Abstract
Enzootic bovine leukosis (EBL) is a retroviral infection that causes persistent lymphocytosis and lymphosarcoma in cattle. The economic importance of infection by bovine leukemia virus (BLV) is due to several factors, including losses in exportation, treatment of secondary infection, and reduction in dairy production. To facilitate the development of a national test that is sensitive, simple, and applicable on a large scale, this work aimed to produce and characterize monoclonal antibodies (mAbs) against gp51 protein from BLV for use in an enzyme-linked immunosorbent assay (ELISA) test. Two hundred seventy-four hybridomas were generated, from which 37 were mAbs secretory clones screened by indirect ELISA. The specificity of the mAbs generated against gp51 was verified by Western blot analysis, and the isotypes were characterized for isotyping in IgG1 and IgM. To evaluate the test, 250 sera were tested by agar gel immunodiffusion and mAb-ELISA. The values obtained for the mAb-ELISA test were 95% sensitivity and 90% specificity.
Key words: bovine leukemia virus, ELISA test, enzootic bovine leukosis, monoclonal antibodies, sensitivity, specificity
Introduction
Enzootic bovine leukosis (EBL) is a chronic disease caused by bovine leukemia virus (BLV). BLV is an exogenous type C retrovirus that belongs to the family Retroviridae, subfamily Orthoretrovirinae, genus Deltaretrovirus, and primarily infects B lymphocytes.1
The proviral genome of BLV consists of 8714 bp,2 while the extremities contain a sequence denominated long terminal repeat, which is composed of three consecutive regions named U3, R, and U5. At least seven alternatively spliced RNAs have been identified, together with eight open reading frames designated gag, prt, pol, env, tax, rex, RIII, and GIV.3 The viral envelope is formed by a cell-derived lipid bilayer into which proteins encoded by the env region are inserted; specifically, a transmembrane protein (gp30) and a surface protein (gp51). The proteins are directly involved in infectivity events and, like the p24 major structural protein, can elicit a strong immune response in infected cattle.4 The gp51 protein ensures the recognition of the cellular viral receptor, and monoclonal antibodies (mAbs) have been used to identify antigenic sites.2
The infection of cattle by BLV is characterized by persistent lymphocytosis and the occurrence of antibodies against viral structural proteins, and lymphoid tumors can appear after a long period in some infected animals.6 EBL is an important animal health problem in Brazil because infected cattle present immune system disorders that increase their susceptibility to other infectious diseases.7,8 This widespread infection presents varying levels of prevalence among herds and shows higher prevalence in dairy cattle.9
The economic importance of BLV infection is due to several factors: loss of export markets that require infection-free animals, the cost of diagnosis and treatment, the sacrifice or premature death of cattle, and the condemnation of carcasses.10
EBL is commonly diagnosed through different methods by the detection of specific antibodies in serum or milk samples. The antibodies are detected in bovine serum between 2 and 8 weeks after infection. The infected cattle develop a humoral response against viral proteins, particularly gp51 and p24.11 Diagnostic tests for BLV have important applications in veterinary medicine, including research, epidemiological surveillance, certification of areas free of disease, and prevalence studies. Several diagnostic methods are used, including enzyme-linked immunosorbent assay (ELISA), Western blot, dot-blot, radioimmunoassay, radioimmunoprecipitation assay, syncytium inhibition, polymerase chain reaction, and agar gel immunodiffusion (AGID).12,13
The ELISA test is based on the use of partially purified viral proteins, and the development of these purified inputs for this technique has an important impact on the specificity of the diagnosis. Monoclonal antibodies have been used extensively in numerous experiments and diagnostic studies of human and veterinary sciences, which has increased test specificity.5 One example is the mAbs for the proteins gp51 and p24, used either as input or as a component in the purification of BLV.6,14,15,29
The main objective of this work was to produce and characterize mAbs against the protein gp51. These could be used for the development of mAb-ELISA, in order to increase the specificity and sensitivity of the test. Diagnosis can reduce associated disease mortality rate and directly help to control and eradicate the infection.16
Materials and Methods
Antigen production and immunization
For BLV virus production, FLK-BLV (TECPAR) cells were cultivated in F10-199 (SIGMA) media supplemented with 10% fetal calf serum (FCS) (GIBCO) and maintained in 5% CO2 at 37°C. The virus from tissue culture fluid was concentrated through tangent filtering (Labscale, 30 kDa) and purified by sucrose gradient centrifugation, after which the BLV precipitates were resuspended in TEN buffer (10 mM Tris-HCl, 1 mM EDTA, 100 mM NaCl, pH 8.0).
The BLV antigen preparation used in this study was treated with a lysis buffer (0.15 M NaCl, 0.05 M Tris-HCl pH 7.2, 0.1% SDS, 1% Triton X-100, 1% sodium deoxycholate) and incubated overnight in ice. The lysate BLV (LYS) was then centrifuged at 35,000 g at 4°C for 1 h.
Two female BALB/c Swiss inbred mice, aged 4–6 weeks, were immunized intraperitoneally four times with 1 mg of LYS antigen. Complete Freund's adjuvant was used in the first application and incomplete Freund's adjuvant in subsequent immunizations. Following each immunization, mice sera were tested using the AGID kit provided by the Parana Institute of Technology (Instituto de Tecnologia do Paraná, TECPAR).
Hybridomas production
Hybridoma cells producing antibodies against BLV proteins were prepared according to technique previously described.17 Several modifications, proposed by Llames et al.18–20 and Shahhosseni et al.,21 were included to simplify the procedure, which increases the yield of antibody-producing cells.
The mouse myeloma cell line SP2-0/Ag14 was used as a fusion partner. This cell line was grown in RPMI 1640 supplemented with 5% FCS, followed by 8-azaguanine. The feeder cells used were BALB/c macrophages, obtained by peritoneal wash with 0.3 M sucrose, distributed in five plates (480 wells).
Following the final immunization, the spleen was removed and macerated to obtain B lymphocytes. Red blood cells were lysed for 5 min on ice using 5 mL of lysis solution (166 mM NH4Cl, 9 μM EDTA, and 95 mM NaHCO3). The cell suspension was then washed twice with RPMI medium. Next, myeloma and spleen cells were fused at a ratio of 1:5, respectively. This protocol involved 1 mL of 50% (w/w) polyethylene glycol/dimethylsulfoxide for 1 min, washed with RPMI medium for 5 min. Following fusion, the hybrid cells were resuspended in RPMI medium, 20% FCS containing antibiotics (40 μg/mL gentamicin and 1.25 μg/mL of amphotericin B), 2 mM of glutamine, and 1 mM of sodium pyruvate, and were placed in 456 wells at a concentration of 2.2×104 cells/well, and the remaining 24 wells were used as control, containing only hypoxanthine-aminopterin-thymidine (HAT) medium and myeloma cells. After 48 h of growth, in RPMI medium 20% FCS at 5% CO2 and 37°C, HAT medium, 20% FCS was then replaced for 10 days, the period required for death of nonfused cells. The antibody-secreting hybridomas were select by screening methods over a period of 15 days. Hybridomas of interest were expanded in RPMI medium, 20% FCS.
Indirect ELISA for selection the positive hybridomas
ELISA plates (Microloan 600, high binding F- Bottom) were coated overnight at 4°C with 200 μL/well of 1 μg LYS antigen in coating buffer (0.5 M carbonate-bicarbonate, pH 9.6). Then, the unbound antigens were discarded and the wells were washed four times with washing buffer (50 mM Tris, 0.2% Tween-20, pH 7.5). The wells were incubated at 37°C for 1 h with blocking buffer (3% bovine serum albumin [BSA], 50 mM Tris-HCl, pH 7.4). The hybridoma supernatants diluted in blocking buffer (1:2) were incubated at 37°C for 1 h, followed by incubation with peroxidase-conjugated anti-mouse IgG (1:12,800) at 37°C for 1 h. The reaction was revealed by adding 100 μL/well of freshly prepared chromogenic substrate (8 mg ortho-phenylenediamine dihydrochloride, dissolved in 5 mL of 0.05 M citrate-phosphate buffer pH 4.5) in the presence of 0.01% hydrogen peroxide. The absorbance was read at 492 nm.
Stability test
The positive hybridomas were selected by screening and subjected to two consecutive cycles of freezing-thawing. Frozen first at −20°C for 2 h, then at −70°C for another 2 h, and stored in liquid nitrogen for 48 h; the cycle was finished by thawing the hybridomas. The clones were tested again by indirect ELISA, and the remaining positives were expanded.
Characterization of mAbs and isotyping
Western blot analysis was performed to confirm the specificity of the mAbs. LYS antigen were separated in 15% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred passively to polyvinyl difluoride for 24 h. The membranes were blocked with blocking buffer for 60 min and subsequently incubated with supernatant of mAbs (1:2) and peroxidase-conjugated anti-mouse IgG (1:2000), with washing between these processes. The reaction was revealed using the 4-chloro-1-naphthol substrate.
The isotyping was realized with the mouse monoclonal antibody isotyping ELISA kit (Sigma) and was conducted according to the manufacturer's recommendations.
Production of hybridoma from ascitic fluid
The production of hybridomas from ascitic fluid was performed according to the protocol described by Harlow and Lane.22 The hybridomas that showed the best results by indirect ELISA were selected for mice inoculation to produce ascitic fluid. The ascitic fluid containing hybridomas was centrifuged at 3000 g, the pellet was washed five times to remove the impurities, and the hybridomas present in the pellet were cultured in RPMI medium, 20% FCS, and expanded until the complete removal of macrophages, leaving only the hybridomas. The hybridomas were then cultured and the supernatant collected for mAbs purification.
Purification and selection of mAbs
The mAbs were purified by chromatography on Protein G-Sepharose gel columns (HiTrap Protein G HP, GE) in accordance with the manufacturer's recommendations. The quantity of proteins of the mAbs was verified using the technique of Bradford (BIO-Rad protein assay kit), and the hybridoma which generated the mAb with best result were selected for mAb-ELISA.
Monoclonal antibody–based ELISA
MAb-ELISA was performed as described: 250 μg of the selected mAb was diluted in 50 μL of coating buffer per well. The plate was incubated overnight at 4°C, blocked with 3% BSA, and incubated at 37°C for 1 h. Subsequently, 1 μg of LYS antigen was diluted in 50 μL of blocking buffer per well and incubated at 37°C for 2 h. The serum samples to be tested were diluted in 1:50 of blocking buffer and incubated at 37°C for 1 h, followed by peroxidase-conjugated anti-bovine IgG (1:12,800). After each incubation step, the wells were washed four times with washing buffer. The reaction was revealed and the absorbance was read.
Agar gel immunodiffusion
A commercial AGID kit from TECPAR was used in accordance with the manufacturer's recommendations. The slides were incubated for 72 h at 25°C in a humid atmosphere. The serum samples were tested and compared with the standard serum in the kit. Those that presented reactions were scored as weak positive or positive, and those that were not reactive were scored negative.
Results
Screening of hybridoma supernatants and selection of mAbs
In this fusion, 274 hybridomas were obtained and screened using the indirect ELISA method with BLV and LYS antigen preparation. Three to four weeks after the fusion, all the hybridomas were tested seven times by indirect ELISA at different stages of growth.
The cut-off was defined by the average optical density in five standard positive and five negative sera, plus 2 standard deviations. The absorbance (A)490<0.2 was considered as negative, A490≥0.3 as positive, and A490>0.3 up to 1.0 as strongly positive.
Among the clones tested, 37 hybridomas were considered positive, and among these, only 18 strongly positive hybridomas were selected. Following screening, the 18 hybridomas were evaluated in a stability test, and among these, 10 hybridomas remained unaltered, maintaining cell growth and secretion of antibodies, three presented approximately 40% reduction in antibody secretion, and five presented no antibody secretion, becoming negative in the ELISA test.
Characterization of mAbs
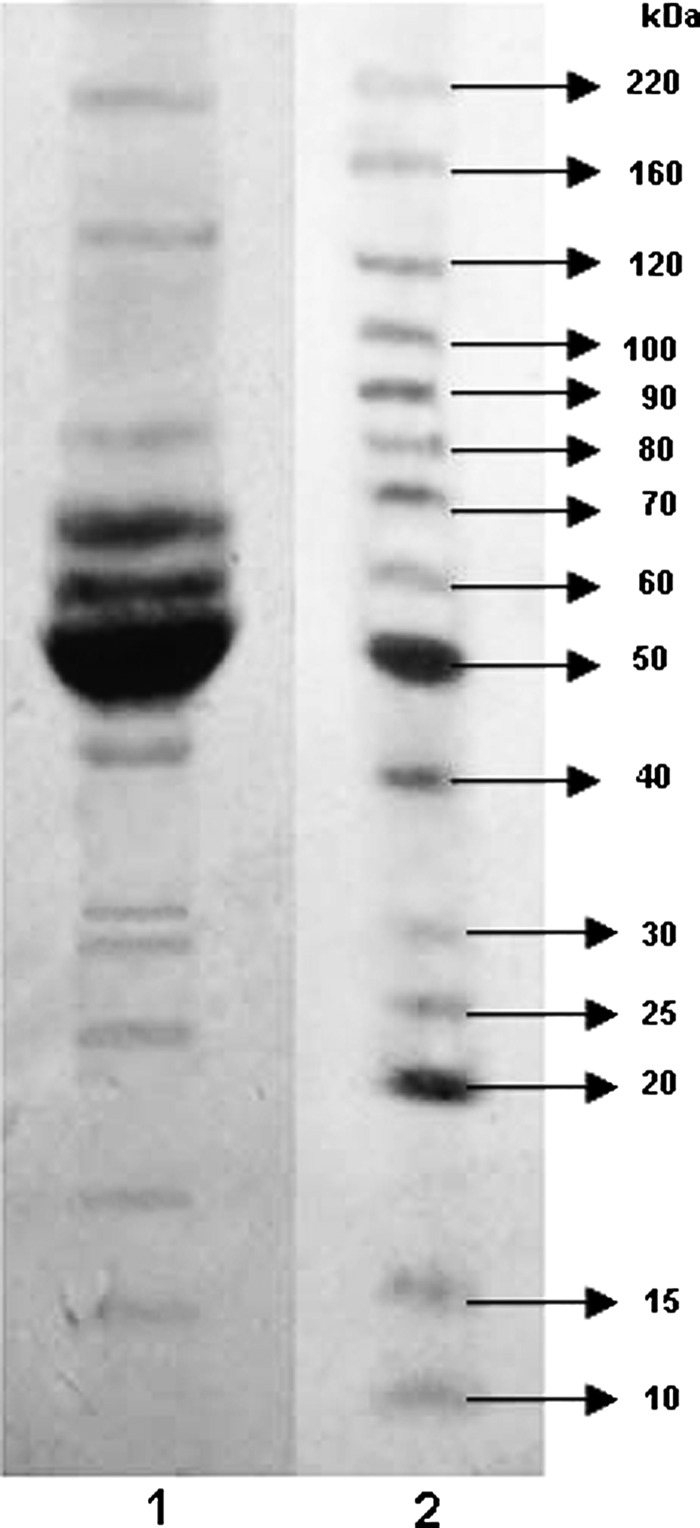
Immunoblots are essential to truly establish the specificity of the antibodies for the protein of interest. The specificity of the obtained mAbs anti-gp51 was studied by immunoblot analysis using a LYS antigen separated by SDS-PAGE (Fig. 1).
FIG. 1.
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) analysis of the LYS antigen. Lane 2 is molecular weight BenchMarck™ molecular weight ladder.
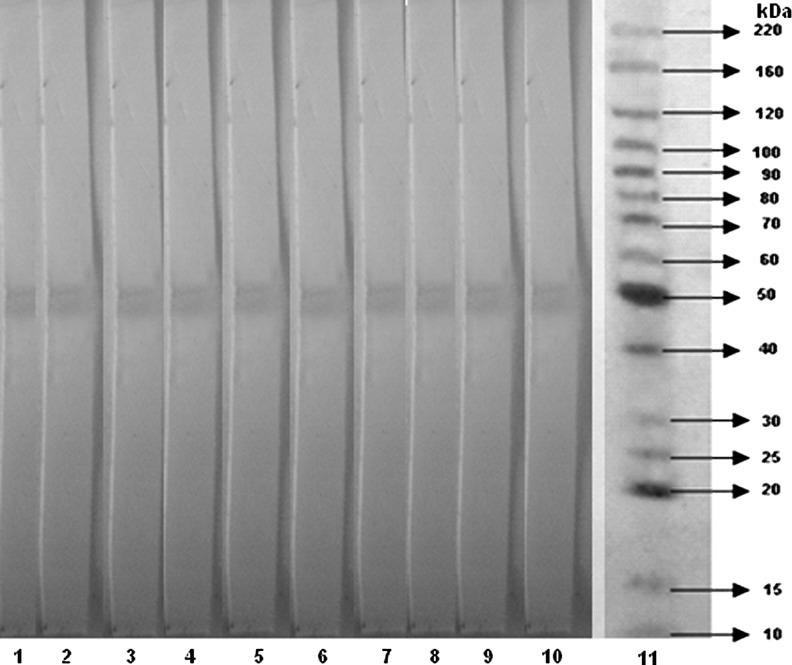
The results showed that all 10 clones recognized an immunogenic band with a molecular weight of approximately 51 kDa from protein gp51 of BLV. No cross-reacting or nonspecific bands were observed, indicating their specificity (Fig. 2). The determination of the class and subclass of antibody is useful in predicting the properties of the antibody and is needed for their proper use. The isotyping (class and subclass) of the produced mAbs was determined by an isotyping kit, confirming clones anti-gp51 of the IgM class (6 mAbs) and IgG subclass IgG1 (4 mAbs) (Table 1).
FIG. 2.
Western blot analysis of anti-gp51 using 10 monoclonal antibodies (mAbs). Lanes 1–10 are mAbs and lane 11 is the molecular weight BenchMarck™ molecular weight ladder.
Table 1.
Isotypes of the Monoclonal Antibodies Produced
| Number | Hybridoma name | Anti- | Isotype |
|---|---|---|---|
| 1 | BLVgp51-1/B3 | gp51 | IgG1, κ |
| 2 | BLVgp51-1/C2 | gp51 | IgM, κ |
| 3 | BLVgp51-3/D4 | gp51 | IgM, κ |
| 4 | BLVgp51-3/E3 | gp51 | IgM, κ |
| 5 | BLVgp51-3/E4 | gp51 | IgG1, κ |
| 6 | BLVgp51-4/E10 | gp51 | IgG1, κ |
| 7 | BLVgp51-5/F1 | gp51 | IgM, κ |
| 8 | BLVgp51-5/F5 | gp51 | IgG1, κ |
| 9 | BLVgp51-5/F11 | gp51 | IgM, κ |
| 10 | BLVgp51-5/G8 | gp51 | IgM, κ |
BLV, bovine leukemia virus.
Hybridomas from ascitic fluid
The positive hybridomas cultured from ascitic fluid presented increased secretion of gp51 antibodies compared with the respective hybridomas derived from fusion. For larger scale production of anti-gp51 BLV mAbs, four hybridomas were selected. Three ascitic clones presented an increase of approximately 4-fold in the secretion of mAbs and one clone, a 2-fold increase.
Comparison of mAb-ELISA and AGID
A total of 250 serum samples from cattle aged more than 1 year were used for this comparison study. They were randomly selected from a bank of diagnostic samples established at TECPAR, and two positive and negative controls were used as reference. These serum samples were previously tested by the AGID technique and subjected to the mAb-ELISA. The results of the serological test performed on 250 serum samples by two evaluation techniques are presented in Table 2. Out of the 119 serum samples that presented negative results in the AGID test, 11 (4.4%) were positive by the mAb-ELISA. From the 131 serum samples that were positive in the AGID, 125 (50%) were positive by mAb-ELISA, and 6 (2.4%) were negative. Each weakly positive serum sample was retested in duplicate with its respective assay to confirm its positive status. The mAb-ELISA test resulted in sensitivity 95% and specificity 90%.
Table 2.
Comparison of Results Obtained with 250 Serum Samples Using Agar Gel Immunodiffusion and Monoclonal Antibody–Enzyme-Linked Immunosorbent Assay
| AGID+ | AGID− | Total | |
|---|---|---|---|
| ELISA+ | 125 (50%) | 11 (4,4%) | 136 |
| ELISA− | 6 (2.4%) | 108 (43.2%) | 114 |
| Total | 131 | 119 | 250 |
AGID, agar gel immunodiffusion; ELISA, enzyme-linked immunosorbent assay.
Discussion
Our protocol for mAb production resulted in a fusion frequency of 60% (274/456). Among these hybridomas, 37 were positive (13.5%, 37/274). Harlow and Lane22 reported that the frequency of nonsecreting colonies is related to errors in the stages of mitosis and interphase of fused cells. The division process of chromosomes is not always equivalent and they can lose their applicability. This irregular process results in the inability of hybridomas to produce specific antibodies or to make arrangements of immunoglobulin chains. Even in the most efficient hybridoma fusions, only about 1% of the starting cells are fused and only about 1 in 100,000 form viable hybrids.
The main difficulty with fusion protocols is the stabilization of mAb secretion, in which only 10 of the 18 hybridomas subjected to the stability testing continued to secrete antibodies. Clark and Milstein,23 observed similar results when they reported that about 50% of the hybridomas they generated, which initially expressed antibodies against a desired antigen, lost the ability to express certain antibodies when subjected to freezing-thawing cycles.
Characterization of the mAbs produced (Table 1, Fig. 2) verified the specificity of the mAbs against gp51, suggesting that this is the most immunogenic protein of BLV and that the isotype IgM (60%) is predominant. Our results corroborate those reported by Llames et al.,19 who showed that 66.1% of the hybridomas they produced reacted to gp51 of BLV and 42.3% were the isotype IgM, using different antigen preparations.
The hybridomas derived from ascitic fluid cultured in vitro showed an increase in the production mAbs up to 4-fold greater when compared to the production of mAbs hybridomas not subject to ascitic fluid. The hybridomas also showed superiority in morphology, absence of cell debris, and improvement in growth performance. Clark and Milstein23 reported that hybridoma culture from ascitic secretion increased up to 10-fold. These increases occur due to the inflammatory process in the inoculated animal and the flux of macrophages. This process promotes a more stable hybridoma strain.
The current method used to detect BLV infected bovine in Brazil is the AGID test, which is specific and inexpensive, but not very sensitive and generally used for individual serum or plasma samples. This methodology is a good screening test for identifying infected animals or herds, and it is recognized by most governments as the official standard for testing imported animals.24 To conduct epidemiological studies and control programs in Brazil, in which a large number of samples are used, it is necessary to develop a highly specific and sensitive ELISA test capable of detecting antibodies in serum and milk.
The preparation of viral antigens of sufficient purity and in large quantities represents the main problem in diagnostic kits for screening purposes. The use of mAbs against BLV antigens substantially solves this problem.6,25 Due to the lack of more efficient methodologies to diagnose BLV nationally, in this study, we developed an ELISA using mAbs. This type of mAb-ELISA offers distinct advantages. The antibodies utilized are homogeneous, with specificity for a single epitope or a small protein region. They are also less likely to interact with closely related proteins, and cross-reactivity of secondary antibodies is eliminated.
Comparing our mAb-ELISA and AGID tests results (Table 2) of the 250 serum samples tested, 108 (43.2%) were negative by both AGID and mAb-ELISA and were considered nonreactive. Another 120 samples (49.2%) were reactive in both assays and were therefore considered positive. However, 11 samples (4.4%) presented discordant results, in that they were positive only by ELISA.
Thus, it is possible to affirm that the sensitivity of the mAb-ELISA test against BLV was 95% and the specificity was 90%, which is favorable compared to AGID (93% sensitivity, 90% specificity). The results obtained by this methodology were better due to the higher sensitivity of the mAb-ELISA test. The calculation was based on the methodology proposed by Menezes and Santos.26 Results similar to those obtained in this experiment have been reported by other authors.27,28
In summary, this work was developed in order to meet the needs of national testing that would be accurate and easy to apply in the area of animal health. We demonstrated that the mAb-ELISA test developed here shows greater sensitivity and specificity, generates quantitative results, requires minimal time for setup, and is easy to interpret compared with the AGID. We obtained good yields in the production of mAbs anti-gp 51, which promote reductions in the cost to produce diagnostic kits, thus increasing its accessibility. In order to effectively eradicate BLV infection in cattle, it is necessary to evaluate all infected bovine, which is currently not feasible in Brazil because the standard technique used is the AGID. Our technique could be incorporated as an alternative test in monitoring and control programs.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Van Regenmortel MHV. Fauquet CM. Bishop DHL, et al. Virus Taxonomy: The Classification and Nomenclature of Viruses, Vol. 61. Family retroviridae. San Diego: Academic Press; 2000. [Google Scholar]
- 2.Sagata N. Yasunaga T. Tsuzuku-Kawamura J, et al. Complete nucleotide sequence of the genome of bovine leukemia virus: its evolutionary relationship to other retroviruses. Proc Natl Acad Sci USA. 1985;82:677–681. doi: 10.1073/pnas.82.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dube S. Dolcini G. Abbott L, et al. The complete genomic sequence of a BLV strain from a Holstein cow from Argentina. Virology. 2000;277:379–386. doi: 10.1006/viro.2000.0622. [DOI] [PubMed] [Google Scholar]
- 4.Van der Maaten M. Miller JM. Bovine Leukosis Virus. Amsterdam: Elsevier Science Publishers B.V; 1990. [Google Scholar]
- 5.Bruck C. Mathot S. Portetelle D, et al. Monoclonal antibodies define eight independent antigenic regions on the bovine leukemia virus (BLV) envelope glycoprotein gp51. Virology. 1982;122:342–352. doi: 10.1016/0042-6822(82)90234-3. [DOI] [PubMed] [Google Scholar]
- 6.Ban J. Gieciova E. Orlik O. Altaner C. Use of monoclonal antibodies in an ELISA for the diagnosis of bovine leukaemia virus infection. J Virol Methods. 1990;30:79–87. doi: 10.1016/0166-0934(90)90045-h. [DOI] [PubMed] [Google Scholar]
- 7.Ferrer JF. Bovine lymphosarcoma. Adv Vet Sci Comp Med. 1980;24:01–68. [PubMed] [Google Scholar]
- 8.Meas S. Ruas J. Farias NA, et al. Seroprevalence and molecular evidence for the presence of bovine immunodeficiency virus in Brazilian cattle. Jpn J Vet Res. 2002;50:9–16. [PubMed] [Google Scholar]
- 9.Ravazzolo AP. Da Costa UM. Retroviridae. In: Flores EF, editor. Virologia Veterinária. Vol. 31. Rio Grande do sul; 2007. pp. 819–823. [Google Scholar]
- 10.DiGiacomo RF. Symposium on bovine leukemia virus infection: the epidemiology and control of bovine leukemia virus infection. Vet Med. 1992;87:248–257. [Google Scholar]
- 11.Cowley JA. Molloy JB. Dimmock CK, et al. Infectivity of bovine leukaemia virus infected cattle: an ELISA for detecting antigens expressed in in vitro cultured lymphocytes. Vet Microbiol. 1992;30:137–150. doi: 10.1016/0378-1135(92)90109-7. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen VK. Maes RF. Evaluation of an enzyme-linked immunosorbent assay for detection of antibodies to bovine leukemia virus in serum and milk. J Clin Microbiol. 1993;31:979–981. doi: 10.1128/jcm.31.4.979-981.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Radostits OM. Gay CC. Blood DC. Kenneth HW. Clinica Veterinaria: um Tratado de Doenças dos Bovinos Ovinos Suínos Caprinos e Equinos. 9th. Rio de Janeiro; Guanabara Koogan: 2002. [Google Scholar]
- 14.De Giuseppe A. Feliziani F. Rutili D. De Mia GM. Expression of the bovine leukemia virus envelope glycoprotein (gp51) by recombinant baculovirus and its use in an enzyme-linked immunosorbent assay. Clin Diagn Lab Immunol. 2004;11:147–151. doi: 10.1128/CDLI.11.1.147-151.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Portetelle D. Mammerickx M. Burny A. Use of two monoclonal antibodies in an ELISA test for the detection of antibodies to bovine leukaemia virus envelope protein gp51. J Virol Methods. 1989;23:211–222. doi: 10.1016/0166-0934(89)90135-3. [DOI] [PubMed] [Google Scholar]
- 16.Deren W. Szewczyk-Sadowska A. Rulka J. The eradication of enzootic bovine leucosis in a large farm population. Pol J Vet Sci. 2003;6:12–14. [PubMed] [Google Scholar]
- 17.Köhler G. Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity 1975. J Immunol. 2005;174:2453–2455. [PubMed] [Google Scholar]
- 18.Llames L. Goyache J. Domenech A, et al. Rapid detection of specific polyclonal and monoclonal antibodies against bovine leukemia virus. J Virol Methods. 1999;82:129–136. doi: 10.1016/s0166-0934(99)00092-0. [DOI] [PubMed] [Google Scholar]
- 19.Llames L. Gomez-Lucia E. Domenech A, et al. Production and characterization of monoclonal antibodies against bovine leukaemia virus using various crude antigen preparations: a comparative study. J Vet Med B Infect Dis Vet Public Health. 2000;47:387–397. doi: 10.1046/j.1439-0450.2000.00359.x. [DOI] [PubMed] [Google Scholar]
- 20.Llames L. Goyache J. Domenech A, et al. Evaluation of virus excretion by cells persistently infected with the bovine leukaemia virus (BLV) using monoclonal antibodies. Journal of clinical virology : the official publication of the Pan American Soc Clin Virol. 2001;22:31–39. doi: 10.1016/s1386-6532(01)00154-8. [DOI] [PubMed] [Google Scholar]
- 21.Shahhosseini S. Das D. Qiu X, et al. Production and characterization of monoclonal antibodies against different epitopes of Ebola virus antigens. J Virol Methods. 2007;143:29–37. doi: 10.1016/j.jviromet.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 22.Harlow ED. Lane D. Antibodies: A Laboratory Manual. New York: Cold Spring Harbor Laboratory; 1988. [Google Scholar]
- 23.Clark MR. Milstein C. Expression of spleen cell immunoglobulin phenotype in hybrids with myeloma cell lines. Somat Cell Genet. 1981;7:657–666. doi: 10.1007/BF01538755. [DOI] [PubMed] [Google Scholar]
- 24.(OIE) Organisation for Animal Health. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals (Mammals, Birds, Bees) Vol. 2. OIE: Paris: 2008. pp. 729–738. [Google Scholar]
- 25.Camargos MF. Feliziani F. Giuseppe AD, et al. Evaluation of diagnostic test to bovine leukaemia virus. Revista Portuguesa de Ciências Veterinária. 2007;102:561–562. [Google Scholar]
- 26.Menezes AMB Santos. Curso de epidemiologia básica para pneumologistas. 4a parte - Epidemiologia clínica. Jornal de Pneumologia. 1999;25:321–326. [Google Scholar]
- 27.Knapen K. Kerkhofs P. Thiry E. Mammerickx M. Epidemiological evaluation of a monoclonal ELISA detecting antibodies against bovine leukaemia virus in serum pools. Epidemiol Infect. 1994;113:563–569. doi: 10.1017/s0950268800068588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van den Heuvel M. Portetelle D. Jefferson B. Jacobs RM. Adaptation of a sandwich enzyme-linked immunosorbent assay to determine the concentration of bovine leukemia virus p24 and optimal conditions for p24 expression in short-term cultures of peripheral blood mononuclear cells. J Virol Methods. 2003;111:61–67. doi: 10.1016/s0166-0934(03)00148-4. [DOI] [PubMed] [Google Scholar]
- 29.Portetelle D. Bruck C. Mammerickx M. Burny A. Use of monoclonal antibody in an ELISA test for the detection of antibodies to bovine leukaemia virus. J Virol Methods. 1983;6:19–29. doi: 10.1016/0166-0934(83)90064-2. [DOI] [PubMed] [Google Scholar]