Abstract
Induction of an effective immune response that can target and eliminate malignant cells or virus-infected cells requires the stimulation of antigen-specific effector T cells. A productive and long-lasting memory response requires 2 signals: a specific signal provided by antigen recognition through engagement of the T cell receptor and a secondary signal via engagement of costimulatory molecules (such as OX40) on these newly activated T cells. The OX40–OX40-ligand interaction is critical for the generation of productive effector and memory T cell functions. Thus agonistic antibodies that stimulate OX40 on activated T cells have been used as adjuvants to augment immune responses. We previously demonstrated that an aptamer modified to stimulate murine OX40 enhanced vaccine-mediated immune responses in a murine melanoma model. In this study, we describe the development of an agonistic aptamer that targets human OX40 (hOX40). This hOX40 aptamer was isolated using systematic evolution of ligands by exponential enrichment and binds the target purified protein with high affinity [dissociation constants (Kd)<10 nM]. Moreover, the hOX40 aptamer–streptavidin complex has an apparent binding affinity of ∼50 nM for hOX40 on activated T cells as determined by flow cytometry and specifically binds activated human T cells. A multivalent version of the aptamer, but not a mutant version of the aptamer, was able to stimulate OX40 on T cells and enhance cell proliferation and interferon-gamma production. Future studies will assess the therapeutic potential of hOX40 aptamers for ex vivo stimulation of antigen specific T cells in conjunction with dendritic cell-based vaccines for adoptive cellular therapy.
Introduction
OX40 is a costimulatory receptor expressed primarily on activated CD4+ T cells approximately 18–24 hours after initial stimulation via the T cell receptor. OX40's cognate ligand, OX40-L, is expressed on antigen presenting cells (APCs) such as dendritic cells and B lymphocytes and binds to OX40, inducing an agonistic response in T lymphocytes that results in cell proliferation, increased cytokine production, and long-term survival of T lymphocytes. OX40-OX40L–mediated signaling events have been shown to augment immune responses to cancer and foreign entities (such as viruses) via adjuvant-based immunotherapy (JUNE, 2007; Restifo et al., 2012). However, in certain cases, OX40-mediated signaling has been shown to prolong the effects of inflammation, leading to deleterious outcomes (Croft et al., 2009; CROFT, 2010). Approaches that can finely control such stimulation may therefore prove useful in exploiting OX40 as an adjuvant effector molecule.
Nucleic acid ligands (aptamers) have been selected against a vast array of biological molecules ranging from small molecules such as adenosine triphosphate (ATP) (Huizenga and Szostak, 1995) and estradiol (Kim et al., 2007) to more complex molecules such as cell surface receptors (Lupold et al., 2002; Dollins et al., 2008; McNamara et al., 2008; Li et al., 2011; Roth et al., 2012) and targeted cell types (lymphomas and breast cancer) (Mallikaratchy et al., 2007; Shangguan et al., 2007; Shangguan et al., 2008; Zhao et al., 2009; Zhang et al., 2012). Aptamers have been identified that can inhibit target proteins of the clotting cascade (Rusconi et al., 2004), bind cell surface receptors to inhibit cell proliferation (Li et al., 2011; Roth et al., 2012), and activate signaling proteins to induce an immune response (Dollins et al., 2008; McNamara et al., 2008). Aptamers are advantageous as a therapeutic due to their high affinity and specificity for the intended target and their ability to be conjugated to molecules such as drugs or toxins (Chu et al., 2006) without a loss in affinity for the intended target when compared with antibody-based therapeutics. Another distinct advantage for nucleic acid-based therapeutics is the ability to reverse or terminate the activity of an aptamer with either an antisense oligonucleotide or a polymer (Nimjee et al., 2006; Oney et al., 2009).
Previously, our lab demonstrated the ability to identify an effective agonistic aptamer against murine OX40 (mOX40) that enhanced the effects of the vaccine-mediated immune responses in an established melanoma murine model (Dollins et al. 2008). This mOX40 aptamer does not cross react with the human protein. Our current report demonstrates that aptamers selected against human OX40 (hOX40) can specifically bind hOX40 on activated T cells in a heterogenous sample of peripheral blood mononuclear cells (PBMCs) and can be engineered into an agonistic stimulatory molecule that competes with OX40-L for its natural binding site. Finally, we demonstrate that the hOX40 aptamer agonist increases both the proliferation of T lymphocytes and the production of interferon-gamma (IFN-γ), a cytokine associated with CD4 Th1 lymphocytes.
Materials and Methods
In vitro selection of hOX40 2′-fluoropyrimidine RNA aptamers
DNA library for the hOX40 selection consisted of a 40-nucleotide region (N40) flanked by two constant regions, 5′-TCGGGCGAGTCGTCTG-N40-CCGCATCGTCCTCCCTA-3′, that was used as a template for Exo Klenow Polymerase with a forward primer containing the T7 promoter, 5′-GGGGGAATTCTAATACGACTCACTATAGGGAGGACGATGCGG-3′ (underlined portion binds to the DNA template). An initial 2′-fluoro (2′F) modified RNA pool was generated by transcribing the double-stranded DNA (dsDNA) templates with a mutant T7 RNA polymerase (Y639F) that allows for incorporation of the modified nucleotides. The dsDNA template was removed from the in vitro transcribed (IVT) RNAs by treatment with DNase 1 (Roche). RNA was purified by polyacrylamide gel electroporation (PAGE).
For selection rounds, 2′F RNA was folded by heating to 70°C for 3 minutes and then cooled at room temperature for 5 minutes in 250 μL of selection buffer E/F [20 mM (4-(2-hydroxyethyl)-1-piperazine ethane sulfonic acid) (HEPES) pH 7.5, 100 mM NaCl, 2 mM CaCl2, and 0.01% bovine serum albumin (BSA)]. The initial round of selection contained ∼2.5 nmol 2′F RNA and 834 pmol human immunoglobulin 1 incubated in 500 μL of selection buffer E/F for implementing negative selection and insured pre-clearing against the fragment crystallizable (Fc) domain of the recombinant protein. Pre-clearing was followed by selection for hOX40 recombinant protein with 250 pmol of hOX40 that had been previously incubated with protein G beads (Dynal) in 200 μL of selection buffer E/F. 2′F RNA was incubated with the hOX40-protein G beads for 20 minutes at 37°C, and then the beads were washed 3 times with 500 μL of wash buffer E/F (20 mM HEPES, 100 mM NaCl, and 2 mM CaCl2). RNA was eluted from the hOX40 beads via phenol:chloroform:IAA extraction for 30 minutes at room temperature. Isolated 2′F RNA was extracted from phenol-chloroform and ethanol precipitated. Eluted RNA was reverse transcribed with avian myeloblastosis virus reverse transcription with the reverse primer, 5′-TCGGGCGAGTCGTCTG-3′ and amplified using polymerase chain reaction (PCR) with platinum Taq polymerase and both forward (5′-GGGGGAATTCTAATACGACTCACTATAGGGAGGACGATGCGG-3′) and reverse (5′-TCGGGCGAGTCGTCTG-3′) DNA primers. The DNA pools from rounds 9 and 11 of the selection were cloned into pUC19 using EcoR1 contained within our forward primer and a reverse primer that contains a BamH1 cut site (5′-CGCGGATCCTCGGGCGAGTCGTCTG-3′) and sequenced (Eton Biosciences). Sequences obtained from Eton Biosciences were analyzed using MacVector.
Binding specificity and dissociation constant
Human OX40 aptamers, 9C7, 11F11, 9D9 (9C7mut), were in vitro transcribed with Y639F T7 RNA polymerase and were truncated based on the m-fold program and blocking oligonucleotide studies. Human OX40 aptamers were incubated with a titration of hOX40 protein (R&D Systems) from 0 to 1,000 nM in selection buffer F (20 mM HEPES, 150 mM NaCl, 2 mM CaCl2, and 0.01% BSA) for 20 minutes. Dissociation constant binding assays were performed with [γ-32P]-ATP labeled aptamers and hOX40 recombinant protein that were loaded onto a vacuum manifold (Schleicher & Schuell) containing nitrocellulose and nylon filters (Wong and Lohman, 1993). Sample wells were washed with 200 μL of wash buffer F (20 mM HEPES, 150 mM NaCl, and 2 mM CaCl2), and the nitrocellulose and nylon filters were visualized using Storm Phosphoimager (GE Healthcare). Data was analyzed using GraphPad Prism to determine dissociation constant (Kd) (Layzer and Sullenger, 2007).
Biotinylation of truncated RNAs
Double-stranded DNA templates for truncated RNAs were generated by using the Exo Klenow Polymerase and the following DNA oligos (IDT), 9C7T: 5′-GGGGGAATTCTAATACGACTCACTATA GGGATGCGGAAAAAAGAACACT-3′ and 5′-GTCTGCGGCCGTTAGGGTGGGGCCCTAATCGGAAGTG TTCTTTTTTCCGCATC-3′, and 9C7T mut (9D9T): 5′-GGGGGAATTCTAATACGACTCACTATAGGGAT GCGGAAAAACAACAC-3′ and 5′-GGCTGCGGCCGTTAGGGTGGGCCCTATCGGCGTGTTGTTTTTCC GCACC-3′. 5′-biotinylated 2′F RNA truncates (9C7T and 9C7T mut) were generated by performing IVT 2′F RNA transcriptions with the incorporation of 5′-biotin G-monophosphate (GMP; Trilink) at 3 mM (Bompiani et al., 2012). Transcribed 5′-biotinylated RNAs were purified via denaturing PAGE for cellular staining experiments. Incorporation of biotin at the 5′ end of the IVT aptamers was verified by electrophoretic mobility shift assays (EMSA). 5′-biotinylated RNAs that had been radiolabeled at the 3′ end with [γ-32P] pCp were incubated in the presence or absence of SA, 50 nM hOX40 protein (R & D Systems), or both SA and hOX40 then analyzed on a 12% native PAGE at 10 W, 4°C.
Cellular binding assays
PBMCs were isolated via standard Ficoll (Ficoll-Plaque Plus, GE Healthcare) density gradient centrifugation from commercially available de-identified buffy coats of healthy volunteers. Freshly isolated PBMCs were stimulated at 1–2×106 cells per well with either CD3 mAb (2 μg/mL, BioLegend) or CD3/CD28 monoclonal antibodies (mAbs) CD3 (2 μg/mL; BioLegend) and CD28 (5 μg/mL; BioLegend)) at 37°C and 5% CO2 in a 6-well plate containing 2 mL per well AIM V medium (Gibco-BRL). CD4+ T cells were isolated from PBMCs using a negative selection protocol with a T cell isolation kit (Invitrogen). Stimulated CD4+ T cells were examined for the expression of hOX40 by flow cytometry 48 hours after initial stimulation via CD3/CD28. Cellular staining with antibodies was conducted by incubating stimulated CD4+ T cells (2.5×105 cells) with Act35-PE mAb (5 μL) in 100 μL phosphate-buffered saline (PBS) containing 4% FBS. Aptamer staining of CD4+ T cells was performed using 20 pmol of biotinylated aptamers (200 nM) mixed with 10 pmol SA-PE (Prozyme, PJ39S) (100 nM) in 100 μL PBS containing 5 mM MgCl2 and complexes were incubated with CD4+ T cells (2.5×105 cells) for 30 minutes at 37°C. Cells were then washed 2 times with 500 μL PBS containing 5 mM MgCl2. hOX40 aptamer complexes (SA-5′Bio-9C7T) were examined for their affinity to activated CD4+ T cells by performing a titration of the complex from 0 to 500 nM utilizing the previously described cellular binding protocol (Li et al., 2009). Cells were then examined by flow cytometry on a FACscalibur (BD Biosciences). Data acquired was analyzed using CellQuest.
Costaining of PBMCs
Freshly isolated PBMCs were stimulated with either CD3 mAb (2 μg/mL) or CD3 mAb (2 μg/mL) and CD28 mAb (5 μg/mL) in AIM V media as described above. After 48 hours of stimulation, the PBMCs (2.5×105 cells) were stained first with either 200 nM PE-SA-Bio-9C7T complex or 200 nM PE-SA-Bio-9C7Tmut complex in PBS containing 5 mM MgCl2 at 37°C for 30 minutes, then the PBMCs were washed with PBS containing 4% FBS. PBMCs not stained with SA-aptamer complexes were stained with PE-labeled hOX40 mAbs (Act35, eBioscience). PBMCs previously stained with either hOX40 mAbs or hOX40 aptamer–SA complexes were then stained with either CD4-fluorescein isothiocyanate (FITC) or Iso-FITC mAbs for 30 minutes at 4°C.
Functional assays
For carboxyfluorescein N-succinimidyl ester (CFSE) proliferation assays, the isolated CD4+ T cells (106 cells) were incubated with 5 μM CFSE for 3 minutes in the dark before activation with CD3/CD28 stimulation, as described above. CFSE-labeled PBMCs (2×105 PBMCs) were treated with stimulation alone (CD3/CD28), stimulation plus 500 nM SA-Bio-9C7T aptamer complexes, or stimulation plus 500 nM SA-Bio-9C7Tmut. The 5′-biotinylated aptamers were folded by heating in binding buffer F (20 mM HEPES, pH 7.4, 150 mM NaCl, 2 mM CaCl2, and 0.01% BSA) at 70°C for 5 minutes then transferring to 37°C for 10 minutes. After folding, biotinylated aptamers, 250 nM of SA was added to the biotinylated aptamer and incubated at 37°C for 20 minutes. Aptamer complexes were added to CD4+ T cells, 18 hours after initial stimulation with anti-CD3/CD28, in a 96-well round bottom plate. Seven days after initial CD3/CD28 stimulation of the CD4+ T cells (6 days post-incubation with aptamer complexes), the supernatants of each treatment were saved for analysis. CFSE-labeled CD4+ T cells were examined for proliferation by FACscalibur and analyzed using CellQuest. Supernatants obtained from CFSE-labeled CD4+ T cells were examined for secreted IFN- γ. Human IFN-γ enzyme-linked immunosorbent assay (eBioscience) was performed following manufacturer's protocol.
Results
Isolation and characterization of hOX40 aptamer sequences
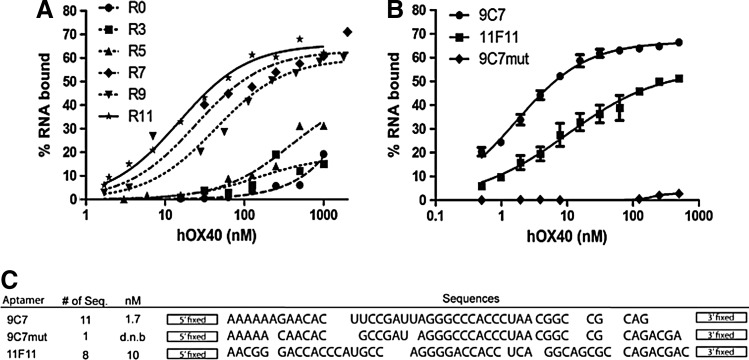
RNA aptamers that bind specifically to hOX40, a costimulatory T cell receptor, were isolated via systematic evolution of ligands by exponential enrichment (Ellington and Szostak, 1990; Burke and Gold, 1997). During the positive selection procedure, a fusion protein containing the extracellular domain of hOX40 and a human IgG fragment-crystallizable (Fc) domain was coupled to protein G-coated beads to facilitate positive selection of RNA aptamers that bind specifically to hOX40. The incorporation of negative selection, incubating the RNA sequences of the selection against hIgG before positive selection, was employed to remove nonspecific RNA sequences that would bind hIgG Fc. Our initial RNA library pool (R0) was generated from a dsDNA template containing a 40-nucleotide random region (N40). A full “selection round” incorporated a pre-clearing step and the positive selection step that isolated hOX40-binding RNAs, which were then amplified by reverse transcription and PCR. The amplified DNA sequences were in vitro transcribed to generate the enriched library of RNA sequences that were later used in the subsequent “selection round.” For the hOX40 selection, the selection round was reiterated 13 times with the stringency being enhanced by increasing the concentration of monovalent ions (100 mM to 150 mM) in the selection buffer and increasing the RNA to protein ratios. Progress of the hOX40 selection was monitored by differential filter binding assays between the multiple selection rounds (Fig. 1A). Sequences of the isolated aptamers were cloned and identified from rounds 9 and 11, since later selection rounds failed to demonstrate an increase in binding affinity and reached a plateau for overall binding activity. As shown in Fig. 1B, two selected aptamers, 9C7 and 11F11, had the best binding affinities overall (Kd ranging from 2 nM to10 nM) and were the two most prominent clones represented in rounds 9 and 11 (Fig. 1C). A mutant version of 9C7 aptamer (9C7mut), used as a control, is an orphan sequence identified from round 9 of the hOX40 selection that did not show any binding affinity for hOX40 but shared 83.3% identity with the identified 9C7 aptamer.
FIG. 1.
Characterization, isolation, and binding of hOX40 aptamers to recombinant human OX40 (hOX40) protein. (A) The binding affinities of representative rounds of systematic evolution of ligands by exponential enrichment against human OX40–immunoglobin G (IgG) fragment crystallizable (Fc) fusion protein (R & D Systems) were determined by differential filter binding: initial library (R0, •); round 3 (R3, ■); round 5 (R5, ▲); round 7 (R7, ♦); round 9 (R9, ▼); and round 11 (R11, ★). (B) The binding affinities for 3 different aptamers selected against hOX40 were isolated from rounds 9 and 11. Aptamers 9C7 (•) and 9C7mut (♦) were isolated from round 9, and aptamer 11F11 (■) was isolated from round 11. (C) Summary of aptamer sequences isolated against hOX40, number of sequences represented in each round, and their binding affinities. The sequence of the 5′ fixed region, 5′-GGGAGGACGATGCGG-3′ and the sequence for the 3′ fixed region, 5′-CAGACGACTCGCCCGA-3′.
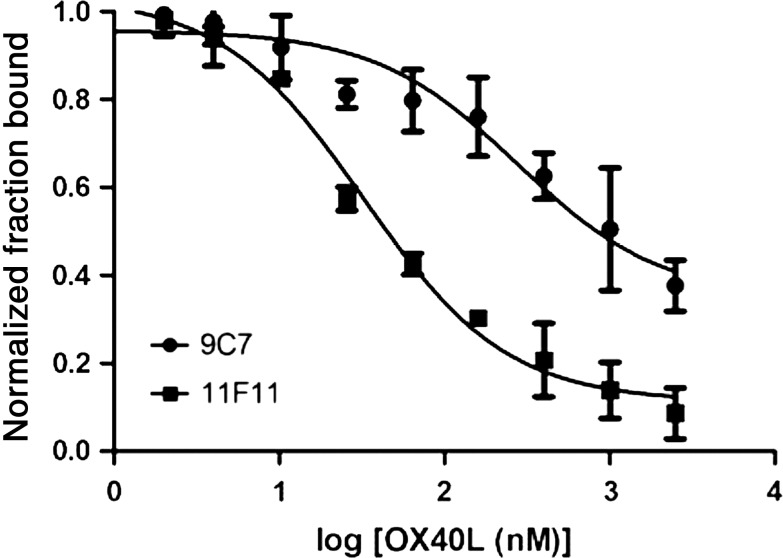
To determine if aptamers would compete with hOX40-L for the identical binding site on hOX40, we performed in vitro competition binding assays between the different hOX40 aptamers (9C7 and 11F11) and a monovalent form of hOX40-L (Fig. 2). Aptamer 9C7 directly competed with hOX40-L for the same binding site on hOX40 protein demonstrating an inhibition constant (Ki) of ∼213± 14 nM, when compared with the Kd of 190 nM for the soluble hOX40-hOX40L interaction (Al-Shamkhani et al., 1997), whereas the 11F11 aptamer bound to a different site on hOX40 and actually improved the binding affinity between hOX40 and its natural ligand, hOX40-L, with a Ki of 30±2 nM. We postulate that the 11F11 aptamer may improve the affinity of hOX40 for its natural ligand, hOX40-L, by stabilizing the protein structure and their mutual interaction. Since 9C7 appears to compete with hOX40-L for its natural binding site all subsequent analysis was performed using the 9C7 aptamer.
FIG. 2.
Evaluating ligand competition between hOX40 aptamer and hOX40 ligand (hOX40-L). OX40 competition assay between hOX40 aptamers (9C7 and 11F11) and hOX40-L for the hOX40 protein binding (n=3). The inhibition constant (Ki) for 9C7 was determined to be 213±14 nM, and Ki for 11F11 was 30±2 nM.
Characterizing cellular binding of hOX40 aptamers
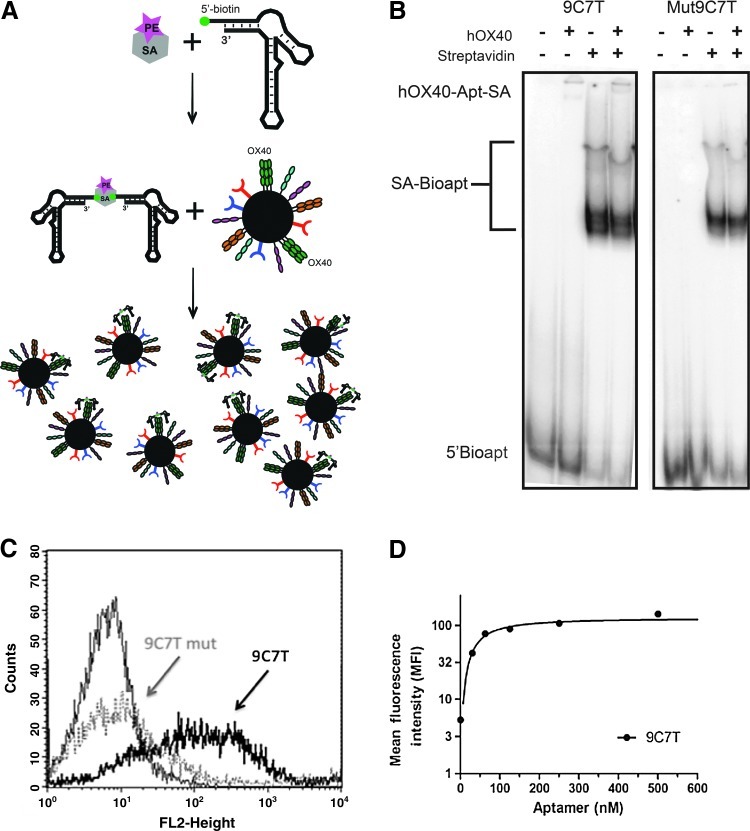
Alterations identified in one of the key binding stems of 9C7mut (Fig. 3A) corresponded with a significant loss in binding activity for both the recombinant form of hOX40 (Fig. 3B) and the cellular surface bound form of hOX40 (Fig. 3C). Full-length aptamers for 9C7 and 9C7mut were truncated from the full length of 72 nucleotides to 52 and 54 nucleotides respectively, (Fig. 3A) without a significant loss in binding activity for the 9C7 aptamer with an observed Kd of ∼29 nM and a maximum binding of RNA (aptamer) (Bmax) of ∼70% (Fig. 3B). For cellular binding activities, we assessed fluorescence binding activity by incorporating a biotin moiety at the 5′ end of our truncated aptamers via IVT with 5′-biotin-GMP that was later covalently linked to SA-labeled PE to form SA-aptamer complexes (Fig. 4A). To verify the incorporation of biotin at the 5′ end of the aptamer truncates, we analyzed the formation of SA-biotin complexes by EMSA via native gel. Both 5′-bio9C7T and 5′-bio9C7Tmut were able to bind to SA, but only 5′-bio9C7T-SA was able to bind hOX40 protein (Fig. 4B). SA-PE labeled aptamer complexes were then used to stain the activated CD4+ T cells and naïve T cells for the presence of OX40 after 48 hours of stimulation. Aptamer complexes (9C7T and 9C7Tmut) did not bind to naïve T cells (data not shown), but the 9C7T aptamer complexes did bind to activated CD4+ T lymphocytes (Fig. 4C). Expression of OX40 on activated T cells was confirmed using a commercially available antibody clone Act35. For Fig. 4D, we calculated the apparent cellular binding affinity by plotting the mean fluorescence of the activated CD4+ T cells against the concentration of the hOX40 aptamer:SA-PE complexes (Davis et al., 1998). Apparent cellular binding affinity or functional affinity of the truncated 9C7 aptamer (SA-bio-9C7T) complexes was ∼50 nM (Fig. 4D) for OX40 on activated CD4+ T cells. Aptamer:SA-PE complexes are multimeric forms of the hOX40 aptamer bound to the SA-PE molecule creating conditions of both polyvalency and avidity by increasing the number of molecular interactions between aptamer and protein, which will have an influence on the binding affinity of the aptamer to cell surface OX40. We observed a 5-fold difference in binding affinity between “in solution” hOX40 protein binding (Kd<10 nM) using nitrocellulose filter binding and cell surface binding on OX40-expressing cells (apparent Kd ∼ 50 nM). The increase in cell surface binding affinity could be affected by nonspecific binding and the conformational differences between cell surface proteins and soluble fusion proteins. Since multiple receptors are expressed on activated T lymphocytes we confirmed the specificity of OX40 aptamer to OX40 using HeLa cells transiently expressing hOX40. The OX40 aptamer (PE-bio9C7T) bound OX40+HeLa cells (Supplementary Fig. S1C; Supplementary Data are available online at www.liebertpub.com/nat) and not OX40−HeLa cells (Supplementary Fig. 1D) as determined by flow cytometry.
FIG. 3.
Truncation and binding of hOX40 aptamers to hOX40. (A) RNA secondary structures of truncated aptamers 9C7T (52 nt) and 9C7Tmut (54 nt) based on RNA structure folding program m-fold (Zuker, 2003). (B) Binding affinities of the truncated aptamers 9C7T and 9C7Tmut. Data representative of 3 independent experiments.
FIG. 4.
Characterizing cellular binding of hOX40 aptamers. (A) Diagram representing the incorporation of biotin to the 5′ end of the aptamer and the formulation of streptavidin (SA)-bioaptamer complexes. Streptavidin-phycoerythrin [SA-PE (Prozyme), pink star] was covalently linked to 5′-biotinylated (green circle) aptamer truncates in selection binding buffer. SA-PE aptamer complexes were then incubated with activated T lymphocytes and were bound to hOX40 receptor. (B) Electrophoretic mobility shift assay of hOX40 aptamers in the presence (+) or absence (−) of SA and hOX40 (50 nM). Samples were run on a 12% native polyacrylamide gel electroporation. Gel representative of one experiment out of three independent experiments performed before functional assays were conducted. (C) Flow cytometry of truncated RNA aptamers containing 5′-biotin bound to PE-streptavidin on activated CD4+ T lymphocytes 48 hours after CD3 (2 μg/mL) and CD28 (5 μg/mL) stimulation. (D) Titration of SA-Bio-9C7 aptamer complexes on activated CD4+ T lymphocytes resulted in an approximate affinity of 50 nM.
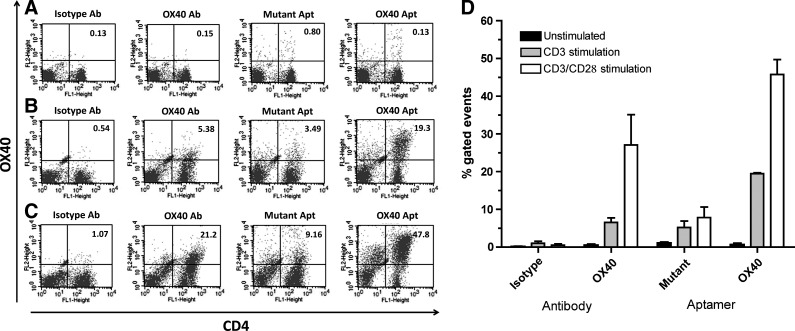
Isolated hOX40 aptamers were assessed for their specificity for OX40 by using them to identify OX40+ cells in a heterogenous sample by costaining activated peripheral blood mononuclear cells (PBMCs) with OX40 aptamer and CD4 antibody to identify CD4+OX40+ T lymphocytes. PBMCs were stimulated with either CD3 antibody alone (2 μg/mL; Fig. 5B) or CD3 (2 μg/mL) and CD28 (5 μg/mL) (CD3/CD28) antibodies simultaneously (Fig. 5C) for 48 hours and were costained for CD4+OX40+ cells. OX40 aptamers labeled with SA-PE were able to specifically identify the CD4+OX40+ cells in this mixed cell population. Notably, a higher number of CD4+OX40+ cells were identified using the OX40 aptamer as compared with the commercially available OX40 (Act35) antibody—3.6-fold higher for CD3-stimulated PBMCs and 2.3-fold higher for CD3/CD28-stimulated PBMCs (Fig. 5D).
FIG. 5.
Dual staining of peripheral blood mononuclear cells (PBMCs) with OX40 aptamer and CD4 antibody. (A) Unstimulated PBMCs costained with either isotype IgG mAb-PE, OX40 (Act35) mAb-PE, mutant aptamer (Apt) SA-PE (9C7Tmut) or OX40 Apt SA-PE (9C7T), and CD4-fluorescein isothiocyanate (FITC). (B) CD3 (5 μg/mL) stimulated PBMCs costained with OX40 (PE) and CD4 (FITC) as stated in (A). (C) CD3 (2 μg/mL) and CD28 (5 μg/mL) stimulated PBMCs costained with OX40 (PE) and CD4 (FITC) as stated in (A). (D) % of PBMCS expressing CD4 and OX40 based on the standard error of the mean (SEM) of 3 independent experiments compared to antibody staining.
We surmised that an aptamer that bound OX40 at the hOX40-L binding site would have a higher stimulatory capacity than an aptamer that bound to an alternate or inert site on hOX40. To develop an agonistic aptamer against hOX40, it was therefore necessary to identify an aptamer that demonstrated the following criteria: compete with hOX40-L's natural binding site, specifically identify hOX40+ T cells in a mixed lymphocyte sample, enhance cellular proliferation, and increase CD4 Th1 cytokine production. We decided to further pursue the development of 9C7T aptamer because it fulfilled 2 of the initial requirements for agonistic function: directly competing with hOX40-L for the natural binding site on OX40 (Fig. 2) and high binding affinity in a mixed lymphocyte sample of activated PBMCs (Fig. 5D).
Functional activity of hOX40 aptamer
Functional experiments for the agonistic activity of OX40 aptamer assessed the ability of the multimerized aptamer to increase T cell proliferation and enhance T cell survival. Human OX40 aptamer (9C7T) needed to be multimerized as a complex to have an agonistic effect on the activated CD4+ T cells and did not display any agonistic function when used as a monomer (data not shown). OX40 aptamer complexes induced an increase in cellular proliferation based on the loss of CFSE on day 7 when compared with CD4+ T cells that were stimulated with CD3 and CD28 antibodies alone (CD3/CD28) or the combination of both CD3/CD28 stimulation and 9C7Tmut aptamer complexes (Fig. 6A). The multimerized hOX40 aptamers increased cellular proliferation by ∼70% over baseline on day 7 (Fig. 6B). Supernatants harvested from activated CD4+ T lymphocytes stimulated with the combination of CD3/CD28 and multimerized hOX40 aptamer complexes (9C7T) increased IFN-γ production by 2.44-fold over CD4+ T cells stimulated with CD3/CD28 antibodies alone or combined with multimerized mutant hOX40 aptamer complexes (Fig. 6C). These results clearly demonstrate that a multivalent version of the hOX40 aptamer can provide a secondary signal to activated T cells.
FIG. 6.
Functional activity of hOX40 aptamers. (A) Histogram of carboxyfluorescein N-succinimidyl ester (CFSE) proliferation on day 7 for CD3/CD28 stimulated CD4+ T cells, CD3/CD28 stimulated CD4+ T cells and multimerized mutant OX40 aptamer (9C7T mut), and CD3/CD28 stimulated CD4+ T cells and multimerized OX40 aptamer (9C7T). Figure representative of CFSE proliferation assay, n=4. (B) Fold difference of CFSE proliferation between OX40 multimerized aptamer (Apt), mutant OX40 multimerized Apt, and stimulated CD4+ T cells. Error bars represent SEM (n=4) with a P value of 0.0014 between +OX40 Apt and the +mut OX40 Apt sample, P value of 0.0067 between +OX40 Apt and stimulated T cells, and P value of 0.0793 between +mut OX40 Apt and stimulated T cells based using a paired, 2-tailed t-test. (C) Assessment of interferon-gamma (IFN- γ) production in the supernatant of CD4+ T cells, same treatment stated in (A). Error bars represent SEM (n=3) with a P value of 0.0041 between +OX40 Apt and +mut OX40 Apt, P value of 0.0033 between +OX40 Apt and stimulated T cells, and P value of 0.0928 between +mut OX40 Apt and stimulated T cells based using a paired, 2-tailed t-test. IFN-γ enzyme-linked immunosorbent assay data is representative of at least 2 independent experiments performed in triplicate. Abbreviations: Apt=aptamer; mut=mutant; +OX40 Apt=stimulation plus OX40 aptamer; +mut OX40 Apt=stimulation plus mutant OX40 aptamer.
Discussion
OX40 expression has been implicated to play a role in a variety of diseases ranging from inflammatory diseases to the treatment of cancer and has been highlighted as a suitable target for immune intervention because of its temporal expression (Cavanagh and Hussell, 2008). A variety of aptamers that recognize specific cell surface receptors expressed on either B or T lymphocytes (Davis et al., 1998; Dollins et al., 2008; McNamara et al., 2008; Zhang et al., 2009; Zhang et al., 2012) such as CD30, mOX40, and m4-1BB have been identified. In this study, we identified a hOX40 aptamer (9C7) that recognizes both the recombinant form of hOX40 and OX40 expressed on activated human T lymphocytes. The 9C7 aptamer was truncated from 72 nucleotides to 52 nucleotides without a significant loss in activity, and this truncated version was used for cellular and functional analyses. To multimerize OX40 aptamers the 5′ end of the aptamer was biotinylated via in vitro transcription and combined with a fluorophore-labeled SA, thus also generating an efficient reporting tool for OX40 expression. This OX40 aptamer complex was effective at identifying OX40-expressing cells in the heterogenous PBMC population. The identified aptamer, 9C7T, did not interfere with the staining of the CD4 antibody when costaining was performed, and the aptamer efficiently bound CD4+OX40+ cells at 4°C or 37°C. Previously, Lieberman and colleagues identified a modified 2′F RNA aptamer that recognizes CD4 on T lymphocytes (Davis et al., 1998); a single aptamer complex of the CD4 aptamer with the 9C7 OX40 aptamer might be useful for simultaneous identification of activated CD4+OX40+ T lymphocytes in a heterogenous cellular population.
The OX40 aptamer we have developed has a high affinity for its receptor and can potentially be used as a diagnostic to identify OX40-expressing cancer cells. OX40 expression has been identified in or surrounding fibroadenomas obtained from breast tissues (Weinberg et al., 2000; Xie et al., 2010) and infiltrating adult T cell leukemia/lymphoma cells (Imura et al., 1997), implying OX40 may be a predictive marker for malignancy and may play a role in tumor metastasis. Human OX40 aptamers could be used as a screening tool to monitor the expression of OX40 on infiltrating cancerous cells and to ascertain whether OX40 may play a role in progressive metastatic disease.
The OX40-mediated signaling pathway has been implicated to play a role in maintaining high levels of anti-apoptotic proteins within T cells and extending the long-term survival of effector T cells. OX40 is an attractive immunomodulatory target for cancer immunotherapy because it can stimulate a Th1 cytokine cascade on activated T cells that results in enhancement of an antitumor immune response without potential life-threatening toxicity toward the patient (i.e., CD28 agonistic antibodies) (Nada and Somberg, 2007; Kennedy and Celis, 2008). Currently, the only other agonistic reagent against hOX40 being developed is an anti-murine hOX40 Ab (9B12) by the Providence Cancer Center group that has recently completed phase 1 trials (Weinberg et al., 2011). Second-generation agonists of 9B12 are currently being developed by the Providence group to improve efficacy, affinity, and to enable repeat dosing, since the anti-murine hOX40 Ab was not an effective therapeutic and could only be administered once. T cell agonistic antibodies have been developed against other costimulatory tumor necrosis factor receptor family members (CD27, 4-1BB, GITR) and anti-4-1BB Ab is currently being evaluated in clinical studies (Melero et al., 1997; Melero et al., 2007; Weinberg et al., 2011).
Our truncated, multimerized version of the hOX40 aptamer (9C7T) demonstrated agonistic function by increasing cell proliferation in vitro and increasing the production of IFN-γ for activated human T cells over T cells stimulated with CD3 and CD28 mAbs alone. The ability of these aptamers to activate downstream events during OX40-OX40L signaling suggests that this aptamer-SA complex could potentially be developed into an artificial APC. Inclusion of this OX40 aptamer into a stimulation cocktail or along with antigen-presenting dendritic cells during in vitro culture of tumor-specific T cells may have an adjuvant effect and stimulate the clonal expansion of functional effector T cells that persist in vivo for adoptive cellular therapy for cancer immunotherapy (JUNE, 2007; CROFT, 2010; Hombach and Abken, 2011; Restifo et al., 2012). Artificial APCs are currently being developed for the clinical expansion of tumor reactive T cells for adoptive cellular therapy against a vast array of cancer cell types (Turtle and Riddell, 2010; Hombach and Abken, 2011). Combining OX40 aptamers onto a magnetic bead with CD3 and CD28 mAbs (Trickett and Kwan, 2003) may prove to be an efficient method to expand tumor reactive T cells replacing repetitive cell stimulations during long-term cell culture ex vivo and promoting the expansion of T cells that through OX40-OX40L signaling have acquired the ability to persist in vivo.
OX40-mediated signaling plays an important role in exacerbating the effects of certain inflammatory diseases such as experimental autoimmune encephalomyelitis, collagen-induced arthritis, inflammatory bowel disease, and graft-versus-host disease by activating OX40 signaling cascade and prolonging the inflammation response through the increased cytokine production of interleukin 2 and increased cell survival of CD4+OX40+ T cells. Blocking of OX40-OX40L by an aptamer, an antibody, or another small molecule may help to alleviate the symptoms associated with inflammation found in patients suffering from rheumatoid arthritis, experimental autoimmune encephalomyelitis, and other inflammatory diseases. An engineered monomeric form of the OX40 aptamer that does not trigger the signaling cascade associated with inflammation could potentially be used to prevent the OX40-OX40L interaction and as a potential treatment of such inflammatory disorders.
Supplementary Material
Acknowledgments
This work was supported by a National Institutes of Health (NIH) T32 Postdoctoral Fellowship Training Grant to E.D.P. and an NIH R01 grant to B.A.S. (R01CA129190).
Author Disclosure Statement
No competing financial interests exist.
References
- AL-SHAMKHANI A. MALLETT S. BROWN M.H. JAMES W. BARCLAY A.N. Affinity and kinetics of the interaction between soluble trimeric OX40 ligand, a member of the tumor necrosis factor superfamily, and its receptor OX40 on activated T cells. J. Biol. Chem. 1997;272:5275–5282. doi: 10.1074/jbc.272.8.5275. [DOI] [PubMed] [Google Scholar]
- BOMPIANI K.M. MONROE D.M. CHURCH F.C. SULLENGER B.A. A high affinity, antidote-controllable prothrombin and thrombin-binding RNA aptamer inhibits thrombin generation and thrombin activity. J. Thromb. Haemost. 2012;10:870–880. doi: 10.1111/j.1538-7836.2012.04679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURKE D.H. GOLD L. RNA aptamers to the adenosine moiety of S-adenosyl methionine: structural inferences from variations on a theme and the reproducibility of SELEX. Nucleic Acids Res. 1997;25:2020–2024. doi: 10.1093/nar/25.10.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAVANAGH M.M. HUSSELL T. Is it wise to target the late costimulatory molecule OX40 as a therapeutic target? Arch. Immunol. Ther. Exp. (Warsz.) 2008;56:291–297. doi: 10.1007/s00005-008-0032-3. [DOI] [PubMed] [Google Scholar]
- CHU T.C. MARKS J.W., 3rd LAVERY L.A. FAULKNER S. ROSENBLUM M.G. ELLINGTON A.D. LEVY M. Aptamer:toxin conjugates that specifically target prostate tumor cells. Cancer Res. 2006;66:5989–5992. doi: 10.1158/0008-5472.CAN-05-4583. [DOI] [PubMed] [Google Scholar]
- CROFT M. Control of immunity by the TNFR-related molecule OX40 (CD134) Annu. Rev. Immunol. 2010;28:57–78. doi: 10.1146/annurev-immunol-030409-101243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROFT M. SO T. DUAN W. SOROOSH P. The significance of OX40 and OX40L to T-cell biology and immune disease. Immunol. Rev. 2009;229:173–191. doi: 10.1111/j.1600-065X.2009.00766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS K.A. LIN Y. ABRAMS B. JAYASENA S.D. Staining of cell surface human CD4 with 2′-F-pyrimidine-containing RNA aptamers for flow cytometry. Nucleic Acids Res. 1998;26:3915–3924. doi: 10.1093/nar/26.17.3915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOLLINS C.M. NAIR S. BOCZKOWSKI D. LEE J. LAYZER J.M. GILBOA E. SULLENGER B.A. Assembling OX40 aptamers on a molecular scaffold to create a receptor-activating aptamer. Chem Biol. 2008;15:675–82. doi: 10.1016/j.chembiol.2008.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLINGTON A.D. SZOSTAK J.W. In vitro selection of RNA molecules that bind specific ligands. Nature. 1990;346:818–822. doi: 10.1038/346818a0. [DOI] [PubMed] [Google Scholar]
- HOMBACH A.A. ABKEN H. Costimulation by chimeric antigen receptors revisited the T cell antitumor response benefits from combined CD28-OX40 signalling. Int. J. Cancer. 2011;129:2935–2944. doi: 10.1002/ijc.25960. [DOI] [PubMed] [Google Scholar]
- HUIZENGA D.E. SZOSTAK J.W. A DNA aptamer that binds adenosine and ATP. Biochemistry. 1995;34:656–665. doi: 10.1021/bi00002a033. [DOI] [PubMed] [Google Scholar]
- IMURA A. HORI T. IMADA K. KAWAMATA S. TANAKA Y. IMAMURA S. UCHIYAMA T. OX40 expressed on fresh leukemic cells from adult T-cell leukemia patients mediates cell adhesion to vascular endothelial cells: implication for the possible involvement of OX40 in leukemic cell infiltration. Blood. 1997;89:2951–2958. [PubMed] [Google Scholar]
- JUNE C.H. Adoptive T cell therapy for cancer in the clinic. J. Clin. Invest. 2007;117:1466–1476. doi: 10.1172/JCI32446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENNEDY R. CELIS E. Multiple roles for CD4+ T cells in anti-tumor immune responses. Immunol. Rev. 2008;222:129–144. doi: 10.1111/j.1600-065X.2008.00616.x. [DOI] [PubMed] [Google Scholar]
- KIM Y.S. JUNG H.S. MATSUURA T. LEE H.Y. KAWAI T. GU M.B. Electrochemical detection of 17beta-estradiol using DNA aptamer immobilized gold electrode chip. Biosens. Bioelectron. 2007;22:2525–2531. doi: 10.1016/j.bios.2006.10.004. [DOI] [PubMed] [Google Scholar]
- LAYZER J.M. SULLENGER B.A. Simultaneous generation of aptamers to multiple gamma-carboxyglutamic acid proteins from a focused aptamer library using DeSELEX and convergent selection. Oligonucleotides. 2007;17:1–11. doi: 10.1089/oli.2006.0059. [DOI] [PubMed] [Google Scholar]
- LI N. EBRIGHT J.N. STOVALL G.M. CHEN X. NGUYEN H.H. SINGH A. SYRETT A. ELLINGTON A.D. Technical and biological issues relevant to cell typing with aptamers. J. Proteome Res. 2009;8:2438–2448. doi: 10.1021/pr801048z. [DOI] [PubMed] [Google Scholar]
- LI N. NGUYEN H.H. BYROM M. ELLINGTON A.D. Inhibition of cell proliferation by an anti-EGFR aptamer. PLoS One. 2011;6:e20299. doi: 10.1371/journal.pone.0020299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUPOLD S.E. HICKE B.J. LIN Y. COFFEY D.S. Identification and characterization of nuclease-stabilized RNA molecules that bind human prostate cancer cells via the prostate-specific membrane antigen. Cancer Res. 2002;62:4029–4033. [PubMed] [Google Scholar]
- MALLIKARATCHY P. TANG Z. KWAME S. MENG L. SHANGGUAN D. TAN W. Aptamer directly evolved from live cells recognizes membrane bound immunoglobin heavy mu chain in Burkitt's lymphoma cells. Mol. Cell Proteomics. 2007;6:2230–2238. doi: 10.1074/mcp.M700026-MCP200. [DOI] [PubMed] [Google Scholar]
- MCNAMARA J.O. KOLONIAS D. PASTOR F. MITTLER R.S. CHEN L. GIANGRANDE P.H. SULLENGER B. GILBOA E. Multivalent 4-1BB binding aptamers costimulate CD8+ T cells and inhibit tumor growth in mice. J. Clin. Invest. 2008;118:376–386. doi: 10.1172/JCI33365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MELERO I. HERVAS-STUBBS S. GLENNIE M. PARDOLL D.M. CHEN L. Immunostimulatory monoclonal antibodies for cancer therapy. Nat. Rev. Cancer. 2007;7:95–106. doi: 10.1038/nrc2051. [DOI] [PubMed] [Google Scholar]
- MELERO I. SHUFORD W.W. NEWBY S.A. ARUFFO A. LEDBETTER J.A. HELLSTROM K.E. MITTLER R.S. CHEN L. Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors. Nat. Med. 1997;3:682–685. doi: 10.1038/nm0697-682. [DOI] [PubMed] [Google Scholar]
- NADA A. SOMBERG J. First-in-man (FIM) clinical trials post-TeGenero: a review of the impact of the TeGenero trial on the design, conduct, and ethics of FIM trials. Am. J. Ther. 2007;14:594–604. doi: 10.1097/MJT.0b013e31813737dd. [DOI] [PubMed] [Google Scholar]
- NIMJEE S.M. KEYS J.R. PITOC G.A. QUICK G. RUSCONI C.P. SULLENGER B.A. A novel antidote-controlled anticoagulant reduces thrombin generation and inflammation and improves cardiac function in cardiopulmonary bypass surgery. Mol. Ther. 2006;14:408–415. doi: 10.1016/j.ymthe.2006.04.006. [DOI] [PubMed] [Google Scholar]
- ONEY S. LAM R.T. BOMPIANI K.M. BLAKE C.M. QUICK G. HEIDEL J.D. LIU J.Y. MACK B.C. DAVIS M.E. LEONG K.W., et al. Development of universal antidotes to control aptamer activity. Nat. Med. 2009;15:1224–1228. doi: 10.1038/nm.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RESTIFO N.P. DUDLEY M.E. ROSENBERG S.A. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat. Rev. Immunol. 2012;12:269–281. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROTH F. DE LA FUENTE A.C. VELLA J.L. ZOSO A. INVERARDI L. SERAFINI P. Aptamer-mediated blockade of IL4Ralpha triggers apoptosis of MDSCs and limits tumor progression. Cancer Res. 2012;72:1373–1383. doi: 10.1158/0008-5472.CAN-11-2772. [DOI] [PubMed] [Google Scholar]
- RUSCONI C.P. ROBERTS J.D. PITOC G.A. NIMJEE S.M. WHITE R.R. QUICK G., JR. SCARDINO E. FAY W.P. SULLENGER B.A. Antidote-mediated control of an anticoagulant aptamer in vivo. Nat. Biotechnol. 2004;22:1423–1428. doi: 10.1038/nbt1023. [DOI] [PubMed] [Google Scholar]
- SHANGGUAN D. CAO Z. MENG L. MALLIKARATCHY P. SEFAH K. WANG H. LI Y. TAN W. Cell-specific aptamer probes for membrane protein elucidation in cancer cells. J. Proteome Res. 2008;7:2133–2139. doi: 10.1021/pr700894d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHANGGUAN D. CAO Z.C. LI Y. TAN W. Aptamers evolved from cultured cancer cells reveal molecular differences of cancer cells in patient samples. Clin. Chem. 2007;53:1153–1155. doi: 10.1373/clinchem.2006.083246. [DOI] [PubMed] [Google Scholar]
- TRICKETT A. KWAN Y.L. T cell stimulation and expansion using anti-CD3/CD28 beads. J. Immunol. Methods. 2003;275:251–255. doi: 10.1016/s0022-1759(03)00010-3. [DOI] [PubMed] [Google Scholar]
- TURTLE C.J. RIDDELL S.R. Artificial antigen-presenting cells for use in adoptive immunotherapy. Cancer J. 2010;16:374–381. doi: 10.1097/PPO.0b013e3181eb33a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEINBERG A.D. MORRIS N.P. KOVACSOVICS-BANKOWSKI M. URBA W.J. CURTI B.D. Science gone translational: the OX40 agonist story. Immunol. Rev. 2011;244:218–231. doi: 10.1111/j.1600-065X.2011.01069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEINBERG A.D. RIVERA M.M. PRELL R. MORRIS A. RAMSTAD T. VETTO J.T. URBA W.J. ALVORD G. BUNCE C. SHIELDS J. Engagement of the OX-40 receptor in vivo enhances antitumor immunity. J. Immunol. 2000;164:2160–2169. doi: 10.4049/jimmunol.164.4.2160. [DOI] [PubMed] [Google Scholar]
- WONG I. LOHMAN T.M. A double-filter method for nitrocellulose-filter binding: application to protein-nucleic acid interactions. Proc. Natl. Acad. Sci. U. S. A. 1993;90:5428–5232. doi: 10.1073/pnas.90.12.5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XIE F. WANG Q. CHEN Y. GU Y. MAO H. ZENG W. ZHANG X. Costimulatory molecule OX40/OX40L expression in ductal carcinoma in situ and invasive ductal carcinoma of breast: an immunohistochemistry-based pilot study. Pathol. Res. Pract. 2010;206:735–739. doi: 10.1016/j.prp.2010.05.016. [DOI] [PubMed] [Google Scholar]
- ZHANG K. SEFAH K. TANG L. ZHAO Z. ZHU G. YE M. SUN W. GOODISON S. TAN W. A novel aptamer developed for breast cancer cell internalization. ChemMedChem. 2012;7:79–84. doi: 10.1002/cmdc.201100457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG P. ZHAO N. ZENG Z. FENG Y. TUNG C.-H. CHANG C.-C. ZU Y. Using an RNA aptamer probe for flow cytometry detection of CD30-expressing lymphoma cells. Lab. Invest. 2009;89:1423–1432. doi: 10.1038/labinvest.2009.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHAO Z. XU L. SHI X. TAN W. FANG X. SHANGGUAN D. Recognition of subtype non-small cell lung cancer by DNA aptamers selected from living cells. Analyst. 2009;134:1808–1814. doi: 10.1039/b904476k. [DOI] [PubMed] [Google Scholar]
- ZUKER M. Mfold web server for nucleic acid folding and hybridization prediction. Nucl. Acids Res. 2003;31:3406–3415. doi: 10.1093/nar/gkg595. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.