Abstract
Interactions between dopamine and N-methyl-d-aspartate receptors (NMDARs) in prefrontal cortex (PFC) and other brain regions are believed to play an important role in normal mental function and neuropsychiatric disorders. In this study, we examined the regulation of NMDAR currents by the dopamine D1 receptor in PFC pyramidal neurons. Application of the D1 receptor agonist SKF81297 caused a prominent increase of the steady-state NMDA-evoked current in acutely isolated PFC pyramidal neurons. The D1 effect on NMDARs was independent of protein kinase A or protein phosphatase 1, but was abolished by incubation of neurons in Ca2+-free medium. Intracellular application of the Ca2+ chelator, calmodulin, or calmodulin inhibitors largely prevented the D1 modulation of NMDAR currents. Moreover, inhibiting PKC activity or disrupting PKC association with its anchoring protein also significantly reduced the D1 effect on NMDAR currents. This upregulation of NMDAR activity by dopamine D1 receptors and the previous finding on up-regulation of dopamine D1 receptors by NMDAR activation provide a cellular mechanism for the reciprocal interactions between D1 and NMDARs. These interactions may play an important role in modulating synaptic plasticity and thus in cognitive and emotional processes.
Prefrontal cortex (PFC), a brain area highly associated with the control of cognition and emotion (1), is one of the most prominent regions affected by schizophrenia (2, 3). Schizophrenics often exhibit deficits in cognitive tasks requiring working memory subserved by PFC circuits (4). Despite the unclear etiology of schizophrenia, aberrations of several neurotransmitter systems have been implicated in this disorder. Among them, dysfunctions of the dopaminergic and glutamatergic systems in PFC are considered to be major contributing factors to the pathophysiology of schizophrenia (5).
It has long been recognized that dopamine receptors play a key role in the modulation of PFC working memory functions (1). Dopamine depletion in PFC results in impaired performances in PFC cognitive tasks, and these deficits can be ameliorated by the mixed dopamine receptor agonist apomorphine (6). The role of D1 receptors in this process has been extensively explored. Iotophoresis of low concentrations of D1 receptor antagonists enhances memory-related neuronal responses in monkeys performing challenging working memory tasks (7). On the other hand, administration of low, but not high, doses of D1 receptor agonists improves spatial working memory performance (8). These results suggest that optimal levels of D1 receptor stimulation are important for maintaining PFC functions.
Increasing evidence has suggested that, in addition to dopaminergic dysfunction, hypofunction of N-methyl-d-aspartate receptors (NMDARs) also plays a key role in schizophrenia (9). Administration of noncompetitive NMDAR antagonists in humans and animals produces behavioral symptoms that are remarkably similar to schizophrenia (10, 11). Mutant mice expressing 5% of the normal level of NR1 (12) exhibit a series of schizophrenia-like behaviors, which are ameliorated by administration of antipsychotic drugs that target dopamine receptors (12). To begin to understand how the “dopamine hypothesis” and “glutamate hypothesis” of schizophrenia may be mechanistically linked, we examined the interactions between D1 and NMDARs in PFC pyramidal neurons. A previous study has shown that dopamine D1 signaling can be regulated by NMDAR activation (13). In this study, we provide evidence showing that NMDAR activity can be regulated by dopamine D1 signaling.
Materials and Methods
Acute-Dissociation Procedure. PFC neurons from young adult (3–5 weeks postnatal) rats were acutely dissociated by using procedures similar to those described in ref. 14. All experiments were carried out with the approval of the State University of New York at Buffalo Animal Care Committee. After incubation of brain slices in a NaHCO3-buffered saline, PFC was dissected and placed in an oxygenated Cell-Stir chamber containing papain (Sigma, 0.4 mg/ml) in Hepes-buffered Hanks' balanced salt solution at 35°C. After 20–40 min of enzyme digestion, tissue was rinsed three times in the low Ca2+, Hepes-buffered saline and mechanically dissociated with a graded series of fire-polished Pasteur pipettes. The cell suspension was then plated into a 35-mm Lux Petri dish, which was then placed on the stage of a Zeiss inverted microscope.
Whole-Cell Recordings. Whole-cell recordings of ion channel currents used standard voltage clamp techniques (15, 16). The internal solution consisted of 180 mM N-methyl-d-glucamine, 40 mM Hepes, 4 mM MgCl2, 0.1 mM 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetate (BAPTA), 12 mM phosphocreatine, 2 mM Na2ATP, 0.2 mM Na3GTP, and 0.1 mM leupeptin, pH 7.2–7.3, osmolarity of 265–270. The external solution consisted of 127 mM NaCl, 20 mM CsCl, 10 mM Hepes, 1 mM CaCl2, 5 mM BaCl2, 12 mM glucose, 0.001 mM Tetrodotoxin, and 0.02 mM glycine, pH 7.3–7.4, osmolarity of 300–305. Recordings were obtained with an Axon Instruments (Foster City, CA) 200B patch-clamp amplifier that was controlled and monitored with an IBM PC running pclamp (version 8) with a DigiData 1320 series interface (Axon Instruments). Electrode resistances were typically 2–4 MΩ in the bath. After seal rupture, series resistance (4–10 MΩ) was compensated (70–90%) and periodically monitored. The cell membrane potential was held at -60 mV. NMDA (500 μM) was applied for 2 s every 30 s to minimize desensitization-induced decrease of current amplitude. The application of NMDA evoked a partially desensitizing inward current. Steady-state current amplitudes were measured at the end of a 2-s application of NMDA for generating the plot as a function of time and drug application. Drugs were applied with a gravity-fed “sewer pipe” system. The array of application capillaries (≈150 μm i.d.) was positioned a few hundred microns from the cell under study. Solution changes were effected by the SF-77B fast-step solution stimulus delivery device (Warner Instruments, Hamden, CT).
Dopamine receptor ligands SKF81297 and SCH23390 (Sigma), as well as second messenger reagents PKI6–22, myristoylated PKI14–22, okadaic acid (OA), calmodulin (CaM), calmidazolium (CDZ), myosin light chain kinase (MLCK) peptide, KN-93, cyclosporin A (CsA), PKC19–36, bisindolylmaleimide I, U73122, heparin, wortmannin, and genistein (Calbiochem) were made up as concentrated stocks and stored at -20°C. The final DMSO concentration in all applied solutions was <0.1%. Stocks were thawed and diluted immediately before use. The amino acid sequence for the protein phosphatase 1 (PP1)-anchoring inhibitory peptide Gm[63–75] is GRRVSFADNFGFN.
Data analyses were performed with axograph (Axon Instruments), kaleidagraph (Albeck, Reading, PA), origin 6 (OriginLab, Northampton, MA), and statview (Abacus Concepts, Berkeley, CA). For analysis of statistical significance, Mann–Whitney U tests were performed to compare the current amplitudes in the presence or absence of agonists. ANOVA tests were performed to compare the differential degrees of current modulation between groups subjected to different treatment.
Results
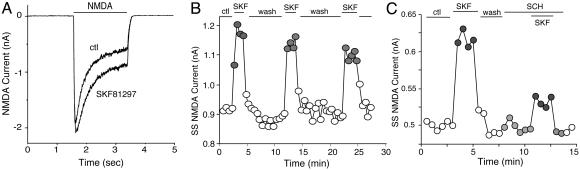
Activation of D1 Receptors Enhances NMDA-Evoked Currents in Dissociated PFC Pyramidal Neurons. To test the potential impact of D1 receptors on NMDA signaling, we examined the effect of SKF81297, a selective D1 receptor agonist, on NMDAR-mediated currents in acutely isolated PFC pyramidal neurons. As shown in Fig. 1 A and B, application of NMDA (500 μM) evoked a partially desensitizing inward current, and SKF81297 (10 μM) caused a potent and reversible increase in the amplitude of NMDA-evoked currents in the PFC pyramidal neuron. Moreover, the enhancing effect of SKF81297 was most prominent in the steady-state component of NMDA-evoked currents, suggesting a suppression of desensitization or inactivation of NMDARs. In a sample of PFC pyramidal neurons we tested, SKF81297 produced a 19.8 ± 5.4% (n = 98, P <0.01, Mann–Whitney) enhancement of steady-state NMDAR currents (Iss).
Fig. 1.
Activation of D1 receptors reversibly enhanced NMDAR currents in acutely dissociated PFC pyramidal neurons. (A) Current traces taken from a representative neuron showing the effect of SKF81297 (10 μM) on NMDA (500 μM)-evoked currents. (B) Plot of the steady-state NMDAR current (Iss) as a function of time and agonist application. (C) Plot of Iss showing that the selective D1 antagonist SCH23390 (SCH) (10 μM) largely blocked the effect of SKF81297 (SKF).
To verify that D1 receptors were responsible for the modulation seen with SKF81297, we examined the ability of SCH23390, a selective D1 receptor antagonist, to prevent the action of SKF81297. As shown in Fig. 1C, SKF81297 (10 μM) produced a reversible enhancement of the steady-state NMDAR current in the PFC neuron, and this effect was greatly reduced by SCH23390 (10 μM). In a sample of cells we examined, SKF81297 had a significantly (P <0.005, ANOVA) smaller effect on steady-state NMDAR currents in the presence of SCH23390 (9.8 ± 3.8%, n = 4), compared to the effect of SKF81297 in the absence of SCH23390 (22.3 ± 2.0%, n = 4). These results suggest D1 as the receptor underlying the SKF81297-induced potentiation of NMDAR currents.
The D1 Enhancement of NMDAR Currents in PFC Pyramidal Neurons Is Independent of Protein Kinase A (PKA)/PP1 but Involves Ca2+/CaM. We next examined the signal transduction pathway mediating the D1 potentiation of NMDAR currents in PFC pyramidal neurons. The “classical” signaling cascade of D1 receptors is to stimulate adenylate cyclase and cAMP formation. The D1-induced activation of PKA could directly modulate NMDAR currents through increased phosphorylation of NR1 subunits on the PKA sites (17). Alternatively, the activation of PKA could cause the inhibition of PP1 via increased phosphorylation of regulatory proteins, such as dopamine and cAMP-regulated phosphoprotein DARPP-32 (18) and the PP1 inhibitory protein I-1, leading to the decreased dephosphorylation of NMDAR subunits by PP1 (19) and up-regulation of NMDAR currents. To evaluate these potential signaling mechanisms, we examined the effect of SKF81297 on NMDARs in the presence of PKA or PP1 inhibitors.
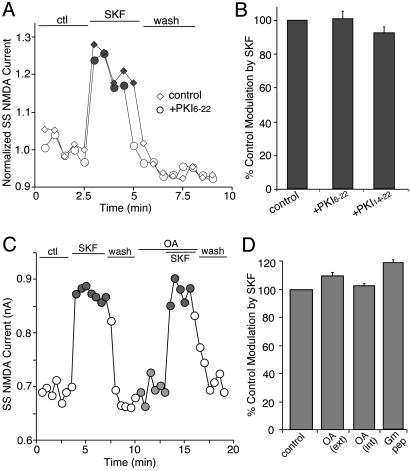
As shown in Fig. 2 A and B, dialysis with the PKA inhibitory peptide PKI6–22 (20 μM) failed to block the SKF81297-induced increase of NMDAR currents (101.3 ± 9.7% of control modulation, n = 15). The effect of SKF81297 was also intact in the presence of the membrane-permeable PKA inhibitory peptide, myristoylated PKI14–22 (0.2 μM, 91.9 ± 4.3% of control modulation, n = 10, Fig. 2B). Moreover, bath application of the PP1 inhibitor OA (0.1 μM) did not affect the ability of SKF81297 to enhance NMDAR currents (Fig. 2C). As summarized in Fig. 2D, inhibiting PP1 catalytic activity with external or internal application of OA failed to block the SKF81297 enhancement of NMDAR currents (external OA: 109.2 ± 2.3% of control modulation, n = 8; internal OA: 102.2 ± 1.9% of control modulation, n = 23). The effect of SKF81297 on NMDAR currents was also unaffected when PP1 targeting was disrupted by 20 μM of peptide Gm[63–75] (20) (118.9 ± 2.5% of control modulation, n = 20). These results suggest that the classical PKA/PP1 cascade does not link D1 receptors to the potentiation of NMDAR currents in PFC pyramidal neurons, at least under the experimental conditions of the present study.
Fig. 2.
The effect of SKF81297 (SKF) on NMDAR currents was independent of PKA/PP1. (A) Plot of Iss showing that dialysis with the PKA inhibitory peptide PKI6–22 (20 μM) did not prevent the SKF81297-induced potentiation of NMDAR currents. (B) Cumulative data (mean ± SE) showing the percentage control modulation of Iss by SKF81297 in the absence (n = 16) or presence of PKI6–22 (n = 15), or the membrane-permeable myristoylated PKA inhibitor PKI14–22 (0.2 μM, n = 10). (C) Plot of Iss showing that inhibiting PP1 activity with OA (0.1 μM) failed to affect the SKF81297-induced potentiation of NMDAR currents. (D) Cumulative data (mean ± SE) showing the percentage control modulation of Iss by SKF81297 in the absence (n = 34) or presence of OA (external application: 0.1 μM, n = 8; internal application: 1 μM, n = 23) or the PP1-anchoring inhibitory peptide Gm (20 μM, n = 20).
The SKF81297-induced potentiation of peak NMDAR current (Ip) is relatively small in saturating concentrations of NMDA and glycine, whereas the enhancement of steady-state NMDAR current (Iss) is prominent, suggesting that the major effect of D1 receptors on NMDA-evoked response in PFC pyramidal neurons is the inhibition of Ca2+-dependent inactivation of NMDAR channels (21–23). We therefore examined the role of Ca2+ in D1 modulation of NMDAR currents in PFC. At first we incubated neurons in a Ca2+-free solution (with 10 μM EGTA), and examined NMDAR currents in the absence and presence of SKF81297 under this condition. NMDA (500 μM)-evoked currents in neurons under the Ca2+-free condition had much smaller inactivation, as calculated by the ratio of Iss to Ipeak (Iss/Ipeak; Ca2+-free: 0.59 ± 0.06, n = 7; Ca2+-containing: 0.32 ± 0.07, n = 32). As shown in Fig. 3 A and B, SKF81297 completely lost the ability to enhance NMDAR currents in the neuron treated with the Ca2+-free solution. We then dialyzed neurons with a high concentration (10 mM) of BAPTA, a potent and rapid Ca2+ chelator, and examined D1 modulation of NMDAR currents under this condition. Dialysis with high BAPTA reduced the inactivation of NMDAR currents (Iss/Ipeak; high BAPTA: 0.42 ± 0.06, n = 13; low BAPTA: 0.32 ± 0.07, n = 32), and substantially attenuated the effect of SKF81297 on NMDAR currents (Fig. 3C). In a set of neurons we tested (Fig. 3D), the enhancing effect of D1 on NMDAR currents was eliminated under the Ca2+-free condition (-6.0 ± 2.0% of control modulation, n = 7) and was significantly (P <0.001, ANOVA) diminished by buffering intracellular Ca2+ with BAPTA in the patch pipette (39.0 ± 5.8% of control modulation, n = 15).
Fig. 3.
The effect of SKF81297 (SKF) on NMDAR currents depended on Ca2+. (A) Plot of Iss as a function of time and agonist application in neurons perfused in a Ca2+-free solution (10 μM EGTA added) or in the normal external solution (containing 1 mM Ca2+). (B) Representative current traces taken from the records used to construct A (at time points denoted by #). (Scale bars: 0.2 nA, 0.5 s.) (C) Plot of Iss as a function of time and agonist application in neurons dialyzed with the high BAPTA internal solution (10 mM) or the normal internal solution (containing 0.5 mM BAPTA). (D) Cumulative data (mean ± SE) showing the percentage control modulation of Iss by SKF81297 in normal conditions (n = 20), in the Ca2+-free solution (n = 7), or in neurons dialyzed with high BAPTA (n = 15). *, P <0.001, ANOVA.
The binding of Ca2+/CaM to the NR1 subunit is thought to underlie Ca2+ inactivation of NMDARs (23–25). Thus, we examined the role of CaM in D1 modulation of NMDAR currents in PFC pyramidal neurons. Intracellular infusion of active CaM (10 μM) caused more inactivation of NMDA-evoked currents (Iss/Ipeak;with CaM, 0.22 ± 0.06, n = 9; without CaM: 0.32 ± 0.07, n = 32), and substantially blocked the SKF81297-induced potentiation of NMDAR currents (Fig. 4 A and B). On the other hand, inclusion of the CaM antagonist CDZ (20 μM) or the CaM inhibitory peptide MLCK peptide (26) in patch electrodes reduced the inactivation of NMDAR currents (Iss/Ipeak; with CaM inhibitors: 0.41 ± 0.04, n = 19; without CaM inhibitors: 0.32 ± 0.07, n = 32), and markedly attenuated the enhancing effect of SKF81297 (Fig. 4C). In a sample of neurons we tested (Fig. 4D), the SKF81297 enhancement of steady-state NMDAR currents was significantly (P <0.001, ANOVA) smaller in neurons dialyzed with CaM (39.4 ± 4.5% of control modulation, n = 7), CDZ (40.6 ± 4.2% of control modulation, n = 14), or the CaM inhibitory peptide MLCK peptide (33.8 ± 3.6% of control modulation, n = 12).
Fig. 4.
The effect of SKF81297 (SKF) on NMDAR currents depended on CaM. (A) Plot of Iss as a function of time and agonist application in neurons loaded with or without CaM (10 μM). (B) Representative current traces taken from the records used to construct A (at time points denoted by #). (Scale bars: 0.2 nA, 0.5 s.) (C) Plot of Iss as a function of time and agonist application in neurons dialyzed with or without the CaM antagonist CDZ (20 μM). (D) Cumulative data (mean ± SE) showing the percentage control modulation of Iss by SKF81297 in normal conditions (n = 10) or in neurons dialyzed with CaM (n = 7), CDZ (n = 14) or the CaM inhibitory peptide MLCK peptide (MLCKP) (n = 12), or in the presence of the CaM-dependent protein kinase II inhibitor KN-93 (10 μM, n = 14) or the calcineurin inhibitor CsA (50 μM, n = 5). *, P <0.001, ANOVA.
We also examined the effect of SKF81297 on NMDAR currents in the presence of the Ca2+/CaM-dependent protein kinase II inhibitor KN-93 or the calcineurin inhibitor CsA, to determine whether these Ca2+-dependent enzymes were involved. The presence of KN-93 (10 μM) or CsA (50 μM) failed to reduce the SKF81297-induced potentiation of NMDAR currents (Fig. 4D, KN-93: 105.8 ± 3.6% of control modulation, n = 14; CsA: 104.4 ± 2.7% of control modulation, n = 5). Collectively, these results suggest that the D1 potentiation of NMDAR currents in PFC pyramidal neurons is caused by suppression of Ca2+/CaM-dependent inactivation of NMDARs.
The D1 Enhancement of NMDAR Currents in PFC Pyramidal Neurons Is Through a Mechanism Involving PKC. Previous studies in hippocampal neurons have shown that PKC activation enhances Ca2+/CaM-dependent inactivation of NMDAR channels (27), presumably because of a phosphorylation-dependent regulation of the interactions between NMDAR subunits, CaM, or other postsynaptic density proteins (27). We therefore examined the role of PKC in D1 modulation of NMDAR currents in PFC pyramidal neurons. As shown in Fig. 5 A and B, intracellular infusion of the PKC inhibitory peptide PKC19–36 (4 μM) substantially diminished the SKF81297-induced potentiation of NMDAR currents. In the presence of bisindolylmaleimide (1 μM), a membrane-permeable selective PKC inhibitor, SKF81297 also had a much smaller effect on NMDAR currents (Fig. 5C). As summarized in Fig. 5D, the SKF81297 enhancement of steady-state NMDAR currents was significantly (P <0.001, ANOVA) reduced in neurons dialyzed with PKC19–36 (29.3 ± 5.3% of control modulation, n = 17) or treated with bisindolylmaleimide (43.9 ± 4.2% of control modulation, n = 21).
Fig. 5.
The effect of SKF81297 (SKF) on NMDAR currents was attenuated by inhibiting PKC. (A) Plot of Iss as a function of time and agonist application in neurons loaded with or without the PKC inhibitory peptide PKC19–36 (4 μM). (B) Representative current traces taken from the records used to construct A (at time points denoted by #). (Scale bars: 0.2 nA, 0.5 s.) (C) Plot of Iss showing that the enhancing effect of SKF81297 was largely diminished in the presence of the PKC inhibitor bisindolylmaleimide (Bis, 1 μM). (D) Cumulative data (mean ± SE) showing the percentage control modulation of Iss by SKF81297 in normal conditions (n = 11) or in neurons dialyzed with PKC19–36 (n = 17), or in the presence of bisindolylmaleimide (Bis, n = 21). *, P <0.001, ANOVA.
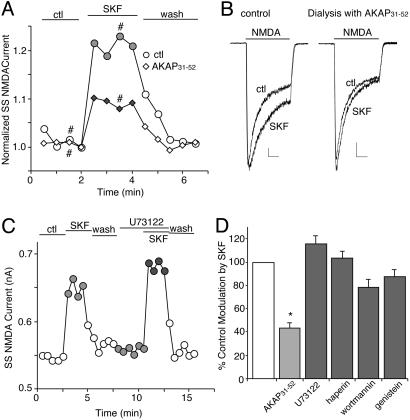
Given the involvement of both Ca2+/CaM and PKC in the D1 modulation of NMDAR currents in PFC pyramidal neurons, we examined their possible interconnections. It has been shown that Ca2+/CaM competes with PKC for binding to A-kinase anchoring protein (AKAP) 79, releasing the inhibited PKC from its association with the anchoring protein (28). Thus, we dialyzed neurons with a peptide AKAP31–52 (29) to disrupt the interaction of PKC with AKAP79, followed by the examination of SKF81297 effects on NMDAR currents. As shown in Fig. 6 A and B, the SKF81297-induced potentiation of NMDAR currents was markedly reduced in neurons loaded with the AKAP31–52 peptide (40 μM, 43.6 ± 4.1% of control modulation, n = 15, P <0.001, ANOVA, Fig. 6D). On the other hand, application of U73122 (1 μM), a phospholipase C (PLC) inhibitor, failed to affect SKF81297 enhancement of NMDAR currents (Fig. 6C, 115.8 ± 6.1% of control modulation, n = 4, Fig. 6D), indicating that the D1 regulation of NMDARs was independent of the PLC pathway.
Fig. 6.
The effect of SKF81297 (SKF) on NMDAR currents was reduced by disrupting the PKC/AKAP interaction but was not affected by inhibiting phospholipase C (PLC) or other signaling molecules. (A) Plot of Iss as a function of time and agonist application in neurons loaded with or without the peptide AKAP31–52 (40 μM). (B) Representative current traces taken from the records used to construct A (at time points denoted by #). (Scale bars: 0.2 nA, 0.5 s.) (C) Plot of Iss showing that the enhancing effect of SKF81297 was intact in the presence of the PLC inhibitor U73122 (1 μM). (D) Cumulative data (mean ± SE) showing the percentage control modulation of Iss by SKF81297 in normal conditions (n = 10) or in neurons dialyzed with AKAP31–52 (n = 15), or in the presence of U73122 (n = 4), the inositol-1-4-5-triphosphate receptor antagonist heparin (2 mg/ml, n = 4), the phosphoinositide 3-kinase inhibitor wortmannin (3 μM, n = 12), or the protein tyrosine kinase inhibitor genistein (100 μM, n = 16). *, P <0.001, ANOVA.
The potential role of several other signaling molecules in the D1 regulation of NMDARs in PFC pyramidal neurons was also examined. As summarized in Fig. 6D, the enhancing effect of SKF81297 was intact in the presence of the inositol-1-4-5-triphosphate receptor antagonist heparin (2 mg/ml, 103.5 ± 5.5% of control modulation, n = 4), the phosphoinositide 3-kinase inhibitor wortmannin (3 μM, 78.3 ± 6.7% of control modulation, n = 12), or the broad-spectrum protein tyrosine kinase inhibitor genistein (100 μM, 87.1 ± 4.0% of control modulation, n = 16), indicating the lack of involvement of these molecules. Taken together, these results suggest that the D1 potentiation of NMDAR currents in PFC pyramidal neurons is through a mechanism involving PKC that is regulated by CaM.
Discussion
Because the schizophrenia-like behaviors induced by administration of NMDAR antagonists (10, 11) or NMDAR knockdown (12) have implicated NMDARs in mental disorders associated with PFC dysfunction, it is conceivable that D1 receptors may influence PFC functions through the regulation of NMDAR channels. In agreement with this, D1 and NMDARs are enriched in dendritic spines (30–33). This synaptic organization enables interactions between dopamine and glutamate signaling in the same neuron. In this study, we demonstrated that activation of D1 receptors significantly enhanced NMDAR currents in acutely dissociated PFC pyramidal neurons, consistent with previous findings on D1 potentiation of NMDAR-mediated excitatory postsynaptic currents in PFC slices (34, 35), and D1 increase of NMDAR currents in medium spiny neostriatal neurons (36). Our data suggest that the postsynaptic NMDAR is a potential key target of D1 receptors. On the other hand, application of NMDA in cultured striatal neurons increases the recruitment of D1 receptors to dendritic spines in a Ca2+-dependent manner (13), suggesting that NMDARs can also regulate D1 signaling. The reciprocal positive interactions between D1 and NMDAR channels could play a significant role in regulating the cognitive and emotional status.
NMDAR channel activity can be regulated by a wide array of extracellular agents, including Mg2+, glycine, and Zn2+ (37), intracellular agents like Ca2+ and CaM (21, 24), and protein kinases/phosphatases, such as PKA, PKC, and PP1 (19, 38, 39). Our results show that the D1 enhancement of steady-state NMDAR currents in PFC pyramidal neurons is through the suppression of Ca2+/CaM-dependent inactivation of NMDAR channels, but not via the classical PKA/PP1 cascade as found in striatal neurons (36, 40). Unlike the D4-mediated down-regulation of NMDAR channels in PFC pyramidal neurons (16), Ca2+/CaM-dependent protein kinase II is not involved in this D1 action. Inhibiting PKC activity or disrupting PKC association with its anchoring protein AKAP79 attenuated the D1 effect on NMDAR channels, suggesting the involvement of PKC in this modulation. Ca2+ entry from NMDAR channels may regulate NMDAR inactivation by two mechanisms. One is through CaM binding to NR1 subunits directly (23, 24). The other is through CaM competing with PKC for binding to AKAP79, leading to the release and disinhibition of PKC (28) and PKC-dependent potentiation of NMDAR inactivation (27). By inhibiting Ca2+/CaM through an unknown mechanism, activation of D1 receptors suppresses the NMDAR inactivation, resulting in an enhanced steady-state NMDAR current. Given the key role of D1 and NMDARs in schizophrenia and other neuropsychiatric disorders, the present results provide a possible cellular mechanism that could underlie the D1 regulation of cognitive functions associated with PFC.
Acknowledgments
We thank Dr. Karima Chergui for reading the manuscript. This work was supported by National Institutes of Health Grants MH-63128 and AG-21923 (to Z.Y.), National Science Foundation Grant IBN-0117026 (to Z.Y.), and Public Health Service Grants MH-40899 and DA-10044 (to P.G.).
Abbreviations: AKAP, A-kinase anchoring protein; BAPTA, 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetate; CaM, calmodulin; CDZ, calmidazolium; CsA, cyclosporin A; MLCK, myosin light chain kinase; NMDA, N-methyl-d-aspartate; NMDAR, NMDA receptor; OA, okadaic acid; PFC, prefrontal cortex; PKA, protein kinase A; PP1, protein phosphatase 1.
References
- 1.Goldman-Rakic, P. S. (1995) Neuron 14, 477-485. [DOI] [PubMed] [Google Scholar]
- 2.Andreasen, N. C., O'Leary, D. S., Flaum, M., Nopoulos, P., Watkins, G. L., Boles Ponto, L. L. & Hichwa, R. D. (1997) Lancet 349, 1730-1734. [DOI] [PubMed] [Google Scholar]
- 3.Lewis, D. A. & Lieberman, J. A. (2000) Neuron 28, 325-334. [DOI] [PubMed] [Google Scholar]
- 4.Goldman-Rakic, P. S. (1994) J. Neuropsychiatry Clin. Neurosci. 6, 348-357. [DOI] [PubMed] [Google Scholar]
- 5.Carlsson, A., Waters, N., Holm-Waters, S., Tedroff, J., Nilsson, M. & Carlsson, M. L. (2001) Annu. Rev. Pharmacol. Toxicol. 41, 237-260. [DOI] [PubMed] [Google Scholar]
- 6.Brozoski, T. J., Brown, R. M., Rosvold, H. E. & Goldman, P. S. (1979) Science 205, 929-932. [DOI] [PubMed] [Google Scholar]
- 7.Williams, G. V. & Goldman-Rakic, P. S. (1995) Nature 376, 572-575. [DOI] [PubMed] [Google Scholar]
- 8.Arnsten, A. F., Cai, J. X., Murphy, B. L. & Goldman-Rakic, P. S. (1994) Psychopharmacology 116, 143-151. [DOI] [PubMed] [Google Scholar]
- 9.Tsai, G. & Coyle, J. T. (2002) Annu. Rev. Pharmacol. Toxicol. 42, 165-179. [DOI] [PubMed] [Google Scholar]
- 10.Javitt, D. C. & Zukin, S. R. (1991) Am. J. Psychiatry 148, 1301-1308. [DOI] [PubMed] [Google Scholar]
- 11.Jentsch, J. D. & Roth, R. H. (1999) Neuropsychopharmacology 20, 201-225. [DOI] [PubMed] [Google Scholar]
- 12.Mohn, A. R., Gainetdinov, R. R., Caron, M. G. & Koller, B. H. (1999) Cell 98, 427-436. [DOI] [PubMed] [Google Scholar]
- 13.Scott, L., Kruse, M. S., Forssberg, H., Brismar, H., Greengard, P. & Aperia, A. (2002) Proc. Natl. Acad. Sci. USA 99, 1661-1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feng, J., Cai, X., Zhao, J. H. & Yan, Z. (2001) J. Neurosci. 21, 6502-6511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yan, Z., Hsieh-Wilson, L., Feng, J., Tomizawa, K., Allen, P. B., Fienberg, A. A., Nairn, A. C. & Greengard, P. (1999) Nat. Neurosci. 2, 13-17. [DOI] [PubMed] [Google Scholar]
- 16.Wang, X., Zhong, P., Gu, Z. & Yan, Z. (2003) J. Neurosci. 23, 9852-9861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tingley, W. G., Ehlers, M. D., Kameyama, K., Doherty, C., Ptak, J. B., Riley, C. T. & Huganir, R. L. (1997) J. Biol. Chem. 272, 5157-5166. [DOI] [PubMed] [Google Scholar]
- 18.Greengard, P., Allen, P. B. & Nairn, A. C. (1999) Neuron 23, 435-447. [DOI] [PubMed] [Google Scholar]
- 19.Westphal, R. S., Tavalin, S. J., Lin, J. W., Alto, N. M., Fraser, I. D., Langeberg, L. K., Sheng, M. & Scott, J. D. (1999) Science 285, 93-96. [DOI] [PubMed] [Google Scholar]
- 20.Egloff, M. P., Johnson, D. F., Moorhead, G., Cohen, P. T., Cohen, P. & Barford, D. (1997) EMBO J. 16, 1876-1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenmund, C., Feltz, A. & Westbrook, G. L. (1995) J. Neurophysiol. 73, 427-430. [DOI] [PubMed] [Google Scholar]
- 22.Kyrozis, A., Albuquerque, C., Gu, J. & MacDermott, A. B. (1996) J. Physiol. (London) 495, 449-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang, S., Ehlers, M. D., Bernhardt, J. P., Su, C. T. & Huganir, R. L. (1998) Neuron 21, 443-453. [DOI] [PubMed] [Google Scholar]
- 24.Ehlers, M. D., Zhang, S., Bernhadt, J. P. & Huganir, R. L. (1996) Cell 84, 745-755. [DOI] [PubMed] [Google Scholar]
- 25.Krupp, J. J., Vissel, B., Thomas, C. G., Heinemann, S. F. & Westbrook, G. L. (1999) J. Neurosci. 19, 1165-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Torok, K. & Trentham, D. R. (1994) Biochemistry 33, 12807-12820. [DOI] [PubMed] [Google Scholar]
- 27.Lu, W. Y., Jackson, M. F., Bai, D., Orser, B. A. & MacDonald, J. F. (2000) J. Neurosci. 20, 4452-4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Faux, M. C. & Scott, J. D. (1997) J. Biol. Chem. 272, 17038-17044. [DOI] [PubMed] [Google Scholar]
- 29.Klauck, T. M., Faux, M. C., Labudda, K., Langeberg, L. K., Jaken, S. & Scott, J. D. (1996) Science 271, 1589-1592. [DOI] [PubMed] [Google Scholar]
- 30.Vincent, S. L., Khan, Y. & Benes, F. M. (1993) J. Neurosci. 13, 2551-2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smiley, J. F., Levey, A. I., Ciliax, B. J. & Goldman-Rakic, P. S. (1994) Proc. Natl. Acad. Sci. USA 91, 5720-5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goldman-Rakic, P. S. (1999) Ann. N.Y. Acad. Sci. 868, 13-26. [DOI] [PubMed] [Google Scholar]
- 33.Petralia, R. S., Yokotani, N. & Wenthold, R. J. (1994) J. Neurosci. 14, 667-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seamans, J. K., Durstewitz, D., Christie, B. R., Stevens, C. F. & Sejnowski, T. J. (2001) Proc. Natl. Acad. Sci. USA 98, 301-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gonzalez-Islas, C. & Hablitz, J. J. (2003) J. Neurosci. 23, 867-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Flores-Hernandez, J., Hernandez, S., Snyder, G. L., Yan, Z., Fienberg, A. A., Moss, S. J., Greengard, P. & Surmeier, D. J. (2000) J. Neurophysiol. 83, 2996-3004. [DOI] [PubMed] [Google Scholar]
- 37.Dingledine, R., Borges, K., Bowie, D. & Traynelis, S. F. (1999) Pharmacol. Rev. 51, 7-61. [PubMed] [Google Scholar]
- 38.Lieberman, D. N. & Mody, I. (1994) Nature 369, 235-239. [DOI] [PubMed] [Google Scholar]
- 39.Roche, K. W., Tingley, W. G. & Huganir, R. L. (1994) Curr. Opin. Neurobiol. 4, 383-388. [DOI] [PubMed] [Google Scholar]
- 40.Snyder, G. L., Fienberg, A. A., Huganir, R. L. & Greengard, P. (1998) J. Neurosci. 18, 10297-10303. [DOI] [PMC free article] [PubMed] [Google Scholar]