Abstract
Synaptotagmin 1 (Syt 1) functions as an essential Ca2+ sensor for the fast but not slow component of Ca2+-triggered exocytosis. One hypothesis to account for this selective function, based on the close homology of Syt 1 with synaptotagmin 9 (Syt 9), is that these Syts are redundant for the slow but not the fast component of release. We now show, however, that Syt 9 has unique properties that set it apart from Syt 1. Different from Syt 1, endogenous Syt 9 does not associate Ca2+ dependently or independently with soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNARE) protein complexes, and the Syt 9 C2B domain does not form Ca2+/phospholipid complexes, whereas such complexes are essential for Syt 1 function. Nevertheless, the C2A domain of Syt 9 functions as a Ca2+-binding module, suggesting that Syts 1 and 9 are Ca2+ sensors with similar Ca2+-binding sequences but distinct properties that indicate nonoverlapping functions.
Keywords: C2 domain, exocytosis, neurotransmitter release, synaptic plasticity, membrane fusion
Ca2+ influx into presynaptic nerve terminals and neuroendocrine cells triggers exocytosis of synaptic and secretory vesicles (1). Ca2+ activates at least two components of exocytosis, a fast synchronous and a slow asynchronous component (2). Among the Ca2+-binding proteins that are potential Ca2+ sensors for exocytosis, Synaptotagmins (Syts) are currently the best candidates (3, 4). All Syts are composed of an N-terminal transmembrane region, a linker sequence, and two C-terminal C2 domains (referred to as C2A and C2B domains). Syts 1 and 2 are abundant synaptic vesicle proteins that are highly homologous but differentially distributed (5–7). In Syt 1, the C2A domain binds three Ca2+ ions and the C2B domain two Ca2+ ions (8, 9). Both C2 domains exhibit low intrinsic Ca2+ affinities (>500 μM Ca2+) that are dramatically increased in the presence of phospholipids (to ≈5–50 μM Ca2+ depending on phospholipid composition; refs. 9 and 10), probably because the negatively charged phospholipid headgroups provide additional coordination sites for bound Ca2+ ions. The Syt C2 domains also bind to soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNARE) complexes by Ca2+-dependent and Ca2+-independent mechanisms (11–14).
Syt 1 functions as a Ca2+ sensor that mediates the fast but not the slow component of Ca2+-triggered synaptic vesicle exocytosis (15, 16) and dense core vesicle exocytosis (17). Direct evidence for this conclusion was derived from knock-in mice carrying a point mutation (R233Q) in Syt 1. These mice exhibited an ≈2-fold reduction in the overall Ca2+ affinity of Syt 1 and a similar decrease in the apparent Ca2+ affinity of exocytosis (10, 18). The Ca2+-binding sites of both the C2A and C2B domains of Syt 1 contribute to its Ca2+ sensor function, but are not functionally equivalent. Whereas the C2A domain Ca2+-binding sites set the overall Ca2+ affinity of exocytosis, the C2B domain Ca2+-binding sites are absolutely essential (14, 19–21).
In contrast to synapses, in neuroendocrine cells, measurements of exocytosis over longer time periods showed that the loss of Syt 1 had no apparent effect (22). Two hypotheses were advanced to explain this observation: (i) neuroendocrine cells express relatively large amounts of Syt 9, which is closely related to Syt 1, suggesting that Syt 9 is functionally redundant with Syt 1 in neuroendocrine cells but not in synapses (23, 24); and (ii)Syt 1 is essential only for fast exocytosis, and the lack of an effect of the Syt 1 deletion on the total amount of Ca2+-triggered exocytosis simply reflects the continued operation of the slow component of exocytosis (17). Recent results demonstrating that recombinant fragments of Syt 9 form Ca2+-dependent phospholipid complexes and pull-down brain SNARE proteins supported the first hypothesis (24). In contrast, the finding that Syt 1 is also essential only for the fast but not the slow component of release in neuroendocrine cells similar to synapses (17) provided strong evidence for the second hypothesis. To assess the potential of Syt 9 to serve as an exocytotic Ca2+ sensor that replaces Syt 1, we have now examined its Ca2+-binding properties. We find that the C2B domain of Syt 9 is unable to from Ca2+/phospholipid complexes, whereas in Syt 1, such complexes are essential for its Ca2+ sensor function. Furthermore, we show that endogenous Syt 9 does not associate Ca2+ dependently or independently with SNARE complexes.
Our results indicate that the unique Ca2+ binding properties of Syt 9 suggest a biological role that must differ substantially from that of Syt 1. This conclusion is consistent with the selective requirement of Syt 1 for the fast component of release in synapses and in neuroendocrine cells (15–18).
Materials and Methods
Plasmids. Bacterial GST fusion proteins were expressed with pGEX-KG encoding the following residues (in parentheses): pGEX-Syt 1 C2A (140–270), pGEX-Syt 1 C2B (271–421), pGEX-Syt 1 C2A/B (140–421), pGEX-Syt 9 C2A (107–237), and pGEX-Syt 9 C2B (238–386). pFastBac vector (Invitrogen) was used for baculoviral production of a GST fusion protein of Syt 9 C2A/B fragment containing residues 107–237. Syt 1/9C2B-domain hybrids were generated by using site-directed mutagenesis (Stratagene); all plasmids were verified by DNA sequencing.
Ca2+-Dependent Phospholipid Binding Assays. These assays were carried out by measuring the copurification of recombinant proteins with liposomes containing a defined phospholipid composition by using the centrifugation assay as described (9, 14).
Circular Dichroism Spectra. Circular dichroism spectra were recorded in a 1-mm cell in an Aviv model 62DS spectropolarimeter with 20 μM C2 domains in 40 mM Tris·HCl, pH 8.2/0.1 M NaCl/0.5 mM EGTA, without or with 10 mM Ca2+ or Mg2+.
Immunoprecipitations. Immunoprecipitations were performed with monoclonal antibodies against Syntaxin 1 (HPC-1; 15 μl of ascites) or Synaptobrevin 2 (CL69.1; 15 μl of ascites) essentially as described (14).
Subcellular Fractionations of PC12 Cells. PC12 cells were hypotonically lysed for 5 min in ice-cold water, cell homogenates were centrifuged at 800 × g, and the postnuclear supernatant was loaded on the top of a step sucrose-density gradient (2.0–0.25 M). Subcellular fractions were separated by centrifugation for 2.5 h at 35,000 rpm in a Beckman SW41 rotor and were analyzed by immunoblotting.
Cracked PC12 Cell Secretion Assays. These assays were carried out with freeze–thaw permeabilization of PC 12 cells (14, 26). Seventy percent confluent PC12 cells were loaded with 3H-norepinephrine for 24 h, were washed with physiological saline (145 mM NaCl/5.6 mM KCl/2.2 mM CaCl2/0.5 mM MgCl2/5.6 mM glucose/15 mM Hepes-NaOH, pH 7.4), were harvested by pipetting a stream of Ca2+-free ice-cold buffer A (120 mM potassium glutamate/20 mM potassium acetate/2 mM EGTA/20 mM Hepes-NaOH, pH 7.2), and were washed twice with the same buffer. Cell ghosts were prepared by freezing cells overnight at -80°C, thawing on ice for 2 h, and washing the “ghosts” three times with 6 ml of buffer A containing 1% BSA. Standard secretion reactions (≈20 reactions per 100-mm plate; 0.1 ml total volume in 1.5-ml tubes) containing washed cell ghosts, 2 mM ATP, 2 mM MgCl2, 10 μl of rat brain cytosol (10 g/liter) in buffer A, 6 μM recombinant C2-domain protein with various concentrations of Ca2+ or Sr2+ were used to obtain the indicated free concentrations (calculated by eqcal; Biosoft, Ferguson, MO). Reactions were incubated for 30 min at 30°C, terminated on ice, and samples were centrifuged at 4°C for 3 min at 20,800 × g. Supernatants and pellets solubilized in 1% Triton X-100 were analyzed by liquid scintillation counting.
Ca2+-Triggered Human Growth Hormone (hGH) Secretion in Transfected PC12 Cells. These procedures were carried out essentially as described (25). hGH secretion was assayed 3 d after transfection with FuGENE (Roche Diagnostics, Indianapolis), and the amounts of hGH in the medium and the cell extracts were quantified by an RIA (Nichols Institute Diagnostics, San Clemente, CA).
Miscellaneous Procedures. Polyclonal antibodies to Syt 9 were raised in rabbits against the purified recombinant GST fusion rat Syt 9 linker domain (residues 50–106). Subcellular fractionations were carried out as described (26). SDS/PAGE and immunoblotting were performed by using standard procedures, and immunoblots were developed by enhanced chemiluminescence (Amersham Pharmacia).
Results
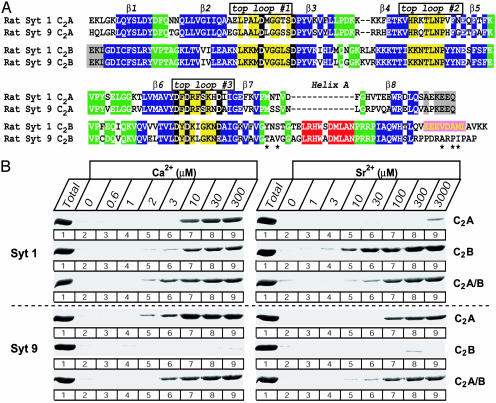
Phospholipid Binding to the Syt 9 C2 Domains. The Syt 1 and 9 C2A and C2B domains are very similar (69% and 81% sequence identity, respectively; Fig. 1A). In particular, the sequences of their top Ca2+-binding loops are nearly identical, indicating that they may exhibit equivalent Ca2+-dependent properties. To test this theory, we investigated whether the C2 domains of Syts 1 and 9 form similar Ca2+-dependent complexes with phospholipid membranes (Fig. 1B). We measured the apparent Ca2+ affinity of the phospholipid complexes by using a centrifugation assay in which Ca2+-dependent binding of recombinant C2 domains to liposomes is monitored (9, 27). In addition to Ca2+, we examined Sr2+, because earlier studies had shown that Sr2+ triggers release similar to Ca2+, albeit less efficiently (14). In these studies, we used purified GST fusion proteins of the isolated Syts 1 and 9 C2A and C2B domains and of the double C2A/B-domain fragments. All of these proteins were produced by bacterial expression, except for the Syt 9 double C2A/B-domain fragment, which was produced with a baculovirus expression system because bacterially expressed double C2A/B-domain fragment was insoluble (data not shown).
Fig. 1.
Comparison of the Ca2+-dependent properties of Syts 1 and 9. (A) Alignment of the sequences for the C2A and C2B domains of rat Syts 1 and 9. Identical residues are highlighted in a color code that reflects the atomic structures of the Syt 1 C2A and C2B domains (8, 9): yellow, top loops; green, bottom loops; blue, β-strands; and red, α-helix A in the bottom loop. In addition, the B α-helix that is present in the Syt 1, but not in the 9 C2B, domain (9) is highlighted in pink. Residues in top loops #1 and #3 that coordinate the Ca2+ ions are shown in white on a black background. Asterisks indicate amino acids that are not conserved in Syt 9 C2B domains (mouse, T347A; human, T347A, V349A, A380V, P382L, and I383L). (B) Phospholipid binding to the C2A, C2B, and double C2A/B domains of Syts 1 and 9 studied by Ca2+- and Sr2+-dependent cosedimentation with liposomes. Purified GST fusion proteins containing the indicated C2 domains were incubated with liposomes composed of 25% phosphatidylserine/75% phosphatidylcholine in presence of free Ca2+ or Sr2+ at the indicated concentrations clamped by Ca2+/EGTA or Sr2+/EGTA buffers. Liposomes were centrifuged and washed, and bound proteins were estimated by SDS/PAGE and Coomassie blue staining.
Our data demonstrate that the C2A domain of Syt 9 efficiently binds to negatively charged phospholipid membranes as a function of Ca2+ with a slightly higher apparent Ca2+ affinity (≈6 μM Ca2+) than the Syt 1 C2A domain (≈10 μM Ca2+, Fig. 1B). Sr2+ exhibited a considerably higher apparent affinity for the Syt 9 C2A domain (≈100 μM Sr2+) than for the Syt 1 C2A domain (>3 mM). Surprisingly, no Ca2+- or Sr2+-dependent phospholipid binding by the Syt 9 C2B domain was detected, whereas the C2B domain of Syt 1 was activated efficiently by both Ca2+ and Sr2+ (Fig. 1B). The inactivity of the Syt 9 C2B domain was not due to a misfolding of the Syt 9 C2B domain, because circular dichroism spectroscopy showed that the recombinant domain was composed of the stable β-stranded structure (data not shown).
Why Do the Ca2+-Binding Properties of the Syts 1 and 9 C2B Domains Differ? The differential Ca2+-binding properties of the Syts 1 and 9 C2B domains are puzzling because only 26 residues in these domains are not identical but are mostly similar (Fig. 1 A). To determine the molecular basis for these differential Ca2+-binding properties, we produced chimeric proteins from the Syt 1 and 9 C2B domains and measured their Ca2+-dependent phospholipid-binding properties (Fig. 2A). Replacements of increasingly shorter Syt 9 sequences with the corresponding Syt 1 sequences revealed that a short sequence (29 residues) in the C-terminal half of the Syt 1 C2B domain, when introduced into the Syt 9 C2B domain, was sufficient to enable Ca2+/phospholipid binding by the Syt 9 C2B domain. This sequence makes up loop #3 and flanking β-strands of the C2B domain (Fig. 2 A), and confers a normal apparent Ca2+ affinity on the Syt 9 C2B domain (Fig. 2B). These results suggest that a few evolutionarily conserved amino acid changes inactivate Ca2+ binding to the C2B domain of Syt 9.
Fig. 2.
Identification of the sequence in the synaptotagmin 9 C2B domain that inactivates Ca2+ binding. (A) Diagram of the hybrid Syt 1 and 9 C2B domains (Left) and Ca2+-dependent phospholipid binding by the respective proteins (Right). The 29-residue sequence in the bottom chimera that is sufficient to convert the Syt 9 C2B domain from a Ca2+-independent into a Ca2+-dependent C2 domain is shown in the sequence alignment below the chimeric structures. (B) Comparison of the apparent Ca2+ affinity of the Syt 1 C2B domain and the chimeric Syt 9/1 C2B domain by Ca2+ titration of phospholipid binding.
Association of Syt 9 with SNARE Complexes. Syt 1 associates with SNARE complexes Ca2+-dependently and -independently (11, 12). GST pull-downs suggested that Syt 9 may also interact with SNARE complexes (24). However, recombinant C2 domains tend to be sticky, and GST pull-downs are prone to artifacts, prompting us to employ immunoprecipitations to test whether endogenous Syt 9 associates with SNARE complexes. For this purpose, we raised polyclonal antibodies to Syt 9 that were directed to the linker sequence separating the transmembrane region from the C2 domains. We chose the linker region because, in previous experiments, we had raised antibodies to the Syt 9 C2 domains (commonly used as epitope for Syt antibodies), and found that it was nearly impossible to render these antibodies specific for Syt 9 vs. the very similar Syt 1 (data not shown). In contrast, immunoblotting analysis of transfected COS cells confirmed that the newly generated antibodies to the Syt 9 linker region were indeed specific for Syt 9, compared with other Syts (Fig. 3A).
Fig. 3.
Comparison of Ca2+-dependent and -independent association of endogenous brain Syts 1 and 9 with SNARE complexes. (A) Characterization of Syt 9 antibodies. COS cells transfected with the indicated Syts were examined by immunoblotting with antibodies to the Syts listed on the right. VCP, vasolin-containing protein; loading control. (B) Immunoprecipitations of SNARE complexes from brain to probe for associated Syts. SNAREs were immunoprecipitated in the presence of Ca2+ from rat brain with antibodies to Syntaxin 1 (Left) or Synaptobrevin 2 (Right). Immunoprecipitates were eluted with EGTA, and the eluted proteins and remaining bound proteins were analyzed by immunoblotting for Syts 1 and 9, and control proteins such as complexins, as a marker of assembled SNARE complexes. Syn 1, Synapsin 1, used as a negative control.
We next probed the association of SNAREs with Syt 9 in brain. We immunoprecipitated SNARE complexes from brain homogenates in the presence or absence of Ca2+, eluted the immunoprecipitates with EGTA, and analyzed the eluates and precipitates by immunoblotting (Fig. 3B). To ensure that a lack of association was not due to antibody interference, we used two different antibodies (to Syntaxin 1 and to Synaptobrevin/vesicle-associated membrane protein 2) for immunoprecipitations, and confirmed that SNARE complexes were isolated by probing the resulting immunoprecipitates with antibodies to complexins that only bind to assembled SNARE complexes (26). We found that, as described (14), Syt 1 exhibited both a Ca2+-dependent and a Ca2+-independent association with SNARE complexes (Fig. 3B). However, we detected no Syt 9 in the EGTA eluates or the residual immunoprecipitates. The high degree of enrichment of complexins confirmed that SNARE complexes were immunoprecipitated, thus demonstrating that endogenous Syt 9 does not associate with SNARE complexes.
Potential Role of Syt 9 in PC12 Cell Exocytosis. The Ca2+-binding properties of Syt 9 demonstrate that its C2A domain has a slightly higher apparent Ca2+ affinity than that of Syt 1, but that its overall Ca2+-binding properties are more restricted than those of Syt 1 (i.e., no Ca2+-dependent phospholipid binding by the C2B domain, and no association with SNARE complexes). These properties argue against a function for Syt 9 equivalent to that of Syt 1 in the fast component of release, but do not allow conclusions about a possible function for Syt 9 in the slow component of exocytosis, which is supported by its particularly high expression levels in chromaffin PC12 cells (23). To test this idea, we first examined the localization of Syt 9 in PC12 cells by using sucrose gradient centrifugation (Fig. 4). Consistent with earlier studies (23), we found that Syt 9 in PC12 cells homogenates copurifies with secretory granule markers such as Syt 1, Synaptobrevin 2, Rab3, or synaptophysin (Fig. 4). In contrast, the plasma membrane SNARE proteins Syntaxin 1A or SNAP-25 were distributed in a broader peak on the gradient than Syt 9, and the cytosolic proteins GDI and intersectin were found at a different position in the sucrose gradients (Fig. 4). These data are consistent with a localization of Syt 9 to secretory vesicles.
Fig. 4.
Analysis of the localization of Syts 1 and 9 in PC12 cells by subcellular fractionation. Subcellular components of PC12 cell homogenates were separated by sucrose gradient centrifugation, and fractions (left is the top and right is the bottom of the gradient) were examined by immunoblotting with antibodies to the proteins listed on the right (Syp, synaptophysin; Syb 2, Synaptobrevin 2; Synt. 1A, Syntaxin 1A).
We then used permeabilized PC12 cells to determine whether soluble recombinant C2 domains from Syt 9 could alter Ca2+-induced exocytosis. In this experiment, total Ca2+-dependent release of norepinephrine is measured from PC12 cell ghosts, which were obtained by freeze–thawing. The Ca2+ concentration and composition of the medium in these experiments can be directly controlled, making it possible to probe the effects of recombinant proteins on Ca2+-induced secretion over a long time frame that only monitors total release mediated by the sum of fast and slow components of exocytosis. Previous experiments (28) had demonstrated that under these conditions, Ca2+ triggers release with a bell-shaped concentration dependence, and that release can be effectively abolished by micromolar concentrations of recombinant C2 domains from Syts 3 and 7, which exhibit a relatively high apparent Ca2+ affinity, but not by recombinant C2 domains from Syt 1, which display a relatively low apparent Ca2+ affinity (27). In addition to examining Ca2+-induced release, we also probed Sr2+-stimulated release to correlate the divalent cation-binding properties of Syt 9 (Fig. 1) with its effects on PC12 cell exocytosis (Fig. 5). We found that the isolated C2A domain, the isolated C2B domain, or the double C2A/B-domain fragment of Syt 9 had no major effect on Ca2+-induced secretion. In contrast, the isolated C2A domain and the double C2A/B-domain fragment caused a moderate inhibition of secretion stimulated by high Sr2+ concentrations (Fig. 5).
Fig. 5.
Effect of recombinant C2 domains from Syt 9 on Ca2+-triggered exocytosis in permeabilized PC12 cells. Cracked PC12 cells prelabeled with 3H-norepinephrine were preincubated with the purified recombinant proteins listed (6 μM), and norepinephrine release was triggered by the addition of Ca2+ or Sr2+ at the indicated free concentrations. The C2A and C2B domain and the double C2A/B-domain fragment of Syt 9 were added as GST fusion proteins; purified GST alone was used as a control that was included in each series of experiments. Data shown are from a representative experiment that was independently repeated three times.
The data of Fig. 5 suggest that the apparent Ca2+ affinity of Syt 9 is too low to mediate the slow component of release that dominates Ca2+-triggered secretion in permeabilized PC12 cells. To probe the possible participation of Syt 9 in PC12 cell exocytosis by an independent approach, we tested the effect of transfected wild-type or mutant Syts 1 and 9. We used mutants of Syts 1 and 9 in which the C2 domains were deleted and replaced with enhanced yellow fluorescent protein (EYFP) (Fig. 6A) because previous studies had shown that truncated Syt 1 is a powerful inhibitor of endocytosis in transfected cells (29). We cotransfected the various Syt constructs with hGH, and measured the effect of cotransfected proteins on the release of hGH under basal conditions or after K+ depolarization. In control transfected PC12 cells, K+ depolarization induced a 4-fold increase in hGH release from cells (Fig. 6B). Transfection of full-length Syt 1 increased the basal rate and the induced rate of release, but had no effect on the net induced secretion (Fig. 6C). Truncated Syt 1 fused to EYFP caused dramatic inhibition of the net Ca2+-triggered hGH secretion. The wild-type or the truncated Syt 9, however, had no major effect on any secretory parameter (Fig. 6 B and C).
Fig. 6.
Effect of transfected wild-type and mutant Syts 1 and 9 on Ca2+-triggered hGH secretion in transfected PC12 cells. (A) Diagram of the structures of wild-type Syts 1 and 9 and of truncation mutants of these Syts (Syt 1ΔC-EYFP, residue 1–85 of Syt 1 followed by EYFP; Syt 9ΔC-EYFP, residue 1–58 of Syt 9 followed by EYFP). (B) Ca2+-induced hGH secretion from intact PC12 cells that were cotransfected with plasmids encoding hGH and the Syt proteins described in A. Seventy-two hours after transfection, cells were stimulated with control medium (gray bar) or with 56 mM KCl (black bar) for 15 min, and the amount of hGH release was determined as the fraction of total hGH present in the extracellular medium, normalized for the amount of hGH release obtained without stimulation under control conditions. Results shown are means ± SEM from three independent experiments performed in duplicate. (C) Normalized evoked hGH secretion, calculated as the difference of secretion between control and depolarizing medium. Statistical analyses were performed by one-way ANOVA (*, P < 0.05).
Discussion
Extensive biophysical and genetic studies revealed that Syt 1 is a Ca2+ sensor that is selectively required for the fast, synchronous component of exocytosis, but not for the delayed, asynchronous component (10, 15–18, 30). A principal question is whether the slow component is entirely mediated by other Ca2+ sensors, for example, other Syts (3), or whether Syt 1 also mediates the slow component, but is functionally redundant with other Ca2+ sensors for this component. The second hypothesis was proposed in the characterization of Syt 9, a Syt isoform that is most closely related to Syts 1 and 2 and is coexpressed with Syt 1 in brain and PC12 cells. Four findings support the second hypothesis: (i) Syt 9 is up-regulated in PC12 cells that lack Syt 1 (23); (ii) the C2A domain of Syt 9 binds Ca2+ in a similar manner to Syt 1 (23); (iii) Syt 9 C2 domains are capable of pulling down SNAREs (24); and (iv) antibodies to Syts 1 and 9 and protein fragments from these Syts inhibit exocytosis in permeabilized PC12 cells (23, 24). However, these findings also raise important questions. In these studies, the Ca2+-binding properties of the C2B domain of Syt 9 were not characterized, presumably because its high degree of sequence identity with that of Syt 1 (81% identity) suggested that they should be functionally similar. However, for a comparison of the physiological properties of Syts 1 and 9, the Ca2+-binding properties of the C2B domain are particularly important because, in Syt 1, Ca2+ binding to the C2B domain is critical for function (19). Only the binding of GST-fusion proteins but not of native Syt 9 to SNAREs was not tested, which is important because the general stickiness of recombinant C2 domains makes it difficult to interpret pull-downs. Finally, it is unclear how antibodies to Syts 1 and 9 and protein fragments from these Syts can inhibit slow exocytosis in neuroendocrine cells (23, 24), because Syt 1 is only required for the fast component of exocytosis. In neuroendocrine cells, deletion of Syt 1 has no effect on overall exocytosis measured over seconds to minutes (17, 21), the time frame during which antibodies appeared to inhibit exocytosis (23), suggesting that the antibodies may have produced a steric but not a functional inhibition.
In addressing these questions, we now show that although Syt 9 is a Ca2+-binding protein, its properties are surprisingly different from those of Syt 1: its C2B domain does not form Ca2+-dependent phospholipid complexes, and endogenous Syt 9 does not associate with SNARE complexes in the absence or presence of Ca2+. Thus, Syts 1 and 9 have distinct functional characteristics, despite their high degree of sequence homology. The C2 domains of Syts 1 and 9, however, are similar in that both sets of C2 domains do not have a large effect on Ca2+-triggered exocytosis in permeabilized PC12 cells (Fig. 5), in contrast to the C2 domains from Syts 3, 6, and 7, which exhibit a higher apparent Ca2+ affinity (9, 27, 31). Our data do not rule out a role for Syt 9 as a Ca2+ sensor in exocytosis but exclude a function as a less abundant, redundant “sidekick” for Syt 1, and suggest that the apparent Ca2+ affinity is too low for a major Ca2+ sensor in the slow component of release. Furthermore, if Syt 9 does act as a Ca2+ sensor in exocytosis, it is unlikely to carry either the fast component (because it cannot support this component in the absence of Syt 1; ref. 17) or the slow component (because its apparent Ca2+ affinity is too low to interfere with this type of exocytosis in permeabilized PC12 cells). Together with the lack of an effect of wild-type or mutant Syt 9 in transfected PC12 cells (Fig. 6), these results are more consistent with a role for Syt 9 in secretion as a Ca2+-dependent modulator instead of an exocytotic Ca2+ sensor.
The lack of efficient Ca2+ binding by the Syt 9 C2B domain is surprising, considering its similarity to the Syt 1 C2B domain (Fig. 1 A). Hybrid analysis revealed that a short sequence surrounding Ca2+-binding loop #3 is responsible for the inability of the Syt 9 C2B domain to form Ca2+/phospholipid complexes (Fig. 2). Because the Ca2+-binding loops of Syts 1 and 9 are almost identical, this result shows that the sequences of the Ca2+-binding loops of C2 domains are not sufficient to predict their Ca2+-binding properties. One possibility is that not only the loops themselves, but also their precise orientations, determined by the exact positions of the β-strands from which they emerge, may dictate the Ca2+-binding properties of a C2 domain.
An interesting property of Syt 1 is its interaction with the SNARE complex. Previous studies (14) showed that the Ca2+-triggered interaction of Syt 1 with the synaptic SNARE complex is not essential for fast Ca2+-triggered release, but raised the possibility that the Ca2+-independent interaction of Syt 1 with the synaptic SNARE complex may function to position Syt 1 at the site of exocytosis. Although recombinant Syt 9 was shown to pull down SNARE proteins (24), we found that immunoprecipitations of SNARE complexes fail to coisolate endogenous Syt 9 from brain (Fig. 3B). To exclude a potential false-negative result, we performed immunoprecipitations with antibodies to two different SNARE proteins, and showed that both Syt 1 and complexins (which only bind to assembled SNARE complexes; 26) were coimmunoprecipitated. At least two potential explanations can account for the discrepancy between the immunoprecipitation results shown here and the previous pull-down results (23, 24): (i) it is possible that the binding of Syt 9 to the SNARE complex is too weak to be detectable by immunoprecipitations. In this case, the binding would have to substantially different from that of Syt 1, which tightly binds in the presence and absence of Ca2+; and (ii) the Ca2+-dependent binding of Syt 9 to SNARE complexes is a pull-down artifact. Support for this hypothesis is derived from the many artifactual Ca2+-dependent interactions that at least Syt 1 is prone to in pull-downs (32).
In summary, our results show that Syt 9 is a Ca2+-binding protein with properties that set it apart from Syt 1. These data are most consistent with a modulatory Ca2+-dependent function, although they do not rule out a potential role of Syt 9 in the slow component of exocytosis. Clearly, Syts form a diverse family of proteins in which individual members may have different characteristics and functions. Elucidating how precisely Ca2+ signaling works through Syts thus continues to be a challenge that depends on a combination of genetics and biophysics, and cannot be achieved by in vitro approaches only.
Acknowledgments
We thank Ms. I. Kornblum, A. Roth, and E. Borowicz for technical assistance, and Dr. R. Jahn (Max Planck Institute for Biophysical Chemistry, Goettingen, Germany) for the gift of antibodies. This study was supported by National Institutes of Health Grant NS40944 (to J.R.).
Abbreviations: Syt, synaptotagmin; SNARE, soluble N-ethylmaleimide-sensitive factor attachment protein receptors; hGH, human growth hormone; EYFP, enhanced yellow fluorescent protein.
References
- 1.Katz, B. (1969) The Release of Neural Transmitter Substances (Liverpool Univ. Press, Liverpool, U.K.)
- 2.Goda, Y. & Stevens, C. F. (1994) Proc. Natl. Acad. Sci. USA 91, 12942-12946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Südhof, T. C. (2002) J. Biol. Chem. 277, 7629-7632. [DOI] [PubMed] [Google Scholar]
- 4.Yoshihara, M., Adolfsen, B. & Littleton, J. T. (2003) Curr. Opin. Neurobiol. 13, 315-323. [DOI] [PubMed] [Google Scholar]
- 5.Matthew, W. D., Tsavaler, L. & Reichardt, L. F. (1981) J. Cell Biol. 91, 257-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perin, M. S., Fried, V. A., Mignery, G. A., Jahn, R. & Südhof, T. C. (1990) Nature 345, 260-263. [DOI] [PubMed] [Google Scholar]
- 7.Geppert, M., Archer, B. T., III, & Südhof, T. C. (1991) J. Biol. Chem. 266, 13548-13552. [PubMed] [Google Scholar]
- 8.Ubach, J., Zhang, X., Shao, X., Südhof, T. C. & Rizo, J. (1998) EMBO J. 17, 3921-3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandez, I., Arac, D., Ubach, J., Gerber, S. H., Shin, O., Gao, Y., Anderson, R. G. W., Südhof, T. C. & Rizo, J. (2001) Neuron 32, 1057-1069. [DOI] [PubMed] [Google Scholar]
- 10.Fernández-Chacón, R., Königstorfer, A., Gerber, S. H., García, J., Matos, M. F., Stevens, C. F., Brose, N., Rizo, J., Rosenmund, C. & Südhof, T. C. (2001) Nature 410, 41-49. [DOI] [PubMed] [Google Scholar]
- 11.Bennett, M. K., Calakos, N. & Scheller, R. H. (1992) Science 257, 255-259. [DOI] [PubMed] [Google Scholar]
- 12.Li, C., Ullrich, B., Zhang, J. Z., Anderson, R. G. W., Brose, N. & Südhof, T. C. (1995) Nature 375, 594-599. [DOI] [PubMed] [Google Scholar]
- 13.Rickman, C. & Davletov, B. (2003) J. Biol. Chem. 278, 5501-5504. [DOI] [PubMed] [Google Scholar]
- 14.Shin, O. H., Rhee, J. S., Tang, J., Sugita, S., Rosenmund, C. & Südhof, T. C. (2003) Neuron 37, 99-108. [DOI] [PubMed] [Google Scholar]
- 15.Geppert, M., Goda, Y., Hammer, R. E., Li, C., Rosahl, T. W., Stevens, C. F. & Südhof, T. C. (1994) Cell 79, 717-727. [DOI] [PubMed] [Google Scholar]
- 16.Yoshihara, M. & Littleton, J. T. (2002) Neuron 36, 897-908. [DOI] [PubMed] [Google Scholar]
- 17.Voets, T., Moser, T., Lund, P. E., Chow, R. H., Geppert, M., Südhof, T. C. & Neher, E. (2001) Proc. Natl. Acad. Sci. USA 98, 11680-11685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sorensen, J. B., Fernandez-Chacon, R., Südhof, T. C. & Neher, E. (2003) J. Gen. Physiol. 122, 265-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernández-Chacón, R., Shin, O. H., Königstorfer, A., Matos, M. F., Meyer, A. C., Garcia, J., Gerber, S. H., Rizo, J., Südhof, T. C. & Rosenmund, C. (2002) J. Neurosci. 22, 8438-8446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mackler, J. M., Drummond, J. A., Loewen, C. A., Robinson, I. M. & Reist, N. E. (2002) Nature 418, 340-344. [DOI] [PubMed] [Google Scholar]
- 21.Tokuoka, H. & Goda, Y. (2003) Neuron 38, 521-524. [DOI] [PubMed] [Google Scholar]
- 22.Shoji-Kasai, Y., Yoshida, A., Sato, K., Hoshino, T., Ogura, A., Kondo, S., Fujimoto, Y., Kuwahara, R., Kato, R. & Takahashi, M. (1992) Science 256, 1821-1823. [DOI] [PubMed] [Google Scholar]
- 23.Fukuda, M., Kowalchyk, J. A., Zhang, X., Martin, T. F. J. & Mikoshiba, K. (2002) J. Biol. Chem. 277, 4601-4604. [DOI] [PubMed] [Google Scholar]
- 24.Tucker, W. C., Edwardson, J. M., Bai, J., Kim, H. J., Martin, T. F. J. & Chapman, E. R. (2003) J. Cell Biol. 162, 199-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schlüter, O. M., Khvotchev, M., Jahn, R. & Südhof, T. C. (2002) J. Biol. Chem. 277, 40919-40929. [DOI] [PubMed] [Google Scholar]
- 26.McMahon, H. T., Missler, M., Li, C. & Südhof, T. C. (1995) Cell 83, 111-119. [DOI] [PubMed] [Google Scholar]
- 27.Sugita, S., Shin, O. H., Han, W., Lao, Y. & Südhof, T. C. (2002) EMBO J. 21, 270-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klenchin, V. A., Kowalchyk, J. A. & Martin, T. F. (1998) Methods 16, 204-208. [DOI] [PubMed] [Google Scholar]
- 29.von Poser, C., Zhang, J. Z., Mineo, C., Ding, W., Ying, Y., Südhof, T. C. & Anderson, R. G. W. (2000) J. Biol. Chem. 275, 30916-30924. [DOI] [PubMed] [Google Scholar]
- 30.Marek, K. W. & Davis, G. W. (2002) Neuron 36, 805-813. [DOI] [PubMed] [Google Scholar]
- 31.Sugita, S., Han, W., Butz, S., Liu, X., Fernandez-Chacon, R., Lao, Y. & Südhof, T. C. (2001) Neuron 30, 459-473. [DOI] [PubMed] [Google Scholar]
- 32.Sugita, S. & Südhof, T. C. (2000) Biochemistry 39, 2940-2949. [DOI] [PubMed] [Google Scholar]