Baseler, M., Lloyd, A. L., Nowak, M. A. & Fauci, A. S. (1997) Proc. Natl. Acad. Sci. USA 94, 13193-13197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finzi, D., Blankson, J., Siliciano, J. D., Margolick, J. B., Chadwick, K., Pierson, T., Smith, K., Lisziewicz, J., Lori, F., Flexner, C., et al. (1999) Nat. Med. 5, 512-517. [DOI] [PubMed] [Google Scholar]
- 4.Siliciano, J. D., Kajdas, J., Finzi, D., Quinn, T. C., Chadwick, K., Margolick, J. B., Kovacs, C., Gange, S. J. & Siliciano, R. F. (2003) Nat. Med. 9, 727-728. [DOI] [PubMed] [Google Scholar]
- 5.Siliciano, R. F. (1999) AIDS 13 Suppl. A, S49-S58. [PubMed] [Google Scholar]
- 6.Derdeyn, C. A., Kilby, J. M., Miralles, G. D., Li, L. F., Sfakianos, G., Saag, M. S., Hockett, R. D. & Bucy, R. P. (1999) J. Infect. Dis. 180, 1851-1862. [DOI] [PubMed] [Google Scholar]
- 7.Persaud, D., Zhou, Y., Siliciano, J. M. & Siliciano, R. F. (2003) J. Virol. 77, 1659-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davey, R. T., Jr., Bhat, N., Yoder, C., Chun, T. W., Metcalf, J. A., Dewar, R., Natarajan, V., Lempicki, R. A., Adelsberger, J. W., Miller, K. D., et al. (1999) Proc. Natl. Acad. Sci. USA 96, 15109-15114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davey, R. T., Jr., Murphy, R. L., Graziano, F. M., Boswell, S. L., Pavia, A. T., Cancio, M., Nadler, J. P., Chaitt, D. G., Dewar, R. L., Sahner, D. K., et al. (2000) J. Am. Med. Assoc. 284, 183-189. [DOI] [PubMed] [Google Scholar]
- 10.Saavedra-Lozano, J., McCoig, C., Xu, J., Cao, Y., Keiser, P., Ghetie, V., Siliciano, R. F., Siliciano, J. D., Picker, L. J., Ramilo, O., et al. (2002) J. Infect. Dis. 185, 306-314. [DOI] [PubMed] [Google Scholar]
- 11.McCoig, C., Van Dyke, G., Chou, C. S., Picker, L. J., Ramilo, O. & Vitetta, E. S. (1999) Proc. Natl. Acad. Sci. USA 96, 11482-11485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Macken, C. (1999) J. Immunol. Methods 222, 13-29. [DOI] [PubMed] [Google Scholar]
- 13.Layton, J. E., Vitetta, E. S., Uhr, J. W. & Krammer, P. H. (1984) J. Exp. Med. 160, 1850-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonnefoix, T., Bonnefoix, P., Verdiel, P. & Sotto, J. J. (1996) J. Immunol. Methods 194, 113-119. [DOI] [PubMed] [Google Scholar]
- 15.Ghetie, V., Thorpe, P., Ghetie, M. A., Knowles, P., Uhr, J. W. & Vitetta, E. S. (1991) J. Immunol. Methods 142, 223-230. [DOI] [PubMed] [Google Scholar]
- 16.Smith, S. H., Brown, M. H., Rowe, D., Callard, R. E. & Beverley, P. C. (1986) Immunology 58, 63-70. [PMC free article] [PubMed] [Google Scholar]
- 17.Appay, V., Dunbar, P. R., Callan, M., Klenerman, P., Gillespie, G. M., Papagno, L., Ogg, G. S., King, A., Lechner, F., Spina, C. A., et al. (2002) Nat. Med. 8, 379-385. [DOI] [PubMed] [Google Scholar]
- 18.Maecker, H. T., Maino, V. C. & Picker, L. J. (2000) J. Clin. Immunol. 20, 391-399. [DOI] [PubMed] [Google Scholar]
- 19.Folks, T. M., Powell, D., Lightfoote, M., Koenig, S., Fauci, A. S., Benn, S., Rabson, A., Daugherty, D., Gendelman, H. E., Hoggan, M. D., et al. (1986) J. Exp. Med. 164, 280-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hermankova, M., Siliciano, J. D., Zhou, Y., Monie, D., Chadwick, K., Margolick, J. B., Quinn, T. C. & Siliciano, R. F. (2003) J. Virol. 77, 7383-7392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chun, T. W., Carruth, L., Finzi, D., Shen, X., DiGiuseppe, J. A., Taylor, H., Hermankova, M., Chadwick, K., Margolick, J., Quinn, T. C., et al. (1997) Nature 387, 183-188. [DOI] [PubMed] [Google Scholar]
- 22.Zhang, L., Ramratnam, B., Tenner-Racz, K., He, Y., Vesanen, M., Lewin, S., Talal, A., Racz, P., Perelson, A. S., Korber, B. T., et al. (1999) N. Engl. J. Med. 340, 1605-1613. [DOI] [PubMed] [Google Scholar]
- 23.Garcia, F., Plana, M., Vidal, C., Cruceta, A., O'Brien, W. A., Pantaleo, G., Pumarola, T., Gallart, T., Miro, J. M. & Gatell, J. M. (1999) AIDS 13, F79-F86. [DOI] [PubMed] [Google Scholar]
- 24.Bukrinsky, M. I., Stanwick, T. L., Dempsey, M. P. & Stevenson, M. (1991) Science 254, 423-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blankson, J. & Siliciano, R. F. (2000) J. Am. Med. Assoc. 284, 236-238. [DOI] [PubMed] [Google Scholar]
- 26.Chun, T. W., Engel, D., Mizell, S. B., Hallahan, C. W., Fischette, M., Park, S., Davey, R. T., Jr., Dybul, M., Kovacs, J. A., Metcalf, J. A., et al. (1999) Nat. Med. 5, 651-655. [DOI] [PubMed] [Google Scholar]
- 27.Hengge, U. R., Goos, M., Esser, S., Exner, V., Dotterer, H., Wiehler, H., Borchard, C., Muller, K., Beckmann, A., Eppner, M. T., et al. (1998) AIDS 12, F225-F234. [PubMed] [Google Scholar]
- 28.Saag, M. S. & Kilby, J. M. (1999) Nat. Med. 5, 609-611. [DOI] [PubMed] [Google Scholar]
- 29.de Boer, A. W., Markowitz, N., Lane, H. C., Saravolatz, L. D., Koletar, S. L., Donabedian, H., Yoshizawa, C., Duliege, A. M., Fyfe, G. & Mitsuyasu, R. T. (2003) Clin. Immunol. 106, 188-196. [DOI] [PubMed] [Google Scholar]
- 30.Brooks, D. G., Hamer, D. H., Arlen, P. A., Gao, L., Bristol, G., Kitchen, C. M., Berger, E. A. & Zack, J. A. (2003) Immunity 19, 413-423. [DOI] [PubMed] [Google Scholar]
- 31.Kulkosky, J., Culnan, D. M., Roman, J., Dornadula, G., Schnell, M., Boyd, M. R. & Pomerantz, R. J. (2001) Blood 98, 3006-3015. [DOI] [PubMed] [Google Scholar]
- 32.Grossman, Z., Polis, M., Feinberg, M. B., Levi, I., Jankelevich, S., Yarchoan, R., Boon, J., de Wolf, F., Lange, J. M., Goudsmit, J., et al. (1999) Nat. Med. 5, 1099-1104. [DOI] [PubMed] [Google Scholar]
- 33.Hel, Z., Venzon, D., Poudyal, M., Tsai, W. P., Giuliani, L., Woodward, R., Chougnet, C., Shearer, G., Altman, J. D., Watkins, D., et al. (2000) Nat. Med. 6, 1140-1146. [DOI] [PubMed] [Google Scholar]
- 34.Rosenberg, E. S., Altfeld, M., Poon, S. H., Phillips, M. N., Wilkes, B. M., Eldridge, R. L., Robbins, G. K., D'Aquila, R. T., Goulder, P. J. & Walker, B. D. (2000) Nature 407, 523-526. [DOI] [PubMed] [Google Scholar]
- 35.Scripture-Adams, D. D., Brooks, D. G., Korin, Y. D. & Zack, J. A. (2002) J. Virol. 76, 13077-13082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Korin, Y. D., Brooks, D. G., Brown, S., Korotzer, A. & Zack, J. A. (2002) J. Virol. 76, 8118-8123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chun, T. W., Justement, J. S., Lempicki, R. A., Yang, J., Dennis, G., Jr., Hallahan, C. W., Sanford, C., Pandya, P., Liu, S., McLaughlin, M., et al. (2003) Proc. Natl. Acad. Sci. USA 100, 1908-1913. [DOI] [PMC free article] [PubMed] [Google Scholar]