Abstract
Proteins of the ezrin-radixin-moesin family are ubiquitous constituents of the submembrane cortex, especially in epithelial cells. Earlier biochemical results suggested that a protein of this family occurs in the hair bundle, the cluster of actin-filled stereocilia that serves as the mechanoreceptive organelle of each hair cell in the inner ear. We prepared antipeptide antisera directed against chicken radixin and ezrin and demonstrated their specificity and absence of crossreactivity. When used in immunocytochemical studies of isolated hair cells, anti-radixin produced an intense band of labeling at the bases of hair bundles from the chicken, frog, mouse, and zebrafish. Electron microscopic immunocytochemistry disclosed that radixin labeling commenced in the stereociliary taper, peaked in the lower stereociliary shaft, and declined progressively toward the hair bundle's top. Labeling with anti-ezrin produced no signal in hair bundles. Radixin is thus a prominent constituent of stereocilia, where it may participate in anchoring the “pointed” ends of actin filaments to the membrane.
The hair bundle is the mechanoreceptive organelle essential for the senses of hearing and equilibrium throughout the vertebrates. Projecting from the apical surface of a hair cell, the bundle comprises some 20–300 rigid, cylindrical stereocilia from ≈1 to ≈50 μm in length. The stereocilia grow successively longer along one axis of the hexagonal array in which they are packed, so that a bundle displays a beveled top (1). In the developing ear, a single kinocilium endowed with an axoneme marks the center of the bundle's tall edge. This organelle is not essential for mechanoelectrical transduction, however, for it degenerates in the mature mammalian cochlea and can be dissected away without an obvious effect on mechanosensitivity (2).
The normal hair bundle plays an essential role in the process of mechanoelectrical transduction (3). Moreover, many forms of genetic, traumatic, pharmacological, and geriatric deafness and dysequilibrium stem from bundle degeneration. Identification of the hair bundle's biochemical constituents is therefore essential for a complete understanding of normal and pathological hearing and balance. Although a hair bundle contains hundreds of proteins (4), only 20 or so have been identified. Biochemical, immunocytochemical, genetic, and physiological techniques are therefore being applied intensively to recognize the components essential to mechanoelectrical transduction.
In the course of analyzing the proteins isolated from hair bundles of the bullfrog's sacculus by PAGE, we noted one with an unusual feature (4). A molecule of an apparent molecular mass of ≈77 kDa seemed to occur at only a modest concentration when assayed by silver staining. When bundle components were instead detected after labeling with N-hydroxysulfosuccinimidobiotin; however, the protein became prominent. Because N-hydroxysuccinimides attack amino groups, this behavior suggested that the protein is especially rich in lysine residues. The labeling pattern of the substance indicated that it is an intracellular protein poorly extracted by nonionic detergent (4), as might be expected for a component of the cytoskeleton or the sub-membrane cortex. Although these features are scarcely diagnostic, they are characteristic of members of the ezrin-radixinmoesin (ERM) protein family (5–8). Moreover, proteins of this family are often constituents of epithelial cells, and especially of their apical protrusions such as the microvilli from which stereocilia originate (1). We have therefore inquired in the present study whether hair cells express an ERM protein that occurs in hair bundles.
Materials and Methods
Production of Antisera. For the production of antisera, we selected portions of chicken ezrin and radixin corresponding to regions in the human proteins that had previously proven useful in eliciting specific immune responses (9). Antisera 1041 and 1029 were raised against two distinct portions of chicken radixin (GenBank accession no. CAB59977), NH2-478-VIPPTENEHDEHDENN-COOH and NH2-400-KAALAKQAADQMKN-COOH, respectively. These sequences are specific for radixin, with little homology to avian ezrin or to the moesins of other species. Antiserum 1028 was raised against a peptide from chicken ezrin (GenBank accession no. BAA75497) NH2-476-IYEPVNYH-VHDNLHDEGSEY-COOH, with minimal homology to avian radixin or to moesins. The synthesis of each peptide, conjugation to keyhole limpet hemocyanin by an amino-terminal cysteine residue, purification, and serum production in rabbits were performed commercially (Covance Research Products). To demonstrate immunoreactivity, preliminary and production bleeds were tested against preimmune sera by immunoblotting. Preimmune sera displayed only weak, nonspecific binding to isolated brain and cochlear proteins, whereas antisera 1041, 1028, and 1029 showed little background but strong reactions with ERM proteins. Although both antisera against chicken radixin produced similar results in immunoblotting and immunocytochemical labeling, antiserum 1041 generally yielded stronger signals. This antiserum was therefore used in all of the immunocytochemical experiments reported here, save for labeling of zebrafish hair bundles, for which antiserum 1029 proved superior. All antisera were used without further purification.
Immunoblotting. Precast 4–20% Tris-glycine gels were used for the initial characterization of sera. Peptide competition and immunoprecipitation experiments involved 6% Tris-glycine gels. Electrophoresis was conducted at 4°C for 2 h at 125 V with Tris/glycine/SDS running buffer containing 1 mM EDTA. Proteins were transferred at 4°C for 80 min at 85 V to nitrocellulose membranes, which were washed in cold PBS containing 0.1% Tween 20, the medium used in all subsequent steps. After having been blocked with 5% nonfat dry milk, membranes were incubated for 3 h at room temperature with the appropriate antisera. They were then washed and exposed for 1 h to horseradish peroxidase-conjugated donkey anti-rabbit immunoglobin (Amersham Pharmacia Biosciences) at a dilution of 1:10,000. Finally, the membranes were washed, developed, and exposed.
Peptide Competition Experiments. To prepare samples for immunoblotting, brains were dissected from chickens 1–2 weeks of age and ≈1 g of tissue was treated with 10 ml of lysis solution containing 150 mM NaCl, 1% Triton X-100, and 40 mM Tris, pH 7.4, with a protease inhibitor mixture (EDTA-free Complete, Roche). Thirty chicken cochleae were treated with 200 μl of lysis solution. Brush borders for protein extraction were obtained by following a published protocol (10) that was simplified by omission of the sucrose gradient and use of intestinal villi as a crude sample. Approximately 1 ml of sedimented intestinal villi was resuspended in 10 ml of lysis solution. Solubilization was performed by dispersing each sample with a glass homogenizer and incubating for 30 min at 4°C. Each sample was then centrifuged for 10 min at 4°C at 12,000 × g and the supernatant was retained. After protein concentrations had been determined, 25 μg of brush-border, brain, or cochlear protein was loaded per lane on two identical gels for each antiserum to be tested. After electrophoresis and transfer had been conducted as described above, membranes were blocked overnight. For each pair of identical membranes, two solutions were prepared: one containing antiserum at a dilution of 1:2,500 in PBS containing 5% nonfat dry milk and 0.1% Tween 20, the other with the same constituents plus 100 μg/ml peptide 1041, 1028, or 1029. Solutions were incubated at room temperature for 1 h before application to membranes and incubation for 3 h at room temperature with mild agitation. The immunoblots were then washed and developed as described above.
Immunoprecipitation and Immunoblotting. We immunoprecipitated proteins from the brain or cochlea with antisera 1041, 1028, and 1029, ran three identical gels loaded with the complete protein sample and with protein from each of the immunoprecipitations, and then blotted the membranes with each antiserum. Proteins for immunoprecipitation were obtained as described above, but were solubilized in lysis solution with 0.5% Nonidet P-40 substituted for Triton X-100. After having been cleared for 1 h at 4°C with beads (ImmunoPure Immobilized Protein A/G, Pierce Biotechnology), 200 μg of brain protein or 250 μg of cochlear protein was incubated with 5 μl of antiserum 1041, 1028, or 1029, and 20 μl of beads. The following day, the beads were washed, resuspended in 40 μl of loading buffer, heated to 70°C for 10 min, and sedimented; 12 μl of solution was then loaded on each of three Tris-glycine gels. Immunoblotting was performed as described above with each membrane exposed to antiserum 1041, 1028, or 1029.
Isolation of Cells. Hair cells were isolated from the cochleae of chickens (Gallus gallus) 2–3 weeks of age by use of a published procedure (11) without BSA. Adult bullfrog (Rana catesbeiana) saccular hair cells were isolated as described (12). The lagenae of adult zebrafish (Danio rerio) were dissected in solutions identical to those used for bullfrogs; hair cells were dissociated by a similar enzymatic treatment extended to 40 min. Cochlear and utricular hair cells were isolated from mice (Mus musculus) 1–2 weeks of age according to a published protocol (13), save that the dissection and enzymatic digestion were performed in Hanks' balanced salt solution.
For use in control experiments, epithelial cells were isolated from the small intestines of chickens and frogs by a published procedure (10).
Light Microscopic Immunocytochemistry. Isolated cells were allowed to settle onto coverslips previously exposed to 1 mg/ml Con A. After fixation for 20 min with 4% paraformaldehyde in PBS (137 mM NaCl/2.7 mM KCl/10 mM sodium phosphate, pH 7.4), cells were permeabilized for 15 min with 0.05% Triton X-100 in PBS and exposed for 30 min to blocking solution containing 0.05% Triton X-100 and 1% BSA in PBS. They were then incubated overnight at 4°C in blocking solution with the primary rabbit antiserum at a dilution of 1:1,000. After a 30-min wash, cells were incubated for 2 h in blocking solution containing fluorescein isothiocyanate-labeled donkey anti-rabbit IgG (Jackson ImmunoResearch) at a dilution of 1:200. Except for the incubation with primary antiserum, all steps were conducted at room temperature. After a 30-min wash in PBS, cells were observed in PBS with a laser-scanning confocal microscope.
For labeling of filamentous actin, Alexa Fluor 568-phalloidin (Molecular Probes) was added at a concentration of 50 ng/ml during the final 30 min of the incubation with secondary antibodies. For staining of nuclei in preparations of intestinal epithelial cells, TO-PRO-3 iodide (Molecular Probes) was included at a concentration of 1 μg/ml throughout the secondary antibody incubation.
Electron Microscopic Immunocytochemistry. Cochleae from chickens ≈3 weeks old were dissected in artificial avian perilymph consisting of 145 mM Na+, 2 mM K+, 2 mM Ca2+, 2 mM Mg2+, 120 mM Cl-, 22 mM d-glucuronate, 11 mM HCO3-, 0.1 mM HPO42-, 5 mM sucrose, 5 mM d-glucose, 1 mM sodium pyruvate, 1 mM creatine, and 5 mM Hepes, pH 7.4. After removal of the tegmentum vasculosum, each specimen was incubated for 30 min at room temperature in 75 μg/ml protease (type XXIV; Sigma) in artificial perilymph solution to facilitate removal of its tectorial membrane. Specimens were then fixed for 2 h at room temperature in 400 mM formaldehyde/25 mM sucrose/4 mM CaCl2/120 mM sodium cacodylate, pH 7.4. After a 30-min permeabilization in PBS containing 1% Triton X-100 and 1% BSA, specimens were exposed for 12 h at 4°C to primary sera at a dilution of 1:1,000 in PBS containing 0.05% Triton X-100 and 1% BSA. After another 30-min wash at the same temperature, cochleae were incubated for 120 min at 4°C with affinity-purified goat anti-rabbit IgG labeled with 12-nm colloidal gold particles (Jackson ImmunoResearch) at a dilution of 1:20 in the same solution. The specimens were then washed and were subjected to secondary fixation by immersion for 2 h at 4°C in 200 mM glutaraldehyde in 120 mM sodium cacodylate, pH 7.4. After another wash, the cochleae were postfixed for 2 h at 4°C with 50 mM OsO4 and 10 mM K+ ferrocyanide in the same buffer solution. Finally, specimens were dehydrated in graded ethanol concentrations, stained en bloc with uranyl acetate, and embedded in epoxy resin. Sections were cut at a thickness of 70 nm, stained with lead citrate, and examined in an electron microscope operated at an accelerating voltage of 80 kV.
Morpholino Injections. The zebrafish genome contains at least two putative radixin genes. The cDNA sequence encoding a protein with an amino terminus typical of ERM proteins, here designated radixin A, occurs in contigs 12502 and 24222 of the zebrafish genomic assembly (Welcome Trust Sanger Institute, Hinxton, U.K.). By interrogating the assembly with those sequences, we identified in contigs 10123 and 11837 part of a second gene encoding radixin B, whose unusual amino terminus we defined by sequencing a 5′-RACE product. We then obtained a morpholino targeted at the start codon of the mRNA for each protein (GeneTools) and injected one-cell embryos as described (14) with ≈20 ng each. The morpholino directed against radixin A had the sequence 5′-CACTGATCGTTTTCGGCATTTT-GTC-3′; the sequence of the mismatched control morpholino was 5′-CAgTGtTCcTTTTCGGgATTTTcTC-3′. For radixin B, the corresponding sequences were 5′-AGCTGACCAGTGTCT-TCTTGTAGAG-3′ and 5′-AGgTGAgCAGTGTgTTCTTcTA-cAG-3′.
Results
Characterization of Antisera. Because the structure and development of hair cells have been extensively characterized in the chicken, we sought ERM proteins in the hair bundles of that species. Moesin has not been identified in the chicken. We therefore produced antipeptide antisera against divergent portions of the remaining ERM proteins: two antisera, 1041 and 1029, were raised against avian radixin, and one, antiserum 1028, against avian ezrin.
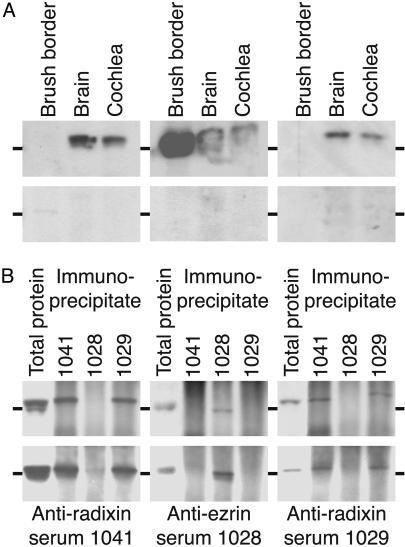
We conducted immunoblotting experiments to confirm the specificity of the antisera. When used on a sample of proteins from the chicken's brain or cochlea, the two antisera directed against radixin strongly labeled a single protein with an apparent molecular mass slightly exceeding 75 kDa (Fig. 1A), a value consistent with that expected for radixin. These antisera, 1041 and 1029, produced no signal in immunoblots of brush-border proteins from the intestine, which include large amounts of ezrin. By contrast, the antiserum raised against an ezrin peptide produced a robust signal when applied to intestinal proteins. This antiserum also detected proteins from the brain and cochlea, each of which includes vascular, epithelial, and connective tissues that might harbor ezrin. The reactivity of each of the three antisera was abolished by competition with the peptide used in immunization (Fig. 1 A).
Fig. 1.
Demonstration of the specificity of antisera. Each of the 12 illustrations represents an immunoblot incubated with the antiserum indicated below it. The horizontal marker beside each illustration represents 75 kDa. (A) Peptide competition experiments with anti-radixin and anti-ezrin sera on brush border, brain, and cochlear proteins. (Upper) The anti-radixin sera 1041 and 1029 recognize a protein found in the brain and cochlea, but not in brush borders from intestinal epithelial cells. Anti-ezrin serum 1028 reacts strongly with brush-border protein, moderately with brain protein, and weakly with cochlear protein. (Lower) Probing with antisera in the presence of the corresponding peptides used for immunization eliminates immunoreactivity. (B) Immunoprecipitation and blotting of brain and cochlear proteins to demonstrate the specificity of the anti-radixin and anti-ezrin sera. (Upper) Probing the protein immunoprecipitated from the brain by each of the three antisera demonstrates that the anti-radixin and anti-ezrin sera display negligible cross-reactivity. (Lower) The corresponding experiment for proteins immunopreciptated from the cochlea. In each instance, total brain or cochlear protein is loaded in the first lane as a control.
As a more stringent test of potential crossreactivity between the antisera raised against radixin and ezrin, we immunoprecipitated proteins from the cochlea and brain with each of the three antisera, then probed these proteins on immunoblots. In positive control experiments, each antiserum detected total cochlear protein as well as the protein that the same antiserum had previously precipitated (Fig. 1B). Antiserum 1041 additionally detected the protein immunoprecipitated by the other anti-radixin antiserum, 1029, and vice versa. Neither of these antisera detected the protein precipitated by the putative antiezrin. Finally, antiserum 1028 raised against ezrin produced no signal when applied to the protein precipitated by antiserum 1041 or 1029. We conclude that the antisera raised against radixin and against ezrin recognize the corresponding proteins with negligible cross-reactivity.
Light Microscopic Immunocytochemistry. In an immunofluorescence analysis of hair cells from the chicken's cochlea, antiserum directed against radixin produced a highly consistent pattern of labeling. Hair bundles were strongly labeled, with the greatest signal restricted to the lower portions of the stereocilia (Fig. 2 A and B). Although labeling clearly declined in the upper reaches of a hair bundle, it appeared that some signal persisted to the stereociliary tips, especially in preparations without counterlabeling of actin (data not shown). No anti-radixin signal was usually evident elsewhere in the hair cells, including their somata, kinocilia, and apical surfaces. Similar results were obtained on hair cells throughout the cochlea.
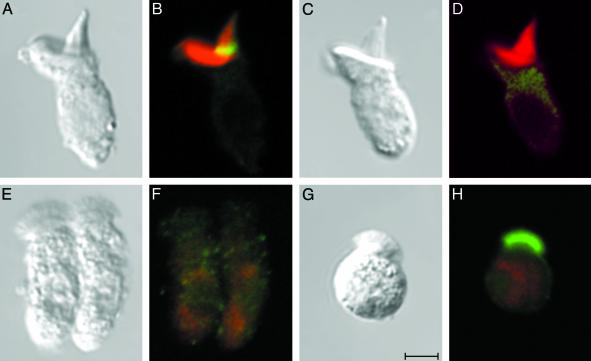
Fig. 2.
Specific immunocytochemical labeling of radixin in avian hair bundles. (A) A hair cell of intermediate length was isolated from the chicken's cochlea and was observed with differential-interference-contrast optics. (B) Labeling of filamentous actin with phalloidin conjugated to Alexa Fluor 568 (red) produces a strong signal throughout the same hair bundle and the subjacent cuticular plate. The partial overlap of this signal with immunolabeling by anti-radixin (green) results in a yellow signal at the bundle's base. (C and D) Immunolabeling with anti-ezrin produces no signal in a hair bundle labeled with fluorescent phalloidin (red). (E and F) In a negative control experiment, immunolabeling with the anti-radixin antiserum used in B produces a negligible signal in the brush borders of isolated intestinal epithelial cells. (G and H) In a positive control experiment, immunolabeling with the anti-ezrin antiserum used in D robustly labels brush-border microvilli. The cellular nuclei in F and H are weakly stained with TO-PRO-3 iodide (red). (Scale bar in G, 5 μm; applies to all images.)
Several control experiments were conducted to confirm the specificity of the observed immunolabeling. No fluorescence signal was observed if the primary anti-radixin antiserum was omitted from the procedure or replaced with preimmune serum (data not shown). Anti-ezrin labeling produced no signal in avian hair cells (Fig. 2 C and D). Anti-radixin immunolabeling conversely yielded no fluorescent signal when performed under identical conditions on avian or anuran intestinal epithelial cells (Fig. 2 E and F), whose brush-border microvilli are known to contain another ERM protein, ezrin. The anti-ezrin antiserum, in contrast, strongly immunolabeled the brush borders of intestinal epithelial cells (Fig. 2 G and H).
Similar patterns of anti-radixin immunolabeling occurred in hair cells isolated from animals of other vertebrate classes. Cells of the bullfrog's sacculus displayed strong labeling at stereociliary bases and only weak signals near the stereociliary tips (Fig. 3 A, B, G, and H). Hair cells from the zebrafish's lagena showed comparable immunofluorescence in the bundles, as well as weak somatic labeling (Fig. 3 C and D). Hair cells of the mouse's utriculus were labeled modestly, but again displayed the strongest anti-radixin signal at the stereociliary bases (Fig. 3 E and F). A similar pattern was observed with cochlear hair cells (data not shown).
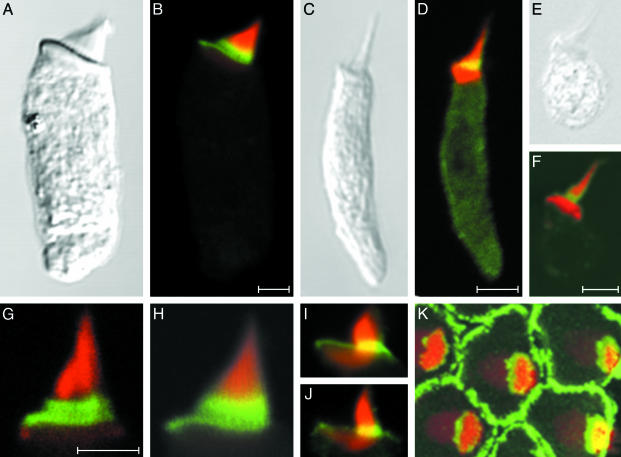
Fig. 3.
Anti-radixin immunolabeling of hair bundles from various vertebrate classes. (A and B) A hair cell isolated from the bullfrog's sacculus displays strong anti-radixin labeling (green) at the base of the hair bundle; the yellow signal results from partial overlap of this labeling with that by fluorescent phalloidin (red). (C and D) A hair cell from the lagena of the zebrafish exhibits anti-radixin labeling at the hair bundle's base. (E and F) Anti-radixin immunolabeling occurs at the hair bundle's base in a hair cell isolated from the utriculus of a mouse. (G and H) Higher-magnification views of two hair bundles from the frog's sacculus demonstrate the most intense anti-radixin labeling at the stereociliary bases. (I and J) In higher-magnification views of hair bundles from short hair cells of the chicken's cochlea, anti-radixin labeling is again strongest at the stereociliary bases. The complex shape of the cuticular plate is also apparent. (K) A surface view of a cluster of short hair cells isolated from the chicken's cochlea demonstrates intense anti-radixin labeling of the supporting cells surrounding each hair cell. An additional immunofluorescence signal is evident at the bases of the stereocilia, which are seen in a stack of 73 images acquired at 0.18-μm intervals. All preparations have additionally been labeled with phalloidin conjugated to Alexa Fluor 568 (red). (Scale bars, 5 μm; bar in B applies also to A; bar in D applies also to C, I, J, and K; bar in F applies also to E; bar in G applies also to H.)
Anti-radixin immunolabeling was occasionally observed at the apical peripheries of avian hair cells, near the sites of their junctional complexes (Fig. 3 I and J). Because the supporting cells that surround each hair cell bear extensive microvilli, we examined patches of sensory epithelium from the chicken's cochlea for labeling of supporting cells. In surface views of such preparations, the narrow apical surfaces of supporting cells were strongly labeled (Fig. 3K). These specimens also demonstrated the absence of anti-radixin labeling on apical hair cell surfaces.
Electron Microscopic Immunocytochemistry. To investigate the distribution of radixin at higher resolution, we conducted electron microscopic immunolabeling of hair bundles from the chicken's cochlea. ImmunoGold particles conjugated to secondary antibodies, which labeled primary antibodies directed against radixin, were concentrated at the bases of the stereocilia (Fig. 4 A and B). Labeling also occurred in the stereociliary tapers themselves. Anti-radixin immunoreactivity declined progressively toward the distal tips of the stereocilia (Fig. 4C). Almost no ImmunoGold particles were encountered on the flattened apical surfaces of the hair cells.
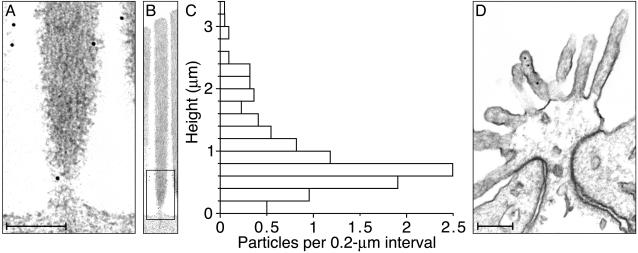
Fig. 4.
Electron microscopic immunolabeling of anti-radixin activity in the chicken's cochlea. (A) A high-magnification electron micrograph depicts the base of a typical stereocilium bearing two ImmunoGold particles, one on the lower shaft and the other on the stereociliary taper. Additional particles adorn the surfaces of two neighboring stereocilia. (B) A lower-magnification micrograph displays the entire stereocilium whose base is depicted in A; the rectangular box indicates the enlarged region. All immunolabeling in this instance is confined to the lowest 0.6 μm of the stereocilium. (C) Each bin of the histogram depicts the average number of particles per hair bundle encountered in 0.2-μm intervals measured axially along the stereocilia from their basal insertions. A total of 227 gold particles was measured from stereocilia in 22 hair bundles ranging in height from 3.0 to 4.2 μm (3.6 ± 0.3 μm, mean ± SD). All stereocilia were oriented within 15° of the plane of section, so the measurement error owing to obliquity was negligible. Because no attempt was made to compensate for shrinkage during specimen preparation, however, the distance measurements are probably underestimates. (D) An electron micrograph of the apical surface of a supporting cell, which is attached to each of the neighboring hair cells by a junctional complex comprising a tight junction (zonula occludens) and an intermediate junction (zonula adherens). Three ImmunoGold particles occur on one microvillus. (Scale bars in A and D, 0.2 μm; the ordinate of the histogram in C calibrates the aligned micrograph in B.)
Consistent with the expected localization of radixin to the submembrane cortex, ImmunoGold particles occurred uniformly near the surfaces of the demembranated stereocilia. Essentially no labeling was encountered within the stereociliary cores. Permeabilization of stereocilia with only 0.1% Triton X-100 failed to remove the plasmalemma completely, perhaps because stereociliary membranes are rich in cholesterol (15) and phosphatidylinositides (16). Preparations treated in this way displayed substantially lower levels of immunolabeling and were not analyzed in detail. When the primary antibody was omitted in control preparations, no ImmunoGold particles were encountered (data not shown).
The hair cells of acousticolateralis organs rarely contact one another (15) but are ordinarily separated by supporting cells. In the cochleae of archosaurs such as birds, however, the apical surfaces of the supporting cells are reduced to narrow strips crowded with microvilli. As anticipated from the light microscopic results, we encountered extensive immunolabeling of these microvilli with antiserum directed against radixin (Fig. 4D). Aside from these structures and the stereocilia, no other organelles were significantly labeled.
Morpholino Experiments. In an attempt to assess the importance of radixin in the development of hair bundles, we used the morpholino “knockdown” technique to interfere with expression of the protein in zebrafish larvae. We injected embryos with morpholinos directed against mRNA transcribed from either of the two putative radixin genes in the zebrafish. To assess the development of functional hair bundles, we analyzed the number of hair cells in neuromasts of the lateral-line system labeled with a fluorescent compound that traverses the transduction channels (14).
Each of the morpholinos reduced the number of labeled neuromasts. In many instances, only two neuromasts could be detected in the posterior lateral-line organ on each side of an animal at 2.5 days of development, when seven pairs are usually present (data not shown). Because the suppression of neuromast development after morpholino injection exceeded that after injection of a mismatched control morpholino at the same concentration, the injection procedure itself was not responsible for the effect. However, morpholino-injected larvae were developmentally delayed in comparison to uninjected or control-injected animals. The interpretation of these experiments is therefore uncertain: although a reduction in radixin expression might suppress hair cell development, the observed retardation might equally stem from the general effect of diminished radixin expression on larval development.
Discussion
The present results indicate that radixin is a ubiquitous constituent of the mechanoreceptive hair bundles of vertebrate hair cells. Immunolabeling of hair cells isolated from fish, amphibians, birds, and mammals disclosed radixin at its highest concentration in the lower shaft of each stereocilium, just above its basal taper. More detailed examination of immunolabeling at the electron microscopic level indicated that, at least for the chicken's hair bundle, radixin also occurs in the tapers themselves. The concentration of radixin does not fall abruptly above the basal shaft; the protein instead diminishes gradually in prevalence toward the stereociliary tip. Radixin appears to be absent from the hair cell's flattened apical surface.
At least in the chicken's cochlea, hair bundles evidently lack ezrin. In the absence of an antibody against moesin, the present results do not directly address the presence of that protein in hair cells. The peptide against which antiserum 1029 was raised differs greatly from the corresponding region in the moesins of other species, however, so immunolabeling with that antiserum was unlikely to have detected any moesin.
In keeping with the role of the ERM proteins in general, radixin in the hair bundle probably links the actin cores of stereocilia to one or more membrane proteins, whose identities are as yet unknown. Radixin may therefore form some of the filamentous connections extending between the stereociliary cytoskeleton and plasmalemma (17). The connection of actin filaments to the surface membrane is of particular importance in the stereociliary taper. As the proteins in each stereocilium turn over, newly synthesized actin monomers are added at its distal end (18). For the stereocilium to maintain a constant length, actin monomers presumably must dissociate at a similar rate from the microfilaments' pointed ends, which largely terminate against the membrane in the stereociliary taper. Radixin may help to anchor actin in this region.
Hair cells are among the body's most mechanically active components: at the base of the human cochlea, for example, each stereocilium may bend as many as 20,000 times per second. It is scarcely surprising that hair bundles, which cannot escape the trauma of repeated flexion, degenerate in many forms of heritable deafness and dysequilibrium. Deficiencies in radixin might be responsible for some of these conditions; the deafness locus DFNB24, for example, occurs near the radixin gene in chromosome region 11q23 (www.uia.ac.be/dnalab/hhh). Mechanical stress is concentrated at the base of each stereocilium, where the miniscule lever pivots about its insertion. Perhaps because of this stress, the stereociliary taper and the region just above it, the bottom of the stereociliary shaft, are endowed with a complex of interacting proteins: harmonin, cadherin 23, myosin VIIA, Sans, and vezatin (19, 20). The presence of radixin in this region implies that the stereociliary base may prove a useful model system in which to examine, as well the functional contribution of radixin and hence of other proteins in the ERM family.
Acknowledgments
We thank S. Balt and Y. Chelliah for performing preliminary experiments; P. Mangeat, F. Solomon, and S. Tsukita for antisera and monoclonal antibodies used in the initial immunohistochemical studies; S. Jani for the injection of morpholinos; E. Fuchs and H. A. Pasolli for access to electron microscopic facilities; A. Sinha and C. J. Starr for cloning a cDNA encoding a portion of the zebrafish radixin gene and assisting with 5-RACE experiments, sequence alignments, and morpholino design; and D. Bozovic, E. Chiappe, P. G. Gillespie, S. Heller, and F. Lesage for providing helpful comments on the manuscript. This research was supported by National Institutes of Health Grant DC00241. A.J.H. is an Investigator of Howard Hughes Medical Institute.
Abbreviation: ERM, ezrin-radixin-moesin.
References
- 1.Tilney, L. G., Tilney, M. S. & DeRosier, D. J. (1992) Annu. Rev. Cell Biol. 8, 257-274. [DOI] [PubMed] [Google Scholar]
- 2.Hudspeth, A. J. & Jacobs, R. (1979) Proc. Natl. Acad. Sci. USA 76, 1506-1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hudspeth, A. J. (1989) Nature 341, 397-404. [DOI] [PubMed] [Google Scholar]
- 4.Gillespie, P. G. & Hudspeth, A. J. (1991) J. Cell Biol. 112, 625-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bretscher, A. (1999) Curr. Opin. Cell Biol. 11, 109-116. [DOI] [PubMed] [Google Scholar]
- 6.Mangeat, P., Roy, C. & Martin, M. (1999) Trends Cell Biol. 9, 187-192. [DOI] [PubMed] [Google Scholar]
- 7.Tsukita, S. & Yonemura, S. (1999) J. Biol. Chem. 274, 34507-34510. [DOI] [PubMed] [Google Scholar]
- 8.Bretscher, A., Chambers, D., Nguyen, R. & Reczek, D. (2000) Annu. Rev. Cell Dev. Biol. 16, 113-143. [DOI] [PubMed] [Google Scholar]
- 9.Winckler, B., Agosti, C. G., Magendantz, M. & Solomon, F. (1994) J. Cell Sci. 107, 2523-2534. [DOI] [PubMed] [Google Scholar]
- 10.Bretscher, A. & Weber, K. (1978) Exp. Cell Res. 116, 397-407. [DOI] [PubMed] [Google Scholar]
- 11.Zidanic, M. & Fuchs, P. A. (1995) Biophys. J. 68, 1323-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lumpkin, E. A. & Hudspeth, A. J. (1995) Proc. Natl. Acad. Sci. USA 92, 10297-10301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He, D. Z. Z., Zheng, J., Edge, R. & Dallos, P. (2000) Hear. Res. 145, 156-160. [DOI] [PubMed] [Google Scholar]
- 14.Starr, C. J., Kappler, J. A., Chan, D. K., Kollmar, R. & Hudspeth, A. J. (2004) Proc. Natl. Acad. Sci. USA 101, 2572-2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobs, R. A. & Hudspeth, A. J. (1990) Cold Spring Harbor Symp. Quant. Biol. 55, 547-561. [DOI] [PubMed] [Google Scholar]
- 16.Horikoshi, T., Yanagisawa, K. & Yoshioka, T. (1984) Proc. Japan Acad. B 60, 157-160. [Google Scholar]
- 17.Hirokawa, N. & Tilney, L. G. (1982) J. Cell Biol. 95, 249-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schneider, M. E., Belyantseva, I. A., Azevedo, R. B. & Kachar, B. (2002) Nature 418, 837-838. [DOI] [PubMed] [Google Scholar]
- 19.Boëda, B., El-Amraoui, A., Bahloul, A., Goodyear, R., Daviet, L., Blanchard, S., Perfettini, I., Fath, K. R., Shorte, S., Reiners, J., et al. (2002) EMBO J. 21, 6689-6699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siemens, J., Kazmierczak, P., Reynolds, A., Sticker, M., Littlewood-Evans, A. & Müller, U. (2002) Proc. Natl. Acad. Sci. USA 99, 14946-14951. [DOI] [PMC free article] [PubMed] [Google Scholar]