Abstract
Long non-coding RNAs (lncRNAs) have emerged as one of the largest and more diverse classes of cellular transcripts. The growing evidence suggests that lncRNAs are key regulatory molecules present at virtually every level of cellular physiology, and their alterations are associated with multiple human diseases. Here we provide a general overview of the known roles of lncRNAs in different diseases, as well as their imminent application as biomarkers and therapeutic targets. We also discuss the challenges and possible strategies for these clinical applications. It is unquestionable that our knowledge of lncRNAs not only adds a new dimension to the molecular architecture of human disease, but also opens up a whole new range of opportunities for treatment.
Introduction
Historically the search for the origin of diseases has focused on coding genes that are mutated or genetically altered (amplifications, deletions, translocation, or epigenetic modifications). Nevertheless there has been a recent paradigm shift boosted by the transcriptomic and genomic technological advances. In fact, we know now that while less than 2% of the genome encodes for proteins, at least 75% of its total is actively transcribed into non-coding RNAs (Djebali et al., 2012). However, it remains unclear how much of this transcriptional muddle is functional, and while a body of work has demonstrated that microRNAs act as posttranscriptional regulators with important roles in cellular differentiation and development, much less is known about the larger and heterogeneous group of long non-coding RNAs (lncRNAs).
LncRNAs are transcripts longer than 200 nt that lack functional open reading frames (Dinger et al., 2008). They are highly diverse and actively present in virtually every aspect of cell biology, including cellular differentiation, proliferation, DNA damage response, dosage compensation, and chromosomal imprinting among others. Only a handful of lncRNAs have been studied in some depth, showing their important roles in many physiological processes that involve gene regulation. For instance, lncRNAs have been shown to act as molecular scaffolds that hold and guide chromatin complexes, to enhance gene transcription, to interfere with the transcriptional machinery, or even to maintain the structure of nuclear speckles (Mercer et al., 2009; Wang and Chang, 2011; Rinn and Chang, 2012). Additionally, lncRNAs have been shown to work post-transcriptionally as regulators of splicing, messenger RNA (mRNA) decay, protein translation, or as molecular decoys for microRNAs (Yoon et al., 2012). It is not surprising then that alterations in the expression of lncRNAs are found in multiple diseases, making these RNAs emerge as attractive therapeutic targets (see Table 1) (ESTELLER, 2011; Wapinski and Chang, 2011).
Table 1.
Long Non-Coding RNAs Linked to Disease
| Name | Function | Disease | References |
|---|---|---|---|
| MIAT | Function still unknown | Cardiovascular disease | (Ishii et al., 2006) |
| FMR4 | Antiapoptotic function | X-fragile syndrome | (Khalil et al., 2008) |
| SCA8 | Down regulates KLHL1 expression through an antisense mechanism | Spinocerebellar ataxia | (Chen et al., 2008) |
| BACE1-AS | Regulates expression of BACE1 mRNA stability and generates additional BACE1 through a post-transcriptional feed-forward mechanism | Alzheimer's disease | (Faghihi et al., 2008) |
| MALAT1 | Metabolism and splicing. Metastasis. Overexpressed in various tumor types | Cancer | (Lin et al., 2007; Tripathi et al., 2010) |
| H19 | Oncogene and potential new target for antitumor therapy | Cancer | (Matouk et al., 2007) |
| HOTAIR | Gene silencing in trans. Metastasis in breast cancer and colon cancer | Cancer | (Kogo et al., 2011; Yang et al., 2011) |
| DISC2 | Regulates DISC1 by antisense mechanism | Schizophrenia | (Chubb et al., 2008) |
| PINK1-AS | Regulated by the insulin signaling PTEN | Diabetes | (Scheele et al., 2007) |
| ANRIL | Gene silencing of CDKN2A, ARF and CDKN2B | Cancer | (Cunnington et al., 2010) |
| PCA3 | Overexpressed in prostate cancer | Prostate cancer | (Lee et al., 2011) |
| PRINS | Protective role in cells exposed to stress | Psoriasis | (Sonkoly et al., 2005) |
BACE1, beta-secretase 1; DISC1, disrupted in schizophrenia 1; CDKN2A, cyclin-dependent kinase inhibitor 2A; ARF, alternative reading frame; KLHL1, kelch-like 1; CDKN2B, cyclin-dependent kinase inhibitor 2B; and PTEN, phosphatase and tensin homolog.
Long non-coding RNAs and Cancer
As discussed above, many lncRNAs play important roles in multiple physiological processes that involve gene regulation. Therefore, it seems likely that lncRNA deregulation constitutes one of the underlying causes of human diseases.
In particular, lncRNAs are emerging as new players in the cancer paradigm, having regulatory functions in both oncogenic and tumor-suppressive pathways such as the p53, MYC, and NF-κB pathways (Huarte et al., 2010; Hung et al., 2011). Indeed, lncRNAs are an active part of the cellular response concomitant to different cues and stresses. For instance, following DNA damage or oncogenic stress, the transcription factor p53—one of the most commonly mutated tumor suppressors in cancer—initiates a program that involves the induction of many genes, including dozens of lncRNAs. One in particular, the long intergenic noncoding RNA (lincRNA)-p21, has been shown to mediate global gene repression and to induce apoptosis in a p53-dependent manner (Huarte et al., 2010). However, several other lncRNAs are part of the p53 network (Huarte et al., 2010; Hung et al., 2011), and it is predictable that they play an active role in the tumor suppressor response affecting cancer progression.
The epigenetic alterations that tumor cells undergo are also a determinant factor for cancer development. A number of studies suggest that many lncRNAs interface with the epigenetic machinery, being active components of chromatin complexes (Guttman and RINN, 2012). An example that illustrates the importance of lncRNAs in tumor invasion and metastasis formation is given by the lncRNA hox antisense intergenic RNA (HOTAIR). It has been shown to be deregulated in different types of cancer (Kogo et al., 2011; Yang et al., 2011) and its expression levels in primary breast tumors positively correlate with metastasis and poor outcome (Gupta et al., 2010). HOTAIR interacts with the polycomb repressive complex 2 (PRC2) and the lysine-specific demethylase 1 (LSD1), forming a macromolecular repressor complex that silences specific gene loci leading to metastasis (Tsai et al., 2011). Besides HOTAIR, other lncRNAs have been suggested to regulate the epithelial-to-mesenchymal transition. For instance, a strong association has been demonstrated between the expression of a particular natural antisense transcript (NAT) of the Zeb2 gene and human tumors with low E-cadherin expression (Beltran et al., 2008). Zeb2 NAT overlaps with an intronic 5' splice site of the Zeb2 gene and prevents its splicing resulting in higher levels of Zeb2 protein, which can subsequently function as a transcriptional repressor of E-cadherin (Beltran et al., 2008). Another example of lncRNA involved in cancer malignancy is metastasis associated lung adenocarcinoma transcript 1 (MALAT1), widely expressed in normal human tissues (Ji et al., 2003) but upregulated in different types of cancer (Lin et al., 2007). More recently, its involvement in the regulation of cell proliferation and invasion has been shown (Hudson et al., 2010) and has been identified as a nuclear-retained lncRNA that regulates the alternative splicing of a set of pre-mRNAs (Tripathi et al., 2010). Interestingly, despite the high conservation between human and murine MALAT1 sequences, MALAT1 knockout mice do not present any apparent phenotype (EIßMANN, 2012). Although this could be due to compensatory mechanisms, it may reflect a species-specific functional specialization of lncRNAs.
Apart from the described examples, several other lncRNAs have been observed to be involved in cancer progression (Gupta et al., 2010; Kogo et al., 2011), and they probably represent only the tip of the iceberg. Nowadays, many research groups are reporting lncRNAs whose expression is altered in different cancer types, such as glioblastoma (Han et al., 2012), primary and metastatic pancreatic cancer (Tahira et al., 2011), or oral premalignant lesions (Gibb et al., 2011), correlating their studies with the presence of genetic alterations associated with malignancy. In conclusion, the catalogue of cancer-associated lncRNAs is rapidly increasing and it seems clear that the future research will allow the identification of the best targets for diagnostics and therapies.
LncRNAs and Neurodegenerative and Psychiatric Diseases
LncRNAs are especially abundant in the nervous system, and it has been hypothesized that the brain complexity requires a high number of regulatory RNAs. Supporting this idea, multiple lncRNAs playing roles in this context have being identified and their malfunction linked to neural disorders. For instance, a recent study has detected over 200 differentially expressed lncRNAs in autism disorders, enriched for genomic regions containing genes related to neurodevelopment and psychiatric disease (Ziats and Rennert, 2012), while another recent work has identified lncRNAs deregulated in Huntington's disease (JOHNSON, 2012).
Interestingly, several lncRNAs have been identified to be transcribed antisense of genes with important roles in the nervous system and to modulate the expression of their sense counterparts. Among them is BACE1 antisense RNA (BACE1-AS), a lncRNA transcribed antisense of BACE1 gene. BACE1 encodes for an integral membrane peptidase A1 glycoprotein that plays a pivotal role in the accumulation of β-amyloid plaques characterizing the Alzheimer's disease. BACE1-AS has been shown to regulate the expression of BACE1 by increasing BACE1 mRNA stability and therefore BACE1 protein level through a post-transcriptional feed-forward mechanism. Moreover, BACE1-AS concentration was found increased in subjects with Alzheimer's disease and in amyloid precursor protein transgenic mice, supporting the involvement of the lncRNA in the disease progression (Faghihi et al., 2008).
Other examples of neuron-specific antisense lncRNAs are DISC2 (disrupted in schizophrenia 2), antisense of DISC1 gene, linked to schizophrenia and several other disorders (Chubb et al., 2008); human accelerated region 1 (HAR1), transcribed antisense of rheelin (RELN) gene and related to schizophrenia and attention deficit disorder (Li et al., 2011); and ubiquitin carboxyl-terminal esterase L1 (Uchl1) antisense, which positively regulates UCHL1 protein, possibly involved in Parkinson's and Alzheimer's diseases (Carrieri et al., 2012). In summary, it seems that evolution has created in the nervous system a network of sense-antisense transcriptional crosstalk able to fine-tune gene expression.
LncRNAs and Other Diseases
LncRNAs are essential components of cellular regulatory networks and can therefore affect every aspect of physiology. This is consistent with the growing number of malignancies in which lncRNAs are known to play a role, including systemic lupus erythaematosus (Kino et al., 2010), psoriasis (Sonkoly et al., 2005), uremia (Sui et al., 2012), brachydactyly (Maass et al., 2012), facioscapulohumeral muscular dystrophy (Cabianca et al., 2012), α-Talasemia (Tufarelli et al., 2003), and immune response to viral infection (Peng et al., 2010).
LncRNAs are likely involved in the regulatory processes that control metabolic functions, but little is known about the role of lncRNAs in endocrine disease. A recent and comprehensive study has shown that lncRNAs are components of the β cell differentiation and maturation program, and many of them have been shown to be deregulated in type 2 diabetes (Dunham et al., 2012). A previously known lncRNA with a role in diabetes is PINK1. LncRNA antisense of PTEN induced putative kinase 1 gene (PINK1-AS) is induced by the important inhibitor of insulin signaling phosphatase and tensin homolog (PTEN), and its depletion has been associated with diabetes (Scheele et al., 2007). Similarly, the H19/insulin-like growth factor 2 (IGF2) and thyroid growth receptor α2 (ERBα2) loci harbor known antisense transcripts that have the potential to regulate their endocrine and metabolic function (Lottin et al., 2002). Besides diabetes, lncRNAs have been implicated in other physiological aspects of metabolism, such as appetite control (Seim et al., 2007), although it is unclear what mechanisms may be involved.
Moreover, lncRNAs have been observed to influence cardiovascular diseases and hypertension. The altered expressions of antisense noncoding RNA in the INK4 locus (ANRIL) and myocardial infarction associated transcript (MIAT) lncRNAs have been correlated with stroke risk and recurrence and myocardial infarction risk respectively (Ishii et al., 2006; Zhang et al., 2012)). Finally, a role for lncRNAs has also been shown in hypertension, where 7 blood pressure candidate genes (ADD3, NPPA, ATP1A1, NPR2, CYP17A1, ACSM3, and SLC14A2) have been found to be regulated in cis by a lncRNA transcript (Annilo et al., 2009).
LncRNAS as Diagnostic and Therapeutic Tools
The discovery of deregulated lncRNAs not only represents a new layer of complexity in the molecular architecture of human diseases, but it also opens up the possibility to use them as diagnostic markers and therapeutic targets. LncRNAs can offer advantages as biomarkers, especially when they can be readily detected in biological fluids. Furthermore, as opposed to mRNA, the lncRNA itself is a functional molecule and its expression level may be a better indicator of the disease. Additionally, the highly specific expression patterns of lncRNAs suggest that their expression signatures could successfully be used for accurate disease diagnostics and classification. Although this application is still under development, the use of individual lncRNAs in clinical medicine has already started. PCA3, a prostate-specific lncRNA markedly overexpressed in prostate cancer, has been reported to be clinically used in a diagnostic assay for prostate cancer detection (Lee et al., 2011). However, the emerging application of circulating lncRNAs for disease diagnosis is restrained by the limited knowledge that we have of their biology. For instance, it remains unclear whether lncRNAs contribute to the disease or if they become altered as a consequence of the disease itself. In any case, in order to make use of their potential as markers, some other questions should be considered. For instance, taking into account their nature as long RNA molecules: how stable are circulating lncRNAs? Is their stability altered in various disease states? Answering these questions will help the implementation of lncRNA as biomarkers.
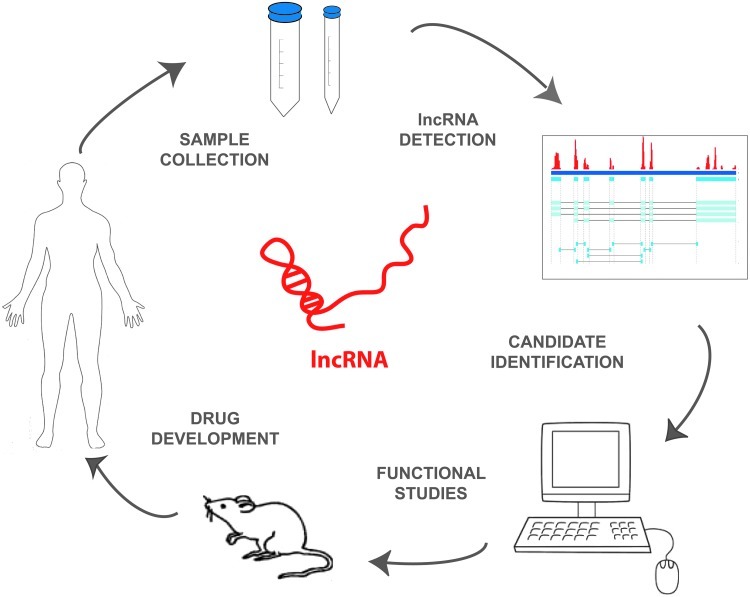
In addition to the imminent use of lncRNAs for diagnosis and prognosis, the targeting of lncRNAs for therapy is already being explored (Fig. 1). Companies and organizations such as the Allen Institution for Brain Science, CuRNA, Regulus Therapeutics, Miragen Therapeutics, and Santaris Pharma among others are developing ncRNA-based strategies against cancer, cardiovascular, neurological, and muscular diseases.
FIG. 1.
General overview of the process of long non-coding RNA (lncRNA) detection and analysis for clinical application. Samples are collected from patients, from either tumor tissue or body fluids. For lncRNA detection, extracted RNA is analyzed by microarray, next-generation RNA sequencing, or real time PCR, and computational analyses determine differentially expressed lncRNAs. Selected lncRNA candidates are subjected to functional studies that can lead to the development of new drugs or other clinical applications.
In principle, there are several possible approaches for targeting lncRNAs:
(1) Silencing of lncRNAs. LncRNA expression levels can be inhibited through RNAi technology, although in some cases this may not be possible due to their secondary structure or intracellular localization. Alternatively, antisense oligonucleotides (ASOs) that block lncRNA activity, or induce their degradation by RNaseH can be applied for lncRNA downregulation and silencing. An alternative strategy for silencing of lncRNAs that avoids the off target effects of oligonucleotides is the genomic integration of RNA destabilizing elements. However, its potential application on patients is more limited as it involves targeted genetic manipulation (Gutschner et al., 2011).
(2) Functional block of lncRNAs. LncRNAS can be impaired by blocking their molecular interactions. This could be possible via application of small molecule inhibitors that mask the binding site in protein interacting partners or antagonistic oligonucleotides that bind to the ncRNA and inhibit the protein binding. Although our understanding of the molecular mechanisms of lncRNA function is limited, some known features of lncRNAs, including structural scaffolds for protein complexes and complex RNA structural motifs, could be targeted. For instance, preventing the interaction of HOTAIR with the PRC2 or LSD1 complexes using small molecular inhibitors may limit the metastatic potential of breast cancer (Tsai et al., 2011).
(3) Structure disruption. Small molecule inhibitors could be designed to bind to lncRNAs and change or mimic their secondary structure in order to compete for their binding partners. Alternatively, oligonucleotides could be used to target a specific region of the ncRNA and block its correct folding. In line with this, undergoing studies are developing small molecules to adapt to the RNA secondary structure motifs in disease (Colley and Leedman, 2009).
Besides the targeting of harmful lncRNAs, gene therapy can be applied for the delivery of beneficial lncRNAs to specific cells, such as tumor suppressor lncRNAs. Additionally, some strategies attempt to take advantage of the highly specific expression of some lncRNAs in tumor cells to reduce the risk of affecting normal tissues during treatment. For example, the plasmid BC-819 (DTA-H19) has been developed to make use of tumor-specific expression of H19 lncRNA. This plasmid carries the gene for the A subunit of diphtheria toxin under the regulation of H19 promoter. The intratumoral injection of this plasmid induces the expression of high levels of diphtheria toxin in the tumor, resulting in a reduction of tumor size in human trials in a broad range of carcinomas (Mizrahi et al., 2009).
The potential use of lncRNAs for therapies is tremendous since it can take advantage of the multiple functional facets of lncRNAs. However, before we might be able to use these new therapeutic options routinely, more functional and structural studies are necessary to better understand the biology of lncRNAs.
It is unquestionable that the more we learn about lncRNA expression patterns in disease, the higher the chances for an improved diagnosis and better prognosis will be. Likewise, the understanding of the mechanisms by which lncRNAs function will inevitably help us to design better therapeutic agents. Hopefully, the next future will satisfy our expectations and bring more challenges to the application of lncRNA research to human health.
Acknowledgments
We thank the members of Maite Huarte's lab for critical reading of the manuscript. Our research is supported by European Research Council Starting Grant 281877 and Spanish Ministry of Science Grants BFU2011-23485 and SRYC1100I008347XV0.
Author Disclosure Statement
No competing financial interests exist.
References
- ANNILO T. KEPP K. LAAN M. Natural antisense transcript of natriuretic peptide precursor A (NPPA): structural organization and modulation of NPPA expression. BMC Mol. Biol. 2009;10:81. doi: 10.1186/1471-2199-10-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BELTRAN M. PUIG I. PENA C. GARCIA J.M. ALVAREZ A.B. PENA R. BONILLA F. DE HERREROS A.G. A natural antisense transcript regulates Zeb2/Sip1 gene expression during Snail1-induced epithelial-mesenchymal transition. Genes Dev. 2008;22:756–769. doi: 10.1101/gad.455708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CABIANCA D. S. CASA V. GABELLINI D. A novel molecular mechanism in human genetic disease: A DNA repeat-derived lncRNA point-of-view. RNA Biol. 2012;9:1211–1217. doi: 10.4161/rna.21922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARRIERI C. CIMATTI L. BIAGIOLI M. BEUGNET A. ZUCCHELLI S. FEDELE S. PESCE E. FERRER I. COLLAVIN L. SANTORO C., et al. Long non-coding antisense RNA controls Uchl1 translation through an embedded SINEB2 repeat. Nature. 2012;491:454–457. doi: 10.1038/nature11508. [DOI] [PubMed] [Google Scholar]
- CHEN W. L. LIN J. W. HUANG H. J. WANG S. M. SU M. T. LEE-CHEN G. J. CHEN C. M. HSIEH-LI H. M. SCA8 mRNA expression suggests an antisense regulation of KLHL1 and correlates to SCA8 pathology. Brain Res. 2008;1233:176–184. doi: 10.1016/j.brainres.2008.07.096. [DOI] [PubMed] [Google Scholar]
- CHUBB J.E. BRADSHAW N.J. SOARES D.C. PORTEOUS D.J. MILLAR J.K. The DISC locus in psychiatric illness. Mol. Psychiatry. 2008;13:36–64. doi: 10.1038/sj.mp.4002106. [DOI] [PubMed] [Google Scholar]
- COLLEY S.M. LEEDMAN P.J. SRA and its binding partners: an expanding role for RNA-binding coregulators in nuclear receptor-mediated gene regulation. Crit. Rev. Biochem. Mol. Biol. 2009;44:25–33. doi: 10.1080/10409230802661719. [DOI] [PubMed] [Google Scholar]
- CUNNINGTON M. S. SANTIBANEZ KOREF M. MAYOSI B. M. BURN J. KEAVNEY B. Chromosome 9p21 SNPs associated with multiple disease phenotypes correlate with ANRIL expression. PLoS Genet. 2010;6:e1000899. doi: 10.1371/journal.pgen.1000899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DINGER M.E. PANG K.C. MERCER T.R. MATTICK J.S. Differentiating protein-coding and noncoding RNA: challenges and ambiguities. PLoS Comput. Biol. 2008;4:e1000176. doi: 10.1371/journal.pcbi.1000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DJEBALI S. DAVIS C.A. MERKEL A. DOBIN A. LASSMANN T. MORTAZAVI A. TANZER A. LAGARDE J. LIN W. SCHLESINGER F., et al. Landscape of transcription in human cells. Nature. 2012;489:101–108. doi: 10.1038/nature11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUNHAM I. KUNDAJE A. ALDRED S.F. COLLINS P.J. DAVIS C.A. DOYLE F. EPSTEIN C.B. FRIETZE S. HARROW J. KAUL R., et al. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489:57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EIßMANN M. Gutschner T. HÄMMERLE M. GÜNTHER S. Caudron-Herger M. Groß M. Schirmacher P. Rippe K. Braun T. Zörnig M. Diederichs S. Loss of the abundant nuclear non-coding RNA MALAT1 is compatible with life and development. RNA Biol. 2012;9:1076–1087. doi: 10.4161/rna.21089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ESTELLER M. Non-coding RNAs in human disease. Nat. Rev. Genet. 2011;12:861–874. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- FAGHIHI M.A. MODARRESI F. KHALIL A.M. WOOD D.E. SAHAGAN B.G. MORGAN T.E. FINCH C.E. ST LAURENT G., 3rd KENNY P.J. WAHLESTEDT C. Expression of a noncoding RNA is elevated in Alzheimer's disease and drives rapid feed-forward regulation of beta-secretase. Nat. Med. 2008;14:723–730. doi: 10.1038/nm1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBB E.A. ENFIELD K.S. STEWART G.L. LONERGAN K.M. CHARI R. NG R.T. ZHANG L. MACAULAY C.E. ROSIN M.P. LAM W.L. Long non-coding RNAs are expressed in oral mucosa and altered in oral premalignant lesions. Oral Oncol. 2011;47:1055–1061. doi: 10.1016/j.oraloncology.2011.07.008. [DOI] [PubMed] [Google Scholar]
- GUPTA R.A. SHAH N. WANG K.C. KIM J. HORLINGS H.M. WONG D.J. TSAI M.C. HUNG T. ARGANI P. RINN J.L., et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464:1071–1076. doi: 10.1038/nature08975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUTSCHNER T. BAAS M. DIEDERICHS S. Noncoding RNA gene silencing through genomic integration of RNA destabilizing elements using zinc finger nucleases. Genome Res. 2011;21:1944–1954. doi: 10.1101/gr.122358.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUTTMAN M. RINN J.L. Modular regulatory principles of large non-coding RNAs. Nature. 2012;482:339–346. doi: 10.1038/nature10887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAN L. ZHANG K. SHI Z. ZHANG J. ZHU J. ZHU S. ZHANG A. JIA Z. WANG G. YU S., et al. LncRNA pro fi le of glioblastoma reveals the potential role of lncRNAs in contributing to glioblastoma pathogenesis. Int. J. Oncol. 2012;40:2004–2012. doi: 10.3892/ijo.2012.1413. [DOI] [PubMed] [Google Scholar]
- HUARTE M. GUTTMAN M. FELDSER D. GARBER M. KOZIOL M.J. KENZELMANN-BROZ D. KHALIL A.M. ZUK O. AMIT I. RABANI M., et al. A large intergenic noncoding RNA induced by p53 mediates global gene repression in the p53 response. Cell. 2010;142:409–419. doi: 10.1016/j.cell.2010.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUDSON T.J. ANDERSON W. ARTEZ A. BARKER A.D. BELL C. BERNABE R.R. BHAN M.K. CALVO F. EEROLA I. GERHARD D.S., et al. International network of cancer genome projects. Nature. 2010;464:993–998. doi: 10.1038/nature08987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNG T. WANG Y. LIN M.F. KOEGEL A.K. KOTAKE Y. GRANT G.D. HORLINGS H.M. SHAH N. UMBRICHT C. WANG P., et al. Extensive and coordinated transcription of noncoding RNAs within cell-cycle promoters. Nat. Genet. 2011;43:621–629. doi: 10.1038/ng.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISHII N. OZAKI K. SATO H. MIZUNO H. SAITO S. TAKAHASHI A. MIYAMOTO Y. IKEGAWA S. KAMATANI N. HORI M., et al. Identification of a novel non-coding RNA, MIAT, that confers risk of myocardial infarction. J. Hum Genet. 2006;51:1087–1099. doi: 10.1007/s10038-006-0070-9. [DOI] [PubMed] [Google Scholar]
- JI P. DIEDERICHS S. WANG W. BOING S. METZGER R. SCHNEIDER P.M. TIDOW N. BRANDT B. BUERGER H. BULK E., et al. MALAT-1, a novel noncoding RNA, and thymosin beta4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene. 2003;22:8031–8041. doi: 10.1038/sj.onc.1206928. [DOI] [PubMed] [Google Scholar]
- JOHNSON R. Long non-coding RNAs in Huntington's disease neurodegeneration. Neurobiol. Dis. 2012;46:245–254. doi: 10.1016/j.nbd.2011.12.006. [DOI] [PubMed] [Google Scholar]
- KHALIL A. M. FAGHIHI M. A. MODARRESI F. BROTHERS S. P. WAHLESTEDT C. A novel RNA transcript with antiapoptotic function is silenced in fragile X syndrome. PLoS One. 2008;3:e1486. doi: 10.1371/journal.pone.0001486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KINO T. HURT D.E. ICHIJO T. NADER N. CHROUSOS G.P. Noncoding RNA gas5 is a growth arrest- and starvation-associated repressor of the glucocorticoid receptor. Sci. Signal. 2010;3:ra8. doi: 10.1126/scisignal.2000568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOGO R. SHIMAMURA T. MIMORI K. KAWAHARA K. IMOTO S. SUDO T. TANAKA F. SHIBATA K. SUZUKI A. KOMUNE S., et al. Long noncoding RNA HOTAIR regulates polycomb-dependent chromatin modification and is associated with poor prognosis in colorectal cancers. Cancer Res. 2011;71:6320–6326. doi: 10.1158/0008-5472.CAN-11-1021. [DOI] [PubMed] [Google Scholar]
- LEE G.L. DOBI A. SRIVASTAVA S. Prostate cancer: diagnostic performance of the PCA3 urine test. Nat. Rev. Urol. 2011;8:123–124. doi: 10.1038/nrurol.2011.10. [DOI] [PubMed] [Google Scholar]
- LI X. ISONO K. YAMADA D. ENDO T.A. ENDOH M. SHINGA J. MIZUTANI-KOSEKI Y. OTTE A.P. CASANOVA M., et al. Mammalian polycomb-like Pcl2/Mtf2 is a novel regulatory component of PRC2 that can differentially modulate polycomb activity both at the Hox gene cluster and at Cdkn2a genes. Mol. Cell Biol. 2011;31:351–364. doi: 10.1128/MCB.00259-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIN R. MAEDA S. LIU C. KARIN M. EDGINGTON T.S. A large noncoding RNA is a marker for murine hepatocellular carcinomas and a spectrum of human carcinomas. Oncogene. 2007;26:851–858. doi: 10.1038/sj.onc.1209846. [DOI] [PubMed] [Google Scholar]
- LOTTIN S. ADRIAENSSENS E. DUPRESSOIR T. BERTEAUX N. MONTPELLIER C. COLL J. DUGIMONT T. CURGY J.J. Overexpression of an ectopic H19 gene enhances the tumorigenic properties of breast cancer cells. Carcinogenesis. 2002;23:1885–1895. doi: 10.1093/carcin/23.11.1885. [DOI] [PubMed] [Google Scholar]
- MAASS P.G. RUMP A. SCHULZ H. STRICKER S. SCHULZE L. PLATZER K. AYDIN A. TINSCHERT S. GOLDRING M.B. LUFT F.C. BAHRING S. A misplaced lncRNA causes brachydactyly in humans. J. Clin. Invest. 2012;122:3990–4002. doi: 10.1172/JCI65508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATOUK I. J. DEGROOT N. MEZAN S. AYESH S. ABU-LAIL R. HOCHBERG A. GALUN E. The H19 non-coding RNA is essential for human tumor growth. PLoS One. 2007;2:e845. doi: 10.1371/journal.pone.0000845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MERCER T.R. DINGER M.E. MATTICK J.S. Long non-coding RNAs: insights into functions. Nat. Rev. Genet. 2009;10:155–159. doi: 10.1038/nrg2521. [DOI] [PubMed] [Google Scholar]
- MIZRAHI A. CZERNIAK A. LEVY T. AMIUR S. GALLULA J. MATOUK I. ABU-LAIL R. SORIN V. BIRMAN T. DE GROOT N., et al. Development of targeted therapy for ovarian cancer mediated by a plasmid expressing diphtheria toxin under the control of H19 regulatory sequences. J. Transl. Med. 2009;7:69. doi: 10.1186/1479-5876-7-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PENG X. GRALINSKI L. ARMOUR C. D. FERRIS M. T. THOMAS M. J. PROLL S. BRADEL-TRETHEWAY B. G. KORTH M. J. CASTLE J. C. BIERY M. C., et al. Unique signatures of long noncoding RNA expression in response to virus infection and altered innate immune signaling. MBio. 2010;1 doi: 10.1128/mBio.00206-00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RINN J.L. CHANG H.Y. Genome Regulation by Long Noncoding RNAs. Annu. Rev. Biochem. 2012;81:145–166. doi: 10.1146/annurev-biochem-051410-092902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHEELE C. NIELSEN A. R. WALDEN T. B. SEWELL D. A. FISCHER C. P. BROGAN R. J. PETROVIC N. LARSSON O. TESCH P. A. WENNMALM K., et al. Altered regulation of the PINK1 locus: a link between type 2 diabetes and neurodegeneration? Faseb J. 2007;21:3653–3665. doi: 10.1096/fj.07-8520com. [DOI] [PubMed] [Google Scholar]
- SEIM I. COLLET C. HERINGTON A.C. CHOPIN L.K. Revised genomic structure of the human ghrelin gene and identification of novel exons, alternative splice variants and natural antisense transcripts. BMC Genomics. 2007;8:298. doi: 10.1186/1471-2164-8-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SONKOLY E. BATA-CSORGO Z. PIVARCSI A. POLYANKA H. KENDERESSY-SZABO A. MOLNAR G. SZENTPALI K. BARI L. MEGYERI K. MANDI Y., et al. Identification and characterization of a novel, psoriasis susceptibility-related noncoding RNA gene, PRINS. J. Biol. Chem. 2005;280:24159–24167. doi: 10.1074/jbc.M501704200. [DOI] [PubMed] [Google Scholar]
- SUI W. YAN Q. LI H. LIU J. CHEN J. LI L. DAI Y. Genome-wide analysis of long noncoding RNA expression in peripheral blood mononuclear cells of uremia patients. J Nephrol. 2012 doi: 10.5301/jn.5000201. [DOI] [PubMed] [Google Scholar]
- TAHIRA A.C. KUBRUSLY M.S. FARIA M.F. DAZZANI B. FONSECA R.S. MARACAJA-COUTINHO V. VERJOVSKI-ALMEIDA S. MACHADO M.C. REIS E.M. Long noncoding intronic RNAs are differentially expressed in primary and metastatic pancreatic cancer. Mol. Cancer. 2011;10:141. doi: 10.1186/1476-4598-10-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAMURA Y. KUNUGI H. OHASHI J. HOHJOH H. Epigenetic aberration of the human REELIN gene in psychiatric disorders. Mol Psychiatry. 2007;12:593–600. doi: 10.1038/sj.mp.4002014. [DOI] [PubMed] [Google Scholar]
- TRIPATHI V. ELLIS J.D. SHEN Z. SONG D.Y. PAN Q. WATT A.T. FREIER S.M. BENNETT C.F. SHARMA A. BUBULYA P.A., et al. The nuclear-retained noncoding RNA MALAT1 regulates alternative splicing by modulating SR splicing factor phosphorylation. Mol. Cell. 2010;39:925–938. doi: 10.1016/j.molcel.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSAI M.C. SPITALE R.C. CHANG H.Y. Long intergenic noncoding RNAs: new links in cancer progression. Cancer Res. 2011;71:3–7. doi: 10.1158/0008-5472.CAN-10-2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TUFARELLI C. STANLEY J.A. GARRICK D. SHARPE J.A. AYYUB H. WOOD W.G. HIGGS D.R. Transcription of antisense RNA leading to gene silencing and methylation as a novel cause of human genetic disease. Nat. Genet. 2003;34:157–165. doi: 10.1038/ng1157. [DOI] [PubMed] [Google Scholar]
- WANG K.C. CHANG H.Y. Molecular mechanisms of long noncoding RNAs. Mol. Cell. 2011;43:904–914. doi: 10.1016/j.molcel.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAPINSKI O. CHANG H.Y. Long noncoding RNAs and human disease. Trends Cell Biol. 2011;21:354–356. doi: 10.1016/j.tcb.2011.04.001. [DOI] [PubMed] [Google Scholar]
- YANG Z. ZHOU L. WU L.M. LAI M.C. XIE H.Y. ZHANG F. ZHENG S.S. Overexpression of long non-coding RNA HOTAIR predicts tumor recurrence in hepatocellular carcinoma patients following liver transplantation. Ann. Surg. Oncol. 2011;18:1243–1250. doi: 10.1245/s10434-011-1581-y. [DOI] [PubMed] [Google Scholar]
- YOON J. H. ABDELMOHSEN K. SRIKANTAN S. YANG X. MARTINDALE J. L. DE S. HUARTE M. ZHAN M. BECKER K. G. GOROSPE M. LincRNA-p21 Suppresses Target mRNA Translation. Mol Cell. 2012;47:648–655. doi: 10.1016/j.molcel.2012.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG W. CHEN Y. LIU P. CHEN J. SONG L. TANG Y. WANG Y. LIU J. HU F.B. HUI R. Variants on chromosome 9p21.3 correlated with ANRIL expression contribute to stroke risk and recurrence in a large prospective stroke population. Stroke. 2012;43:14–21. doi: 10.1161/STROKEAHA.111.625442. [DOI] [PubMed] [Google Scholar]
- ZIATS M. N. RENNERT O. M. Aberrant Expression of Long Noncoding RNAs in Autistic Brain. J Mol Neurosci. 2012 doi: 10.1007/s12031-12012-19880-12038. [DOI] [PMC free article] [PubMed] [Google Scholar]