SUMMARY
Iron regulatory proteins 1 and 2 (Irps) post-transcriptionally control the expression of transcripts that contain iron responsive element (IRE) sequences, including ferritin, ferroportin, transferrin receptor and hypoxia inducible factor 2α (HIF2α). We report here that mice with targeted deletion of Irp1 developed pulmonary hypertension and polycythemia that was exacerbated by a low iron diet. Hematocrits increased to 65% in iron-starved mice, and many polycythemic mice died of abdominal hemorrhages. Irp1 deletion enhanced HIF2α protein expression in kidneys of Irp1−/− mice, which led to increased erythropoietin (EPO) expression, polycythemia and concomitant tissue iron deficiency. Increased HIF2α expression in pulmonary endothelial cells induced high expression of endothelin-1, likely contributing to the pulmonary hypertension of Irp1−/− mice. Our results reveal why anemia is an early physiological consequence of iron deficiency, highlight the physiological significance of Irp1 in regulating erythropoiesis and iron distribution, and provide important insights into the molecular pathogenesis of pulmonary hypertension.
INTRODUCTION
Iron is an essential element to virtually all organisms, as it is a crucial component of heme and iron sulfur proteins, and is indispensable for DNA replication, oxygen transport and ATP production. Iron deficiency usually causes anemia, whereas iron overload can generate reactive oxygen species and damage lipids, DNA and proteins in diseases such as hemochromatosis, cancer, cardiovascular and neurodegenerative diseases (reviewed in (Hentze et al., 2010; Rouault, 2006)). Therefore iron homeostasis must be precisely regulated.
Cellular iron homeostasis is mainly controlled by the iron regulatory proteins (Irp)/iron responsive elements (IRE) machinery in mammalian cells. Iron regulatory proteins regulate the expression of target proteins post-transcriptionally by binding to IREs in transcripts that mostly encode iron metabolism proteins, including the iron storage proteins, H- and L-ferritin, iron uptake proteins, transferrin receptor 1 (Tfrc), divalent metal transporter 1 (Dmt1), the iron export protein, ferroportin, and several other transcripts. By inhibiting the translation of proteins involved in iron export, storage and utilization, and stabilizing the transcripts of proteins involved in iron uptake, the Irp/IRE machinery regulates cellular iron homeostasis in response to intracellular iron status (Hentze et al., 2010; Rouault, 2006). Though the two Irps, Irp1 and Irp2, share high sequence similarity, they are regulated by different mechanisms. Irp1 is a bifunctional protein that binds to IREs in iron-deficient conditions when it is an apoprotein, but it converts to cytosolic aconitase in iron-replete conditions upon acquisition of a [4Fe-4S] cluster in the active site cleft that separates domains 1–3 from domain 4 (Dupuy et al., 2006; Walden et al., 2006). Irp2 is active as an IRE-binding protein in iron deficient cells, because it is otherwise rapidly degraded in iron-replete conditions as a result of FBXL5-mediated ubiquitination and subsequent proteasomal degradation (Salahudeen et al., 2009; Vashisht et al., 2009). Previously, we and others reported that mice with a targeted deletion of Irp2 (Irp2−/−) developed adult-onset neurodegeneration and anemia (Cooperman et al., 2005; Galy et al., 2005; LaVaute et al., 2001) as the tissues and cells that were adversely affected were largely dependent on Irp2 for regulation, and residual Irp1 did not appear to compensate for the loss of Irp2 in affected cells, whereas Irp2 could compensate for loss of Irp1 in many cells (Meyron-Holtz et al., 2004a). Complete loss of Irp1 and Irp2 causes early embryonic lethality, implying that Irp1 and Irp2 have redundant functions (Smith et al., 2006). However mice with a targeted deletion of Irp1 (Irp1−/− mice) did not display an easily recognizable phenotype when maintained on a normal iron diet, which raised questions about the physiological role of Irp1 in iron homeostasis.
One of the more recently identified targets of the Irp/IRE system is HIF2α (also known as Endothelial PAS Domain protein 1, EPAS1), a transcription factor that belongs to the hypoxia-inducible factor (HIF) alpha protein family. In response to hypoxia, anemia or iron deficiency, HIF2α heterodimerizes with HIF1β and translocates into the nucleus to regulate the expression of genes involved in the adaptive response to hypoxia, including erythropoietin (EPO) (Semenza, 2012). Under normoxia, prolyl hydroxylase domain protein 2 (PHD2) site-specifically hydroxylates HIF2α, thereby targeting the latter for ubiquitination by the von Hippel–Lindau (VHL) E3 ligase complex and proteasomal degradation (Kaelin and Ratcliffe, 2008). Evidence is growing that HIF2α is the master regulator for erythropoiesis in the adaptive response to hypoxia, and mutations of the proteins in this pathway including HIF2α, PHD2 and VHL, are implicated in dysregulation of erythropoiesis (Majmundar et al., 2010). Recently it was found that HIF2α contains an IRE in its 5′UTR, and Irps could bind and inhibit the translation of HIF2α mRNA in vitro, suggesting that iron homeostasis and erythropoiesis could be potentially linked by HIF2α (Sanchez et al., 2007; Zimmer et al., 2008). However, the relevance of the IRE in the 5′UTR of the HIF2α transcript in mammalian physiology has not been established.
In this study, to investigate the physiological significance of Irp1 in iron metabolism, we challenged Irp1−/− mice with iron deficiency, and we found that deletion of Irp1 increased HIF2α expression, which in turn stimulated EPO and endothelin-1 expression, leading to polycythemia, pulmonary hypertension and early sudden death from hemorrhage.
RESULTS
Irp1−/− mice on low iron diet die early of abdominal hemorrhages
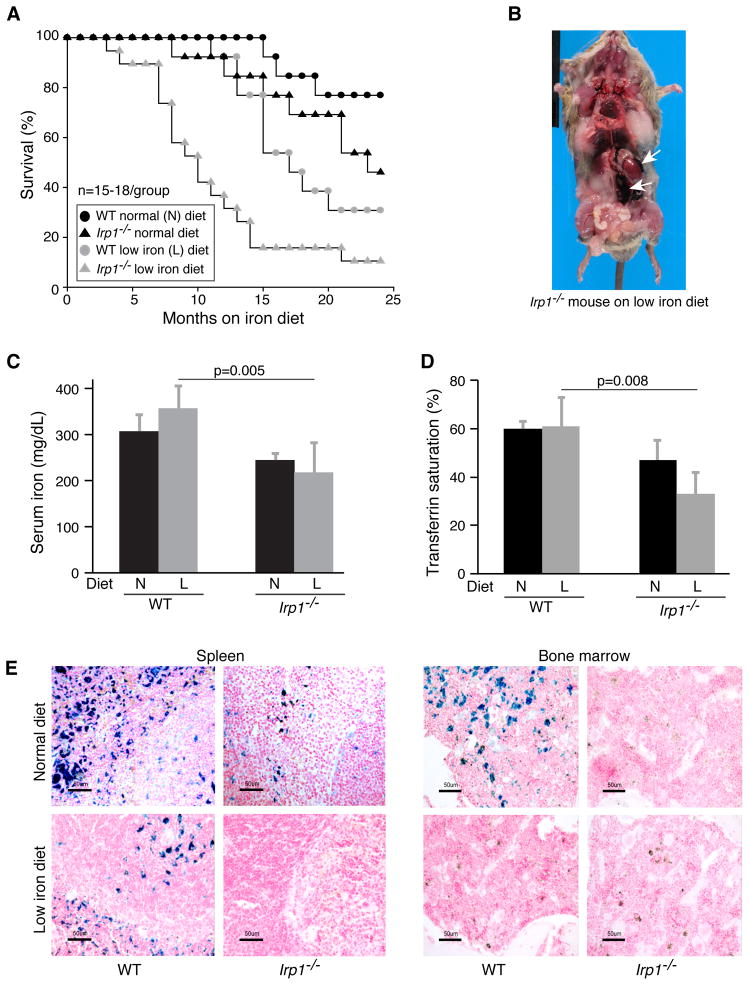
To challenge the mice with iron deficiency, we maintained the wild type (WT) and Irp1−/− mice (15–18 per group) on a low iron diet for up to 24 months. We found that the Irp1−/− mice on the low iron diet were prone to sudden death when they were as young as three months of age (Figures 1A, S1A), and 50% of these mice had died by the age of 10 months. The autopsies revealed gross abdominal hemorrhages as the presumed cause of death for almost all of the Irp1−/− mice that died while on the low iron diet (Figure 1B). To further characterize the causes of sudden death, we evaluated the iron status and tissue pathology of Irp1−/− mice. Compared with their WT counterparts, Irp1−/− mice had low serum iron levels (Figure 1C), lower transferrin saturations (Figure 1D), and low stainable non-heme iron in tissues such as spleen and bone marrow (Figures 1E, S1B), suggestive of systemic iron deficiency. However, low dietary iron uptake was not the cause of iron deficiency in Irp1−/− mice, as the intestinal iron uptake machinery was functional and responsive (Figures S1C–E).
Figure 1.
Reduced life expectancy of Irp1−/− mice is exacerbated by iron deficiency. (A) Survival curves for mice with indicated genotypes and diets. (B) Image of a 3 month old low iron diet Irp1−/− mouse that died from abdominal hemorrhage in the perinephric area (white arrows) (C) Serum iron and (D) Transferrin saturation in one-year old normal diet (N) or low iron diet (L) mice (n = 5 per group) showing iron deficiency in low iron diet Irp1−/− mice. (E) Representative Prussian blue staining of spleen and bone marrow of WT and Irp1−/− mice maintained on normal (N) or low iron (L) diets showing low ferric non-heme iron stores in Irp1−/− mice (n = 4–5 per group). Data are represented as mean±SD. See also Figure S1.
Irp1−/− mice develop polycythemia with extramedullary erythropoiesis
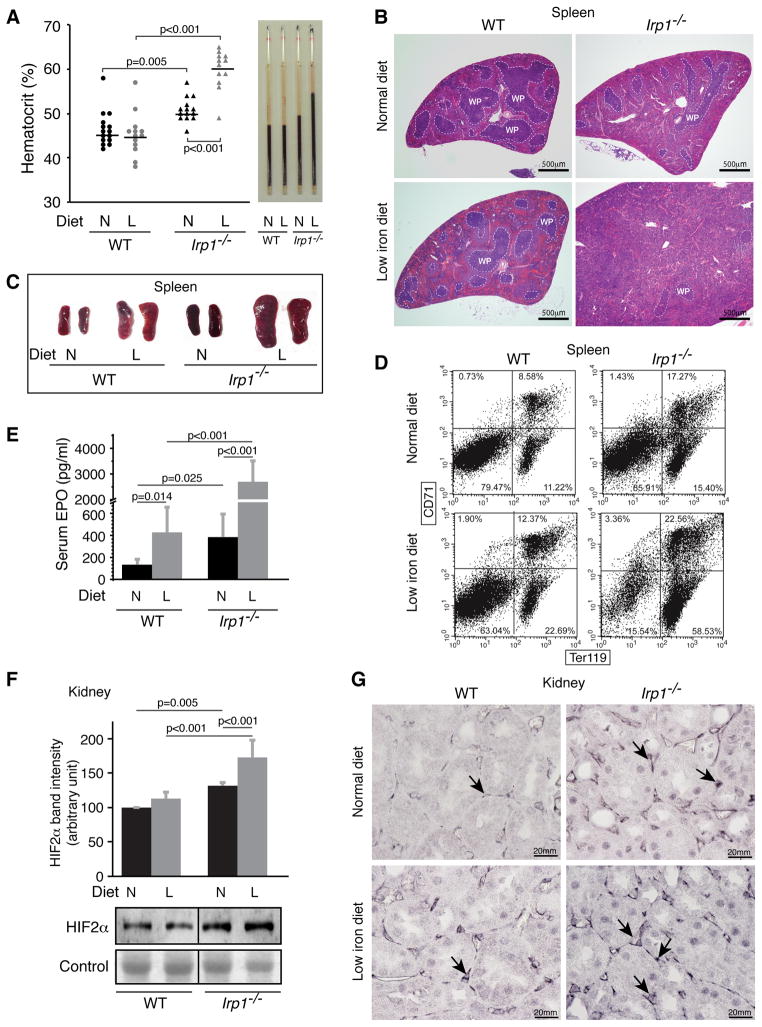
To determine whether abnormal erythropoiesis was responsible for the abdominal hemorrhages that caused early death, we evaluated complete blood counts of Irp1−/− mice. Strikingly, hematocrits of Irp1−/− animals were elevated on the normal diet, and increased further to as high as 65% in Irp1−/− mice on the low iron diet (Figure 2A). The hematocrits were also increased in young Irp1−/− animals (Figure S2A). RBC and hemoglobin levels were higher in Irp1−/− mice, whereas MCV and MCH levels were lower, consistent with systemic iron deficiency in IRP1−/− mice (Table S1). Extramedullary erythropoiesis increased in Irp1−/− mice, especially in the mice maintained on a low iron diet, as evidenced by expansion of splenic red pulp (Figure 2B), gross splenomegaly (Figures 2C, S2B), and increased percentages of Ter119-positive immature erythroid cells as assessed by fluorescence activated cell sorting (FACS) analysis (Figure 2D). We did not see evidence for an expansion of hematopoiesis in bone marrow cross-sections of Irp1−/− mice (Figure S2D). Though the spleen showed less ferric non-heme iron in histologic stains (Figure 1E), total splenic iron measured by ICP-MS was higher in Irp1−/− mice on the low iron diet (Figure S2C), suggesting that splenic iron was mainly incorporated into heme. Thus, increased diversion of iron for expanded erythropoiesis likely caused systemic iron deficiency, as evidenced by low serum transferrin saturations and low tissue iron stains (Figures 1C–E).
Figure 2.
Irp1−/− mice have polycythemia and enhanced extramedullary hematopoiesis, high serum EPO and elevated renal HIF2α expression. (A) Hematocrits of one-year old animals as determined by capillary tube centrifugation were significantly higher in Irp1−/− mice on both normal and low iron diets. (B) Representative H&E staining of spleens from WT and Irp1−/− mice on normal (N) or low iron (L) diets showed increased red pulp and diminished white pulp (WP) in Irp1−/− mice. (C) Spleens of one-year old mice of iron-starved Irp1−/− mice were significantly larger than WT mice demonstrating splenomegaly in Irp1−/− animals maintained on the low iron diet. (D) Representative flow cytometric analyses of splenic cell populations from WT and Irp1−/− mice separated with antibodies to CD71 and Ter119 showed increase of Ter119+ erythroid precursors in Irp1−/− mice. (E) Serum EPO levels measured by ELISA were increased in Irp1−/− mice, particularly in those on the low iron diet (n = 5–7 per group). (F) Immunoblot analysis of nuclear fractions from kidney lysates (n = 5 per group) and (G) renal immunohistochemistry showed increased HIF2α protein expression in Irp1−/− mice that increased on the low iron diets. Arrows point to interstitial fibroblasts in which intense dark purple stain indicates increased HIF2α expression. Data are represented as mean±SD. See also Figure S2.
To determine the cause of polycythemia in Irp1−/− mice, we analyzed levels of EPO and observed that both serum EPO (Figure 2E) and EPO mRNA levels in kidney (Figure S2E) were significantly increased in Irp1−/− mice, especially in mice on the low iron diet. Because EPO is a known transcriptional target of HIF2α (Semenza, 2012), we assessed the HIF2α protein levels in renal lysates of WT and Irp1−/− animals (Figure 2F), and observed that HIF2α protein levels were increased in the EPO-producing interstitial renal fibroblasts (Pan et al., 2011) of Irp1−/− mice, particularly in the mice on low iron diet (Figures 2G, S2F). Since the HIF2α transcript is a known Irp1 target (Sanchez et al., 2007; Zimmer et al., 2008), and HIF2α mRNA levels remained unchanged (Figure S2G), we hypothesized that loss of Irp1 allowed unrepressed translation of HIF2α in interstitial fibroblasts, which activated expression of HIF2α target genes including EPO, and thereby increased red blood cell production, causing polycythemia (Figure 2A) and redistribution of iron from tissues to developing erythroid cells (Figure 1E).
Irp1−/− mice develop pulmonary hypertension
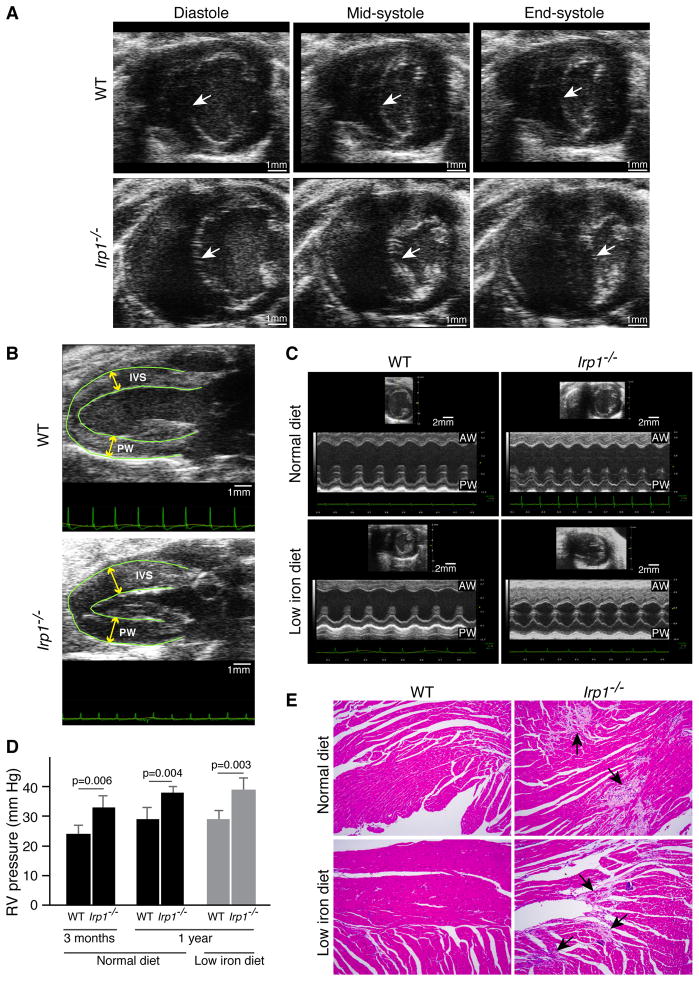
Since high hematocrits stress cardiovascular function, we evaluated the potential contribution of cardiac disease to the phenotype of sudden death with cardiac ultrasound. Abnormal motion of the interventricular wall during systole in Irp1−/− animals suggested that there was pulmonary hypertension (Figure 3A, Supplementary Video). There was also hypertrophy of the cardiac walls (Figures 3B,C). Systolic ejection fractions did not change in Irp1−/− mice, indicating normal cardiac contractility (Figure S3A). Catheterization of the right ventricle revealed that pulmonary pressures were significantly elevated in Irp1−/− animals at both 3 and 12 months of age (Figure 3D), but did not increase further in Irp1−/− mice on low iron diet, in contrast to the polycythemic response. At autopsy, areas of fibrosis and cardiomyocyte degeneration were present throughout the heart (Figures 3E, S3E). However, discernible changes in the pulmonary vasculature (Figures S3B,C) and thromboses were rarely observed (data not shown). Notably, the low iron diet did not induce pulmonary hypertension or cardiac hypertrophy of the WT mice. Moreover, the resistance and tissue damping of Irp1−/− lung measured by FlexiVent were significantly lower (Figure S3D), and the oxygen and carbon dioxide tensions in arterial blood of IRP1−/− were normal (results not shown), suggesting that the pulmonary functions of IRP1−/− mice are within the normal range.
Figure 3.
Irp1−/− mice developed pulmonary hypertension and cardiac fibrosis. (A) Representative cardiac ultrasound images showed abnormal motion of the interventricular septum (IVS) (arrows) during the cardiac cycle. The IVS flattened and was pushed inward into the left ventricle (LV) chamber at the end of systole giving the LV a D-shaped appearance in the low iron diet Irp1−/− mice, suggesting that right ventricular (RV) pressure was high, whereas the IVS maintained a normal concave shape throughout systole in the WT mice on low iron diets (n = 5 per group). (B) In representative parasternal long axis views of the left ventricle, the IVS and posterior walls (PW) of the left ventricle were hypertrophied in the iron-starved Irp1−/− mice compared to the iron-starved wild type mice (n = 5 per group). (C) Representative parasternal short axis M-mode of the left ventricle demonstrated significant hypertrophy of the anterior (AW) and posterior walls (PW) in Irp1−/− mice on low iron diets (n = 5 per group) (D) Right ventricular (RV) pressure measured by catheterization of lightly anesthetized 3 months and one year old WT and Irp1−/− mice showed significantly elevated pressures in Irp1−/− mice. Data are represented as mean ± SD (n = 3 per group). (E) Representative H&E staining of hearts from WT and Irp1−/− mice on normal and low iron diet showed extensive fibrosis in Irp1−/− mice, though cardiac function was not compromised (see text). See also Figure S3, Video.
Pulmonary hypertension in Irp1−/− mice was associated with high expression of HIF2α in pulmonary endothelial cells and transcriptional activation of Endothelin-1
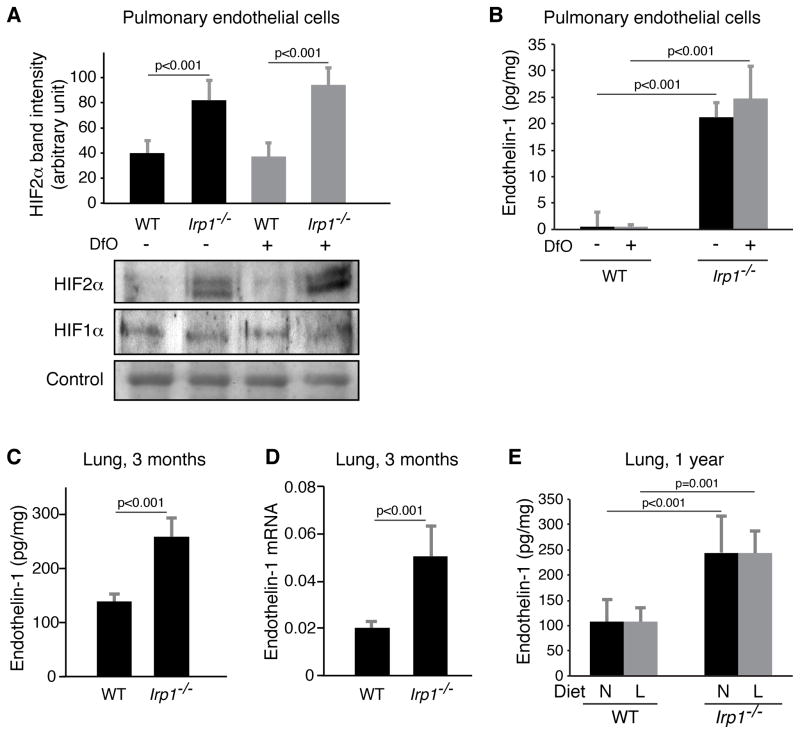
As abnormally high HIF2α expression has been implicated in the pathogenesis of polycythemia (Formenti et al., 2010) and pulmonary hypertension (Hickey et al., 2010), we evaluated the expression of HIF2α in the pulmonary vascular system. Although immunohistochemistry and immunoblots of total lung lysates did not reveal reproducible increases in HIF2α protein levels (data not shown), we found increased HIF2α expression in specific cells of other tissues such as kidney (Figure 2G) and spleen (Figure S4A). Moreover, in primary pulmonary endothelial cells isolated from WT and Irp1−/− animals, and cultured at low oxygen tensions, HIF2α protein levels increased significantly in Irp1−/− cells (Figure 4A) cultured under both normal and low iron conditions, whereas mRNA levels did not increase (Figure S4B). Endothelin-1, a HIF target and potent vasoconstrictor, has been implicated in the pathogenesis of pulmonary hypertension, tissue hypertrophy and fibrosis (Shao et al., 2011). We found that Endothelin-1 expression significantly increased in Irp1−/− pulmonary endothelial cell lysates and cell media (Figures 4B, S4C), but did not increase upon treatment with the iron chelator, desferrioxamine (Dfo). Endothelin-1 also increased in serum (Figure S4D) and lung tissue of Irp1−/− mice at 3 months of age, as determined by ELISA (Figure 4C) and mRNA quantification (Figure 4D), and also at 12 months of age (Figure S4E). As observed in primary pulmonary endothelial cells (Figure 4B), endothelin-1 expression did not increase in the lung tissue lysates of low iron diet Irp1−/− animals (Figure 4E) possibly explaining why pulmonary hypertension was not exacerbated by the low iron diet. Expression of other known HIF targets also increased (Figures S4F–I), including Aldoa, a key glycolytic enzyme, Cxcl12, a chemokine involved in inflammation, Bnip3, a pro-apoptotic factor, and Retnla, also known as Himf (hypoxia-induced mitogenic factor), a member of the resistin family proposed to drive vascular remodeling and pulmonary hypertension in a mouse model of hypoxia-driven pulmonary hypertension (Hickey et al., 2010). In contrast, HIF1α protein levels did not change in Irp1−/− pulmonary endothelial cells (Figure 4A), indicating that the major prolyl hydroxylase-dependent HIF degradation pathway was not altered in Irp1−/− endothelial cells (Kaelin and Ratcliffe, 2008). Therefore, changes in HIF2α expression occurred in the absence of transcriptional changes or changes in degradation, implying that the loss of translational repressor activity caused by loss of Irp1 was the major factor that drove increased HIF2α expression.
Figure 4.
Pulmonary hypertension in Irp1−/− mice was associated with high expression of HIF2α in pulmonary endothelial cells and transcriptional activation of multiple targets (n = 3 per group). (A) HIF2α protein expression increased in the nuclear fractions of Irp1−/− pulmonary endothelial cells relative to WT pulmonary endothelial cells isolated from mice, cultured at 1% oxygen and treated (for 15 hours) with or without the iron chelator, desferrioxamine (Dfo, 100 μM), whereas HIF1α protein levels remained unchanged. (B) Endothelin-1 levels (measured by ELISA) in the lysates of pulmonary endothelial cells isolated from WT and Irp1−/− mice and cultured at 1% oxygen (n = 6 per group). (C) Endothelin-1 levels in lung lysates of 3 months old normal diet mice measured by ELISA. (n = 5–6 per group) (D) Endothelin-1 mRNA levels in lungs of 3 months old normal diet mice. (E) Endothelin-1 levels in lung lysates of one-year old normal and low-iron diet mice measured by ELISA (n = 5 per group). Data are represented as mean ± SD. See also Figure S4.
Differences in tissue-specific expression levels of Irp1 and Irp2 explain why Irp1−/− mice develop polycythemia and pulmonary hypertension, whereas Irp2−/− mice develop neurodegeneration
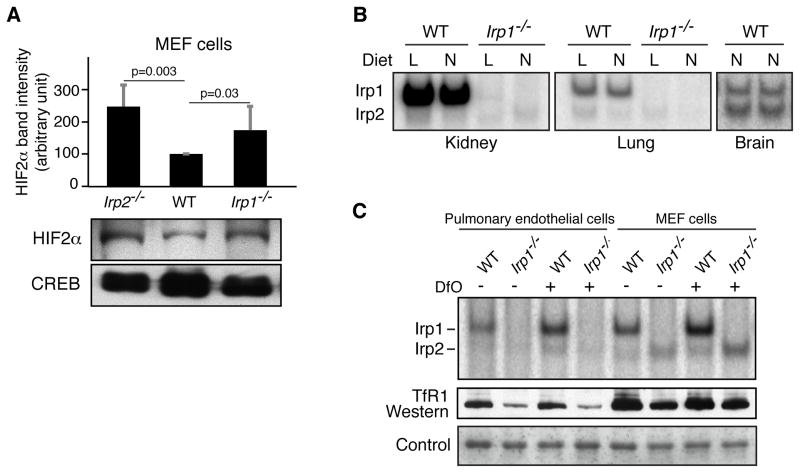
The two major phenotypic features of Irp1−/− animals reported here, namely polycythemia exacerbated by iron deficiency and associated with abdominal hemorrhage, and pulmonary hypertension associated with cardiac fibrosis, occurred respectively in two tissues, kidney and lung, that contained cells in which HIF2α expression increased markedly as a result of loss of Irp1 translational repressor activity. Notably, these phenotypes were not observed in Irp2−/− animals (data not shown). To explore why Irp2−/− mice lacked these phenotypes, we tested whether either Irp1 or Irp2 contributed to regulation of HIF2α expression in Irp2−/− or Irp1−/− mouse embryonic fibroblast (MEF) cells (Figure 5A), and we concluded from our results that both Irp1 and Irp2 can contribute to regulation of HIF2α expression. Then we compared relative contributions of Irp1 and Irp2 to total IRE-binding activity in several different tissues and cell lines. Notably, there was very little contribution from Irp2 in kidney or lung tissue (Figure 5B), whereas there was considerable contribution from Irp2 in brain, as previously reported (Meyron-Holtz et al., 2004a; Meyron-Holtz et al., 2004b). In Irp1−/− pulmonary endothelial cells, there was almost no detectable IRE binding activity (Figure 5C), even when cells were iron-deprived, whereas Irp2 binding activity was readily detected in Irp1−/− embryonic fibroblasts (MEFs). These findings indicated that pulmonary endothelial cells depend almost exclusively on Irp1 to regulate the translation or stability of IRE-containing transcripts.
Figure 5.
Irp1 is the major contributor of IRE-binding activity in kidney and lung tissues and in pulmonary endothelial cells. (A) HIF2α western with nuclear fractions of Irp2−/−, WT and Irp1−/− MEF cells showed that HIF2α expression increased equally upon loss of either Irp1 or Irp2. Data (n = 5 per group) are represented as mean ± SD. (B) IRE-binding activity assays of kidney, lung and forebrain lysates of normal (N) and low iron (L) diet WT and Irp1−/− mice showed that most of the IRE-binding activity in kidney and lung tissues was contributed by Irp1, whereas Irp1 was not the major contributor of IRE-binding activity in brain. (C) IRE binding activity and TfR1 western blots of WT and Irp1−/− mouse pulmonary endothelial cell lysates and MEF cell lysates in the absence and presence of 100 μM Dfo showed that there was very little contribution from Irp2 to the total IRE-binding activity in pulmonary endothelial cells both at normal and low iron conditions (left lanes), whereas Irp2 was readily detectable in MEFs (right lanes).
DISCUSSION
The polycythemia and systemic iron deficiency that developed in Irp1−/− mice reveal that Irp1 plays a pivotal role in balancing erythropoiesis and systemic iron homeostasis. As the master regulator of erythropoietin expression, HIF2α was previously known to be regulated by hypoxia, anemia and iron status through PHD2/VHL mediated degradation (Bond et al., 2011; Gale et al., 2008; Smith et al., 2008). Here, we established that Irp1-mediated translational control plays a previously unrecognized critical role in regulation of HIF2α expression, and loss of Irp1 activity has serious physiological consequences.
Under hypoxic, anemic and iron-deficient conditions, HIF2α protein stabilization induces EPO expression, which increases production of red blood cells (RBC) in a process that consumes large amounts of iron. When erythropoiesis consumes enough iron to cause non-erythroid tissue iron deficiency, synthesis of the iron sulfur cluster of Irp1 is decreased, and Irp1 converts to the apoprotein form that binds to IREs. Apo-Irp1 represses HIF2α translation, decreases erythropoietin production, and thereby diminishes RBC production (Figure 6). Through this Irp1/HIF2α–dependent feedback mechanism, the body balances erythropoietic activity and systemic iron availability to ensure that high RBC production in iron-deficient animals does not deplete iron stores in tissues such as the heart, brain and kidneys and compromise crucial cellular functions such as mitochondrial respiration. In Irp1−/− mice, de-repression of HIF2α translation increases HIF2α protein levels, which stimulates EPO expression, which in turn drives red cell production and concomitant systemic iron deficiency. Moreover, iron deficiency can further stabilize HIF2α protein levels by diminishing activity of the iron-dependent prolyl hydroxylases involved in its degradation and can thereby exacerbate the polycythemia further (Kaelin and Ratcliffe, 2008; Percy et al., 2006). An increase in the total red cell volume can increase the blood viscosity and resistance in vascular tissues, and can thereby endanger the cardiovascular system. As shown in the model in Figure 6, we propose that the extremely high hematocrits of Irp1−/− mice on a low iron diet contribute to sudden death from abdominal hemorrhages, perhaps because the high viscosity leads to localized blood pressure increases. Thus, Irp1−/− mice represent a unique model in which iron deficiency exacerbates a mild polycythemia rather than causing anemia, and in which iron supplementation partially reverses the polycythemia. Irp1−/− mice reveal that the anemia of iron deficiency normally results from a restriction of EPO production mediated by renal Irp1, which represses HIF2α translation and EPO expression when the kidney is iron-deficient.
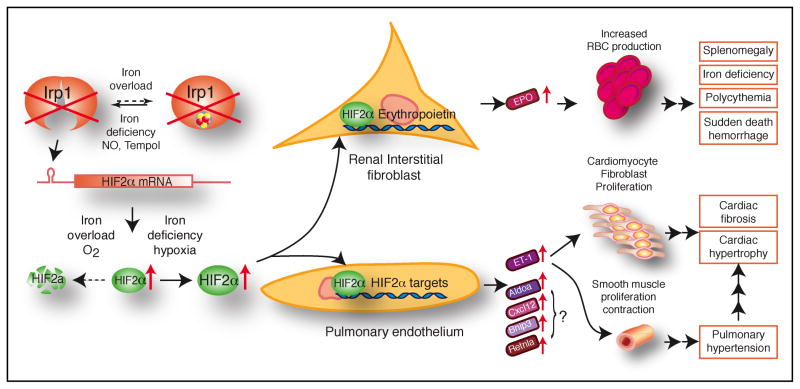
Figure 6.
Model for the molecular pathogenesis of polycythemia and pulmonary hypertension in Irp1−/− mice. In renal interstitial fibroblasts and pulmonary endothelial cells, where IRP1 is the predominant IRE-binding protein, IRP1 deficiency results in increased HIF2α expression through de-repression of HIF2α translation. In renal interstitial fibroblasts, HIF2α induces high expression of EPO, which promotes increased RBC production and consumption of high amounts of iron, thereby causing iron deficiency in non-erythroid tissues. Importantly, the tissue iron deficiency can stabilize HIF2α protein further and exacerbate the polycythemia. The systemic iron deficiency and high hematocrits probably stress the cardiovascular system and lead to sudden death from hemorrhages. In pulmonary endothelial cells, elevated expression of HIF2α leads to increased endothelin-1 (ET-1) expression. By binding to the endothelin-1 receptor type A (ETAR), ET-1 induces the proliferation and contraction of vascular smooth muscle cells, resulting in pulmonary hypertension. ET-1 also induces cardiac fibrosis and hypertrophy when it binds to ETAR. HIF2α also drives increased expression of other HIFα targets, including Aldoa, Cxcl12, Bnip3 and Retnla, and their potential contributions to pulmonary hypertension remain to be investigated in Irp1−/− mice.
Irp1−/− mice develop pulmonary hypertension, and pulmonary endothelial cells represent another tissue in which Irp1 appears to play a dominant role. Since the pulmonary endothelial cells represent a small fraction of the total population of cells in the lung tissue and they form a thin cell layer covering the vascular lumen, we did not consistently detect increased HIF2α expression in western blots or immunohistochemistry. However, we cultured primary pulmonary endothelial cells and found that HIF2α was significantly increased in primary pulmonary endothelial cells from Irp1−/− mice. Increased HIF2α expression was also confirmed by the expression of some well-known HIFα targets including the potent vasoconstrictor, endothelin-1. Endothelin-1 expression is significantly increased in Irp1−/− mice and likely plays a major role in the pathogenesis of pulmonary hypertension. Endothelin-1 is known to bind to endothelin-1 receptor type A (ETAR) on smooth muscle cells in the vascular wall and to induce the contraction of blood vessels (Shao et al., 2011), leading to increased pulmonary hypertension, as we observed. Endothelin-1 also binds to ETAR on cardiomyocytes and fibroblasts and induces their proliferation, leading to cardiac hypertrophy and fibrosis (Thorin and Clozel, 2010). The pulmonary hypertension may also contribute to the cardiac hypertrophy by increasing the workload of the heart as it works against high resistance in the pulmonary vasculature. The spontaneous pulmonary hypertension of Irp1−/− mice warrants further investigation.
An important role for HIF2α in development of polycythemia and pulmonary hypertension was recently reported in a mouse model of Chuvash polycythemia (Hickey et al., 2007; Hickey et al., 2010). In Chuvash polycythemia patients, the VHLR200W mutation leads to highly expressed HIF2α protein, which stimulates EPO production and polycythemia, and also promotes increased endothelin-1 expression and pulmonary hypertension in the lungs (Sable et al., 2012). In a Chuvash mouse model carrying the identical mutation as human patients, mice were protected from developing pulmonary hypertension by heterozygous deletion of HIF2α, whereas HIF1α deletion did not prevent pulmonary hypertension and vascular remodeling, supporting that HIF2α was pivotal in the pathophysiology of polycythemia and pulmonary hypertension in the mouse model, and by inference, in the Chuvash patients. As in Irp1−/− mice, Chuvash patients also show decreased serum iron levels and transferrin saturation, supporting that iron redistributes into RBC production. The HIF2α heterozygous mice were also previously shown to have resistance to pulmonary hypertension caused by chronic hypoxia, supporting the essential role of HIF2α in this process (Brusselmans et al., 2003). Moreover, the HIF2α locus was recently implicated as being important in successful adaptation to chronic hypoxia at high altitude (Bigham et al., 2010; Yi et al., 2010). Irp1−/− mice confirm the central role of HIF2α in regulating erythropoiesis and pulmonary hypertension, emphasize the importance of Irp1 in controlling HIF2α expression, and also suggest a potential treatment for HIF2α related diseases including Chuvash polycythemia. Pharmaceutical activation of the IRE binding activity of Irp1 by chemicals such as Tempol (Ghosh et al., 2008) or nitric oxide could potentially repress HIF2α translation and alleviate the polycythemia and pulmonary hypertension in patients with problems in the Irp/HIF2α axis.
In conclusion, our results in Irp1−/− mice show that Irp1 plays an important function in regulating erythropoiesis and maintaining balance with systemic iron status, and the regulation of Irp1 on HIF2α translation represents an important aspect of fine-tuning of HIF2α expression in physiology. Since human genomes were recently shown to contain at least 20 loss-of-function mutations per genome (MacArthur et al., 2012), and Irp1−/− mice survive to adulthood, we suggest that loss of function mutations of Irp1 may underlie previously unexplained pulmonary hypertension and/or polycythemia in some patients.
EXPERIMENTAL PROCEDURES
Mice
Irp1−/− animals were generated by targeting Aco1 (Irp1), propagated by breeding, and genotyped by Southern blotting using gene specific probes as previously described (Meyron-Holtz et al., 2004a). WT and Irp1−/− mice used in this study have a mixed genetic background consisting of 129S4/SvJae and C57Bl/6. The mice were weaned 3–4 weeks after their birth. Immediately after weaning, mice were maintained on a diet either containing 3.7 ± 0.9 (measured by ICP-MS) mg iron/kg chow (low-iron diet) from Harlan Teklad or normal diet containing 143 ± 18 mg iron/kg (measured by ICP-MS) chow (normal diet). Experiments with blood and tissues were performed with one year old mice unless otherwise noted. Whenever possible, siblings were used in matched study sets to minimize phenotypic variation due to genetic background. Mice of different genotypes and diets were age- and gender matched for statistical comparisons. All the mouse experiments were done following protocols approved by NICHD Animal Care and Use committee and met NIH guidelines for the humane care of animals.
Hematocrit Measurements
Approximately 100 μL of blood was harvested in to a heparin-coated tube (BD microtainer tubes with lithium heparin) from each mouse by mandibular method. Hematocrit tubes were filled with blood to three-quarters full by capillary action, sealed and centrifuged for 5 minutes in a microhematocrit centrifuge (L W Scientific) The hematocrit was measured with the help of a hematocrit reader.
CBC Measurement
Blood was drawn from mice by mandibular method or by cardiac puncture in deeply anesthetized mice prior to euthanizing. CBCs were performed on a Hemavet 1500 (Drew Scientific, Dallas, Texas) analyzer.
Intracardial Pressure Measurements
Intra-ventricular pressures were measured invasively by using a microtip pressure transducer catheter connected to an electrostatic chart recorder (Millar Instruments, Houston). The mice were anesthetized with 1–3% isoflurane, and the right external jugular veins were cannulated. For each mouse the catheter was advanced to the right ventricle. Correct placement was verified according to RV pressure curve and the pressure and volume tracings were recorded. Body temperature of the mice was maintained between 37 °C and 38 °C, and the data were recorded using LabChart software (AD Instruments, Colorado Springs). This was a non-survival procedure. Animals were euthanized, while under anesthesia, by cervical dislocation verified by thoracotomy, or by exsanguination under anesthesia verified by thoracotomy.
Echocardiography
Transthoracic echocardiography was performed with 1-year old mice with a high frequency ultrasound system (VisualSonics, Vevo 2100). All images were acquired using a MS-400 transducer (VisualSonics) with a center operating frequency of 30 MHz, and broadband frequency of 18–38 MHz. The axial resolution of this transducer is 50 μm, and the footprint is 20 mm × 5 mm. 2-D images were obtained for multiple views of the heart. M-mode images of the left ventricle were collected from the parasternal short axis view at the level of the mid-papillary muscles and obtained by rotating the probe 90 degrees clockwise from the parasternal long axis view. From the M-mode images the LV systolic and diastolic posterior and anterior wall thicknesses, as well as end systolic and end diastolic internal LV chamber dimensions (LVIDs, LVIDd) were measured using the leading edge method. The LV functional value of ejection fraction (EF) was calculated from chamber dimension measurements through system software as: %EF = 100 ((LV vol;d-LV vol;s)/LV Vol;d) where LV vol;d = ((7.0/(2.4 + LVID;d)) × LVID;d3 and LV Vol;s = ((7.0/(2.4 × LVID;s)) × LVID;s3.
Flexivent Procedure for measurement of pulmonary function
To measure lung mechanics, mice were anesthetized and a cannula was inserted into the trachea. The mice were then attached to the Scireq FlexiVent system and were mechanically ventilated. The maintenance of tidal volume and respiration rate were controlled (150–200 bpm, 7–9 ml/kg, PEEP 3 cm H20). A few brief sighs were given to the mouse to determine lung volume information but did not exceed 25 cm H20. After that, the mice were euthanized while under anesthesia.
Pulmonary Vessel Thickness Assessment
Quantification of the pulmonary vascular media and intima thickness was performed as previously described (Graham et al., 2010).
Immunohistochemistry
Staining for HIF2α was performed following previously published (Jeong and David, 2006) protocol using rabbit anti-HIF2α antibody (IHC World). Sections were analyzed using a bright field microscope (Nikon Eclipse E600)
Fluorescence-activated cell sorting (FACS)
Freshly isolated spleens were gently poked in cold PBS with forceps to release the cells, and the cell suspensions were filtered with a cell strainer to generate single cell suspensions. The cells were washed in PBS and re-suspended in PBA buffer (1% bovine serum albumin, 0.1% sodium azide in PBS, pH7.4). Cells (1×106) were incubated with FITC-labeled rat anti-mouse CD71 and PE-CY7 labeled rat anti-mouse TER119 antibodies (BD Pharmingen) in PBA buffer at 4 °C for 30 minutes, and then were analyzed by FACS on BD FACS Calibur.
Erythropoietin and Endothelin-1 Measurement by ELISA
Erythropoietin and endothelin-1 were measured by ELISA kit (R&D systems) according to the manufacturer’s protocol. The lung lysates were prepared in RIPA buffer (50 mM Tris, 150 mM NaCl, 0.1 % SDS, 0.5 % Na.Deoxycholate, 1% Triton X-100) and diluted 100 times before being measured by ELISA.
Measurement of Total Serum Iron and Transferrin Saturation
Serum iron and unsaturated iron-binding capacity (UIBC) were measured in non-hemolyzed mouse serum using a Pointe Scientific (Lincoln Park, MI) Iron/TIBC Reagent Set following manufacturer’s instructions. Transferrin saturation was calculated from measured serum iron and UIBC.
Measurement of Iron Concentration in Spleen
Animals were deeply anesthetized and blood was removed by extensively perfusing PBS through the hearts. Spleens were harvested and immediately snap frozen in liquid nitrogen. Total iron concentrations were measured by inductively coupled plasma mass spectroscopy (ICP-MS) as described (Kim et al., 2009).
Cell Culture
Pulmonary endothelial cells: Endothelial cells from lungs of WT and Irp1−/− mice were isolated following the method of Sobczak et al. (Sobczak et al., 2010). Lungs from three 8-day old WT and Irp1−/− pups were used for isolation of pulmonary endothelial cells. The cells were grown at 1% oxygen, and harvested for experiments when they became about 80% confluent. Mouse embryonic fibroblasts (MEF) of 13-day old embryos were isolated and cultured from WT, Irp1−/− and Irp2−/− mice as previously described (Meyron-Holtz et al., 2004a; Meyron-Holtz et al., 2004b).
RNA Mobility Shift Assays
Gel retardation assays were performed as previously described (Meyron-Holtz et al., 2004a) with lysate containing 10 μg of total protein.
Western Blotting and Antibodies
Protein analysis was carried out as previously described (LaVaute et al., 2001). HIF1α and HIF2α westerns were performed with nuclear fractions separated following the protocol of ‘Active Motif’ (www.activemotif.com) from tissues and cells. HIF2α antibody (affinity purified goat anti human) was from R&D systems, and a final concentration of 1.0 μg/ml was used as described in R&D protocol. Purified mouse anti-human HIF1α antibody from BD biosciences was used at 1:1000 dilution. A mouse monoclonal Tfr1 antibody from Zymed was used at 1:2000 dilution. Western blots were treated with secondary peroxidase-conjugated bovine anti-goat IgG antibody from Santa Cruz Biotechnology, Inc. or sheep anti-mouse IgG antibody from GE healthcare at 1:1000 and 1:2000 dilutions respectively. HIF1α and HIF2α western blots were developed using supersignal west femto maximum sensitivity substrate (Thermoscientific). All other western blots were developed using supersignal west pico chemiluminescent substrate (Thermoscientific).
Quantitative Real-time PCR (qRT-PCR)
Total RNA was prepared with TRIzol reagent (Invitrogen), cDNA was prepared with High Capacity cDNA Reverse Transcription kit (Applied Biosystems) with 2 μg total RNA. qRT-PCR experiments were performed with SYBR Green PCR master mixture (Applied Biosystems). The primers for DMT1, FPN1, Dcytb, hepcidin, endothelin-1, erythropoietin, HIF1alpha, HIF2alpha, ALDOA, CCXL12, Bnip3, Retnla and actin are shown in Supplemental Table 1. The results were normalized against actin levels.
Statistical Analyses
Where applicable, data are expressed as the mean ± standard deviation. Pairwise comparisons between two groups were analyzed using the unpaired Student’s t-test. Analyses of multiple groups were performed using two-way ANOVA. P values of less than 0.05 were considered statistically significant. For statistical analysis of mouse survival time, survival curves were plotted using the Kaplan-Meier method and evaluated by log-rank test using the XLSTAT software package from Addinsoft.
Supplementary Material
Highlights.
Irp1 deficiency causes polycythemia and pulmonary hypertension
Irp1 regulates HIF2α translation and expression of EPO and endothelin 1
Irp1 represses erythropoiesis to protect tissue iron levels during iron deficiency
A low iron diet worsens polycythemia and causes sudden death in Irp1−/− animals
Acknowledgments
This work was supported by the intramural programs of NICHD and NHLBI. We thank Gregory Holmes-Hampton for helpful discussions, Michele Allen for her help in mouse studies, Shawn Kozlov for blood gas measurement and Javier Seravalli for performing metal measurements. Rubin Tuder’s research was supported by NIH (RC1HL100849).
Footnotes
AUTHOR CONTRIBUTIONS
M.C.G. and D-L.Z. designed the study, generated data, performed analyses and wrote the paper. S.Y.J. and D.A.S. generated data, performed analyses and wrote the paper. G.K., A.N., D.R.C., B.B.G., Z.Y., M.E. and J.L. generated data and performed analyses. H.O-W., T.T., T.S., G.R., K.R. and A.S. generated data. W-H.T. and R.M.T. provided substantial intellectual contribution. T.A.R. designed the study, performed analyses and wrote the paper.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bigham A, Bauchet M, Pinto D, Mao X, Akey JM, Mei R, Scherer SW, Julian CG, Wilson MJ, Lopez Herraez D, et al. Identifying signatures of natural selection in Tibetan and Andean populations using dense genome scan data. PLoS genetics. 2010:6. doi: 10.1371/journal.pgen.1001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond J, Gale DP, Connor T, Adams S, de Boer J, Gascoyne DM, Williams O, Maxwell PH, Ancliff PJ. Dysregulation of the HIF pathway due to VHL mutation causing severe erythrocytosis and pulmonary arterial hypertension. Blood. 2011;117:3699–3701. doi: 10.1182/blood-2010-12-327569. [DOI] [PubMed] [Google Scholar]
- Brusselmans K, Compernolle V, Tjwa M, Wiesener MS, Maxwell PH, Collen D, Carmeliet P. Heterozygous deficiency of hypoxia-inducible factor-2alpha protects mice against pulmonary hypertension and right ventricular dysfunction during prolonged hypoxia. The Journal of clinical investigation. 2003;111:1519–1527. doi: 10.1172/JCI15496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooperman SS, Meyron-Holtz EG, Olivierre-Wilson H, Ghosh MC, McConnell JP, Rouault TA. Microcytic anemia, erythropoietic protoporphyria, and neurodegeneration in mice with targeted deletion of iron-regulatory protein 2. Blood. 2005;106:1084–1091. doi: 10.1182/blood-2004-12-4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuy J, Volbeda A, Carpentier P, Darnault C, Moulis JM, Fontecilla-Camps JC. Crystal structure of human iron regulatory protein 1 as cytosolic aconitase. Structure. 2006;14:129–139. doi: 10.1016/j.str.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Formenti F, Constantin-Teodosiu D, Emmanuel Y, Cheeseman J, Dorrington KL, Edwards LM, Humphreys SM, Lappin TR, McMullin MF, McNamara CJ, et al. Regulation of human metabolism by hypoxia-inducible factor. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:12722–12727. doi: 10.1073/pnas.1002339107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale DP, Harten SK, Reid CD, Tuddenham EG, Maxwell PH. Autosomal dominant erythrocytosis and pulmonary arterial hypertension associated with an activating HIF2 alpha mutation. Blood. 2008;112:919–921. doi: 10.1182/blood-2008-04-153718. [DOI] [PubMed] [Google Scholar]
- Galy B, Ferring D, Minana B, Bell O, Janser HG, Muckenthaler M, Schumann K, Hentze MW. Altered body iron distribution and microcytosis in mice deficient in iron regulatory protein 2 (IRP2) Blood. 2005;106:2580–2589. doi: 10.1182/blood-2005-04-1365. [DOI] [PubMed] [Google Scholar]
- Ghosh MC, Tong WH, Zhang D, Ollivierre-Wilson H, Singh A, Krishna MC, Mitchell JB, Rouault TA. Tempol-mediated activation of latent iron regulatory protein activity prevents symptoms of neurodegenerative disease in IRP2 knockout mice. Proc Natl Acad Sci U S A. 2008;105:12028–12033. doi: 10.1073/pnas.0805361105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham BB, Mentink-Kane MM, El-Haddad H, Purnell S, Zhang L, Zaiman A, Redente EF, Riches DW, Hassoun PM, Bandeira A, et al. Schistosomiasis-induced experimental pulmonary hypertension: role of interleukin-13 signaling. The American journal of pathology. 2010;177:1549–1561. doi: 10.2353/ajpath.2010.100063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentze MW, Muckenthaler MU, Galy B, Camaschella C. Two to tango: regulation of Mammalian iron metabolism. Cell. 2010;142:24–38. doi: 10.1016/j.cell.2010.06.028. [DOI] [PubMed] [Google Scholar]
- Hickey MM, Lam JC, Bezman NA, Rathmell WK, Simon MC. von Hippel-Lindau mutation in mice recapitulates Chuvash polycythemia via hypoxia-inducible factor-2alpha signaling and splenic erythropoiesis. The Journal of clinical investigation. 2007;117:3879–3889. doi: 10.1172/JCI32614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey MM, Richardson T, Wang T, Mosqueira M, Arguiri E, Yu H, Yu QC, Solomides CC, Morrisey EE, Khurana TS, et al. The von Hippel-Lindau Chuvash mutation promotes pulmonary hypertension and fibrosis in mice. The Journal of clinical investigation. 2010;120:827–839. doi: 10.1172/JCI36362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong SY, David S. Age-related changes in iron homeostasis and cell death in the cerebellum of ceruloplasmin-deficient mice. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2006;26:9810–9819. doi: 10.1523/JNEUROSCI.2922-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaelin WG, Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Molecular cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Kim H, Son HY, Bailey SM, Lee J. Deletion of hepatic Ctr1 reveals its function in copper acquisition and compensatory mechanisms for copper homeostasis. American journal of physiology Gastrointestinal and liver physiology. 2009;296:G356–364. doi: 10.1152/ajpgi.90632.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaVaute T, Smith S, Cooperman S, Iwai K, Land W, Meyron-Holtz E, Drake SK, Miller G, Abu-Asab M, Tsokos M, et al. Targeted deletion of the gene encoding iron regulatory protein-2 causes misregulation of iron metabolism and neurodegenerative disease in mice. Nature genetics. 2001;27:209–214. doi: 10.1038/84859. [DOI] [PubMed] [Google Scholar]
- MacArthur DG, Balasubramanian S, Frankish A, Huang N, Morris J, Walter K, Jostins L, Habegger L, Pickrell JK, Montgomery SB, et al. A systematic survey of loss-of-function variants in human protein-coding genes. Science. 2012;335:823–828. doi: 10.1126/science.1215040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Molecular cell. 2010;40:294–309. doi: 10.1016/j.molcel.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyron-Holtz EG, Ghosh MC, Iwai K, LaVaute T, Brazzolotto X, Berger UV, Land W, Ollivierre-Wilson H, Grinberg A, Love P, et al. Genetic ablations of iron regulatory proteins 1 and 2 reveal why iron regulatory protein 2 dominates iron homeostasis. The EMBO journal. 2004a;23:386–395. doi: 10.1038/sj.emboj.7600041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyron-Holtz EG, Ghosh MC, Rouault TA. Mammalian tissue oxygen levels modulate iron-regulatory protein activities in vivo. Science. 2004b;306:2087–2090. doi: 10.1126/science.1103786. [DOI] [PubMed] [Google Scholar]
- Pan X, Suzuki N, Hirano I, Yamazaki S, Minegishi N, Yamamoto M. Isolation and characterization of renal erythropoietin-producing cells from genetically produced anemia mice. PloS one. 2011;6:e25839. doi: 10.1371/journal.pone.0025839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percy MJ, Zhao Q, Flores A, Harrison C, Lappin TR, Maxwell PH, McMullin MF, Lee FS. A family with erythrocytosis establishes a role for prolyl hydroxylase domain protein 2 in oxygen homeostasis. Proc Natl Acad Sci U S A. 2006;103:654–659. doi: 10.1073/pnas.0508423103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouault TA. The role of iron regulatory proteins in mammalian iron homeostasis and disease. Nature chemical biology. 2006;2:406–414. doi: 10.1038/nchembio807. [DOI] [PubMed] [Google Scholar]
- Sable CA, Aliyu ZY, Dham N, Nouraie M, Sachdev V, Sidenko S, Miasnikova GY, Polyakova LA, Sergueeva AI, Okhotin DJ, et al. Pulmonary artery pressure and iron deficiency in patients with upregulation of hypoxia sensing due to homozygous VHL(R200W) mutation (Chuvash polycythemia) Haematologica. 2012;97:193–200. doi: 10.3324/haematol.2011.051839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salahudeen AA, Thompson JW, Ruiz JC, Ma HW, Kinch LN, Li Q, Grishin NV, Bruick RK. An E3 ligase possessing an iron-responsive hemerythrin domain is a regulator of iron homeostasis. Science. 2009;326:722–726. doi: 10.1126/science.1176326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez M, Galy B, Muckenthaler MU, Hentze MW. Iron-regulatory proteins limit hypoxia-inducible factor-2alpha expression in iron deficiency. Nature structural & molecular biology. 2007;14:420–426. doi: 10.1038/nsmb1222. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Hypoxia-inducible factors in physiology and medicine. Cell. 2012;148:399–408. doi: 10.1016/j.cell.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao D, Park JE, Wort SJ. The role of endothelin-1 in the pathogenesis of pulmonary arterial hypertension. Pharmacological research: the official journal of the Italian Pharmacological Society. 2011;63:504–511. doi: 10.1016/j.phrs.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Smith SR, Ghosh MC, Ollivierre-Wilson H, Hang Tong W, Rouault TA. Complete loss of iron regulatory proteins 1 and 2 prevents viability of murine zygotes beyond the blastocyst stage of embryonic development. Blood cells, molecules & diseases. 2006;36:283–287. doi: 10.1016/j.bcmd.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Smith TG, Brooks JT, Balanos GM, Lappin TR, Layton DM, Leedham DL, Liu C, Maxwell PH, McMullin MF, McNamara CJ, et al. Mutation of the von Hippel-Lindau gene alters human cardiopulmonary physiology. Advances in experimental medicine and biology. 2008;605:51–56. doi: 10.1007/978-0-387-73693-8_9. [DOI] [PubMed] [Google Scholar]
- Sobczak M, Dargatz J, Chrzanowska-Wodnicka M. Isolation and culture of pulmonary endothelial cells from neonatal mice. Journal of visualized experiments: JoVE. 2010 doi: 10.3791/2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorin E, Clozel M. The cardiovascular physiology and pharmacology of endothelin-1. Adv Pharmacol. 2010;60:1–26. doi: 10.1016/B978-0-12-385061-4.00001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vashisht AA, Zumbrennen KB, Huang X, Powers DN, Durazo A, Sun D, Bhaskaran N, Persson A, Uhlen M, Sangfelt O, et al. Control of iron homeostasis by an iron-regulated ubiquitin ligase. Science. 2009;326:718–721. doi: 10.1126/science.1176333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walden WE, Selezneva AI, Dupuy J, Volbeda A, Fontecilla-Camps JC, Theil EC, Volz K. Structure of dual function iron regulatory protein 1 complexed with ferritin IRE-RNA. Science. 2006;314:1903–1908. doi: 10.1126/science.1133116. [DOI] [PubMed] [Google Scholar]
- Yi X, Liang Y, Huerta-Sanchez E, Jin X, Cuo ZX, Pool JE, Xu X, Jiang H, Vinckenbosch N, Korneliussen TS, et al. Sequencing of 50 human exomes reveals adaptation to high altitude. Science. 2010;329:75–78. doi: 10.1126/science.1190371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer M, Ebert BL, Neil C, Brenner K, Papaioannou I, Melas A, Tolliday N, Lamb J, Pantopoulos K, Golub T, et al. Small-molecule inhibitors of HIF-2a translation link its 5′UTR iron-responsive element to oxygen sensing. Molecular cell. 2008;32:838–848. doi: 10.1016/j.molcel.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.