Abstract
Temperature responses of anterior hypothalamic neurons are considered key elements in the regulation of the temperature setpoint of homeotherms. We have investigated the sensitivity to warming of cultured neurons of the AH from mice with electrophysiological and immunocytochemical techniques. In control experiments, only ≈9% of the 3- to 5-week-old cells exhibited changes of their basic firing rate when the temperature was raised from 37°C to 40°C. This ratio was increased to 27% after the cultures were “primed” by adding prostaglandin E2 (PGE2), an endogenous pyrogen, in the extracellular medium. In these neurons the firing rate was significantly increased, and the frequency of the gamma γ-aminobutyric acid (GABA) inhibitory postsynaptic potentials was markedly decreased. In contrast, the resting potential and membrane resistance of the recorded cells remained unchanged. PGE2 was found to decrease the level of phosphorylation of the extracellular signal-regulated kinases 1 and 2 in a subset of GABAergic neurons that express the E-prostanoid receptor type 3. Inhibition of ERK1/2 by U0126 mimicked the effects of PGE2. These data indicate that PGE2 acts primarily on the excitability of GABAergic presynaptic cells, most likely via alterations of voltage-gated K+ channels. Our results also suggest that far from being an inherent property of a specialized class of neurons, the degree of thermosensitivity can be strongly modulated by synaptic activity and is a more adaptive property of hypothalamic neurons than previously thought.
Neurons in the preoptic area (PO) and anterior hypothalamus (AH) are involved in regulating body temperature, as indicated by lesion and thermode implant studies (reviewed in ref. 1). In confirmation, electrophysiological recordings in this region have revealed that some cells, termed warm-sensitive, increase their firing rate when the local temperature is raised. However, numerous complicating factors have hampered their analysis. For example, their definition, which often relies on the increase of the firing rate per °C (impulse s-1/°C) or is expressed as a Q10, varies among authors. Furthermore, their proportion relative to the cold-sensitive and thermo-insensitive neurons is in the rather low range of 20–30% (2–6) with extreme values of 10% (7) to 48% (8), despite the fact that all in vitro studies have been carried out in cells presumed to be highly excitable, because of their unusually high extracellular K+. Another complicating factor is that most hypothalamic warm-sensitive neurons also respond to changes in skin and spinal temperatures (3); therefore, they must be viewed as integrators of thermal information.
The mechanism of warm-sensitivity is not fully understood. It has been suggested that the depolarizing prepotentials which precede an action potential shorten with warming, thus reducing the interspike interval and increasing the firing rate (7, 9). An A-type K+ current, which regulates the firing rate in other neuronal types, could be responsible for the shortening of the prepotential's duration (9), because it activates and inactivates faster at higher temperatures. In opposition to this view, it has been proposed that in warm-sensitive cells the enhanced firing rate is obtained through a temperature-dependent depolarization, which is caused by an increased cationic conductance (10), and that in cell-attached configuration the activity of single cationic channels is correlated with the firing rate of the neuron during warming (11).
To characterize the inherent cellular and synaptic properties of warm-sensitive cells, we have recorded from mouse AH neurons in culture with patch-clamp electrodes. Particular attention was given to the responses of these cells to temperature changes and to the effects of prostaglandin E2 (PGE2) on their thermosensitivity. Inflammatory cytokines such as IL-1 and IL-6, and tumor necrosis factor alpha, are the most potent inducers of PGE2 synthesis. This prostaglandin is synthesized by both glial and neuronal cells through the induction of cyclooxygenase 2 (12); injection of PGE2 into the AH evokes fever responses (13).
PGE2 was previously reported to have excitatory as well as inhibitory actions on PO/AH neurons at 37°C, both in vivo and in slices (4, 5, 8, 14). As shown, a striking finding of the present work is that PGE2 increases the ratio of thermosensitive neurons, a result which raises the question of the functional specificity of “thermosensitive” cells.
Materials and Methods
Anterior Hypothalamic Cultures. Mixed AH cultures (containing neurons and glia) were prepared from Swiss–Webster mice. Briefly, dissociated anterior hypothalami from fetal mice at 13–14 days of gestation were plated at a density of 1–2 anterior hypothalami per ml onto poly(d-lysine)-coated coverslips. Cultures were kept in minimal essential medium (MEM) with Earle's salts, glutamine free, from GIBCO/BRL, supplemented with 5% FBS, 5% horse serum, glucose (20 mM), and glutamine (2 mM). Five days after plating (day in vitro 5), when the glial monolayer had almost reached confluency (>60%), glial division was halted by a 2-day exposure to 10 μM cytosinearabinoside. Cultures were then fed every 4 days with minimal essential medium containing 10% horse serum and as described. They were maintained in a 37°C humidified incubator in a 5% CO2 atmosphere, and used at days in vitro 30–45 for electrophysiological recordings and immunocytochemistry.
Immunocytochemistry and Confocal Imaging. Coverslips were washed by immersion in serum-free medium (HCSS; 120 mM NaCl/5.4 mM KCl/0.8 mM MgCl2/1.8 mM CaCl2/15 mM glucose/20 mM Hepes, pH 7.4) and equilibrated in this medium for 3 h at 37°C to allow recovery from washing. PGE2 (500 nM) was applied for the times indicated, after which the coverslips were washed in PBS, fixed in ice-cold 4% paraformaldehyde for 30 min, and then incubated for 10 min at room temperature in PBS containing 0.25% Triton X-100. Nonspecific sites were blocked by incubation in PBS containing 10% normal goat/horse serum. For double immunostaining, the coverslips were incubated in 2% normal goat serum containing an antibody against EP3 receptors (EP3R) (1:100 dilution, Alpha Diagnostics, San Antonio, TX), and antibodies against phospho-extracellular signal-regulating kinase (pERK) (1:400 dilution, Cell Signaling Technology, Beverly, MA) or against GAD67 (1:1,000 dilution; Chemicon, Temecula, CA), for 2 h at 37°C. Rabbit antibodies against the mouse EP1, EP2, EP3, and EP4 receptors raised against peptide antigens form the N terminus of these GPCRs were from Alpha Diagnostics, and their specificity was tested by the supplier and has not been retested by us. Specific binding was detected by using secondary antibodies conjugated to AlexaFluor dyes (594, red; 488, green; Molecular Probes). Images were collected on a Delta Vision Optical Sectioning Microscope consisting of an Olympus (Melville, NY) IX-70 microscope equipped with a mercury arc lamp. A Photometrics CH 350 cooled CCD camera and a high-precision motorized XYZ stage were used to acquire multiple consecutive optical sections at a 0.2-μm interval for each of the fluorescent probes by using a 60× oil objective. The settings of the confocal were kept constant so that the images could be analyzed by densitometry and the time-dependent changes could be compared. Three independent experiments were performed on different sets of day in vitro 30 AH neurons. For cell imaging, 10 different fields in each coverslip were captured, and all neurons in the pictures were analyzed for their green and red fluorescence content using photoshop software (Adobe Systems, San Jose, CA). Backgrounds were defined as the fluorescence intensity in nonneuronal fields and subtracted from the values obtained for each neuron. Neurons giving mean intensities above background were considered positive.
Patch Clamp Recording. Standard tight seal recording techniques were used with a patch-clamp amplifier (Axopatch 200B, Axon Instruments, Union City, CA). The external recording solution was 155 mM NaCl/3.5 mM KCl/2 mM CaCl2/1.5 mM MgSO4/10 mM glucose/10 mM Hepes (pH 7.4). The osmolarity was 300–305 mOsm. The pipette solution was 130 mM K-gluconate/10 mM KCl/10 mM Hepes/2 mM MgCl2/0.5 mM EGTA (pH 7.4). Whole-cell recordings were achieved by using gramicidin perforated-patch configuration to prevent intracellular washout while leaving the Cl- concentration unaltered. Gramicidin was added to the pipette solution to a final concentration of 200 μg/ml from a stock solution prepared in DMSO (20 mg/ml). After obtaining a gigaseal, electrical access into the neuron was established within 5–10 min. No holding current was applied to neurons when recording spontaneous activity and/or during temperature changes. Glass micropipettes (2–6 MΩ) were pulled with a horizontal puller (P-87, Sutter Instruments, Novato, CA) by using Sutter borosilicate glass capillaries with filament. All voltage measurements were corrected for the liquid junction potential (approximately -12 mV). The temperature of the external solution was controlled with an HCC-100A heating/cooling device (Dagan, Minneapolis), equipped with a Peltier element. The usual temperature during the recordings was 36–37°C. To prevent changes induced in the electrode reference potential, the ground electrode was thermally isolated in a separate bath connected to the recording bath by a filter paper bridge.
Data Acquisition and Analysis. Recordings were filtered at 5 KHz and then digitized by using a Digidata 1320A interface and the pclamp8 software package (Axon Instruments) and stored on the disk of a computer. For assessing temperature-dependence, the activity of neurons was recorded for 2–4 min as a control at 36–37°C. Temperature cycles of at least 37–40°C lasting 2–5 min were performed with or without drug treatment. Synaptic potentials were detected automatically by using the Mini Analysis version 5.6.25 program (Synaptosoft, Decatur, GA). The recorded neurons were classified by comparing the inhibitory postsynaptic potential (IPSP) frequencies before or after PGE2; statistical significance of this parameter was assessed with the Kolmogorov–Smirnov two-sample test (KS test), by using the Mini Analysis program. Only those pairs that yielded positive tests (P < 0.01) were kept for further analysis. A similar procedure was followed for other parameters such as amplitudes and rise and decay times of the postsynaptic potentials (PSPs), and the interaction potential intervals. t tests were also used. The mean frequency of PSPs was determined for stretches of 3–5 min of recordings (number of events/duration) before and after treatment. Neurons that displayed nonreversible firing rate changes after modifications of temperature were excluded from this study. All data are presented as mean ± SD.
Results
Intracellular recordings of adult slices of PO/AH or in vivo material have suggested that warm-sensitive neurons belong to two classes. The first is excitatory PSP (EPSP)-driven (15). The second, most commonly recognized as truly thermosensitive, is pacemaker- or prepotential-driven, with neurons that fire almost rhythmically as illustrated in Fig. 1A (7, 9, 15).
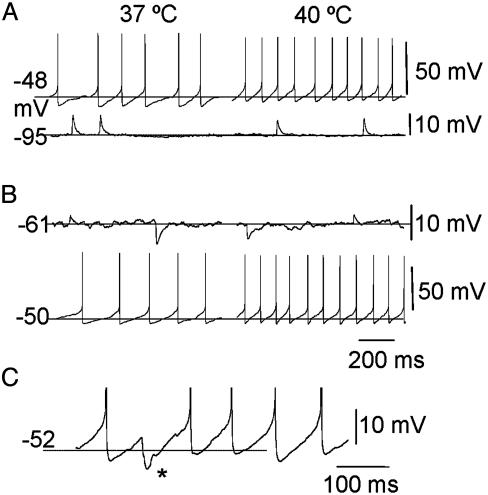
Fig. 1.
Enhancement of thermosensitivity by depolarization. (A) Example of a temperature-sensitive prepotential driven neuron (Upper). The firing rate was increased from 5.2 Hz at 37°C to 9.4 Hz at 40°C, i.e., by ≈1.4 impulse s-1/°C. Hyperpolarization of the cell from -48 mV to -95 mV obtained by injecting negative current (-100 pA) revealed underlying PSPs (Lower). (B) Example of a “silent” neuron that displayed synaptic potentials at resting potential and which was apparently temperature-insensitive (Upper). This cell became temperature-sensitive when it was depolarized from -61 to -50 mV by a current injection of 20 pA. Under these new conditions the firing rate increased from 4.5 Hz at 37°C to 10.4 Hz at 40°C, an increase of ≈2 impulse s-1/°C (Lower). (C) Train of action potentials (truncated) recorded in another cell at the indicated potential. Note that the depolarizing prepotentials can be interrupted by an IPSP (asterisk) which prolongs the interspike interval.
Intracellular recordings of cultured AH neurons have shown us that, surprisingly, the basic properties of warm-sensitive cells reported for adult cells are already present at early stages of development. In addition, the distinction between prepotential- and EPSP-driven neurons is not so clear, because the recorded cells can belong to either class, depending on their membrane potential. Specifically, hyperpolarization of a temperature-sensitive, apparently prepotential-driven neuron from -48 to -95 mV reveals background PSPs (Fig. 1 A), as it did in five neurons tested. In addition, an initially “silent” AH temperature-insensitive neuron, which does not fire spontaneously, can become “prepotential”-driven and temperature-sensitive when depolarizing currents are injected through the patch electrode (Fig. 1B). Yet, in this case, intercalated IPSPs can still be observed (Fig. 1C), as were consistently seen in six neurons tested. Finally, all neurons display prepotentials when a depolarizing current is injected (data not shown), or when the cells are depolarized and made excitable by raising the external K+ from 3.5 to 6.25 mM (data not shown), as was observed in four of seven cells.
These findings already suggest that the thermosensitivity of some AH neurons can be induced by external conditions. Yet as others (10, 15) have seen, we found that, when recorded without manipulation, temperature-sensitive neurons are rather scarce. In our experiments they did not exceed ≈9% (n = 128 total number) of the investigated cells. Therefore, to increase this ratio, we decided to “prime” (or sensitize) AH neurons with PGE2, and hence, come close to pathophysiological conditions leading to fever in vivo. One possible explanation for the low percentage of thermosensitive neurons in these cultures may arise from diluting the PO tissue that is expected to be richer in thermosensitive neurons than the adjacent AH, with anterior hypothalamic tissue as these two areas are hard to separate at the early stage of development represented by E13/E14 mice used in preparation of the cultures.
Effect of PGE2 on Firing Rate and Thermosensitivity. PGE2, a small extracellular lipid messenger, is a key endogenous pyrogen, which is synthesized and released in the PO. Its effects on the core temperature of homeotherms have long been recognized (13). PGE2 affects the firing rates and thermosensitivity of anterior hypothalamic PO neurons in brain slices (5, 8) and in vivo (4). However, the cellular and molecular mechanisms underlying these changes are not understood.
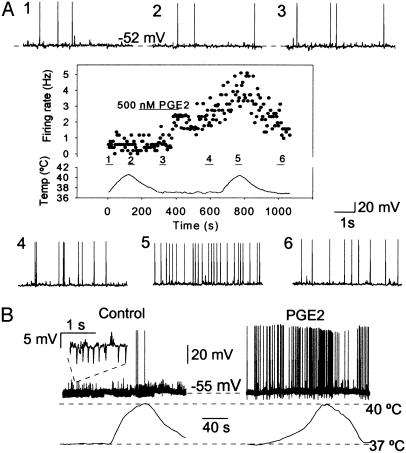
Bath application of PGE2 (500 nM, 3–6 min) increased the firing frequency of 12 of 36 spontaneously active AH neurons and increased the thermosensitivity of 10 of them. Fig. 2A depicts a spontaneously firing cell that was not affected by raising the temperature from 36.5 to 40.5°C alone, but PGE2 application caused this cell to fire at a 2-fold increased rate (from 2.4 to 4.8 Hz) upon a second warming cycle. At ≈37°C, the firing rate was modified from an average of 1.8 ± 2.6 Hz (n = 12) to 2.6 ± 3.0 Hz (n = 12) in absence and presence of PGE2, respectively (P < 0.01, paired t test). Furthermore, in 10 cells, the thermosensitivity shifted from 0.1 ± 0.2 impulse s-1/°C and reached 0.8 ± 1.0 impulse s-1/°C after PGE2. Finally, 2 of 34 silent neurons had their firing altered by the pyrogen. One of them is illustrated in Fig. 2B, which also clearly shows that the frequency of IPSPs was lowered after exposure to PGE2 (as developed below).
Fig. 2.
Conversion of temperature-insensitive neurons by PGE2. (A1–A6) Sample recordings of spontaneous activity obtained from an AH neuron at the indicated membrane potential. The mean firing rate of this cell is plotted in the inside box where variations of temperature versus time are shown (each data point is the average firing rate computed over 5 s of recording time). Sweeps from 1 to 6 were monitored at corresponding periods in the graph. Note that the firing rate of this neuron increased from 0.5 Hz (in control, at 36.5°C) to 2.4 Hz (after PGE2, at 36.5°C) and 4.8 Hz (after PGE2, at 40.5°C), indicating a thermosensitivity of 0.6 impulse s-1/°C in the presence of the pyrogen. (B) Recordings from an initially “silent” neuron collected during a warming cycle before (Left) and after (Right) exposure to PGE2, which produced an increased firing rate indicating an overall thermosensitivity of impulse 0.23 s-1/°C. Note that the frequency of the IPSPs (Inset) was simultaneously reduced by ≈89% (see text).
The recovery after washout most often took 10 min to be complete. In addition, PGE2 treatment did not affect the resting membrane potential -54.5 ± 7.8 mV versus -53.5 ± 9.7 mV after PGE2 (n = 70, paired t test, P < 0.05), or the input resistance of the neurons, which ranged between 260 and 650 MΩ and averaged 437 ± 105 MΩ in both conditions (n = 15, paired t test, P < 0.05).
Ten other spontaneously active neurons decreased their firing rate by ≈60% after PGE2 treatment (data not shown), as their frequency of firing dropped from an average of 6.8 ± 4.3 Hz to 2.9 ± 3.6 Hz (P < 0.01, paired t test). This decrease was not associated with obvious modifications of PSPs.
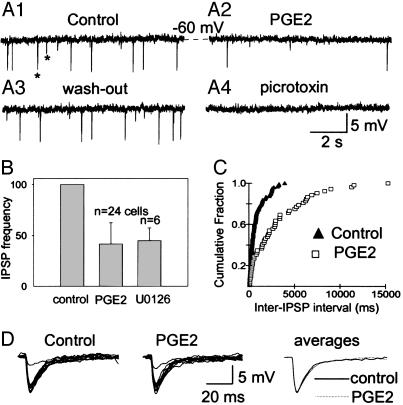
Effect of PGE2 on Synaptic Potentials. The frequency of IPSPs was reduced in neurons treated with PGE2. Fig. 3 shows that this reduction was reversible (Fig. 3 A1–A3) and that IPSPs were blocked by bath application of picrotoxin (Fig. 3A4), indicating that they are generated by activation of γ-aminobutyric acid GABA-A receptors. The PGE2-induced decrease in IPSP frequency was found in 24 of the 70 neurons, its mean averaging 62.3 ± 21.3% (Fig. 3B), and it was manifest in inter-IPSP interval histograms (Fig. 3C). This decrease was consistently seen in all 12 cells that exhibited IPSPs in the absence of PGE2, the thermosensitivity of which was raised by PGE2, as in Fig. 2B.
Fig. 3.
Responses of IPSP frequency and kinetics to PGE2. (A1–A4) Representative membrane voltage traces recorded at -60 mV in resting conditions with spontaneously occurring IPSPs (some are indicated by asterisks) (A1), 5 min after the onset of a perfusion with 500 nM PGE2, which reduced the IPSP frequency from 3.2 Hz to 0.7 Hz (A2), after complete recovery after a 10 min washout (A3), and in presence of 50 μM picrotoxin (A4), which abolished all postsynaptic potentials (A4). (B) Average reduction of IPSP frequency ± SD, calculated using the indicated number (n) of cells. (C) Cumulative histogram of the interevent intervals for the same cell as in A (KS test, P < 0.01). (D) Constancy of IPSP kinetics. Superimposed randomly selected traces (n = 10) from the experiment in A and their overlapping averages.
In contrast to its effects on the frequency of IPSPs, PGE2 was without effect on their kinetics, as shown in Fig. 3D, where two groups of 10 randomly selected events and their superimposed averages can be compared. That is, their rise time (of 1.5–4 ms) and decay times (≈39 ± 14 ms, with a range of 15–56 ms) and their time constant (≈25 ± 8 ms, with a range of 12–36 ms) were not significantly different.
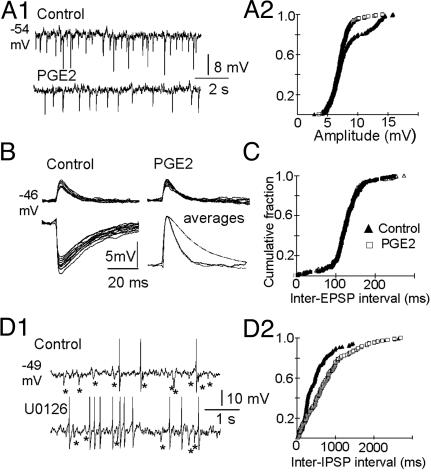
Although PGE2 reduced the frequency of IPSPs, it did not affect their amplitudes, except in 8 of the 70 cells studied (which all belonged to the subset presented in Fig. 3B). Fig. 4A1 and A2 illustrates this reduction of the IPSP amplitude in a cell where it was moderate. Overall the average decrease ranged from 8% to 25% (18.5 ± 6.5%, n = 8).
Fig. 4.
Similarity of PGE2 and U0126 effects. (A1 and A2) Reduction of IPSP amplitudes by PGE2 in a subset of cells. (A1) Voltage traces recorded before (Upper) and after (Lower) PGE2 treatment, with an average decrease from 8.1 to 6.9 mV, a decrease of ≈15%. (A2) Corresponding cumulative histograms of IPSP amplitudes with two statistically different distributions (KS test, P < 0.01). (B and C) Constancy of EPSP amplitudes, kinetics, and frequencies. Superimposition of randomly selected (n = 10) EPSPs (Upper) and IPSPs (Lower Left), and superimposition of their normalized averages (Lower Right), which have been inverted to emphasize the differences between their time course and that of the EPSPs. Cumulative histograms of inter-EPSP intervals, illustrating their similarities before and after PGE2 (KS test, P < 0.001; B and C are from the same cell). (D1 and D2) PGE2-like effect of U0126. (D1) Sample sweeps recorded before (Upper) and after (Lower) exposure of the recorded cell to a solution containing 30 μM U0126. Note the increase of the firing rate from 0.7 to 2.5 Hz and the decreased number of IPSPs (asterisks). (D2) Corresponding inter-IPSP intervals (KS test, P < 0.01).
None of the effects of PGE2 reported here could be attributed to alterations of the IPSP reversal potential, which remained stable. Assessed with hyper- and depolarizing current pulses, its value, which varied from -55 to -79 mV, averaged -65.6 ± 7.6 mV and 65.1 ± 7.8 mV before and after PGE2, respectively, in 14 cells, including four neurons in which the amplitude distributions were affected by PGE2 (paired t test, P < 0.05).
Some AH neurons also presented spontaneous EPSPs, which were sensitive to 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) (data not shown), suggesting that they represent activation of AMPA/kainate receptors. Yet the increased firing rate of PGE2-treated cells could not be accounted for by modified EPSP frequency or amplitude (Fig. 4B). The inter-EPSP interval distribution (Fig. 4C), as well as the EPSP rise time, decay time, and amplitude distributions (data not shown), were not affected by PGE2 in the neurons tested (n = 15).
Prompted by our finding that PGE2 reduces the level of pERK1/2 in AH neurons (see below), we asked whether the reduction in the IPSP frequency and the increase in firing rate of the neurons could be related to decreased activity of ERK1/2. Thus, we used U0126, a potent and selective inhibitor of MAPK kinase 1 (MEK1), which is responsible for the phosphorylation and activation of ERK1/2 (16), expecting that this compound could mimic some of the effects of PGE2. As expected, in 6 of 43 neurons tested, U0126 decreased the frequency of IPSPs within 5–10 min. Fig. 4D1 illustrates recordings from such a neuron. The inter-IPSP interval distributions were significantly different before and after addition of U0126 (Fig. 4D2), whereas the kinetics and amplitudes of the synaptic potentials were not modified (data not shown). Overall, in six neurons, the IPSP frequency averaged 2.4 ± 0.7 Hz in control and 1.0 ± 0.6 Hz after U0126, meaning a decrease of 55.1 ± 12.5%. This value was not significantly different from that obtained with PGE2 (Fig. 3B). In three of these cells there was also an increase of the firing rate averaging 219 ± 116%. Furthermore, and as expected, U0126 increased the thermosensitivity of the same three neurons, which shifted from 0.1 ± 0.1 to 0.7 ± 0.4 impulse s-1/°C.
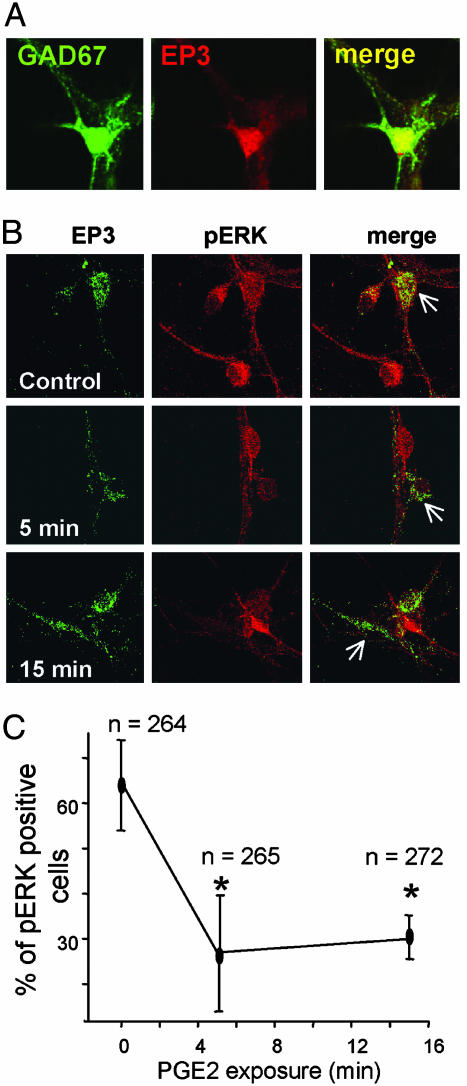
Possible Involvement of ERK1/2. PGE2 binds to four types of G-protein-coupled EP1R–EP4R and, depending on the activated receptor subtype, it has been shown to affect in either direction the intracellular levels of cAMP and Ca2+ (reviewed in ref. 17). The EP3R subtype is the main EPR expressed in the hypothalamus (18). Our experimental material contains a high proportion of GABAergic neurons (81 ± 6%; sample size 330 cells, n = 3 experiments). EP3R was identified by immunohistochemistry; coexpression of GAD67 and EP3R immunoreactivity (Fig. 5A) was detected in 218 of 801 cells (27%). EP1R and EP4R staining was not detectable in the same cultures.
Fig. 5.
Effects of PGE2 on pERK in identified EP3R-expressing GABAergic neurons. (A and B) Confocal images of hypothalamic neurons treated for immunocytochemistry. (A) Staining for GAD67 (Left), for EP3R (Center), and their superimposition (Right, merge) showing coexpression for these two proteins. (B and C). Microphotographs of neurons labeled for EP3R (Left, green), for pERK (Center, red), and their superimposition (Right, merge). They were obtained in control conditions (Top) and after 5 and 15 min exposure to 500 nM PGE2 (Middle and Bottom, respectively). The progressive decrease of pERK is indicated by arrows. (C) Percentage of cells (ordinate) stained for pERK in the EP3R-positive population, observed in three independent experiments, versus time. The numbers (n) refer to the total number of neurons analyzed in each condition. * indicates statistical significance (P < 0.05, ANOVA followed by Tukey test).
EP3Rs have several splice variants in the C-terminal region, which determine their coupling to G-proteins. Most splice variants inhibit cAMP production through a Gi-coupled mechanism, although some of them can couple to Gs and increase cAMP levels, or increase intracellular calcium levels and produce a cAMP-independent activation of the cAMP-responsive element (CRE). Recent studies have shown that EP3R can signal through the small GTPase Rho via activation of tyrosine kinases (19). Except for the reduction in intracellular cAMP levels, all these signaling mechanisms have been shown to activate the ERK 1/2 MAP kinases in neurons (reviewed in ref. 20). Thus, to delineate the signaling pathways activated by EP3R in AH neurons, we analyzed the phosphorylation state of both the ERK1/2 MAP kinases and of the CRE binding protein CREB after PGE2 treatment. We found no evidence for changes in the phosphorylation state of CREB (data not shown). However, a large percentage of the neuronal population (i.e., 70.3 ± 10.7% of the cells) had a high level of pERK1/2 under basal conditions both in the overall neuronal population and in 64.7 ± 8% of the EP3R-expressing neurons (Fig. 5 B and C). This high degree of pERK1/2 was most likely dependent on synaptic activity because it was abolished by tetrodotoxin (1 μM for 20 min). Exposure to PGE2 under similar conditions to those causing a reduction of the frequency of IPSPs (see Fig. 3) led to a time-dependent reduction of the levels of pERK to 30 ± 4.5% of the EP3R-expressing neuronal population (Fig. 5C). [Similar results were obtained in low-K (3.5 mM) medium, but these were not quantified.] The effects of PGE2 on the phosphorylation state and those on temperature sensitivity had similar time courses.
Discussion
The mechanism by which PGE2 affected the firing properties or by which it increased the number of warm-sensitive cells, which rose from ≈9% to 27% (19 of 70) in a series of control experiments with untreated cells in presence of this pyrogen, is not completely understood. This effect occurred without major changes in membrane potential either at rest or during warming (<6 mV). Thus, contrary to early studies of thermosensitivity (21), it is unlikely that a Na+/K+ pump is involved, despite evidence that ouabain perfusion can also induce thermosensitivity in temperature-insensitive cells (22).
Our results provide strong support for the notion that, at least in the presence of PGE2, inhibitory inputs onto AH neurons are in large part responsible for these cells' sensitivity to warming. This observation was made possible because, as indicated by both recordings and immunocytochemistry, neurons studied here formed a dense network where most cells were GABAergic, with but a few intercalated excitatory neurons. We have not investigated the involvement of IPSPs in the thermosensitivity of untreated cultured cells. This issue has been addressed in slices by others, with contrasting results. That is, the “effectiveness” of (GABA)ergic IPSPs (i.e., their duration and kinetics) are reduced at high temperatures, making the cells more warm-sensitive (7). Such is also the case in suprachiasmatic nucleus neurons, as changes in membrane resistance in addition to a block of inhibitory synapses with bicuculline can enhance thermosensitivity (23). However, in the two last preparations, the frequency of IPSPs was reported to increase (23) or to remain unaffected (24) during warming.
Modifications of IPSP frequency in the recorded cells indicate a predominantly presynaptic effect of PGE2 on GABAergic neurons (although a moderate reduction in the IPSPs amplitude was also observed in a subset of cells). Furthermore, in eight experiments, U0126 was added to the pipette solution, and breakthrough whole-cell configuration was used instead of perforated patch. Yet the intracellular diffusion of U0126 never affected IPSP frequency (at least within 10 min), once in whole-cell configuration. The discrepancy between the efficacy of the bath-applied compound and its ineffectiveness when it is applied intracellularly also points to a presynaptic origin of IPSPs alterations. In support, the firing rate was reduced by U0126 in three other cells.
The levels of pERK1/2 can be considered to be the product of balanced actions of excitatory and inhibitory signals in neurons. Thus inputs such as neurotrophins or excitatory neurotransmitters increase the levels of pERK1/2 as a mediator of their long-term effects. Conversely, several types of inhibitory signals can decrease the level of pERK, thus blunting the activatory signals described (reviewed in refs. 20 and 25). In our anterior hypothalamic cultures we found that a large proportion of the EP3R-positive neurons had a high basal level of pERK, which was probably maintained by synaptic activity and was reduced by exposure to PGE2. The main signaling pathway influenced by PGE2 binding to EP3R is that of reduction in cAMP levels (reviewed in ref. 26). Our results indicating a reduction in pERK upon PGE2 treatment suggests that activated EP3Rs in these neurons induce the inhibition of adenylate cyclase. The mechanism of ERK activation by cAMP involves a protein kinase A-mediated activation of the small G-protein Rap1 and subsequential activation of the serine/threonine kinase B-Raf, which in turn activates the MAPK kinase 1 (MEK1) and the ERK1/2 kinase pathway (27, 28). In turn, cAMP-mediated activation of protein kinase A (PKA) and the ERK1/2 kinase pathways was recently shown to control neuronal excitability by reducing dendritic A-type voltage-gated K+-currents in hippocampal neurons (27–30). Hence, a low level of pERK/ERK1/2 activity could result in larger A-type currents causing prolonged interspike intervals (see ref. 31) and therefore a reduction of neuron's firing rate. Although we have not analyzed the signaling elements upstream of ERK1/2 in the PGE2 mediated effects, in vivo data indicate that PGE2 injections in the AH produce a decrease in intracellular cAMP levels (26).
Taken together, our results can be viewed in the context of the network formed by the studied AH-cells. The fact that PGE2 decreases IPSP frequency reflects a decreased firing rate in EP3R-positive GABAergic neurons, most likely via an ERK1/2-mediated presynaptic mechanism. Released from inhibition, their targets become more excitable and exhibit an increased number of action potentials. Both opposite and apparently conflicting effects of PGE2, which are documented in this report, suggest that a subtle balance of neuronal activities is involved in thermosensitivity.
Acknowledgments
We thank Dr. B. Conti for both constant support and comments during this work, Dr. W. Loomis for critically reading the manuscript, and Svetlana Gaidarova for technical help. This work was supported by National Institutes of Health Grant NS043501 and Ellison Foundation Grant AG-SS-06641.
Abbreviations: PSP, postsynaptic potential; EPSP, excitatory PSP; IPSP, inhibitory PSP; GABA, γ-aminobutyric acid; ERK, extracellular signal-regulated kinase; pERK, phospho-ERK; EpnR, E-prostanoid receptor type n; PGE2, prostaglandin E2; PO, preoptic area; AH, anterior hypothalamus; KS test, Kolmogorov–Smirnov two-sample test.
References
- 1.Boulant, J. A. (2000) Clin. Infect. Dis. 31, Suppl. 5, S157-S161. [DOI] [PubMed] [Google Scholar]
- 2.Hardy, J. D., Hellon, R. F. & Sutherland, K. (1964) J. Physiol. 175, 242-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boulant, J. A. & Hardy, J. D. (1974) J. Physiol. 240, 639-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gordon, C. J. & Heath, J. E. (1980) Brain Res. 183, 113-121. [DOI] [PubMed] [Google Scholar]
- 5.Ranels, H. J. & Griffin, J. D. (2003) Brain Res. 964, 42-50. [DOI] [PubMed] [Google Scholar]
- 6.Dean, J. B. & Boulant, J. A. (1989) Am. J. Physiol. 257, R57-R64. [DOI] [PubMed] [Google Scholar]
- 7.Curras, M. C., Kelso, S. R. & Boulant, J. A. (1991) J. Physiol. 440, 257-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsuda, T., Hori, T. & Nakashima, T. (1992) J. Physiol. 454, 197-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Griffin, J. D., Kaple, M. L., Chow, A. R. & Boulant, J. A. (1996) J. Physiol. 492, 231-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kobayashi, S. & Takahashi, T. (1993) Proc. R. Soc. London Ser. B 251, 89-94. [DOI] [PubMed] [Google Scholar]
- 11.Hori, A., Minato, K. & Kobayashi, S. (1999) Neurosci. Lett. 275, 93-96. [DOI] [PubMed] [Google Scholar]
- 12.Coceani, F. & Akarsu, E. S. (1998) Ann. N.Y. Acad. Sci. 856, 76-82. [DOI] [PubMed] [Google Scholar]
- 13.Feldberg, W. & Saxena, P. N. (1971) J. Physiol. 219, 739-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ono, T., Morimoto, A., Watanabe, T. & Murakami, N. (1987) J. Appl. Physiol. 63, 175-180. [DOI] [PubMed] [Google Scholar]
- 15.Nelson, D. O. & Prosser, C. L. (1981) Science 213, 787-789. [DOI] [PubMed] [Google Scholar]
- 16.Favata, M. F., Horiuchi, K. Y., Manos, E. J., Daulerio, A. J., Stradley, D. A., Feeser, W. S., Van Dyk, D. E., Pitts, W. J., Earl, R. A., Hobbs, F., et al. (1998) J. Biol. Chem. 273, 18623-18632. [DOI] [PubMed] [Google Scholar]
- 17.Breyer, R. M., Bagdassarian, C. K., Myers, S. A. & Breyer, M. D. (2001) Annu. Rev. Pharmacol. Toxicol. 41, 661-690. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura, K., Kaneko, T., Yamashita, Y., Hasegawa, H., Katoh, H., Ichikawa, A. & Negishi, M. (1999) Neurosci. Lett. 260, 117-120. [DOI] [PubMed] [Google Scholar]
- 19.Aoki, J., Katoh, H., Yasui, H., Yamaguchi, Y., Nakamura, K., Hasegawa, H., Ichikawa, A. & Negishi, M. (1999) Biochem. J. 340, 365-369. [PMC free article] [PubMed] [Google Scholar]
- 20.Sweatt, J. D. (2001) J. Neurochem. 76, 1-10. [DOI] [PubMed] [Google Scholar]
- 21.Sperelakis, N. (1970) Physiological and Behavioural Temperature Regulation (Thomas, Springfield, IL).
- 22.Curras, M. C. & Boulant, J. A. (1989) Am. J. Physiol. 257, R21-R28. [DOI] [PubMed] [Google Scholar]
- 23.Burgoon, P. W. & Boulant, J. A. (1998) J. Physiol. 512, 793-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Griffin, J. D., Saper, C. B. & Boulant, J. A. (2001) J. Physiol. 537, 521-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grewal, S. S., York, R. D. & Stork, P. J. (1999) Curr. Opin. Neurobiol. 9, 544-553. [DOI] [PubMed] [Google Scholar]
- 26.Steiner, A. A., Antunes-Rodrigues, J. & Branco, L. G. (2002) Brain Res. 944, 135-145. [DOI] [PubMed] [Google Scholar]
- 27.Vossler, M. R., Yao, H., York, R. D., Pan, M. G., Rim, C. S. & Stork, P. J. (1997) Cell 89, 73-82. [DOI] [PubMed] [Google Scholar]
- 28.Morozov, A., Muzzio, I. A., Bourtchouladze, R., Van-Strien, N., Lapidus, K., Yin, D., Winder, D. G., Adams, J. P., Sweatt, J. D. & Kandel, E. R. (2003) Neuron 39, 309-325. [DOI] [PubMed] [Google Scholar]
- 29.Schrader, L. A., Anderson, A. E., Mayne, A., Pfaffinger, P. J. & Sweatt, J. D. (2002) J. Neurosci. 22, 10123-10133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yuan, L. L., Adams, J. P., Swank, M., Sweatt, J. D. & Johnston, D. (2002) J. Neurosci. 22, 4860-4868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hille, B. (2001) Ion Channels of Excitable Membranes (Sinauer, Sunderland, MA).