Abstract
Background
There is variation in the use of radioactive iodine (RAI) as treatment for well-differentiated thyroid cancer. The factors involved in physician decision-making for RAI remain unknown.
Methods
We surveyed physicians involved in postsurgical management of patients with thyroid cancer from 251 hospitals. Respondents were asked to rate the factors important in influencing whether a thyroid cancer patient receives RAI. Multivariable analyses controlling for physician age, gender, specialty, case volume, and whether they personally administer RAI, were performed to determine correlates of importance placed on patients' and physicians' worry about death from cancer and differences between low– versus higher–case-volume physicians.
Results
The survey response rate was 63% (534/853). Extent of disease, adequacy of surgical resection, patients' willingness to receive RAI, and patients' age were the factors physicians were most likely to report as quite or very important in influencing recommendations for RAI to patients with thyroid cancer. Interestingly, both physicians' and patients' worry about death from thyroid cancer were also important in determining RAI use. Physicians with less thyroid cancer cases per year were more likely than higher-volume physicians to report patients' (p<0.001) and physicians' worry about death (p=0.016) as quite or very important in decision-making. Other factors more likely to be of greater importance in determining RAI use for physicians with lower thyroid cancer patient volume versus higher include the accepted standard at the affiliated hospital (p=0.020), beliefs about RAI expressed by colleagues comanaging patients (p=0.003), and patient distance from the nearest facility administering RAI (p=0.012).
Conclusion
In addition to the extent of disease and adequacy of surgical resection, physicians place importance on physician and patient worry about death from thyroid cancer when deciding whether to treat a patient with RAI. The factors important to physician decision-making differ based on physician thyroid-cancer case-volume, with worry about death being more influential for low–case-volume physicians. As the mortality from thyroid cancer is low, the importance placed on death in decision making may be unwarranted.
Introduction
The incidence of well-differentiated thyroid cancer is rising, and the American Cancer Society estimates that there will be 56,450 new cases in the year 2012 (1). Despite the rise in thyroid cancer incidence, there has not been an associated increase in mortality rates (2–5). Five-year survival rates reach 97% for all thyroid cancers and 100% if contained in the thyroid (6).
The use of radioactive iodine (RAI) for remnant ablation following surgical resection for well-differentiated thyroid cancer has increased (7,8), but there is still controversy regarding the effectiveness of postoperative RAI use for reducing mortality and disease-specific survival in the majority of thyroid cancer patients (9–12). Recently, a study found that postoperative RAI used for well-differentiated thyroid cancers does not impact survival of patients designated as low risk after thyroidectomy (13). The increasing use of RAI in thyroid cancer has health implications, as the risk–benefit ratio for RAI use may not be favorable in low-risk patients. Reported adverse effects include eye/nasolacrimal, salivary, pulmonary, gastrointestinal, hematopoietic, and gonadal dysfunction, as well as development of second primary malignancies (8,14–16). Moreover, escalating healthcare costs from unnecessary RAI use in these patients will be unjustifiable. Although our previous work has demonstrated a relationship between surgeon preference for more intensive management and greater subsequent RAI use (17), and between the specialty of the primary decision-maker and RAI use (18), the factors that contribute to physician decision-making for RAI use post-thyroid surgery remain unknown.
In an effort to identify the factors that influence whether a physician recommends that a thyroid cancer patient receive RAI after thyroid surgery, we surveyed nonsurgical physicians involved in thyroid cancer management.
Methods
Data source and study population
As previously described by Haymart et al. (17,18), surgeons from randomly sampled hospitals affiliated with the National Cancer Database were surveyed. The 560 surgeon respondents were then asked to “please list the names, specialties, and hospital affiliations of the physicians who provide care to your thyroid cancer patients or administer RAI when needed.” The 903 physicians identified by the surgeons were the subjects of this second survey study.
The modified Dillman survey method (19) was used to enhance survey response rates. This protocol consists of (i) an initial mailing of an introductory letter, the survey instrument, a postage-paid return envelope, and a small gift, (ii) a postcard reminder 3 weeks later, and (iii) a second survey with a postage-paid return envelope to all nonresponders after another 2 weeks.
Data from the survey were de-identified and the double entry method was used to ensure <1% error. The study was granted exemption by the University of Michigan Institutional Review Board.
Measures
Before administration to targeted physicians, the survey instrument was piloted in a multidisciplinary group of providers at the University of Michigan. The survey instrument was created and reviewed by a multidisciplinary group of providers, consisting of health services researchers involved in survey methodology and providers from the fields of endocrinology, surgery, and nuclear medicine. The instrument was then piloted in a cohort of endocrinologists and nuclear medicine physicians in Ann Arbor, Michigan. After piloting, minor revisions were made before survey administration.
Subsequently, the survey was administered. A 5-point Likert scale was used for the 17 survey items addressing factors involved in RAI decision making, with the following options: 1=not at all important, 2=a little important, 3=somewhat important, 4=quite important, and 5=very important. Respondents were asked to rate the following factors' importance in influencing whether a thyroid cancer patient receives RAI: extent of disease, adequacy of surgical resection, patient age, the accepted standard of care at the affiliate hospital, the beliefs about RAI treatment expressed by colleagues comanaging the patient, patient level of flexibility at work, patient willingness to receive RAI, patient interest in conceiving a child, presence of young children in the patient's home, patient distance from nearest facility that administers RAI, patient ability to tolerate thyroid hormone withdrawal before RAI, patient anxiety about their diagnosis and prognosis, patient worry about complications from RAI, patient worry about death from thyroid cancer, physician worry about litigation, physician worry about complications from RAI, and physician worry about patient death from thyroid cancer.
Statistical analysis
The 5-point Likert scale used to rate the factors influencing use of RAI for thyroid cancer was dichotomized to very and quite important versus not at all, a little, and somewhat important. Descriptive statistics were generated.
Based on the survey, the provider case-volume was categorized in intervals of 0–4, 5–24, 25–49, 50–99, and 100 or more patients with thyroid cancer seen in 1 year. Multivariable logistic regression analysis controlling for gender, age, specialization, provider case volume, and whether providers personally administer RAI, was performed to determine correlates of importance placed on patient's and physician's worry about death. Additional multivariable logistic regression analyses controlling for gender, age, specialization, and whether providers administer RAI, were performed evaluating differences between low versus higher case volume physicians.
All statistical tests were performed using SAS 9.2 (SAS Institute, Inc., Cary, NC). Two-sided tests were used with p<0.05 considered as statistically significant.
Results
Seventeen of 903 surveyed physicians were found to be ineligible for the survey because they were unreachable due to incorrect mailing address and 33 were ineligible because they were deceased, ill, retired, or not treating thyroid cancer patients. Of the 853 response-eligible physicians, 534 (63%) completed the survey; these respondents represent 251 of the sampled hospitals.
Table 1 shows the respondent characteristics. The majority of the respondents were white and male (79% and 74%, respectively), and the mean age was 52 years. Respondents had an average of 20 years in practice; 67% were endocrinologists, 15% nuclear medicine providers, 10% other, 5% oncologists, and 2% radiologists. From the category other, 71% answered radiation oncology as their specialization. In regard to practice setting, 57% were in private practice, 23% in an academic tertiary care center, 15% in a community-based academic affiliate, and 5% in other settings. Of the respondents, 62% answered that they do not routinely administer RAI in their practice.
Table 1.
Provider Characteristics
| n (%) | |
|---|---|
| Gender | |
| Male | 382 (74) |
| Female | 135 (26) |
| Ethnicity | |
| White | 399 (79) |
| African-American | 9 (2) |
| American Indian/Alaska Native | 2 (0) |
| Asian | 81 (16) |
| Hispanic | 19 (4) |
| Other | 17 (3) |
| Years in practice (mean±SD) | 19.76±10.85 |
| Age (mean±SD) | 51.75±11.38 |
| Specialization | |
| Endocrinology | 359 (67) |
| Nuclear medicine | 81 (15) |
| Radiology | 11 (2) |
| Oncology | 27 (5) |
| General practitioner | 2 (0) |
| Other | 52 (10) |
| Practice setting | |
| Private practice | 300 (57) |
| Community-based academic affiliate | 80 (15) |
| Academic tertiary care center | 121 (23) |
| Other | 25 (5) |
| Country region | |
| East North Central | 98 (18) |
| East South Central | 40 (7) |
| Mid-Atlantic | 74 (14) |
| Mountain | 31 (6) |
| New England | 37 (7) |
| Pacific | 67 (13) |
| South Atlantic | 105 (20) |
| West North Central | 51 (10) |
| West South Central | 28 (5) |
| Number of thyroid cancer patients physician sees in 1 year | |
| 0–4 | 18 (4) |
| 5–24 | 143 (28) |
| 25–49 | 137 (27) |
| 50–99 | 112 (22) |
| 100 or more | 98 (19) |
| Physician administers RAI | |
| Yes | 195 (38) |
| No | 316 (62) |
RAI, radioactive iodine.
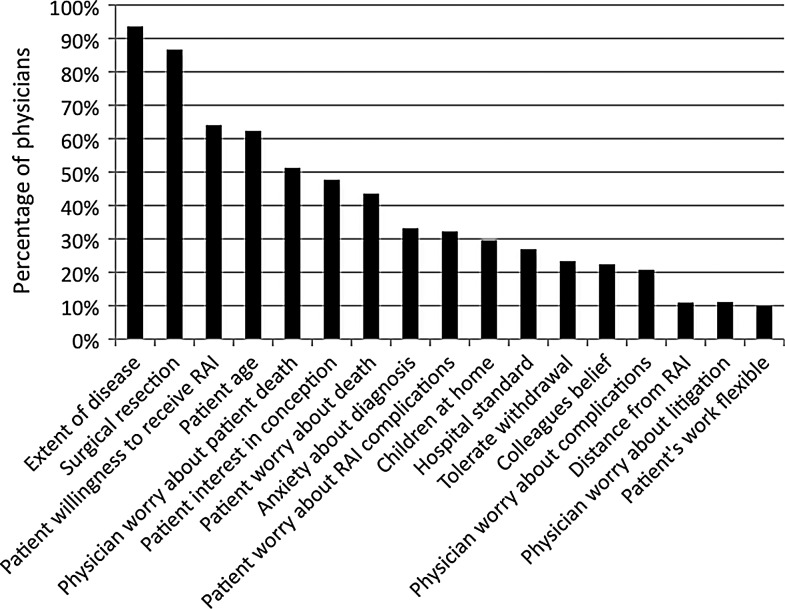
Figure 1 shows that the extent of disease (94%), adequacy of surgical resection (87%), patients' willingness to receive RAI (64%), and patients' age (62%) were reported to be important factors influencing recommendations regarding RAI use for patients with thyroid cancer. Both physicians' (53%) and patients' (45%) worry about death from thyroid cancer were also important in determining RAI use. Physicians with less thyroid cancer cases per year were more likely to report physicians' and patients' worry about death as quite or very important in decision making. The median values for the 17 factors that influence RAI use are listed in Table 2.
FIG. 1.
Factors physicians report as quite or very important in influencing whether a patient receives radioactive iodine (RAI) therapy for treatment of thyroid cancer.
Table 2.
Median Values for the 17 Factors That Influence Radioactive Iodine Use
| Median value | |
|---|---|
| Extent of surgery | 5 |
| Adequacy of surgical resection | 5 |
| Patient's age | 4 |
| Accepted standard at the affiliated hospital | 3 |
| Beliefs about RAI treatment expressed by colleagues comanaging the patient | 3 |
| Patient's level of flexibility at work | 2 |
| Patient willingness to receive RAI | 4 |
| Patient interest in conceiving a child | 3 |
| Presence of young children in the patient's home | 3 |
| Patient distance from nearest facility that administers RAI | 2 |
| Patient ability to tolerate thyroid hormone withdrawal before RAI administration | 3 |
| Patient anxiety about their diagnosis and prognosis | 3 |
| Patient worry about complications from RAI | 3 |
| Patient worry about death from thyroid cancer | 3 |
| Physician worry about litigation | 2 |
| Physician worry about complications from RAI | 3 |
| Physician worry about patient death from thyroid cancer | 4 |
The range for all 17 items was 1–5.
In analyzing responses from providers surveyed by region, we found that there was variability in the degree to which providers rated the importance of hospital standards in their decision making regarding RAI use. The physicians surveyed from the East South Central region placed the least importance, while the physicians surveyed from the Mid-Atlantic region placed the most importance on hospital standards (p-value<0.001). None of the other sixteen survey items differed by region.
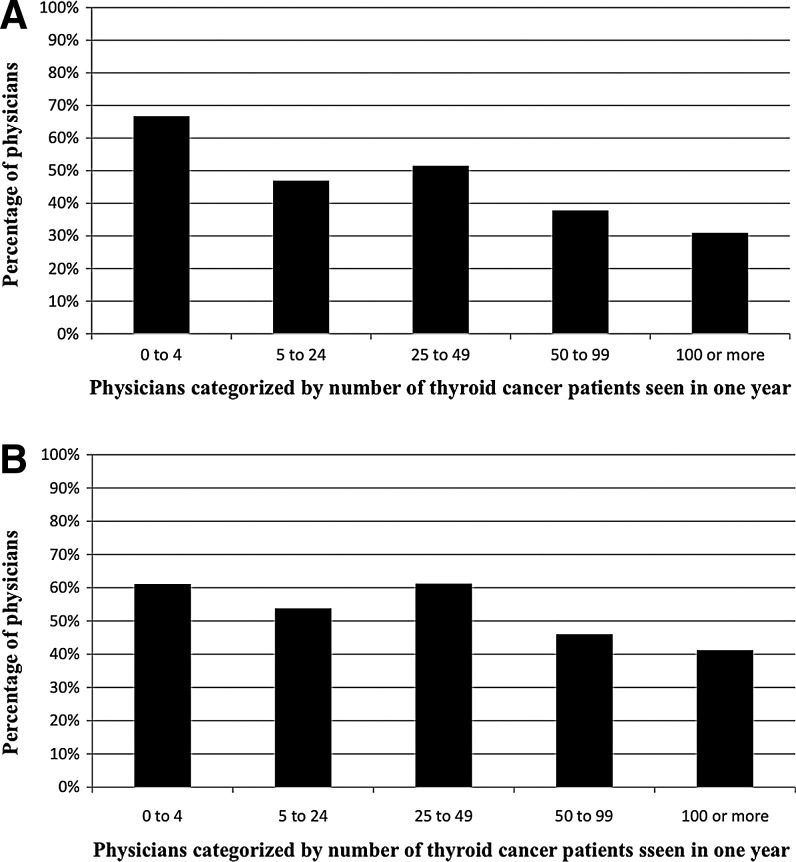
After controlling for physician gender, age, specialization, and whether the provider personally administers RAI, lower volume physicians were statistically more likely to place importance on patients' worry (p<0.001) (Fig. 2A and Table 3) and physicians' worry about death (p=0.016) compared to high-volume physicians (Fig. 2B and Table 4). Other factors more likely to be rated as quite or very important to physicians with lower thyroid cancer patient volume include accepted standards at affiliated hospital (p=0.020), beliefs about RAI expressed by colleagues comanaging patients (p=0.003), and patient distance from the nearest facility administering RAI (p=0.012).
FIG. 2.
(A) Percentage of physicians who felt patient worry about death is quite or very important in influencing whether the patient receives RAI therapy for thyroid cancer. (B) Percentage of physicians who felt physician worry about death is quite or very important in influencing whether the patient receives RAI therapy for thyroid cancer.
Table 3.
Multivariable Analysis of Physician Characteristics Associated with Importance Placed on Patient Worry About Death in Determining Radioactive Iodine Use
| Physicians reporting patient worry about death as quite or very important for decision on RAI use [n (%)] | Multivariable p-value | |
|---|---|---|
| Gender | 0.634 | |
| Male | 169 (43) | |
| Female | 60 (45) | |
| Age | 0.245 | |
| <50 years old | 132 (46) | |
| >50 years old | 97 (41) | |
| Specialization | ||
| Endocrinology | 157 (44) | Ref. |
| Nuclear medicine | 30 (38) | 0.136 |
| Other | 42 (47) | 0.979 |
| Number of thyroid cancer patients seen in 1 year | <0.001 | |
| 0–4 | 12 (67) | |
| 5–24 | 67 (47) | |
| 25–49 | 69 (51) | |
| 50–99 | 42 (38) | |
| 100 or more | 30 (31) | |
| Administer RAI | 0.067 | |
| Yes | 99 (46) | |
| No | 130 (42) | |
Table 4.
Multivariable Analysis of Physician Characteristics Associated with Importance Placed on Physician Worry About Death in Determining Radioactive Iodine Use
| Physicinas reporting physician worry about death as quite or very important for decision on RAI use [n (%)] | Multivariable p-value | |
|---|---|---|
| Gender | 0.214 | |
| Male | 197 (50) | |
| Female | 74 (56) | |
| Age | 0.124 | |
| <50 years old | 116 (49) | |
| >50 years old | 155 (53) | |
| Specialization | ||
| Endocrinology | 185 (52) | Ref. |
| Nuclear medicine | 36 (45) | 0.147 |
| Other | 50 (56) | 0.479 |
| Number of thyroid cancer patients seen in 1 year | 0.016 | |
| 0–4 | 11 (61) | |
| 5–24 | 77 (54) | |
| 25–49 | 82 (61) | |
| 50–99 | 51 (46) | |
| 100 or more | 40 (41) | |
| Administer RAI | 0.448 | |
| Yes | 111 (52) | |
| No | 160 (51) | |
Discussion
The results of this study improve our knowledge of the factors that influence whether a physician recommends RAI for a patient with well-differentiated thyroid cancer. Extent of disease, adequacy of surgical resection, patients' willingness to receive RAI, and patients' age were the most important factors influencing recommendations regarding RAI use for patients with thyroid cancer. Interestingly, patients' and physicians' worry about death also played an important role in decision making. Compared to higher volume physicians, physicians with less thyroid cancer cases each year were more likely to prioritize patients' worry and physicians' worry about death, accepted standards at affiliated hospital, beliefs about RAI expressed by colleagues comanaging patients, and patient distance from the nearest facility administering RAI.
Previous work has shown a significant rise in the proportion of patients with well-differentiated thyroid cancer treated with RAI after surgery, as well as a wide hospital-level variation in RAI use in these patients (7). It is now known that both the specialty of the primary decision maker (18) and the thyroid surgeon's tendency for more aggressive management are associated with greater RAI use in patients with low-risk disease (17). However, the clinical and nonclinical factors influencing physician decision making in RAI use have not been previously studied. As the most recent clinical guidelines leave the use of RAI to provider discretion in a large proportion of patients (20), understanding the clinical and nonclinical factors that providers use in their decision-making process is critical to understanding the rise in RAI use. This knowledge will ultimately encourage tailoring of treatment to disease severity. Our study has shown that significant importance is placed on both physicians' and patients' worry about death. This is remarkable considering that there is unclear survival benefit to RAI use in the majority of thyroid cancer patients as most patients have an excellent prognosis regardless of intervention (10,20–22). A recent study by Schvartz et al., with follow-up of 10.3 years, failed to prove any survival benefit of RAI after surgery in a large cohort of low-risk, well-differentiated thyroid cancer patients (13). Moreover, the benefits of RAI use in these patients may not always exceed the risks (8,14,15).
Several studies have investigated the importance of worry about death from cancer when considering treatment, especially among breast and ovarian cancer patients (23,24). However, these primarily focus on patients with advanced cancer. Our study's results are novel, in that, worry about death appears to influence treatment in a cancer with a generally favorable prognosis.
The strengths of this study include an innovative research question, a large sample size of providers and hospitals, and a high response rate among providers. However, this study has limitations. First, survey studies in general carry a risk for nonresponse bias. Second, it is not known whether the provider report to the survey questions is consistent with provider treatment behavior. Third, although the survey included a comprehensive set of factors that may influence physician decision making, there may be other factors that we did not inquire about. Finally, although this study focuses solely on the physician, the patient is also involved in the decision-making process.
Despite its limitations, this study sheds light onto some of the factors involved in provider decision making in regard to RAI use in patients with well-differentiated thyroid cancer. Surprisingly, importance is placed on worry about death by both patients and providers. As the mortality from well-differentiated thyroid cancer is low, the importance placed on death in decision making may be unwarranted. Fear may be driving the rise in RAI use, suggesting a role for better provider education and further research into the risk–benefit of low-risk thyroid cancer treatments.
Acknowledgments
The authors thank Brittany Gay, Barbara Salem, Ashley Gay, and Kathryn Schuessler for their work in data collection and processing. We also acknowledge the endorsement from the American Thyroid Association and the collaboration with the American College of Surgeons Commission on Cancer. Dr. Papaleontiou is supported by the NIH Institutional National Research Service Award (T32). This study was funded by K07CA154595-02 to Dr. Haymart from the National Institutes of Health, the University of Michigan Comprehensive Cancer Center Idea Award, the Cancer Surveillance and Outcomes Research Team (CanSORT) Pilot of Feasibility Fund, and the Elizabeth Caroline Crosby Fund.
Disclosure Statement
There are no commercial associations or conflicts of interest to be disclosed by any of the authors of this manuscript.
References
- 1.Siegel R. Naishadham D. Jamal A. Cancer statistics. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Davies L. Welch HG. Increasing incidence of thyroid cancer in the United States, 1973–2002. JAMA. 2006;295:2164–2167. doi: 10.1001/jama.295.18.2164. [DOI] [PubMed] [Google Scholar]
- 3.Burke JP. Hay ID. Dignan F. Goellner JR. Achenbach SJ. Oberg AL. Melton LJ., 3rd Long-term trends in thyroid carcinoma: a population-based study in Olmsted County, Minnesota, 1935–1999. Mayo Clin Proc. 2005;80:753–758. doi: 10.1016/S0025-6196(11)61529-2. [DOI] [PubMed] [Google Scholar]
- 4.Hodgson NC. Button J. Solorzano CC. Thyroid cancer: is the incidence still increasing? Ann Surg Oncol. 2004;11:1093–1097. doi: 10.1245/ASO.2004.03.066. [DOI] [PubMed] [Google Scholar]
- 5.Zhu C. Zheng T. Kilfoy BA. Han X. Ma S. Ba Y. Bai Y. Wang R. Zhu Y. Zhang Y. A birth cohort analysis of the incidence of papillary thyroid cancer in the United States, 1973–2004. Thyroid. 2009;19:1061–1066. doi: 10.1089/thy.2008.0342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cancer Facts and Figures 2012 American Cancer Society. www.cancer.org/Research/CancerFactsFigures/ACSPC-031941. [Jun 1;2012 ]. www.cancer.org/Research/CancerFactsFigures/ACSPC-031941
- 7.Haymart MR. Banerjee M. Stewart AK. Koenig RJ. Birkmeyer JD. Griggs J. Use of radioactive iodine for thyroid cancer. JAMA. 2011;306:721–728. doi: 10.1001/jama.2011.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iyer NG. Morris LG. Tuttle RM. Shaha AR. Ganly I. Rising incidence of second cancers in patients with low-risk (T1N0) thyroid cancer who receive radioactive iodine therapy. Cancer. 2011;117:4439–4446. doi: 10.1002/cncr.26070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Podnos YD. Smith DD. Wagman LD. Ellenhorn JD. Survival in patients with papillary thyroid cancer is not affected by the use of radioactive isotope. J Surg Oncol. 2007;96:3–7. doi: 10.1002/jso.20656. [DOI] [PubMed] [Google Scholar]
- 10.Jonklaas J. Sarlis NJ. Litofsky D. Ain KB. Bigos ST. Brierley JD. Cooper DS. Haugen BR. Ladenson PM. Magner J. Robbins J. Ross DS. Skarulis M. Maxon HR. Sherman SI. Outcomes of patients with differentiated thyroid carcinoma following initial therapy. Thyroid. 2006;16:1229–1242. doi: 10.1089/thy.2006.16.1229. [DOI] [PubMed] [Google Scholar]
- 11.Hay ID. McConahey WM. Goellner JR. Managing patients with papillary thyroid carcinoma: insights gained from the Mayo Clinic's experience of treating 2512 consecutive patients during 1940 through 2000. Trans Am Clin Climatol Assoc. 2002;113:241–260. [PMC free article] [PubMed] [Google Scholar]
- 12.Ito Y. Masuoka H. Fukushima M. Inoue H. Kihara M. Tomoda C. Higashiyama T. Takamura Y. Kobayashi K. Miya A. Miyauchi A. Excellent prognosis of patients with solitary T1N0M0 papillary thyroid carcinoma who underwent thyroidectomy and elective lymph node dissection without radioiodine therapy. World J Surg. 2010;34:1285–1290. doi: 10.1007/s00268-009-0356-0. [DOI] [PubMed] [Google Scholar]
- 13.Schvartz C. Bonnetain F. Dabakuyo S. Gauthier M. Cueff A. Fieffe S. Pochart JM. Cochet I. Crevisy E. Dalac A. Papathanasiou D. Toubeau M. Impact on overall survival of radioactive iodine in low-risk differentiated thyroid cancer patients. J Clin Endocrinol Metab. 2012;97:1526–1535. doi: 10.1210/jc.2011-2512. [DOI] [PubMed] [Google Scholar]
- 14.Chow SM. Side effects of high-dose radioactive iodine for ablation or treatment of differentiated thyroid cancer. J HK Coll Radiol. 2005;8:127–135. [Google Scholar]
- 15.Van Nostrand D. The benefits and risks of I-131 therapy in patients with well differentiated thyroid cancer. Thyroid. 2009;19:1381–1391. doi: 10.1089/thy.2009.1611. [DOI] [PubMed] [Google Scholar]
- 16.Sawka AM. Thabane L. Parlea L. Ibrahim-Zada I. Tsang RW. Brierley JD. Straus S. Ezzat S. Goldstein DP. Second primary malignancy risk after radioactive iodine treatment for thyroid cancer: a systematic review and meta-analysis. Thyroid. 2009;19:451–457. doi: 10.1089/thy.2008.0392. [DOI] [PubMed] [Google Scholar]
- 17.Haymart MR. Banerjee M. Yang D. Stewart AK. Doherty GM. Koenig RJ. Griggs JJ. The relationship between extent of thyroid cancer surgery, use of radioactive iodine. Ann Surg. 2012 doi: 10.1097/SLA.0b013e31826e8915. (In press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haymart MR. Banerjee M. Yang D. Stewart AK. Koenig RJ. Griggs JJ. The role of clinicians in determining radioactive iodine use for low-risk thyroid cancer. Cancer. 2012 2012 Jun 28; doi: 10.1002/cncr.27721. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dillman DA. Mail, Internet Surveys: The Tailored Design Method. 2nd. Wiley; New York: 2007. [Google Scholar]
- 20.Cooper DS. Doherty GM. Haugen BR. Kloos RT. Lee SL. Mandel SJ. Mazzaferri EL. McIver B. Pacini F. Schlumberger M. Sherman SI. Steward DL. Tuttle RM. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 21.Gilliland FD. Hunt WC. Morris DM. Key CR. Prognostic factors for thyroid carcinoma: a population- based study of 15 698 cases from the Surveillance, Epidemiology, and End Results (SEER) program 1973–1991. Cancer. 1997;79:564–573. doi: 10.1002/(sici)1097-0142(19970201)79:3<564::aid-cncr20>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 22.Hundahl SA. Fleming ID. Fremgen AM. Menck HR. A National Cancer Data Base report on 53 856 cases of thyroid carcinoma treated in the US, 1985–1995. Cancer. 1998;83:2638–2648. doi: 10.1002/(sici)1097-0142(19981215)83:12<2638::aid-cncr31>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 23.Shnn EH. Taylor CL. Kilgore K. Valentine A. Bodurka DC. Kavanagh J. Sood A. Li Y. Basen-Engquist K. Associations with worry about dying and hopelessness in ambulatory ovarian cancer patients. Palliat Support Care. 2009;7:299–306. doi: 10.1017/S1478951509990228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohaeri BM. Ofl AB. Campbell OB. Relationship of knowledge of psychosocial issues about cancer with psychic distress and adjustment among breast cancer clinic attendees in a Nigerian teaching hospital. Psychooncology. 2012;21:419–426. doi: 10.1002/pon.1914. [DOI] [PubMed] [Google Scholar]