Abstract
Background
Effects of thyroid function status on lipoprotein metabolism may extend into the euthyroid range. Low-density lipoprotein (LDL) metabolism is governed by proprotein convertase subtilisin-kexin type 9 (PCSK9), which down-regulates LDL receptor expression, resulting in higher LDL cholesterol (LDL-C). Here, we tested whether plasma PCSK9 correlates with thyroid function in nonobese and obese euthyroid subjects.
Methods
We assessed the extent to which plasma PCSK9 is determined by thyrotropin (TSH) in 74 euthyroid subjects (31 women; TSH between 0.5 and 4.0 mU/L and free thyroxine [FT4] between 11.0 and 19.5 pM) with varying degrees of obesity (body mass index [BMI] ranging from 20.2 to 40.4 kg/m2).
Results
TSH, FT4, PCSK9, non–high-density lipoprotein cholesterol (non-HDL-C), LDL-C, and apolipoprotein B (apoB) levels were not different between 64 nonobese subjects (BMI<30 kg/m2) and 10 obese subjects (BMI≥30 kg/m2; p>0.20 for each). PCSK9 correlated positively with TSH in nonobese subjects (r=0.285, p=0.023). In contrast, PCSK9 was not associated positively with TSH in obese subjects (r=−0.249, p=0.49). The relationship of PCSK9 with TSH was different between nonobese and obese subjects when taking age, sex, FT4, and the presence of anti-thyroid antibodies into account (multiple linear regression analysis: β=−0.320, p=0.012 for the interaction term between the presence of obesity and TSH on PCSK9), and was also modified by BMI as a continuous trait (β=−0.241, p=0.062 for the interaction term between BMI and TSH on PCSK9). Non-HDL-C, LDL-C, and apoB levels were dependent on PCSK9 in nonobese subjects (p≤0.01 for each), but not in obese subjects (p>0.50), Accordingly, BMI interacted negatively with PCSK9 on non-HDL-C (p=0.028) and apoB (p=0.071).
Conclusions
This study suggests that circulating PCSK9 levels correlate with thyroid function even in the normal range. This relationship appears to be blunted by obesity. Thyroid functional status may influence cholesterol metabolism through the PCSK9 pathway.
Introduction
The impact of thyroid dysfunction on cardiovascular morbidity and mortality is receiving continued interest. Current evidence strongly favors the notion that overt hypothyroidism confers increased cardiovascular risk, but equivocal effects of subclinical hypothyroidism on incident cardiovascular disease have been reported (1–6). More recently, the concept is evolving that higher levels of circulating thyrotropin (TSH) and lower thyroid hormone (TH) levels, even within the euthyroid range, adversely affect cardiovascular risk factors, including plasma levels of pro-atherogenic lipoproteins (7–10), as well as subclinical atherosclerosis (11,12).
It is well established that thyroid functional status has multiple and complex effects on lipoprotein metabolism (13,14). Despite the stimulation of hepatic cholesterol synthesis, TH lowers plasma low-density lipoprotein cholesterol (LDL-C) due to its ability to up-regulate low-density lipoprotein receptor (LDL-R) expression (13–18). Among the other factors involved, TH also affects lipoprotein metabolism via effects on intestinal cholesterol absorption, hepatic lipase, lipoprotein lipase, and cholesteryl ester transfer protein, a lipid transfer protein that specifically transfers cholesteryl esters between lipoproteins in plasma (13,14,19–21). Although it has long been recognized that TH plays a crucial role in the regulation of plasma lipoprotein and cholesterol metabolism (13,14), it was reported only recently that TSH may directly stimulate the expression of 3-hydroxy-3-methyl-glutaryl coenzyme A reductase, the rate-limiting enzyme in hepatic cholesterol synthesis (22).
The discovery that proprotein convertase subtilisin-kexin type 9 (PCSK9) represents a key regulatory pathway for LDL-R degradation has greatly impacted current concepts about the regulation of cholesterol metabolism. PCSK9 is a secreted protease that binds to the extracellular domain of the LDL-R, and targets it for lysosomal degradation after endocytosis (23,24). PCSK9 prevents LDL-R recycling to the cell surface and results in decreased LDL-R availability (23,24). Accordingly, plasma cholesterol is decreased in mice lacking PCSK9 (25). In humans, the fractional catabolic rate of apolipoprotein B (apoB) is correlated negatively with circulating PCSK9 levels, whereas plasma LDL-C, non–high-density lipoprotein cholesterol (non-HDL-C) and apoB levels have been shown to be correlated positively with PCSK9 (26–28). Interindividual differences in plasma PCSK9 concentrations are, therefore, likely to be physiologically relevant (25,26). Importantly, the sterol regulatory element binding protein transcription factor 2 (SREBP2), which stimulates intracellular cholesterol synthesis and promotes LDL-R gene expression, is also able to up-regulate PCSK9 (23,24). Of further relevance, SREBP2 has been identified as a TH target, explaining at least in part the effects of thyroid functional status on LDL-R expression (29). This makes it plausible to postulate that thyroid functional status could play a role in PCSK9 regulation.
In view of recent findings suggesting that the effects of thyroid function on lipoprotein metabolism may extend in the euthyroid range (7–10), we asked the question whether plasma PCSK9 levels are related to thyroid function in subjects without overt or subclinical hypo- and hyperthyroidism. The present study was initiated to determine the extent to which plasma PCSK9 is dependent on TSH or free thyroxine (FT4) in euthyroid subjects with varying degrees of obesity.
Materials and Methods
Patients and methods
Subjects (aged >18 years) were recruited by an advertisement in local newspapers. Eligible subjects were of Caucasian ethnicity, had a negative history of thyroid disease, did not show thyroid abnormality on physical examination, and had serum TSH and FT4 levels within the institutional reference range. Previously diagnosed diabetes mellitus, known hypertension, renal function abnormalities (elevated serum creatinine and/or elevated urinary albumin excretion), liver function abnormalities (transaminase levels >1.5 times the upper normal level), clinically manifest cardiovascular disease, and pregnancy were exclusion criteria. Smokers, subjects who consumed >3 alcoholic drinks daily, and subjects who used lipid-lowering drugs or other medications (except for oral contraceptives) were also excluded. No participant had previously-diagnosed genetically-determined hyperlipidemia, but we did not exclude subjects with high plasma total cholesterol or triglyceride concentrations in order to ensure generalizability of the data obtained as much as possible. Body mass index (BMI) was calculated as weight divided by height squared (kg/m2). Obesity was defined as BMI≥30 kg/m2. After 15 minutes of rest, systolic and diastolic blood pressure were measured thrice with 5-minutes intervals with a digital sphygmomanometer in the supine position. Homeostasis model assessment (HOMAir) was used as a measure of insulin sensitivity using the following equation: fasting plasma insulin (mU/L)×glucose (mM)/22.5 (30). All participants were studied after an overnight fast, and venous blood was obtained between 8 and 10 a.m. The medical ethics committee of the University Medical Center Groningen, The Netherlands, approved the study. All participants provided written informed consent.
Laboratory analyses
Serum and EDTA-anticoagulated plasma samples were stored at −80°C until analysis. Glucose was measured shortly after blood collection. Serum TSH (sandwich principle; Roche Diagnostics GmbH, Mannheim Germany, cat. no. 117314591; reference range 0.5–4.0 mU/L) and FT4 (competition principle; Roche Diagnostics GmbH; cat. no. 11731297; reference range 11.0–19.5 pM) were measured by an electrochemiluminescence immunoassay using a MODULAR ANALYTICS immunoassay analyzer (Roche GmbH, Mannheim, Germany). Anti–thyroid peroxidase (anti-TPO) and anti-thyroglobulin (anti-Tg) autoantibodies were determined using commercially available automated enzyme-linked immunoassays (ImmunoCap cat nos. 14-4508-35 and 14-4507-35, respectively; Phadia, Freiburg, Germany). Thyroid autoantibodies were considered to be positive in case of anti-TPO antibodies >60 IU/mL or anti-Tg antibodies >289 IU/mL, as indicated by the supplier. PCSK9 was measured using a sandwich enzyme-linked immunosorbent assay (25). Plasma cholesterol and triglycerides were assayed by routine enzymatic methods. HDL-C was measured with a homogeneous enzymatic colorimetric test. Non-HDL cholesterol (non-HDL-C) was calculated as the difference between plasma total cholesterol and HDL-C. LDL-C was calculated using the Friedewald formula. ApoB was measured by immunoturbidimetry. Plasma glucose was measured with an APEC glucose analyzer (APEC, Inc., Danvers, MA). Plasma insulin was assayed with a microparticle enzyme immunoassay. All intra- and interassay coefficients of variation were <6%.
Statistical analyses
Data are in median (interquartile range, IQR). Between-group differences were determined by Mann–Whitney U and Chi-square tests. Univariate relationships were calculated using Spearman's rank correlation coefficients. Multiple linear regression analyses were performed to assess the independent contribution of variables. Interaction terms were calculated as the product terms between TSH and BMI (dichotomized in BMI<30 and ≥30 kg/m2, and in addition as a continuous variable). For continuous variables, distributions centered to the mean were made by subtracting the group mean value from the individual value of the variable of interest to account for possible outliers. Interaction terms were considered to be significant at a two-sided p-value <0.10. Otherwise, statistical significance was set at a two-sided p-value<0.05.
Results
Seventy-four subjects participated (Table 1). Sixty-four subjects were nonobese subjects (BMI<30 kg/m2), and 10 were obese subjects (BMI≥30 kg/m2). Two nonobese women used oral contraceptives; other medications were not used. Four of the nonobese and none of the obese subjects had either positive anti-TPO or anti-TG autoantibodies (p=0.59). Age (p=0.78), and sex distribution (p=0.23) were not different between the nonobese and obese subjects. Blood pressure, plasma insulin, and HOMAir were higher in the obese subjects. Plasma total cholesterol was somewhat lower in the obese subjects (range: 4.14–7.08 mM) than in the nonobese subjects (range: 3.71–8.29 mM), which was due to lower HDL-C (Table 1). Non-HDL-C, LDL-C, triglycerides, and apoB levels were not significantly different between the nonobese and obese subjects. TSH, FT4, and PCSK9 levels were also not significantly different between the nonobese and obese subjects (Table 1). In addition, the levels of PCSK9 (131 [109–191] μg/L) and TSH (1.36 [1.02–1.74] mU/L) were lower in women than in men (169 [142–198] μg/L, p=0.043 and 1.80 [1.33–2.22] mU/L, p=0.022, respectively), but FT4 was not different between women and men (p=0.92).
Table 1.
Clinical Characteristics, Plasma Glucose, Insulin, Homeostasis Model Assessment, Lipoproteins, PCSK9, Thyrotropin, and Free Thyroxine in All Subjects Combined, and Separately in Nonobese Subjects and Obese Subjects
| All subjects (n=74) | Nonobese subjects (n=64) | Obese subjects (n=10) | |
|---|---|---|---|
| Age (years) | 55 [48–62] | 54 [48–63] | 57 [44–58] |
| Sex (M/F) | 31/43 | 28/36 | 3/7 |
| Systolic blood pressure (mmHg) | 128 [118–142] | 128 [117–138] | 142 [130–152]** |
| Diastolic blood pressure (mmHg) | 82 [76–88] | 79 [75–86] | 88 [84–94]** |
| BMI (kg/m2) | 25.4 [23.6–27.4] | 24.7 [23.4–26.4] | 33.3 [30.2–35.2]*** |
| Glucose (mM) | 5.7 [5.1–6.1] | 5.7 [5.2–6.1] | 5.8 [5.1–6.4] |
| Insulin (mU/L) | 6.9 [4.8–8.5] | 6.0 [4.4–8.1] | 11.1 [8.4–19.8]** |
| HOMAir (mU mmol/[L2×22.5]) | 1.61 [1.15–2.26] | 1.50 [1.12–2.04] | 3.00 [2.17–3.70]*** |
| Total cholesterol (m) | 5.70 [5.03–6.46] | 5.76 [5.26–6.48] | 4.96 [4.88–5.10]* |
| Non-HDL-C (mM) | 4.10 [3.65–5.03] | 4.21 [3.57–5.08] | 3.82 [3.72–4.11] |
| LDL-C (mM) | 3.51 [2.94–4.22] | 3.64 [2.97–4.27] | 3.16 [2.78–3.44] |
| HDL-C (m) | 1.40 [1.16–1.72] | 1.46 [1.23–1.74] | 1.15 [0.97–1.32]** |
| Triglycerides (mM) | 1.31 [0.90–1.91] | 1.31 [0.89–1.90] | 1.36 [0.97–2.22] |
| ApoB (g/L) | 0.93 [0.80–1.12] | 0.93 [0.79–1.18] | 0.90 [0.80–1.04] |
| PCSK9 (μg/L) | 150 [116–192] | 150 [116–191] | 150 [78–192] |
| TSH (mU/L) | 1.54 [1.25–2.02] | 1.61 [1.14–2.04] | 1.36 [1.27–1.56] |
| FT4 (pM) | 13.34 [12.48–14.69] | 13.38 [12.56–14.73] | 13.17 [12.32–14.03] |
Values are presented as mean IQR.
p≤0.05, **p≤0.01, ***p≤0.001 vs. nonobese subjects.
BMI, body mass index; non-HDL-C, non-HDL cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; ApoB, apolipoprotein B; PCSK9, proprotein convertase subtilisin-kexin type 9; TSH, thyrotropin; FT4, free thyroxine.
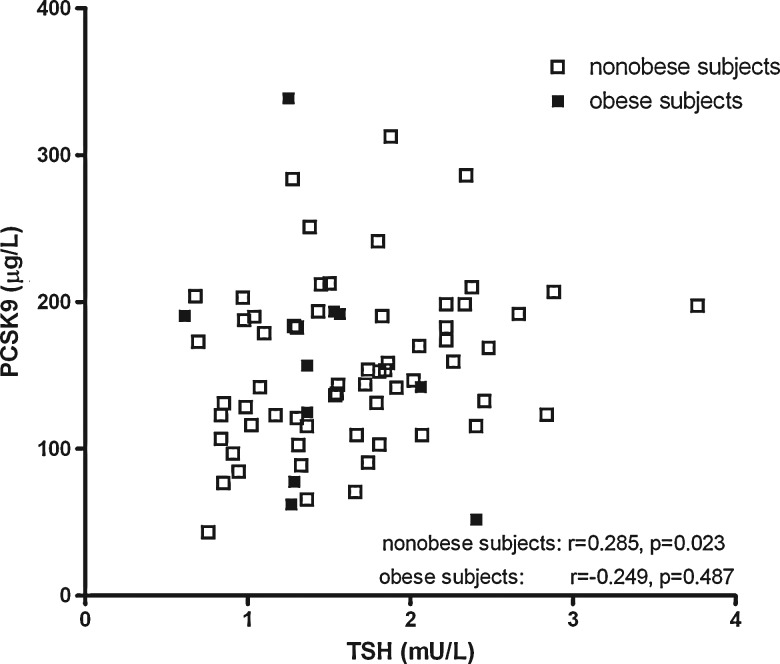
Total cholesterol, non-HDL-C, LDL-C, triglycerides, and apoB levels were correlated positively with PCSK9 in all subjects combined. Total cholesterol, non-HDL-C, LDL-C, triglycerides, and apoB levels were correlated positively with PCSK9 in all subjects combined, and in nonobese subjects, but no such relationships were found in obese subjects (Table 2). PCSK9 was related positively to insulin or HOMAir in all subjects combined and in obese subjects separately, although PCSK9 was not significantly related to BMI. Notably, PCSK9 was correlated positively with TSH in nonobese subjects (r=0.285, p=0.023). In contrast, no positive relationship of PCSK9 with TSH was observed in the obese subjects (r=−0.249, p=0.49). Figure 1 shows the scatter plot of plasma TSH and PCSK9 in nonobese and obese subjects. BMI was correlated positively with TSH in obese subjects, but thyroid function parameters were not significantly related to insulin, HOMAir and lipid variables. FT4 was unrelated to PCSK9 in the nonobese and obese subjects.
Table 2.
Correlation Coefficients Between Thyrotropin, Free Thyroxine, PCSK9, Clinical Characteristics, Plasma Glucose, Insulin, Homeostasis Model Assessment, and Lipoproteins in All Subjects Combined, and Separately in Nonobese Subjects and Obese Subjects
| |
All subjects (n=74), correlation coefficient with |
Nonobese subjects (n=64), correlation coefficient with |
Obese subjects (n=10), correlation coefficient with |
||||||
|---|---|---|---|---|---|---|---|---|---|
| PCSK9 | TSH | FT4 | PCSK9 | TSH | FT4 | PCSK9 | TSH | FT4 | |
| Age | −0.122 | 0.027 | 0.114 | −0.143 | 0.103 | 0.179 | 0.024 | 0.380 | −0.524 |
| BMI | −0.003 | −0.052 | −0.092 | 0.026 | −0.009 | −0.056 | −0.139 | 0.869*** | −0.430 |
| Glucose | −0.151 | 0.046 | −0.003 | −0.105 | 0.109 | 0.047 | −0.326 | −0.198 | −0.265 |
| Insulin | 0.237* | −0.093 | −0.158 | 0.226 | −0.091 | −0.175 | 0.745* | 0.195 | −0.079 |
| HOMAir | 0.216 | −0.085 | −0.138 | 0.185 | −0.062 | −0.124 | 0.855** | 0.097 | −0.261 |
| Total cholesterol | 0.354** | 0.114 | 0.037 | 0.405*** | 0.089 | 0.043 | 0.370 | 0.225 | −0.006 |
| Non-HDL-C | 0.401*** | 0.024 | 0.062 | 0.439*** | 0.026 | 0.070 | 0.224 | −0.091 | −0.103 |
| LDL-C | 0.291* | 0.083 | 0.058 | 0.314** | 0.081 | 0.039 | 0.067 | −0.122 | 0.236 |
| HDL-C | −0.144 | 0.136 | −0.088 | −0.168 | 0.079 | −0.126 | −0.200 | 0.207 | 0.018 |
| Triglycerides | 0.329** | −0.034 | 0.106 | 0.386** | −0.038 | 0.158 | 0.067 | 0.109 | −0.176 |
| ApoB | 0.271* | −0.064 | −0.007 | 0.319** | −0.058 | 0.008 | −0.091 | 0.012 | 0.140 |
| PCSK9 | 0.201 | 0.031 | 0.285* | 0.057 | −0.249 | −0.067 | |||
Spearman's rank correlation coefficients are shown. Statistically significant correlations are shown in bold.
p≤0.05, **p≤0.01, ***p≤0.001.
BMI, body mass index; non-HDL-C, non-HDL cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; ApoB, apolipoprotein B; PCSK9, proprotein convertase subtilisin-kexin type 9; TSH, thyrotropin; FT4, free thyroxine.
FIG. 1.
Scatter plot showing the univariate relationship between thyrotropin (TSH) and proprotein convertase subtilisin-kexin type 9 (PCSK9) in nonobese (n=64) and obese subjects (n=10).
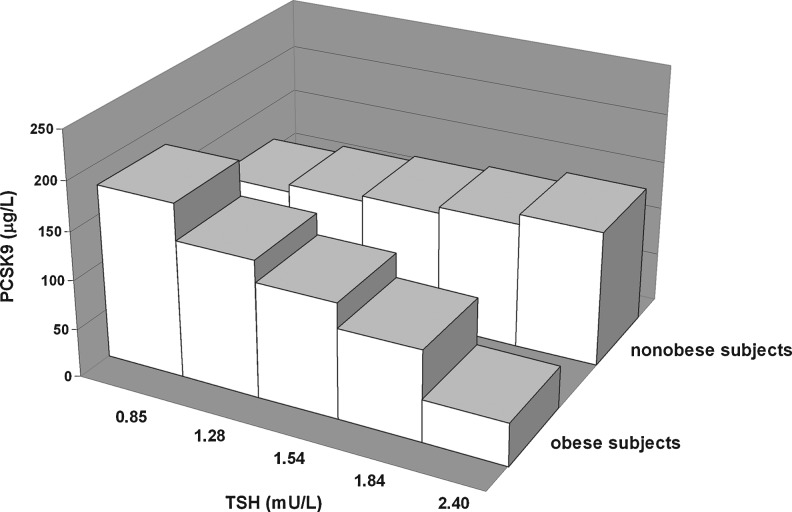
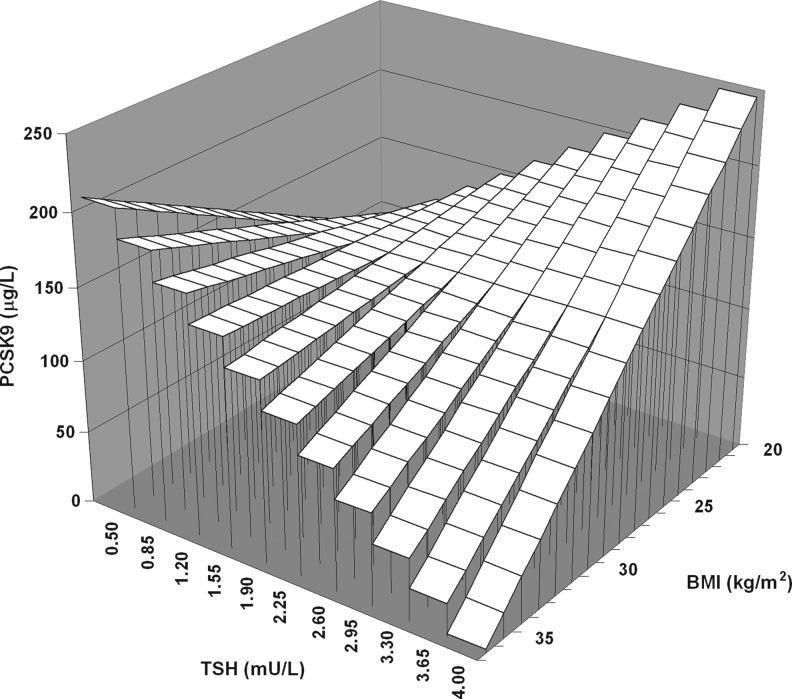
To determine whether the relationship of TSH with PCSK9 was different between the nonobese and obese subjects, multiple linear regression analysis was performed, which included the interaction term between the presence of obesity and TSH levels. In a model that accounted for age, sex, FT4, and the presence of thyroid autoantibodies, a negative interaction between the presence of obesity and TSH on PCSK9 levels was observed (β=−0.320, p=0.012; Table 3, model 1). This interaction was also present after additional adjustment for HOMAir (β=−0.330, p=0.008). In an alternative analysis, which included BMI as continuous trait, BMI was again found to interact with TSH on PCSK9 (β=−0.241, p=0.062; Table 3, model 2). These interactions remained essentially similar after the exclusion of those 4 subjects who were positive for thyroid autoantibodies (model 1: β=−0.325, p=0.015; model 2: β=−0.271, p=0.046). Thus, these analyses were consistent with the possibility that the positive relationship of the PCSK9 level with TSH was blunted by increasing adiposity. Graphical presentations of the influence of obesity and BMI as continuous traits on the relationship of PCSK9 with TSH are shown in Figures 2 and 3. Finally, we assessed whether the relationship of plasma lipoprotein cholesterol with PCSK9 was modified by obesity. Negative interactions between BMI and PCSK9 impacting on both non-HDL-C (β=−0.244, p=0.028) and apoB (β=−0.207, p=0.071) were observed after adjustment for age and sex (data not shown).
Table 3.
Multiple Linear Regression Analyses Demonstrating Interactions Between Obesity, Body Mass Index, and Thyrotropin on PCSK9 in 74 Euthyroid Subjects
| |
Model 1 |
Model 2 |
||
|---|---|---|---|---|
| β | p-Value | β | p-Value | |
| Age | −0.092 | 0.45 | −0.106 | 0.40 |
| Sex (men vs. women) | −0.244 | 0.050 | −0.193 | 0.13 |
| Thyroid autoantibodies (yes vs. no) | −0.114 | 0.32 | −0.144 | 0.22 |
| FT4 | 0.058 | 0.61 | 0.067 | 0.57 |
| TSH | 0.197 | 0.11 | 0.068 | 0.58 |
| Obesity (yes vs. no) | −0.074 | 0.53 | ||
| BMI | 0.012 | 0.92 | ||
| Interaction TSH×obesity (yes vs. no) | −0.320 | 0.012 | ||
| Interaction TSH×BMI (continuous variable) | −0.241 | 0.062 | ||
Obesity is defined as BMI≥30 kg/m2.
β, standardized regression coefficient; FT4, free thyroxine; TSH, thyrotropin; BMI, body mass index.
FIG. 2.
Three-dimensional bar graph showing the negative interaction of obesity (z-axis) with quintiles of thyrotropin (TSH) (x-axis) on proprotein convertase subtilisin-kexin type 9 (PCSK9) (y-axis) in 74 euthyroid subjects. The standardized regression coefficient of the interaction term obtained by the multiple linear regression model 1 (Table 3) is used.
FIG. 3.
Graphical presentation of the negative interaction of body mass index (BMI) as continuous trait (z-axis) with thyrotropin (TSH) (x-axis) on proprotein convertase subtilisin-kexin type 9 (PCSK9) (y-axis) in 74 euthyroid subjects. The standardized regression coefficient of the interaction term obtained by the multiple linear regression model 2 (Table 3) is used.
Discussion
This study demonstrates for the first time that plasma PCSK9 levels are correlated positively with TSH in nonobese euthyroid subjects. In contrast, no positive relationship of PCSK9 with TSH was observed in obese individuals. Accordingly, the effect of TSH on PCSK9 was blunted by increasing adiposity, as inferred from negative interactions of TSH with obesity as a categorical variable, as well as with BMI as a continuous trait. The present data, therefore, support the hypothesis that variations in thyroid functional status within the euthyroid range may influence lipoprotein cholesterol metabolism via the PCSK9 pathway in nonobese subjects. Our results also raise the possibility that this relationship is disturbed in obesity.
As expected (27,28,31), PCSK9 was associated positively with insulin in all subjects combined, and also in obese individuals separately. We did not observe a relationship of PCSK9 with BMI. Previous studies showed no significant (28,31) or weaker (27) associations of PCSK9 with obesity. The currently documented positive relationship of total cholesterol, non-HDL-C, LDL-C, and apoB with PCSK9 levels in nonobese individuals is in line with other data, and underscores the important role of PCSK9 in cholesterol metabolism (23–25,27,28,31,32). Of potential interest, this relationship of apoB-containing lipoproteins with circulating PCSK9 was disturbed in obese subjects. In agreement, PCSK9 interacted negatively with BMI on non-HDL-C and apoB. In comparison, the negative impact of PCSK9 on LDL apoB catabolism was found to be absent in predominantly obese diabetic subjects (33), although a strong positive relationship of apoB-containing lipoproteins with PCSK9 has been observed in diabetes mellitus in another report (32).
Large-scale studies in euthyroid subjects have shown positive relationships of total cholesterol and apoB-containing lipoproteins with TSH (7,10), and negative relationships with FT4 (8). Whether such variable relationships of apoB-containing lipoproteins with either TSH or FT4 would reflect a direct effect of TSH (22), besides the more established direct contributions of TH on cholesterol metabolism (14), is unclear at present. In the current report, only the relationship of PCSK9 with TSH was statistically significant, which we interpret to suggest that variations in thyroid functional status within the euthyroid range influence circulating PCSK9. Obviously, further studies are needed to provide greater insights on the impact of thyroid functional status on intracellular cholesterol homeostasis, and to specifically address the role of SREBP2 and the PCSK9 pathway therein. Remarkably, adiposity was found to interact with TSH on PCSK9 levels in such a way that the effect of TSH on PCSK9 was blunted with increasing adiposity. This interaction remained present after controlling for insulin resistance, which is important because insulin resistance itself has been shown to be related to the TSH level (8), and to modify the effect of TSH on LDL-C (34). The mechanisms responsible for such a modification of the degree of adiposity on the relationship of PCSK9 with TSH are unknown, but seem unrelated to direct the effects of insulin, as prolonged intravenous insulin administration does not affect circulating PCSK9 levels in humans (35). Together with the lack of a positive relation of apoB-containing lipoproteins with PCSK9 in obesity, this finding would suggest that the degree of obesity could interfere with the effects of TH status on cholesterol metabolism via the PCSK9 pathway.
Several methodological aspects and limitations of our study need to be considered. First, we carried out a cross-sectional study, and cause–effect relationships cannot be unequivocally established. Second, the number of obese individuals in the current report was small. Notably, an effect modification of adiposity on the relationship of PCSK9 with TSH was also documented by additional multiple linear regression analyses in which the impact of the interaction of TSH with BMI as a continuous trait on PCSK9 was determined. Third, we took the presence of thyroid autoantibodies in the analyses into account, because autoimmune responses could enhance early atherogenesis even in euthyroid subjects (36). In the current report, the independent relationship of PCSK9 with TSH was not appreciably modified by the presence of thyroid autoantibodies. Fourth, reasoning that changes in FT4 in association with variations in TSH levels within the euthyroid range are more pronounced compared with changes in free triiodothyronine (FT3) (10), we did not measure FT3 levels, Furthermore, TH effects on apoB-lipoproteins during reversal of both hypo- and hyperthyroidism to euthyroidism are adequately documented by FT4 measurement only (18). Thus, a major additional contribution of variations in FT3 to the presently observed relationship of plasma PCSK9 with thyroid functional status seems unlikely. Finally, the present observations in euthyroid subjects warrant determination of the extent to which overt thyroid dysfunction and its treatment affects the PCSK9 pathway.
In conclusion, this study suggests that circulating PCSK9 levels correlate with thyroid function even in the normal range. This effect of thyroid functional status on PCSK9 regulation appears to be blunted by adiposity.
Acknowledgments
This study was supported in part by a grant from the Dutch Diabetes Research Foundation (to R.P.F.D.), a project grant from the NH&MRC (101867), and a Programme Blanc grant from the Agence Nationale de la Recherche (to G.L.). The authors appreciate the contribution of Dr. R. de Vries, MD, PhD (Department of Endocrinology, Ijongerschans Hospital, Heerenveen, The Netherlands), in subject recruitment. The expert technical assistance of F. Petrides (The Heart Research Institute, Sydney, Australia), Dr. C. Roozendaal, PhD (Laboratory Center, University Medical Center, Groningen, The Netherlands), and Dr. B.D. Dikkeschei, PhD (Laboratory of Clinical Chemistry, Isala Clinics, Zwolle, The Netherlands) is greatly appreciated.
Disclosure Statement
The authors state no conflict of interest.
References
- 1.Duntas LH. Wartofsky L. Cardiovascular risk and subclinical hypothyroidism: focus on lipids and new emerging risk factors. What is the evidence? Thyroid. 2007;17:1075–1084. doi: 10.1089/thy.2007.0116. [DOI] [PubMed] [Google Scholar]
- 2.Cappola AR. Fried LP. Arnold AM. Danese MD. Kuller LH. Burke GL. Tracy RP. Ladenson PW. Thyroid status, cardiovascular risk, and mortality in older adults. JAMA. 2006;295:1033–1041. doi: 10.1001/jama.295.9.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hak AE. Pols HA. Visser TJ. Drexhage HA. Hofman A. Witteman JC. Subclinical hypothyroidism is an independent risk factor for atherosclerosis and myocardial infarction in elderly women: the Rotterdam Study. Ann Intern Med. 2000;132:270–278. doi: 10.7326/0003-4819-132-4-200002150-00004. [DOI] [PubMed] [Google Scholar]
- 4.Ochs N. Auer R. Bauer DC. Nanchen D. Gussekloo J. Cornuz J. Rodondi N. Meta-analysis: subclinical thyroid dysfunction and the risk for coronary heart disease and mortality. Ann Intern Med. 2008;148:832–845. doi: 10.7326/0003-4819-148-11-200806030-00225. [DOI] [PubMed] [Google Scholar]
- 5.Rodondi N. den Elzen WP. Bauer DC. Cappola AR. Razvi S. Walsh JP. Asvold BO. Iervasi G. Imaizumi M. Collet TH. Bremner A. Maisonneuve P. Sgarbi JA. Khaw KT. Vanderpump MP. Newman AB. Cornuz J. Franklyn JA. Westendorp RG. Vittinghoff E. Gussekloo J Thyroid Studies Collaboration. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA. 2010;304:1365–1374. doi: 10.1001/jama.2010.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Asvold BO. Bjøro T. Platou C. Vatten LJ. Thyroid function and the risk of coronary heart disease: 12-year follow-up of the HUNT Study in Norway. Clin Endocrinol (Oxf) 2012;77:911–917. doi: 10.1111/j.1365-2265.2012.04477.x. [DOI] [PubMed] [Google Scholar]
- 7.Asvold BO. Vatten LJ. Nilsen TI. Bjøro T. The association between TSH within the reference range and serum lipid concentrations in a population-based study. The HUNT Study. Eur J Endocrinol. 2007;156:181–186. doi: 10.1530/eje.1.02333. [DOI] [PubMed] [Google Scholar]
- 8.Roos A. Bakker SJ. Links TP. Gans ROB. Wolffenbuttel BHR. Thyroid function is associated with components of the metabolic syndrome in euthyroid subjects. J Clin Endocrinol Metab. 2007;92:491–496. doi: 10.1210/jc.2006-1718. [DOI] [PubMed] [Google Scholar]
- 9.de Jesus Garduño-Garcia J. Alvirde-Garcia U. López-Carrasco G. Padilla Mendoza ME. Mehta R. Arellano-Campos O. Choza R. Sauque L. Garay-Sevilla ME. Malacara JM. Gomez-Perez FJ. Aguilar-Salinas CA. TSH and free thyroxine concentrations are associated with differing metabolic markers in euthyroid subjects. Eur J Endocrinol. 2010;163:273–278. doi: 10.1530/EJE-10-0312. [DOI] [PubMed] [Google Scholar]
- 10.Wang F. Tan Y. Wang C. Zhang X. Zhao Y. Song X. Zhang B. Guan Q. Xu J. Zhang J. Zhang D. Lin H. Yu C. Zhao J. Thyroid-stimulating hormone levels within the reference range are associated with serum lipid profiles independent of thyroid hormones. J Clin Endocrinol Metab. 2012;97:2724–2731. doi: 10.1210/jc.2012-1133. [DOI] [PubMed] [Google Scholar]
- 11.Dullaart RPF. de Vries R. Roozendaal C. Kobold AC. Sluiter WJ. Carotid artery intima media thickness is inversely related to serum free thyroxine in euthyroid subjects. Clin Endocrinol (Oxf) 2007;67:668–673. doi: 10.1111/j.1365-2265.2007.02943.x. [DOI] [PubMed] [Google Scholar]
- 12.Takamura N. Akilzhanova A. Hayashida N. Kadota K. Yamasaki H. Usa T. Nakazato M. Maeda T. Ozono Y. Aoyagi K. Thyroid function is associated with carotid intima-media thickness in euthyroid subjects. Atherosclerosis. 2009;204:e77–e81. doi: 10.1016/j.atherosclerosis.2008.09.022. [DOI] [PubMed] [Google Scholar]
- 13.Duntas LH. Thyroid disease and lipids. Thyroid. 2002;12:287–293. doi: 10.1089/10507250252949405. [DOI] [PubMed] [Google Scholar]
- 14.Pearce EN. Update in lipid alterations in subclinical hypothyroidism. J Clin Endocrinol Metab. 2012;97:326–333. doi: 10.1210/jc.2011-2532. [DOI] [PubMed] [Google Scholar]
- 15.Danese MD. Ladenson PW. Meinert CL. Powe NR. Clinical review 115: effect of thyroxine therapy on serum lipoproteins in patients with mild thyroid failure: a quantitative review of the literature. J Clin Endocrinol Metab. 2000;85:2993–3001. doi: 10.1210/jcem.85.9.6841. [DOI] [PubMed] [Google Scholar]
- 16.Chait A. Bierman EL. Albers JJ. Regulatory role of triiodothyronine in the degradation of low density lipoprotein by cultured human skin fibroblasts. J Clin Endocrinol Metab. 1979;48:887–889. doi: 10.1210/jcem-48-5-887. [DOI] [PubMed] [Google Scholar]
- 17.Bakker O. Hudig F. Meijssen S. Wiersinga WM. Effects of triiodothyronine and amiodarone on the promoter of the human LDL receptor gene. Biochem Biophys Res Commun. 1998;249:517–521. doi: 10.1006/bbrc.1998.9174. [DOI] [PubMed] [Google Scholar]
- 18.Diekman MJ. Anghelescu N. Endert E. Bakker O. Wiersinga WM. Changes in plasma low-density lipoprotein (LDL)- and high-density lipoprotein cholesterol in hypo- and hyperthyroid patients are related to changes in free thyroxine, not to polymorphisms in LDL receptor or cholesterol ester transfer protein genes. J Clin Endocrinol Metab. 2000;85:1857–1862. doi: 10.1210/jcem.85.5.6595. [DOI] [PubMed] [Google Scholar]
- 19.Dullaart RPF. Hoogenberg K. Groener JE. Dikkeschei LD. Erkelens DW. Doorenbos H. The activity of cholesteryl ester transfer protein is decreased in hypothyroidism: a possible contribution to alterations in high-density lipoproteins. Eur J Clin Invest. 1990;20:581–587. doi: 10.1111/j.1365-2362.1990.tb01904.x. [DOI] [PubMed] [Google Scholar]
- 20.Tan KC. Shiu SW. Kung AW. Effect of thyroid dysfunction on high-density lipoprotein subfraction metabolism: roles of hepatic lipase and cholesteryl ester transfer protein. J Clin Endocrinol Metab. 1998;83:2921–2924. doi: 10.1210/jcem.83.8.4938. [DOI] [PubMed] [Google Scholar]
- 21.Borggreve SE. De Vries R. Dullaart RPF. Alterations in high-density lipoprotein metabolism and reverse cholesterol transport in insulin resistance and type 2 diabetes mellitus: role of lipolytic enzymes, lecithin:cholesterol acyltransferase and lipid transfer proteins. Eur J Clin Invest. 2003;33:1051–1069. doi: 10.1111/j.1365-2362.2003.01263.x. [DOI] [PubMed] [Google Scholar]
- 22.Tian L. Song Y. Xing M. Zhang W. Ning G. Li X. Yu C. Qin C. Liu J. Tian X. Sun X. Fu R. Zhang L. Zhang X. Lu Y. Zou J. Wang L. Guan Q. Gao L. Zhao J. A novel role for thyroid-stimulating hormone: up-regulation of hepatic 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase expression through the cyclic adenosine monophosphate/protein kinase A/cyclic adenosine monophosphate-responsive element binding protein pathway. Hepatology. 2010;52:1401–1409. doi: 10.1002/hep.23800. [DOI] [PubMed] [Google Scholar]
- 23.Lambert G. Unravelling the functional significance of PCSK9. Curr Opin Lipidol. 2007;18:304–309. doi: 10.1097/MOL.0b013e3281338531. [DOI] [PubMed] [Google Scholar]
- 24.Horton JD. Cohen JC. Hobbs HH. PCSK9: a convertase that coordinates LDL catabolism. J Lipid Res. 2009;50(Suppl):S172–S177. doi: 10.1194/jlr.R800091-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chan DC. Lambert G. Barrett PH. Rye KA. Ooi EM. Watts GF. Plasma proprotein convertase subtilisin/kexin type 9: a marker of LDL apolipoprotein B-100 catabolism? Clin Chem. 2009;55:2049–2052. doi: 10.1373/clinchem.2009.128645. [DOI] [PubMed] [Google Scholar]
- 26.Rashid S. Curtis DE. Garuti R. Anderson NN. Bashmakov Y. Ho YK. Hammer RE. Moon YA. Horton JD. Decreased plasma cholesterol and hypersensitivity to statins in mice lacking Pcsk9. Proc Natl Acad Sci USA. 2005;102:5374–5379. doi: 10.1073/pnas.0501652102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lakoski SG. Lagace TA. Cohen JC. Horton JD. Hobbs HH. Genetic and metabolic determinants of plasma PCSK9 levels. J Clin Endocrinol Metab. 2009;94:2537–2543. doi: 10.1210/jc.2009-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kappelle PJWH. Lambert G. Dullaart RPF. Relationship of plasma apolipoprotein M with proprotein convertase subtilisin-kexin type 9 levels in non-diabetic subjects. Atherosclerosis. 2011;214:492–494. doi: 10.1016/j.atherosclerosis.2010.10.041. [DOI] [PubMed] [Google Scholar]
- 29.Shin DJ. Osborne TF. Thyroid hormone regulation and cholesterol metabolism are connected through sterol regulatory element-binding protein-2 (SREBP-2) J Biol Chem. 2003;278:34114–34118. doi: 10.1074/jbc.M305417200. [DOI] [PubMed] [Google Scholar]
- 30.Matthews DR. Hosker JP. Rudenski AS. Naylor BA. Treacher DF. Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 31.Baass A. Dubuc G. Tremblay M. Delvin EE. O'Loughlin J. Levy E. Davignon J. Lambert M. Plasma PCSK9 is associated with age, sex, and multiple metabolic markers in a population-based sample of children and adolescents. Clin Chem. 2009;55:1637–1645. doi: 10.1373/clinchem.2009.126987. [DOI] [PubMed] [Google Scholar]
- 32.Brouwers MC. Troutt JS. van Greevenbroek MM. Ferreira I. Feskens EJ. van der Kallen CJ. Schaper NC. Schalkwijk CG. Konrad RJ. Stehouwer CD. Plasma proprotein convertase subtilisin kexin type 9 is not altered in subjects with impaired glucose metabolism and type 2 diabetes mellitus, but its relationship with non-HDL cholesterol and apolipoprotein B may be modified by type 2 diabetes mellitus: The CODAM study. Atherosclerosis. 2011;217:263–267. doi: 10.1016/j.atherosclerosis.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 33.Vergès B. Duvillard L. Brindisi MC. Gautier E. Krempf M. Costet P. Cariou B. Lack of association between plasma PCSK9 and LDL-apoB100 catabolism in patients with uncontrolled type 2 diabetes. Atherosclerosis. 2011;219:342–348. doi: 10.1016/j.atherosclerosis.2011.07.098. [DOI] [PubMed] [Google Scholar]
- 34.Bakker SJ. ter Maaten JC. Popp-Snijders C. Slaets JP. Heine RJ. Gans ROB. The relationship between thyrotropin and low density lipoprotein cholesterol is modified by insulin sensitivity in healthy euthyroid subjects. J Clin Endocrinol Metab. 2001;86:1206–1211. doi: 10.1210/jcem.86.3.7324. [DOI] [PubMed] [Google Scholar]
- 35.Kappelle PJ. Lambert G. Dullaart RPF. Plasma proprotein convertase subtilisin-kexin type 9 does not change during 24h insulin infusion in healthy subjects and type 2 diabetic patients. Atherosclerosis. 2011;214:432–435. doi: 10.1016/j.atherosclerosis.2010.10.028. [DOI] [PubMed] [Google Scholar]
- 36.Xiang GD. He YS. Zhao LS. Hou J. Yue L. Xiang HJ. Impairment of endothelium-dependent arterial dilation in Hashimoto's thyroiditis patients with euthyroidism. Clin Endocrinol (Oxf) 2006;64:698–702. doi: 10.1111/j.1365-2265.2006.02531.x. [DOI] [PubMed] [Google Scholar]