In this issue of JAGS Weiss and colleagues present evidence of high variability in resting metabolic rate (RMR) among pre-frail and frail women. The authors should be commended for drawing attention to energetic need and regulation in late life and their association with and potential reflection of aging phenotypes and disease states.
Research on the complex relationship between resting metabolic rate, aging and longevity has profound roots in human research science. In 1892 the American chemist Wilbur Olin Atwater built the first human calorimeter in collaboration with Max Rubner and Francis G. Benedict. In 1908 Max Rubner set forth his "rate-of-living theory", based on the observation that larger animals tend to outlive smaller animals, and proposed that a slower metabolism may be associated with longevity. To this day, Rubner’s theory continues to be debated in the literature 1, 2 which reveals our limited understanding of energy metabolism and its role in human aging, chronic disease progression and differential longevity.
RMR - or the cost of living - accounts for as much as 60–70% of daily cumulative oxygen consumption, which should not be surprising considering the complexity of human existence. It is also known that RMR changes dramatically with age. It is high in early life to support the energetic cost of growth, slows throughout growth and development, declines rapidly up to around the age of 20, and then continues to decline more slowly during mid-to-late life, in part as a consequence of a decline in lean body mass (Figure 1). As previously demonstrated in the Baltimore Longitudinal Study of Aging, this decline in RMR appears consistent with normal aging and a lack of such a decline may be a marker of health deterioration and high risk of premature death 3. At first glance, these findings seem counterintuitive, but upon further examination they make perfect sense. Most chronic conditions, health events, and aging-related phenotypes provoke instabilities in homeostatic equilibrium that require extra energy for recovery, repair and homeostatic restoration. For example, in the presence of chronic respiratory disease the cost of ventilation (acquiring oxygen from the air) may be significantly more energetically costly. Similarly, in diabetes glucose metabolism is dysregulated and considerably more energetically expensive. Hypermetabolism has frequently been described in COPD patients as a strong independent predictor of disease severity progression 4. Finally, in a study recently performed in Pima Indians who are predisposed to metabolic dysregulation, higher energy expenditure was a strong, independent predictor of mortality 6. Overall this evidence signifies persons in poor health status may spend considerably more energy at rest than those who are healthy. Findings reported by Weiss and colleagues indicate that some of the frail women had high RMR and thus support of this hypothesis. However, the finding that some frail women actually had lower RMR than expected compared to non-frail controls is a previously undiscovered challenge that warrants further investigation.
Figure 1.
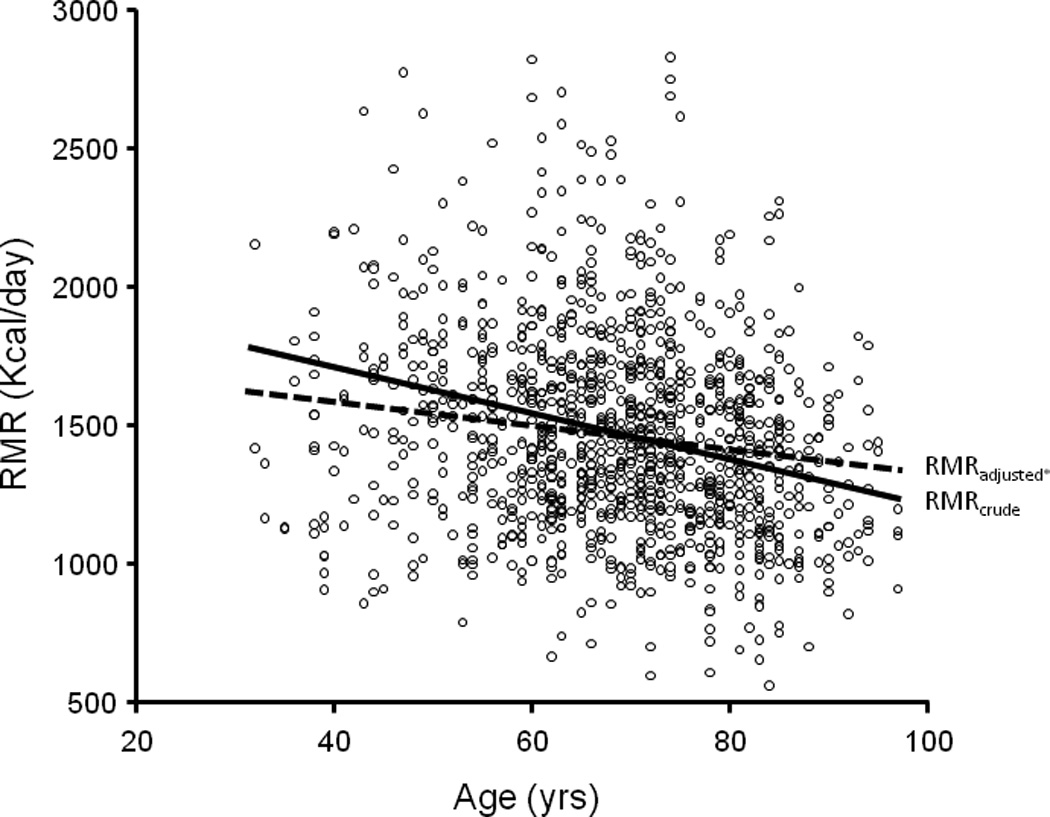
Average changes in RMR with aging. The dashed line in adjusted for sex, lean body mass and fat mass. From cross-sectional data collected in the Baltimore Longitudinal Study of Aging (N=1146)
The first and most logical consideration is that RMR cannot be interpreted by itself. Deciding whether RMR is normal, too high or too low requires a comparison with some “normative value”. Beyond any health difference, variability in RMR is explained by differences in body size, body composition, age and possibly level of physical activity. The Mifflin-St Jeor equation, commonly used to estimate RMR, uses data on sex, age, weight and height to estimate a “reference” RMR. However, the validity of this equation has not been previously tested in the oldest and frailest portion of the population. Weiss and colleagues determined the fit for this equation was poor, especially in the frailest women. This may be due to the lack of consideration for drastic differences in body composition, specifically very low (or poor quality) lean body mass and high levels of fat mass. Even though this profile is inextricably linked with aging, the ratio of lean to fat is quite heterogeneous across individuals and strongly affected by health status. Although Weiss and coll. considered lean body mass in their analysis, this information was acquired using bioelectrical impedance, a technique with limited validity and precision that has not been extensively validated in very old women. Alternatively, when ratios between parenchymatous organs, muscle and fat are dramatically altered, as in the oldest and frailest individuals, the keys to calculating “expected” RMR may change substantially and accounting for lean body mass may not be enough.
Another possibility recently highlighted in the frailty literature notes that older persons often become frail through weight loss that is only partially reflected by malnutrition. With the expansion of the obesity epidemic to the older population, more and more individuals come to be frail through sarcopenic obesity. As brought to light by Weiss et al., connecting the changes in RMR that characterize these two separate pathways to frailty in a unifying hypothesis presents a substantial challenge. In individuals who transition to frailty through “weight loss”, the consideration of fat mass may be irrelevant and hypermetabolism may dominate the clinical picture. In sarcopenic obesity, evaluation of true lean body mass may be problematic due to lipid infiltration of lean tissue and almost unavoidably a substantial part of RMR would result from fat tissue metabolism. It is possible that in in these individuals, we may overestimate the expected energy metabolism, and consequently completely miss the “homeostatic effort”.
Measuring RMR may help assess the overall burden of diseases status, across different chronic conditions and aging phenotypes. Accordingly, excess RMR may be a quantitative measure of the “homeostatic effort” required to cope with a precarious homeostatic equilibrium caused by the synergy of poor health status and accelerated aging. In addition, in non-healthy older persons, the combination of low fitness and high RMR may critically reduce the amount of energy available for other activities and contribute directly and indirectly (through the long term effects of inactivity) to impaired physical function.
The potential for translation of these concepts to clinical practice is enormous and very exciting. RMR has the potential to become the quantitative measure of frailty the field of aging research has been pursuing for decades. For example, in geriatric patients, the traditional approach of clinical evaluation based on signs, symptoms and biomarkers is often not very useful and functional impairments may be detected only at a late stage. Conversely, RMR could be used to assess risk of health deterioration at a very early stage to select the most effective therapeutic strategy within the complexity of comorbidity, and to track the effectiveness of interventions.
Addressing the critical issues outlined in this editorial is tantamount to the translation of RMR to clinical practice. First of all, recognizing the multiple possible sources of measurement interference and bias, criteria for quality assurance of RMR should be established. State-of-the-art measures of RMR, body composition, health status and physical activity should be collected in longitudinal cohorts that include large numbers of both very old frail and healthy participants, and used to derive reference equations. The hypothesis should be tested that those individuals with “inappropriately high” RMR and those who have no decline in RMR with age, tend to be sicker, show more evident aging phenotypes and are more likely to experience adverse health outcomes, such as frailty and death. The provocative data reported by Weiss and colleagues position energy metabolism again at the central focus of aging research and define a rich research agenda aimed with exciting possibilities of translation to the clinic.
Rethinking how “energy for life” changes with aging may be a very fruitful investment. In the end, the flow of energy that actively maintains our “operative system” is a pre-requisite to any physiological function and as such has probably been prioritized by evolution. In Rubner’s words: “Mute and still, by night and by day, labor goes on in the workshops of life” 7.
Acknowledgments
Funding Sources:
This research was supported by the Intramural Research Program of the NIH, National Institute on Aging.
Data for these analyses were obtained from the Baltimore Longitudinal Study of Aging, a study performed by the National Institute on Aging.
References
- 1.Austad SN, Fischer KE. Mammalian aging, metabolism, and ecology: Evidence from the bats and marsupials. Journals of Gerontology. 1991;46(2):B47–B53. doi: 10.1093/geronj/46.2.b47. [DOI] [PubMed] [Google Scholar]
- 2.Speakman JR. Body size, energy metabolism and lifespan. J Exp Biol. 2005;208(9):1717–1730. doi: 10.1242/jeb.01556. [DOI] [PubMed] [Google Scholar]
- 3.Ruggiero C, Metter EJ, Melenovsky V, Cherubini A, Najjar SS, Ble A, Senin U, Longo DL, Ferrucci L. High basal metabolic rate is a risk factor for mortality: The baltimore longitudinal study of aging. J Gerontol A Biol Med Sci 2008 July 1. 2008;63(7):698–706. doi: 10.1093/gerona/63.7.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sergi G, Coin A, Marin S, Vianello A, Manzan A, Peruzza S, Inelmen EM, Busetto L, Mulone S, Enzi G. Body composition and resting energy expenditure in elderly male patients with chronic obstructive pulmonary disease. Respiratory Medicine. 2006 Nov;(11):1918. doi: 10.1016/j.rmed.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Yee-Moon Wang A, Man-Mei Sea M, Tang N, Sanderson JE, Lui S, Kam-Tao Li P, Woo J. Resting energy expenditure and subsequent mortality risk in peritoneal dialysis patients. Journal of the American Society of Nephrology. 2004 Dec 01;15(12):3134–3143. doi: 10.1097/01.ASN.0000144206.29951.B2. [DOI] [PubMed] [Google Scholar]
- 6.Jumpertz R, Hanson RL, Sievers ML, Bennett PH, Nelson RG, Krakoff J. Higher energy expenditure in humans predicts natural mortality. J Clin Endocrinol Metab. 2011 Mar 30; doi: 10.1210/jc.2010-2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rubner M. Machinery of metabolism. JAMA: The Journal of the American Medical Association. 1916;66(24):1879. [Google Scholar]


