Abstract
Inorganic phosphate (Pi) is an essential nutrient for living organisms. It plays a key role in diverse physiological functions, including osteoblast differentiation and skeletal mineralization. Relevantly, Pi is emerging as an important signaling molecule capable of modulating multiple cellular functions by altering signal transduction pathways, gene expression, and protein abundance in many cell types. To our knowledge, the consequences of elevated Pi on behavior of breast cancer cells have been poorly addressed. In this study we investigate the effects of Pi on proliferation of MDA-MB-231 breast cancer cells. We report that Pi inhibits proliferation of MDA-MB-231 cells by slowing cell cycle progression, without apoptosis occurrence. We found that Pi causes cells to accumulate in G1 phase in a time-dependent manner. Accordingly, G1 accumulation was associated with a decrease of cyclin A and cyclin E and an increase of cell cycle inhibitors p21 and p27 protein levels, respectively. Moreover, the Pi-induced antiproliferative effect was dynamically accompanied by profound changes in ERK1/2 and STAT3 protein and phosphorylation levels in response to Pi. Altogether, our data represent the first evidence of Pi acting as a novel signaling molecule in MDA-MB-231 breast cancer cells, capable of eliciting a strong antiproliferative action and suggest that targeting Pi levels at local sites might represent the rationale for developing novel strategies for therapeutic intervention in triple-negative breast cancer.
Key words: ERK1/2, growth inhibition, inorganic phosphate, MDA-MB-231 cells, STAT3
Introduction
Inorganic phosphate (Pi) is an essential nutrient for living organisms. It plays a key role in diverse physiological functions, including osteoblast differentiation and skeletal mineralization.1 Serum Pi level is maintained within a narrow range through a complex interplay between intestinal absorption, exchange with intracellular and bone storage pools, and renal tubular reabsorption and depends mainly on the activity of Na/Pi cotransporters.2 Pi is abundant in the diet, and intestinal absorption of Pi is efficient and minimally regulated. The kidney is a major regulator of Pi homeostasis and can increase or decrease its Pi reabsorptive capacity to accommodate Pi need. Adequate control of Pi homeostasis is crucial because a moderate increase in serum Pi concentration and polymorphisms in genes involved in Pi metabolism may result in bone impairment and influence the aging process and lifespan.3
The amount of Pi in the human diet, and in particular the Western diet, continues to increase.4,5 In addition, new drug delivery systems containing calcium phosphate nanoparticles have been developed. Notably, release of Pi from hydroxyapatite nanoparticles and its retention at local sites are known to occur, thus affecting Pi concentrations locally.6 Thus, it will be important to fully understand the influence of Pi on cell function and the possible relationship to cancer.7,8
Relevantly, Pi is emerging as an important signaling molecule capable of modulating multiple cellular functions by altering signal transduction pathways, gene expression, and protein abundance in many cell types.9 Previously, we showed that Pi inhibits proliferation and aggressiveness of human osteosarcoma U2OS cells, identifying adenylate cyclase, beta3 integrin, Rap1, and ERK1/2 as proteins whose expression and function are relevantly affected by Pi.10,11 In addition, more recently, we demonstrated that Pi is capable of inducing sensitization of osteosarcoma cells to doxorubicin in a p53-dependent manner and through a mechanism involving ERK1/2 down-regulation.12
To our knowledge, no research has been directed at determining the consequences of elevated Pi on proliferation of breast cancer cells. In this study we investigate the possible effects of Pi on proliferation of triple-negative MDA-MB-231 breast cancer cells and on the underlying molecular mechanisms.
Materials and Methods
Materials
All cell culture materials were from Gibco–Life Technologies (Gaithersburg, MD). Anti-tubulin antibodies were obtained from Oncogene-Calbiochem (La Jolla, CA). Anti-p-ERK antibodies were obtained from Cell Signaling Technology (Danvers, MA). All other antibodies were obtained from Santa Cruz Biotechnology (San Diego, CA).
Cell culture and treatments
The MDA-MB-231 human breast cancer cell line was obtained from the American Type Culture Collection (Rockville, MD). MDA-MB-231 cells were grown in Dulbecco's modified Eagle's medium supplemented with 2 mM glutamine, 100 U/mL penicillin, 100 mg/mL streptomycin, and 10% fetal bovine serum and cultured at 37°C in a 5% CO2 humidified atmosphere. Typically, cells were split (5×105/10-cm plate) and grown in 10% serum containing medium. After 24 h, the medium was removed, cells were washed with phosphate-buffered saline (PBS) and cultured again in 10% serum fresh medium supplemented or not (control) with Pi (time 0).
The control medium contained 0.916 mM Pi, and Pi concentrations listed in the figures are the final Pi medium concentrations. Added Pi was in the form of NaPO4, pH 7.4, from Sigma.10–13
Floating cells were recovered from culture medium by centrifugation, and adherent cells were harvested by trypsinization. Both floating and adherent cells were used in experiments aimed to study expression of proteins involved in apoptosis and to perform FACS analysis.14
Cell viability assay
Viable cells were determined by the 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay, as previously described.14,15 Briefly, cells were seeded in 96-multiwell plates at the density of 4×103 cells/well and grown in 10% serum containing medium. After 24 h, the medium was removed, and cells were washed with PBS and cultured again in 10% serum fresh medium supplemented or not (control) with Pi (time 0) for up to 72 h (see the figure legends). Before harvesting, 100 μL of MTT solution (5 mg/mL) was added to each well and incubated at 37°C for 3 h, then the formazan product was solubilized by the addition of 100 μL of 0.04 normality (N) HCl isopropanol. The optical density of each sample was determined by measuring the absorbance at 570 nm versus 650 nm using an enzyme-linked immunosorbent assay reader (Molecular Devices, Downingtown, PA). Cell proliferation assays were performed three times (in replicates of six wells for each data point in each experiment). Data are presented as means±standard deviation for a representative experiment.
Evaluation of cell cycle phases by flow cytometry
After Pi treatment, cells were recovered as described in Cell culture and treatments, fixed by resuspension in 70% ice-cold methanol/PBS, and incubated overnight at 4°C. After fixing, samples were pelleted at 400 g for 5 min, and pellets were washed once with ice-cold PBS and centrifuged for a further 5 min. Pellets were resuspended in 0.5 mL of DNA staining solution (50 μg/mL of propidium iodide [PI] and 100 μg of RNase A in PBS), and incubated at 37°C for 1 h in the dark. Samples were transferred to 5-mL Falcon tubes and stored on ice until assayed. Flow cytometric analysis was performed using a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA) interfaced with a Hewlett-Packard computer (mod. 310) for data analysis performed with the ModiFIT Cell Cycle Analysis software. For the evaluation of intracellular DNA contents, at least 20,000 events for each point were analyzed, and regions were set up to acquire quantitative data of cells that fell into the normal G1, S, and G2 regions and with fragmented DNA (sub-G1 or apoptotic events).12,14
Preparation of cell lysates
Cell extracts were prepared as follows. Briefly, three to five volumes of RIPA buffer (PBS, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS) containing 10 μg/mL aprotinin, leupeptin, and 1 mM phenylmethylsulfonyl fluoride were added to recovered cells. After incubation on ice for 1 h, samples were centrifuged at 18,000 g in an Eppendorf microcentrifuge for 15 min at 4°C and the supernatant (SDS total extract) was recovered. Some aliquots were taken for protein quantification according to Bradford method (Bradford, 1976); others were diluted in 4×Laemmli buffer, boiled, and stored as samples for immunoblotting analysis.16
Immunodetection of proteins
Typically, we employed 20–40 μg of total extracts for immunoblotting. Proteins from cell preparations were separated by SDS-PAGE and transferred onto nitrocellulose sheets (Schleicher & Schuell, Dassel, Germany) by a Mini Trans-Blot apparatus BioRad (Hercules, CA). II Goat anti-rabbit or anti-mouse antibodies, conjugated with horseradish peroxidase (BioRad), were used as a detection system (ECL) according to the manufacturer's instructions (Amersham Biosciences, Amersham, United Kingdom).17
Statistical analysis
Experiments were performed three times with replicate samples, except where otherwise indicated. Data are plotted as mean±SD (standard deviation). The means were compared using analysis of variance (ANOVA) plus Bonferroni's t-test; p values of less than 0.05 were considered significant. National Institutes of Health Image J 1.42Q (NIH, Bethesda, MD) software was used for densitometric analysis.
Results
Pi inhibits proliferation of human MDA-MB-231 breast cancer cells
The triple-negative human breast cancer cell line MDA-MB-231 is a well-established and widely used model system of highly aggressive breast cancer cells.18,19 To evaluate the consequences of elevated Pi on behavior of breast cancer cells, first we looked at the impact of Pi on proliferation of MDA-MB-231 cells. To this purpose, first we performed dose–response experiments. Throughout our experiments, we used a spectrum of final concentration of Pi in agreement with most of the published studies on Pi-triggered effects.9–13
MDA-MB-231 cells were incubated with increasing (2.5, 5, and 10 mM) concentrations of Pi for 72 h, and then cell proliferation was determined by conventional MTT assay and by direct cell number counting. Figure 1A shows that Pi causes a statistically significant reduction of cell viability (p<0.05) in a dose-dependent manner of 12%, 35%, and 40% at 2.5, 5, and 10 mM concentrations, respectively.
FIG. 1.
Effects of inorganic phosphate (Pi) on the proliferation of MDA-MB-231 breast cancer cells. (A) Dose–response. MDA-MB-231 cells were cultured in medium supplemented with 2.5, 5, and 10 mM Pi or not (control) for 72 h. (B) Time-course. MDA-MB-231 cells were cultured in medium supplemented with 5 mM Pi or not (control) for 24, 48, 72 h. Then, cell viability was measured by MTT assay. (C) MDA-MB-231 cells were plated at 5×105/10 cm plate, cultured in medium supplemented with 5 mM Pi or not (control) for 24, 48, 72 h and cell number recorded. Data represent the average of three independent experiments. The means and SD are shown. *p<0.05 vs. control untreated cells.
Next, we performed time-course experiments. MDA-MB-231 cells were exposed to 5 mM Pi (submaximal dose) for up to 72 h, after which cell proliferation was determined by conventional MTT assay and by direct cell number counting. Figure 1B, shows that Pi caused a statistically significant reduction of cell viability (p<0.05) of 12%, 26%, and 40% at 24, 48, and 72 h, respectively.
Parallel direct cell counting and growth curves yielded similar results (Fig. 1C).
The antiproliferative effect induced by Pi in MDA-MB-231 cells is paralleled by consistent changes of relevant cell cycle regulating protein levels
To extend data on the antiproliferative effect of Pi in MDA-MB-231 cells, we studied the expression of some relevant cell cycle regulating proteins.
To this purpose, MDA-MB-231 cells were exposed to 5 mM Pi for 24, 48, and 72 h, after which cell extracts were prepared and Western blotting was used to examine the levels of cyclin-dependent kinase inhibitors p21Cip1 and p27Kip1, and of cyclin A and cyclin E proteins.20,21 Figure 2 shows that at each time point considered (from 24 h up to 72 h) in Pi-treated cells the amount of the positive growth regulators cyclin A and E was decreased compared to untreated control cells, whereas the amount of p21 and p27 cell growth inhibitors was increased. These variations were clearly evident at 48 h and very strong at 72 h of Pi treatment.
FIG. 2.
Effects of Pi on expression levels of some relevant cell growth regulating proteins in MDA-MB-231 cells. Treatments with 5 mM Pi, were carried out for 24, 48, and 72 h. (A) Thirty micrograms of cell extracts were subjected to SDS-PAGE and blotted with antibodies against the indicated proteins (α-tubulin was used as a standard for the equal loading of protein in the lanes). The image is representative of three immunoblotting analysis from three different cellular preparations with similar results. (B) Graphs showing the densitometric intensity of bands ratio are shown. The intensities of signals were expressed as arbitrary units. *p<0.05 vs. control untreated cells.
Pi causes slowing of the cell division cycle but not apoptosis occurrence in MDA-MB-231 cells
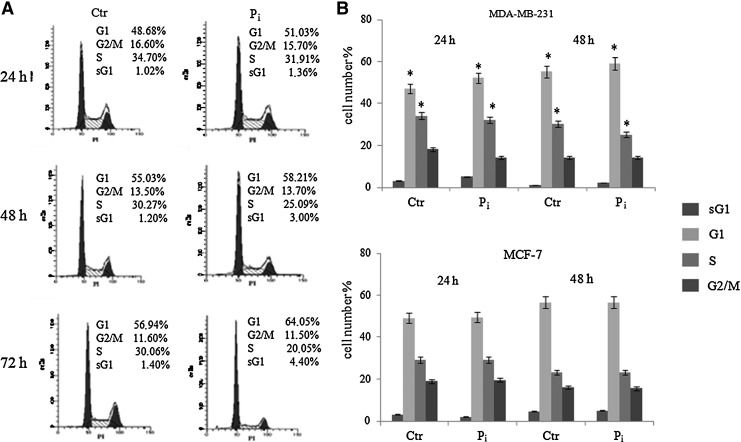
To further explore the inhibitory growth effect of Pi on MDA-MB-231 cells, the distribution of cells in cell cycle phases was evaluated by flow cytometric analysis of PI-stained cells in response to Pi. We also looked at the proportion of cells with hypoploid DNA content (sub-G1 population), characteristic of cells having undergone DNA fragmentation, which is a biochemical hallmark of apoptosis.
Figure 3 shows that at each time point considered (from 24 h up to 72 h) in Pi-treated MDA-MB-231 cells the percentage of G1 phase cells was significantly higher than that of control untreated cells, with a concomitant decrease of S phase. Moreover, no obvious appearance of sub-G1 population in response to Pi was observed up to 72 h.
FIG. 3.
Effects of Pi on the distribution of MDA-MB-231 cells in cell cycle and sub-G1 phases. (A) MDA-MB-231 cells were cultured in medium supplemented with 5 mM Pi or not (control) for 24, 48, and 72 h. Then, FACS analysis of propidium iodide (PI)-stained cells was performed. Representative FACS histograms of PI-stained cells (20,000 events/sample) are shown. (B) Quantitative data indicating the percentage of hypoploid sub-G1, G1, S, and G2/M MDA-MB-231 cells (upper part) and MCF-7 (lower part) from three independent experiments are shown. The means and SD are shown. *p<0.05 vs. control untreated cells.
Interestingly, as shown in Fig. 3B, in contrast to MDA-MB-231 cells, no obvious changes on cell cycle distribution in response to Pi could be seen in MCF-7 breast cancer cells, which are not “triple negative” and express estrogen and progesterone receptors. This strongly suggests that Pi can have discrete effects on cell cycle depending on cell type or cellular background.
Pi relevantly affects ERK1/2 and STAT3 protein and phosphorylation levels
The extracellular-signal-regulated kinase (ERK)-dependent and signal transducer and activator of transcription 3 (STAT3)-dependent signaling pathways are relevant to breast cancer, and several studies demonstrate they are frequently activated.22,23
In order to investigate the possible role of ERK1/2 and STAT3 in the antiproliferative effect of Pi in MDA-MB-231 cells, Western blotting was used to examine the expression and phosphorylation (activation) of ERK1/2 and STAT3 from extracts of cells treated with 5 mM Pi during a time course from 1 h up to 24 h (to look at “early” events) and for 24, 48, and 72 h (to look at “late” events).
Figure 4 shows that Pi induced an early and transient increase in ERK activity at 1 h, rapidly declining after 2–6 h of Pi incubation (Fig. 4A), followed by a strong inhibition starting at 24 h and maintained for up to 72 h of Pi treatment (Fig. 4A, 4B). No obvious variations in the total amount of ERK1/2 protein levels occurred at any time points.
FIG. 4.
Effects of Pi on phosphorylation and levels of ERK1/2 and STAT3 proteins. MDA-MB-231 cells were cultured in medium supplemented with 5 mM Pi or not (control) for 1, 2, 6, and 24 h (left), and for 24, 48, and 72 h (right). (A) The activation (phosphorylation) and levels of ERK1/2 and STAT3 proteins were assessed by Western blotting from 30 μg of cell extracts using antibodies against the indicated proteins. The image is representative of three different experiments with similar results. (B) Graphs showing the densitometric intensity of bands ratio are shown. The intensities of signals were expressed as arbitrary units. *p<0.05 vs. control untreated cells.
As far as STAT3 was concerned, Fig. 4 shows that Pi induced a small increase at 1 and 2 h and a more evident decrease of STAT3 phosphorylation at 6 and 24 h of treatment, respectively (Fig. 4A), and, very surprisingly, a dramatic decrease of the total amount of STAT3 protein with a strong increase of its phosphorylation at 48 and 72 h of Pi treatment (Fig. 4B).
Discussion
Currently, there is no effective therapy for triple-negative breast cancer, and new pharmacological approaches for affected patients are warranted.24,25
In this study we report that in MDA-MB-231 human breast cancer cell line, a well-established model system of highly aggressive triple-negative breast cancer, Pi strongly inhibits proliferation.
Dietary supplements, phytotherapeutic agents, and naturally occurring molecules with antitumor activity and with the least toxicity to normal tissues are suggested as possible candidates to be investigated alone and/or for their synergistic efficacy in combination with antineoplastic drugs 22,26–30
In particular, diet represents an environmental factor that can be easily manipulated and has a profound effects on functional genomics and proteomics; its potential relationship with cancer is well known.31,32 Pi is a common dietary component that may directly alter cell behavior in such a manner.13,33–36
Pi is emerging as an important signaling molecule capable of modulating multiple cellular functions by altering signal transduction pathways, gene expression, and protein abundance in many cell types.9
Additionally, Pi has been shown to stimulate specific signal transduction pathways, including ERK1/2 and Akt, and to increase cell proliferation in some cell types, such as preosteoblastic MC3T3-E1 cells, human lung cells, and epidermal JB6 cells, thereby defining Pi as a novel mitogenic signal for such cells.13,34,37–41
Notably, the proliferative effect of Pi is not a widespread phenomenon affecting all cell types.
Previously, we showed that Pi inhibits proliferation and aggressiveness of human osteosarcoma U2OS cells, identifying adenylate cyclase, beta3 integrin, Rap1, and ERK1/2 as proteins whose expression and function are relevantly affected by Pi.10,11 In addition, more recently, we demonstrated that Pi is capable of inducing sensitization of osteosarcoma cells to doxorubicin in a p53-dependent manner and through a mechanism involving Erk1/2 down-regulation.12 Moreover, apoptosis induction in response to Pi has been reported in MO6-G3 odontoblast-like cells.42
So far, little research has been directed at determining the consequences of elevated Pi on the behavior of breast cancer cells.43,44
In this study, we describe the first evidence of Pi acting as a novel signaling molecule capable of eliciting a strong antiproliferative action in triple-negative MDA-MB-231 breast cancer cells. We report that Pi inhibits proliferation of MDA-MB-231 cells by slowing cell cycle progression, without apoptosis occurrence. We show that Pi time dependently causes the cells to accumulate in G1 phase. Accordingly, G1 accumulation of MDA-MB-231 cells is associated with a decrease of cell cycle positive effectors cyclin A and cyclin E and an increase of cell cycle inhibitors p21 and p27 protein levels, respectively.
Interestingly, in contrast to MDA-MB-231 cells, the antiproliferative effects of Pi do not occur in MCF-7 breast cancer cells, which are not “triple negative” and express estrogen and progesterone receptors, without obvious changes in cell cycle distribution in response to Pi. This strongly suggests that Pi can have discrete effects on cell proliferation depending on cell type and cellular background.
Initial analysis of the underlying molecular mechanisms indicates that the Pi-induced antiproliferative effect in MDA-MB-231 cells is dynamically accompanied by profound changes in phosphorylation status and protein levels of ERK1/2 and STAT3 in response to Pi. The Ras/Raf/Erk signaling pathway is relevant to breast cancer and is frequently activated, and its importance in the control of proliferation is largely known.45–47 A blockade of such signaling pathway is considered a relevant strategy for therapeutic intervention.48,49
Importantly, we found that Pi treatment results in a prolonged inhibition of ERK1/2 phosphorylation (without significant change in the total amount of ERK1/2 protein). On the other hand, multiple lines of evidence place STAT3 at a central node in the development, progression, and maintenance of many human tumors, including breast cancer, and STAT3 has been validated as an anticancer target in several contexts.50–52
Importantly, we found that exposure of MDA-MB-231 cells to Pi resulted in inhibition of phosphorylation of STAT3 (at 6–24 h of treatment), and then in a strong decrease of STAT3 protein abundance with an increase of its phosphorylation as a late effect (at 48–72 h of treatment).
Our data suggest that Pi strongly inhibits proliferation of MDA-MB-231 cells, possibly through inhibition of ERK1/2 and STAT3 signaling pathways within the tumor cells themselves. The detailed molecular mechanism underlying this growth inhibition by Pi is just starting to be understood, and interplay between these pathways (and possibly others) is very likely implicated in the observed slowing of cell cycle progression of MDA-MB-231 cells. However, we do know that further studies and more exhaustive experiments are warranted.
Combination chemotherapy has received more attention to find compounds that could increase the therapeutic index of clinical anticancer drugs.53 Pi is an attractive candidate for investigation.
New drug delivery systems containing calcium phosphate nanoparticles have been developed. Very interestingly, release of Pi from hydroxyapatite nanoparticles and its retention at local sites are known to occur, thus affecting Pi concentrations locally.6
Collectively, our data represent the first evidence of Pi as a signaling molecule in MDA-MB-231 breast cancer cells and indicate that Pi may act as a potent growth suppressor by slowing cell cycle progression, possibly via ERK1/2 and STAT3 inhibition. These findings suggest that targeting Pi levels at local sites could contribute to the development of novel strategies for therapeutic intervention in triple-negative breast cancer.
Acknowledgment
This study was supported by contract grant sponsor PRIN 2009.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Yoshiko Y. Candeliere GA. Maeda N. Aubin JE. Osteoblast autonomous Pi regulation via Pit1 plays a role in bone mineralization. Mol Cell Biol. 2007;27:4465–4474. doi: 10.1128/MCB.00104-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takeda E. Taketani Y. Sawada N, et al. The regulation and function of phosphate in the human body. Biofactors. 2004;21:345–355. doi: 10.1002/biof.552210167. [DOI] [PubMed] [Google Scholar]
- 3.Prié D. Beck L. Urena P. Friedlander G. Recent findings in phosphate homeostasis. Curr Opin Nephrol Hypertens. 2005;14:318–324. doi: 10.1097/01.mnh.0000172716.41853.1e. [DOI] [PubMed] [Google Scholar]
- 4.Harum P. Phosphorus additives. A problem for ESRD patients and the public. Nephrol News Issues. 2012;26:16–17. [PubMed] [Google Scholar]
- 5.Kemi VE. Rita HJ. Kärkkäinen MU, et al. Habitual high phosphorus intakes and foods with phosphate additives negatively affect serum parathyroid hormone concentration: a cross-sectional study on healthy premenopausal women. Public Health Nutr. 2009;12:1885–1892. doi: 10.1017/S1368980009004819. [DOI] [PubMed] [Google Scholar]
- 6.Jun W. Lin L. Yurong C. Juming Y. Recent advances of calcium phosphate nanoparticles for controlled drug delivery. Mini Rev Med Chem. 2012 Jun 13; doi: 10.2174/13895575113139990059. [Epub ahead of print]; PMID: 22697516. [DOI] [PubMed] [Google Scholar]
- 7.Kim YS. Milner JA. Bioactive food components and cancer-specific metabonomic profiles. J Biomed Biotechnol. 2011:721213. doi: 10.1155/2011/721213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Welch AA. Fransen H. Jenab M, et al. Variation in intakes of calcium, phosphorus, magnesium, iron and potassium in 10 countries in the European Prospective Investigation into Cancer and Nutrition study. Eur J Clin Nutr. 2009;63(Suppl 4):S101–121. doi: 10.1038/ejcn.2009.77. [DOI] [PubMed] [Google Scholar]
- 9.Khoshniat S. Bourgine A. Julien M, et al. The emergence of phosphate as a specific signaling molecule in bone and other cell types in mammals. Cell Mol Life Sci. 2011;68:205–218. doi: 10.1007/s00018-010-0527-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naviglio S. Spina A. Chiosi E, et al. Inorganic phosphate inhibits growth of human osteosarcoma U2OS cells via adenylate cyclase/cAMP pathway. J Cell Biochem. 2006;98:1584–1596. doi: 10.1002/jcb.20892. [DOI] [PubMed] [Google Scholar]
- 11.Naviglio S. Di Gesto D. Borrelli V, et al. Novel molecular mechanisms by inorganic phosphate in osteosarcoma U2OS cells. Front Biosci. 2011;E3:1249–1258. doi: 10.2741/e328. [DOI] [PubMed] [Google Scholar]
- 12.Spina A. Sorvillo L. Di Maiolo F, et al. Inorganic phosphate enhances sensitivity of human osteosarcoma U2OS cells to doxorubicin via a p53-dependent pathway. J Cell Physiol. 2013;228:198–206. doi: 10.1002/jcp.24124. [DOI] [PubMed] [Google Scholar]
- 13.Camalier CE. Young MR. Bobe G, et al. Elevated phosphate activates N-ras and promotes cell transformation and skin tumorigenesis. Cancer Prev Res. 2010;3:359–370. doi: 10.1158/1940-6207.CAPR-09-0068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naviglio S. Di Gesto D. Romano M, et al. Leptin enhances growth inhibition by cAMP elevating agents through apoptosis of MDA-MB-231 breast cancer cells. Cancer Biol Ther. 2009;8:1183–1190. doi: 10.4161/cbt.8.12.8562. [DOI] [PubMed] [Google Scholar]
- 15.Naviglio S. Di Gesto D. Illiano F, et al. Leptin potentiates antiproliferative action of cAMP elevation via protein kinase A down-regulation in breast cancer cells. J Cell Physiol. 2010;225:801–809. doi: 10.1002/jcp.22288. [DOI] [PubMed] [Google Scholar]
- 16.Chiosi E. Spina A. Sorrentino A, et al. Change in TNF-alpha receptor expression is a relevant event in doxorubicin-induced H9c2 cardiomyocyte cell death. J Interferon Cytokine Res. 2007;27:589–597. doi: 10.1089/jir.2006.0161. [DOI] [PubMed] [Google Scholar]
- 17.Naviglio S. Spina A. Marra M, et al. Adenylate cyclase/cAMP pathway downmodulation counteracts apoptosis induced by IFN-alpha in human epidermoid cancer cells. J Interferon Cytokine Res. 2007;27:129–136. doi: 10.1089/jir.2006.0101. [DOI] [PubMed] [Google Scholar]
- 18.Radestock Y. Hoang-Vu C. Hombach-Klonisch S. Relaxin reduces xenograft tumour growth of human MDA-MB-231 breast cancer cells. Breast Cancer Res. 2008;10:R71. doi: 10.1186/bcr2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Du J. Sun C. Hu Z, et al. Lysophosphatidic acid induces MDA-MB-231 breast cancer cells migration through activation of PI3K/PAK1/ERK signaling. PLoS One. 2010;30(5):e15940. doi: 10.1371/journal.pone.0015940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu B. Fan Z. Edgerton SM, et al. Metformin induces unique biological and molecular responses in triple negative breast cancer cells. Cell Cycle. 2009;8:2031–2040. doi: 10.4161/cc.8.13.8814. [DOI] [PubMed] [Google Scholar]
- 21.Borriello A. Bencivenga D. Criscuolo M, et al. Targeting p27Kip1 protein: its relevance in the therapy of human cancer. Expert Opin Ther Targets. 2011;15:677–693. doi: 10.1517/14728222.2011.561318. [DOI] [PubMed] [Google Scholar]
- 22.Kim SH. Nagalingam A. Saxena NK, et al. Benzyl isothiocyanate inhibits oncogenic actions of leptin in human breast cancer cells by suppressing activation of signal transducer and activator of transcription 3. Carcinogenesis. 2010;32:359–367. doi: 10.1093/carcin/bgq267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang LH. Ho YJ. Lin JF, et al. Butein inhibits the proliferation of breast cancer cells through generation of reactive oxygen species and modulation of ERK and p38 activities. Mol Med Rep. 2012;6:1126–1132. doi: 10.3892/mmr.2012.1023. [DOI] [PubMed] [Google Scholar]
- 24.Rakha EA. Chan S. Metastatic triple-negative breast cancer. Clin Oncol. 2011;23:587–600. doi: 10.1016/j.clon.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 25.Pal SK. Childs BH. Pegram M. Triple negative breast cancer: unmet medical needs. Breast Cancer Res Treat. 2011;125:627–636. doi: 10.1007/s10549-010-1293-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raina K. Agarwal R. Combinatorial strategies for cancer eradication by silibinin and cytotoxic agents: efficacy and mechanisms. Acta Pharmacol Sin. 2007;28:1466–1475. doi: 10.1111/j.1745-7254.2007.00691.x. [DOI] [PubMed] [Google Scholar]
- 27.Szekeres T. Saiko P. Fritzer-Szekeres M, et al. Chemopreventive effects of resveratrol and resveratrol derivatives. Ann NY Acad Sci. 2011;1215:89–95. doi: 10.1111/j.1749-6632.2010.05864.x. [DOI] [PubMed] [Google Scholar]
- 28.Manu KA. Shanmugam MK. Rajendran P, et al. Plumbagin inhibits invasion and migration of breast and gastric cancer cells by downregulating the expression of chemokine receptor CXCR4. Mol Cancer. 2011;10:107. doi: 10.1186/1476-4598-10-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siebert AE. Sanchez AL. Dinda S. Moudgil VK. Effects of estrogen metabolite 2-methoxyestradiol on tumor suppressor protein p53 and proliferation of breast cancer cells. Syst Biol Reprod Med. 2011;57:279–287. doi: 10.3109/19396368.2011.633152. [DOI] [PubMed] [Google Scholar]
- 30.Chen JT. Fong YC. Li TM, et al. DDTD, an isoflavone derivative, induces cell apoptosis through the reactive oxygen species/apoptosis signal-regulating kinase 1 pathway in human osteosarcoma cells. Eur J Pharmacol. 2008;597:19–26. doi: 10.1016/j.ejphar.2008.08.036. [DOI] [PubMed] [Google Scholar]
- 31.Go VL. Wong DA. Wang Y, et al. Diet and cancer prevention: evidence-based medicine to genomic medicine. J Nutr. 2004;134:3513–3516S. doi: 10.1093/jn/134.12.3513S. [DOI] [PubMed] [Google Scholar]
- 32.Su LJ. 2012. Diet, epigenetics, and cancer. Methods Mol Biol. 2012;863:377–393. doi: 10.1007/978-1-61779-612-8_24. [DOI] [PubMed] [Google Scholar]
- 33.Ichikawa S. Austin AM. Gray AK, et al. Dietary phosphate restriction normalizes biochemical and skeletal abnormalities in a murine model of tumoral calcinosis. Endocrinology. 2011;152:4504–4513. doi: 10.1210/en.2011-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jin H. Xu CX. Lim HT, et al. High dietary inorganic phosphate increases lung tumorigenesis and alters Akt signaling. Am J Respir Crit Care Med. 2009;179:59–68. doi: 10.1164/rccm.200802-306OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu CX. Jin H. Lim HT, et al. Low dietary inorganic phosphate stimulates lung tumorigenesis through altering protein translation and cell cycle in K-ras(LA1) mice. Nutr Cancer. 2010;62:525–532. doi: 10.1080/01635580903532432. [DOI] [PubMed] [Google Scholar]
- 36.Pal D. Banerjee S. Ghosh AK. Dietary-induced cancer prevention: An expanding research arena of emerging diet related to healthcare system. J Adv Pharm Technol Res. 2012;3:16–24. doi: 10.4103/2231-4040.93561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beck GR., Jr Inorganic phosphate as a signaling molecule in osteoblast differentiation. J Cell Biochem. 2003;90:234–243. doi: 10.1002/jcb.10622. [DOI] [PubMed] [Google Scholar]
- 38.Kemi VE. Karkkainen MU. Lamberg-Allardt CJ. High phosphorus intakes acutely and negatively affect Ca and bone metabolism in a dose-dependent manner in healthy young females. Br J Nutr. 2006;96:545–552. [PubMed] [Google Scholar]
- 39.Jin H. Hwang SK. Yu K, et al. A high inorganic phosphate diet perturbs brain growth, alters Akt-ERK signaling, and results in changes in cap-dependent translation. Toxicol Sci. 2006;90:221–229. doi: 10.1093/toxsci/kfj066. [DOI] [PubMed] [Google Scholar]
- 40.Jin H. Chang SH. Xu CX, et al. High dietary inorganic phosphate affects lung through altering protein translation, cell cycle, and angiogenesis in developing mice. Toxicol Sci. 2007;100:215–223. doi: 10.1093/toxsci/kfm202. [DOI] [PubMed] [Google Scholar]
- 41.Xu CX. Jin H. Lim HT, et al. High dietary inorganic phosphate enhances cap-dependent protein translation, cell-cycle progression, and angiogenesis in the livers of young mice. Am J Physiol Gastrointest Liver Physiol. 2008;295:G654–G663. doi: 10.1152/ajpgi.90213.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bourgine A. Beck L. Khoshniat S, et al. Inorganic phosphate stimulates apoptosis in murine MO6-G3 odontoblast-like cells. Arch Oral Biol. 2011;56:977–983. doi: 10.1016/j.archoralbio.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 43.Cox RF. Jenkinson A. Pohl K, et al. Osteomimicry of mammary adenocarcinoma cells in vitro; increased expression of bone matrix proteins and proliferation within a 3D collagen environment. PLoS One. 2012;7:e41679. doi: 10.1371/journal.pone.0041679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cox RF. Hernandez-Santana A. Ramdass S, et al. Microcalcifications in breast cancer: novel insights into the molecular mechanism and functional consequence of mammary mineralisation. Br J Cancer. 2012;106:525–537. doi: 10.1038/bjc.2011.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Santen RJ. Song RX. McPherson R, et al. The role of mitogen-activated protein (MAP) kinase in breast cancer. J Steroid Biochem Mol Biol. 2002;80:239–256. doi: 10.1016/s0960-0760(01)00189-3. [DOI] [PubMed] [Google Scholar]
- 46.Chiaradonna F. Balestrieri C. Gaglio D. Vanoni M. RAS and PKA pathways in cancer: new insight from transcriptional analysis. Front Biosci. 2008;13:5257–5278. doi: 10.2741/3079. [DOI] [PubMed] [Google Scholar]
- 47.Mannello F. Papa S. HER2 and proliferation of wound-induced breast carcinoma. Lancet. 2003;362:1503–1504. doi: 10.1016/S0140-6736(03)14709-5. [DOI] [PubMed] [Google Scholar]
- 48.Alvarez RH. Valero V. Hortobagyi GN. Emerging targeted therapies for breast cancer. J Clin Oncol. 2010;28:3366–3379. doi: 10.1200/JCO.2009.25.4011. [DOI] [PubMed] [Google Scholar]
- 49.Santarpia L. Lippman SM. El-Naggar AK. Targeting the MAPK-RAS-RAF signaling pathway in cancer therapy. Expert Opin Ther Targets. 2012;16:103–119. doi: 10.1517/14728222.2011.645805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jing N. Tweardy DJ. Targeting Stat3 in cancer therapy. Anticancer Drugs. 2005;16:601–607. doi: 10.1097/00001813-200507000-00002. [DOI] [PubMed] [Google Scholar]
- 51.Germain D. Frank DA. Targeting the cytoplasmic and nuclear functions of signal transducers and activators of transcription 3 for cancer therapy. Clin Cancer Res. 2007;13:5665–5669. doi: 10.1158/1078-0432.CCR-06-2491. [DOI] [PubMed] [Google Scholar]
- 52.Johnston PA. Grandis JR. STAT3 signaling: anticancer strategies and challenges. Mol Interv. 2011;11:18–26. doi: 10.1124/mi.11.1.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reddy KB. Triple-negative breast cancers: an updated review on treatment options. Curr Oncol. 2011;18:173–179. doi: 10.3747/co.v18i4.738. [DOI] [PMC free article] [PubMed] [Google Scholar]