Abstract
In the title compound, C14H9F3N2O, the best planes of the benzimidazole group and benzene ring form a dihedral angle of 26.68 (3)°. In the crystal, N—H⋯N hydrogen bonds link the molecules into infinite chains parallel to the c axis. Stacking interactions between the benzimidazole groups [centroid–centroid distance = 3.594 (5) Å] assemble the molecules into layers parallel to (100). The trifluoromethyl group is disordered over three sets of sites with site-occupancy factors of 0.787 (4), 0.107 (7) and 0.106 (7).
Related literature
For therapeutic and medicinal properties of benzimidazole derivatives, see: Chimirri et al. (1991 ▶); Benavides et al. (1995 ▶); Ishihara et al. (1994 ▶); Kubo et al. (1993 ▶). For related structures, see: Jian et al. (2006 ▶); Rashid, Tahir, Yusof et al. (2007 ▶); Rashid, Tahir, Kanwal et al. (2007 ▶).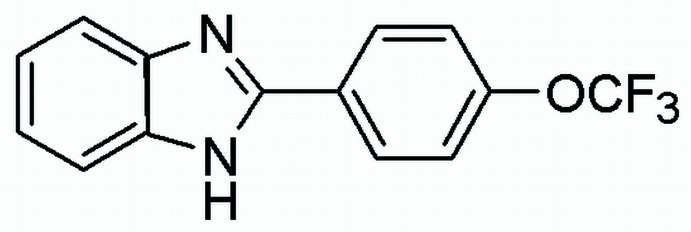
Experimental
Crystal data
C14H9F3N2O
M r = 278.23
Monoclinic,
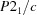
a = 14.476 (6) Å
b = 9.312 (4) Å
c = 9.835 (4) Å
β = 108.192 (8)°
V = 1259.5 (9) Å3
Z = 4
Mo Kα radiation
μ = 0.13 mm−1
T = 296 K
0.18 × 0.16 × 0.16 mm
Data collection
Bruker SMART APEX CCD detector diffractometer
Absorption correction: multi-scan (SADABS; Bruker, 1998 ▶) T min = 0.978, T max = 0.980
6284 measured reflections
2209 independent reflections
1333 reflections with I > 2σ(I)
R int = 0.077
Refinement
R[F 2 > 2σ(F 2)] = 0.064
wR(F 2) = 0.157
S = 1.00
2209 reflections
210 parameters
21 restraints
H-atom parameters constrained
Δρmax = 0.26 e Å−3
Δρmin = −0.22 e Å−3
Data collection: SMART (Bruker, 1998 ▶); cell refinement: SAINT-Plus (Bruker, 1998 ▶); data reduction: SAINT-Plus; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: ORTEP-3 for Windows (Farrugia, 2012 ▶) and CAMERON (Watkin et al., 1996 ▶); software used to prepare material for publication: WinGX (Farrugia, 2012 ▶).
Supplementary Material
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536813001220/gk2550sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536813001220/gk2550Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536813001220/gk2550Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| N2—H21⋯N1i | 0.86 | 2.07 | 2.864 (4) | 154 |
Symmetry code: (i) 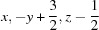 .
.
Acknowledgments
NSB is thankful to the University Grants Commission (UGC), India, for financial assistance.
supplementary crystallographic information
Comment
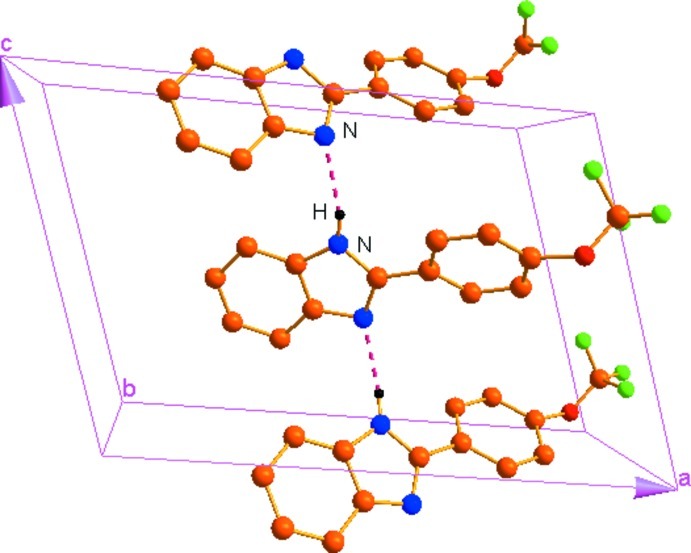
Benzimidazole and its derivatives are known to exhibit a wide variety of pharmacological activities such as anti-HIV (Chimirri et al., 1991), antihistaminic (Benavides et al., 1995), antiulcer (Ishihara et al., 1994), antihypertensive (Kubo et al., 1993). The benzimidazole and phenyl groups form a dihedral angle of 26.68 (3)°. The bond lengths and angles of the benzimidazole moiety in the title compound are in good agreement with those observed in other benzimidazole derivatives (Jian et al., 2006; Rashid, Tahir, Yusof et al., 2007; Rashid, Tahir, Kanwal et al., 2007). The N1—C8 and N2—C8 distances were found to be 1.338 (5) Å and 1.356 (5) Å, respectively. The trifluoromethyl group is disordered over three sites with occupancy factors 0.787 (4), 0.107 (7) and 0.106 (7). The molecules are linked by intermolecular N—H···N hydrogen bonds to form infinite chains parallel to the c axis. Additionally, the crystal packing is further stabilized by π -π stacking interactions between the benzimidazole groups [interplanar distance 3.594 (5) Å].
Experimental
A mixture of 4-(trifluoromethoxy)benzaldehyde (10 mmol, 0.19 g) and o-phenyldiamine (10 mmol, 0.19 g) in benzene (2 ml) was refluxed for 6 h on a water bath. The reaction mixture was cooled. The solid separated, was filtered and dried (yield: 0.26 g, 75% and m.p. 503–508 K). Pale yellow crystals of the title compound were obtained by slow evaporation from a solution in ethyl acetate.
Refinement
All H atoms were included in calculated positions, with C—H bond distances of 0.93 Å and N—H = 0.86 Å and refined in a riding model approximation with Uiso(H) = 1.2Ueq(C,N). The trifluoromethyl group is disordered over three sites with the occupancy factors 0.787 (4), 0.107 (7) and 0.106 (7). The atoms of the minor components were refined isotropically with a common displacement parameter for each group. The geometry of the minor components was restrained to that of the major component with the SAME instruction of SHELXL97 (Sheldrick, 2008).
Figures
Fig. 1.
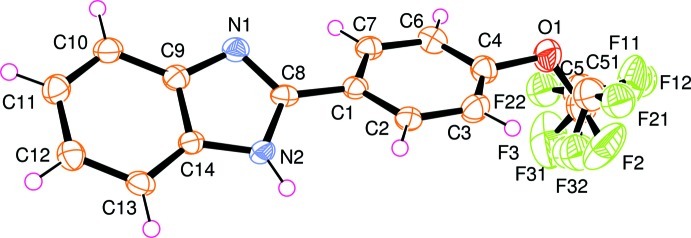
The molecular structure of the title compound with the atom numbering scheme. Displacement ellipsoids are drawn at the 50% probability level. H atoms are presented as small spheres of arbitrary radius.The trifluoromethyl group is disordered over three sites.
Fig. 2.
A view of the intermolecular hydrogen bonds(dotted lines) in the crystal structure of the title compound. H atoms non participating in H-bonding and minor components of the disordered CF3 group were omitted for clarity.
Crystal data
| C14H9F3N2O | F(000) = 568 |
| Mr = 278.23 | Dx = 1.467 Mg m−3 |
| Monoclinic, P21/c | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2ybc | Cell parameters from 2209 reflections |
| a = 14.476 (6) Å | θ = 2.6–25.0° |
| b = 9.312 (4) Å | µ = 0.13 mm−1 |
| c = 9.835 (4) Å | T = 296 K |
| β = 108.192 (8)° | Block, yellow |
| V = 1259.5 (9) Å3 | 0.18 × 0.16 × 0.16 mm |
| Z = 4 |
Data collection
| Bruker SMART APEX CCD detector diffractometer | 2209 independent reflections |
| Radiation source: fine-focus sealed tube | 1333 reflections with I > 2σ(I) |
| Graphite monochromator | Rint = 0.077 |
| ω scans | θmax = 25.0°, θmin = 2.6° |
| Absorption correction: multi-scan (SADABS; Bruker, 1998) | h = −17→15 |
| Tmin = 0.978, Tmax = 0.980 | k = −8→11 |
| 6284 measured reflections | l = −11→11 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.064 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.157 | H-atom parameters constrained |
| S = 1.00 | w = 1/[σ2(Fo2) + (0.0655P)2] where P = (Fo2 + 2Fc2)/3 |
| 2209 reflections | (Δ/σ)max = 0.001 |
| 210 parameters | Δρmax = 0.26 e Å−3 |
| 21 restraints | Δρmin = −0.22 e Å−3 |
Special details
| Geometry. All s.u.'s (except the s.u. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell s.u.'s are taken into account individually in the estimation of s.u.'s in distances, angles and torsion angles; correlations between s.u.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell s.u.'s is used for estimating s.u.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > 2σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | Occ. (<1) | |
| C1 | 0.3459 (2) | 0.8348 (3) | 0.4611 (3) | 0.0246 (8) | |
| C2 | 0.3309 (2) | 0.9562 (3) | 0.3748 (3) | 0.0297 (8) | |
| H2 | 0.3786 | 0.9857 | 0.3359 | 0.036* | |
| C3 | 0.2445 (2) | 1.0344 (4) | 0.3460 (4) | 0.0361 (9) | |
| H3 | 0.2352 | 1.1177 | 0.2912 | 0.043* | |
| C4 | 0.1735 (2) | 0.9856 (4) | 0.4004 (4) | 0.0333 (9) | |
| C6 | 0.1860 (2) | 0.8644 (3) | 0.4854 (3) | 0.0321 (9) | |
| H6 | 0.1368 | 0.8332 | 0.5206 | 0.039* | |
| C7 | 0.2734 (2) | 0.7900 (3) | 0.5172 (3) | 0.0284 (8) | |
| H7 | 0.2836 | 0.7097 | 0.5763 | 0.034* | |
| C8 | 0.4390 (2) | 0.7578 (3) | 0.4969 (3) | 0.0245 (8) | |
| C9 | 0.5675 (2) | 0.6361 (3) | 0.6072 (3) | 0.0260 (8) | |
| C10 | 0.6411 (2) | 0.5565 (3) | 0.7050 (3) | 0.0316 (9) | |
| H10 | 0.6339 | 0.5228 | 0.7901 | 0.038* | |
| C11 | 0.7245 (2) | 0.5305 (3) | 0.6695 (4) | 0.0327 (9) | |
| H11 | 0.7743 | 0.4778 | 0.7323 | 0.039* | |
| C12 | 0.7367 (2) | 0.5806 (3) | 0.5422 (3) | 0.0311 (8) | |
| H12 | 0.7946 | 0.5624 | 0.5233 | 0.037* | |
| C13 | 0.6639 (2) | 0.6567 (3) | 0.4443 (3) | 0.0277 (8) | |
| H13 | 0.6709 | 0.6883 | 0.3583 | 0.033* | |
| C14 | 0.5804 (2) | 0.6840 (3) | 0.4794 (3) | 0.0233 (8) | |
| O1 | 0.08661 (17) | 1.0648 (3) | 0.3804 (3) | 0.0460 (7) | |
| N1 | 0.47847 (18) | 0.6843 (3) | 0.6176 (3) | 0.0272 (7) | |
| N2 | 0.49671 (18) | 0.7606 (3) | 0.4113 (3) | 0.0260 (7) | |
| H21 | 0.4836 | 0.8024 | 0.3295 | 0.031* | |
| C5 | 0.0271 (4) | 1.0787 (7) | 0.2470 (6) | 0.0534 (16) | 0.787 (4) |
| F1 | −0.0537 (4) | 1.1368 (8) | 0.2540 (9) | 0.0740 (15) | 0.787 (4) |
| F2 | 0.0616 (3) | 1.1639 (8) | 0.1658 (6) | 0.0954 (17) | 0.787 (4) |
| F3 | 0.0071 (4) | 0.9548 (6) | 0.1788 (7) | 0.110 (2) | 0.787 (4) |
| C51 | 0.0312 (14) | 1.109 (2) | 0.268 (3) | 0.047 (6)* | 0.107 (7) |
| F11 | −0.0580 (16) | 1.145 (3) | 0.259 (7) | 0.047 (6)* | 0.107 (7) |
| F21 | 0.0781 (14) | 1.2304 (19) | 0.261 (3) | 0.047 (6)* | 0.107 (7) |
| F31 | 0.030 (3) | 1.033 (3) | 0.154 (4) | 0.047 (6)* | 0.107 (7) |
| C52 | 0.010 (2) | 1.062 (3) | 0.270 (3) | 0.056 (7)* | 0.106 (7) |
| F12 | −0.066 (4) | 1.144 (3) | 0.251 (8) | 0.056 (7)* | 0.106 (7) |
| F22 | −0.0187 (16) | 0.928 (2) | 0.282 (3) | 0.056 (7)* | 0.106 (7) |
| F32 | 0.037 (4) | 1.064 (4) | 0.154 (4) | 0.056 (7)* | 0.106 (7) |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| C1 | 0.0277 (18) | 0.0240 (18) | 0.0207 (17) | −0.0013 (14) | 0.0055 (14) | −0.0033 (14) |
| C2 | 0.031 (2) | 0.037 (2) | 0.0256 (18) | 0.0045 (15) | 0.0146 (15) | 0.0039 (16) |
| C3 | 0.040 (2) | 0.038 (2) | 0.033 (2) | 0.0027 (17) | 0.0134 (17) | 0.0087 (17) |
| C4 | 0.030 (2) | 0.035 (2) | 0.032 (2) | 0.0030 (16) | 0.0069 (16) | −0.0047 (17) |
| C6 | 0.030 (2) | 0.033 (2) | 0.035 (2) | −0.0056 (15) | 0.0115 (16) | −0.0059 (17) |
| C7 | 0.033 (2) | 0.0275 (19) | 0.0244 (18) | −0.0029 (15) | 0.0087 (15) | −0.0031 (15) |
| C8 | 0.0320 (19) | 0.0228 (18) | 0.0204 (17) | −0.0048 (14) | 0.0105 (15) | −0.0034 (14) |
| C9 | 0.0305 (19) | 0.0217 (18) | 0.0248 (18) | −0.0018 (14) | 0.0069 (14) | −0.0029 (14) |
| C10 | 0.043 (2) | 0.028 (2) | 0.0243 (19) | 0.0028 (16) | 0.0104 (16) | −0.0006 (15) |
| C11 | 0.038 (2) | 0.0272 (19) | 0.030 (2) | 0.0044 (16) | 0.0060 (16) | −0.0005 (16) |
| C12 | 0.032 (2) | 0.0268 (19) | 0.034 (2) | 0.0023 (15) | 0.0106 (16) | −0.0014 (16) |
| C13 | 0.036 (2) | 0.0242 (18) | 0.0250 (18) | 0.0006 (15) | 0.0132 (15) | −0.0037 (15) |
| C14 | 0.0318 (19) | 0.0179 (17) | 0.0206 (17) | −0.0004 (14) | 0.0087 (14) | −0.0005 (14) |
| O1 | 0.0353 (15) | 0.0582 (18) | 0.0454 (16) | 0.0162 (12) | 0.0137 (13) | 0.0099 (13) |
| N1 | 0.0327 (16) | 0.0248 (15) | 0.0238 (15) | −0.0002 (12) | 0.0085 (12) | −0.0020 (12) |
| N2 | 0.0335 (16) | 0.0241 (15) | 0.0207 (14) | 0.0028 (12) | 0.0089 (12) | 0.0042 (12) |
| C5 | 0.038 (3) | 0.065 (5) | 0.054 (4) | 0.012 (3) | 0.010 (3) | 0.003 (4) |
| F1 | 0.026 (2) | 0.084 (3) | 0.111 (3) | 0.0205 (17) | 0.021 (2) | 0.043 (2) |
| F2 | 0.056 (2) | 0.154 (5) | 0.086 (3) | 0.030 (3) | 0.034 (2) | 0.078 (3) |
| F3 | 0.076 (3) | 0.085 (3) | 0.116 (4) | 0.016 (3) | −0.045 (3) | −0.042 (3) |
Geometric parameters (Å, º)
| C1—C2 | 1.390 (4) | C11—C12 | 1.397 (5) |
| C1—C7 | 1.393 (4) | C11—H11 | 0.9300 |
| C1—C8 | 1.469 (4) | C12—C13 | 1.380 (4) |
| C2—C3 | 1.397 (4) | C12—H12 | 0.9300 |
| C2—H2 | 0.9300 | C13—C14 | 1.380 (4) |
| C3—C4 | 1.376 (5) | C13—H13 | 0.9300 |
| C3—H3 | 0.9300 | C14—N2 | 1.385 (4) |
| C4—C6 | 1.382 (4) | O1—C5 | 1.332 (6) |
| C4—O1 | 1.417 (4) | N2—H21 | 0.8600 |
| C6—C7 | 1.391 (4) | C5—F1 | 1.309 (5) |
| C6—H6 | 0.9300 | C5—F3 | 1.321 (7) |
| C7—H7 | 0.9300 | C5—F2 | 1.329 (8) |
| C8—N1 | 1.334 (4) | C51—F11 | 1.309 (5) |
| C8—N2 | 1.358 (4) | C51—F31 | 1.321 (7) |
| C9—N1 | 1.399 (4) | C51—F21 | 1.329 (8) |
| C9—C14 | 1.400 (4) | C52—F12 | 1.309 (5) |
| C9—C10 | 1.403 (4) | C52—F32 | 1.321 (7) |
| C10—C11 | 1.379 (5) | C52—F22 | 1.329 (8) |
| C10—H10 | 0.9300 | ||
| C2—C1—C7 | 119.5 (3) | C10—C11—H11 | 118.9 |
| C2—C1—C8 | 120.0 (3) | C12—C11—H11 | 118.9 |
| C7—C1—C8 | 120.4 (3) | C13—C12—C11 | 121.0 (3) |
| C1—C2—C3 | 120.5 (3) | C13—C12—H12 | 119.5 |
| C1—C2—H2 | 119.8 | C11—C12—H12 | 119.5 |
| C3—C2—H2 | 119.8 | C14—C13—C12 | 117.2 (3) |
| C4—C3—C2 | 118.6 (3) | C14—C13—H13 | 121.4 |
| C4—C3—H3 | 120.7 | C12—C13—H13 | 121.4 |
| C2—C3—H3 | 120.7 | C13—C14—N2 | 132.6 (3) |
| C3—C4—C6 | 122.1 (3) | C13—C14—C9 | 122.6 (3) |
| C3—C4—O1 | 120.8 (3) | N2—C14—C9 | 104.8 (3) |
| C6—C4—O1 | 116.9 (3) | C5—O1—C4 | 117.5 (3) |
| C4—C6—C7 | 118.7 (3) | C8—N1—C9 | 104.3 (3) |
| C4—C6—H6 | 120.6 | C8—N2—C14 | 107.8 (3) |
| C7—C6—H6 | 120.6 | C8—N2—H21 | 126.1 |
| C6—C7—C1 | 120.4 (3) | C14—N2—H21 | 126.1 |
| C6—C7—H7 | 119.8 | F1—C5—F3 | 109.3 (5) |
| C1—C7—H7 | 119.8 | F1—C5—F2 | 107.1 (5) |
| N1—C8—N2 | 112.7 (3) | F3—C5—F2 | 106.3 (6) |
| N1—C8—C1 | 124.7 (3) | F1—C5—O1 | 107.6 (5) |
| N2—C8—C1 | 122.5 (3) | F3—C5—O1 | 112.8 (5) |
| N1—C9—C14 | 110.4 (3) | F2—C5—O1 | 113.6 (5) |
| N1—C9—C10 | 129.7 (3) | F11—C51—F31 | 109.3 (5) |
| C14—C9—C10 | 119.9 (3) | F11—C51—F21 | 107.1 (5) |
| C11—C10—C9 | 117.1 (3) | F31—C51—F21 | 106.3 (6) |
| C11—C10—H10 | 121.4 | F12—C52—F32 | 109.3 (5) |
| C9—C10—H10 | 121.4 | F12—C52—F22 | 107.1 (5) |
| C10—C11—C12 | 122.2 (3) | F32—C52—F22 | 106.3 (6) |
Hydrogen-bond geometry (Å, º)
| D—H···A | D—H | H···A | D···A | D—H···A |
| N2—H21···N1i | 0.86 | 2.07 | 2.864 (4) | 154 |
Symmetry code: (i) x, −y+3/2, z−1/2.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: GK2550).
References
- Benavides, J., Schoemaker, H., Dana, C., Clausture, Y., Delahaye, M., Prouteau, M., Manoury, P., Allen, J. V., Scatton, B., Langer, S. Z. & Arbilla, S. (1995). Arzneim. Forsch. (Drug. Res.), 45, 551—558. [PubMed]
- Bruker. (1998). SMART, SAINT-Plus and SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
- Chimirri, A., Grasso, S., Monforte, A. M., Monforte, P. & Zappala, M. (1991). Il Farmaco, 46, 925—933. [PubMed]
- Farrugia, L. J. (2012). J. Appl. Cryst. 45, 849–854.
- Ishihara, K., Ichikawa, T., Komuro, Y., Ohara, S. & Hotta, K. (1994). Arzneim. Forsch. (Drug. Res.), 44, 827—830. [PubMed]
- Jian, F.-F., Yu, H.-Q., Qiao, Y.-B., Zhao, P.-S. & Xiao, H.-L. (2006). Acta Cryst. E62, o5194–o5195.
- Kubo, K., Kohara, Y., Imamia, E., Sugiura, Y., Inada, Y., Furukawa, Y., Nishikawa, K. & Naka, T. (1993). J. Med. Chem. 36, 2182–2195. [DOI] [PubMed]
- Rashid, N., Tahir, M. K., Kanwal, S., Yusof, N. M. & Yamin, B. M. (2007). Acta Cryst. E63, o1402–o1403.
- Rashid, N., Tahir, M. K., Yusof, N. M. & Yamin, B. M. (2007). Acta Cryst. E63, o1260–o1261.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Watkin, D. J., Prout, C. K. & Pearce, L. J. (1996). CAMERON Chemical Crystallography Laboratory, University of Oxford, England.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablock(s) global, I. DOI: 10.1107/S1600536813001220/gk2550sup1.cif
Structure factors: contains datablock(s) I. DOI: 10.1107/S1600536813001220/gk2550Isup2.hkl
Supplementary material file. DOI: 10.1107/S1600536813001220/gk2550Isup3.cml
Additional supplementary materials: crystallographic information; 3D view; checkCIF report