Abstract
MicroRNAs (miRNAs) play important roles in tumorigenesis and metastasis. In this study, we investigated miR-200b expression in endometrial adenocarcinomas and normal adjacent tissues and found that miR-200b is more highly expressed in cancer tissues than in normal adjacent tissues. A novel target of miR-200b, tissue inhibitor of metalloproteinase 2 (TIMP2), was predicted using a bioinformatics approach and was confirmed in human endometrial cancer cell line HEC-1A cells by luciferase assay, quantitative real-time polymerase chain reaction, western blotting, and enzyme-linked immunosorbent assay. We found that miR-200b repressed TIMP2 expression at both the messenger RNA and protein levels, although a family member, miR-200a, had no such effect. Using reverse gelatin zymography, we showed that miR-200b enhances matrix metallopeptidase 2 (MMP2) activity by downregulating TIMP2 expression in HEC-1A cells. These data suggest that miR-200b may play an important role in the metastasis of endometrial adenocarcinomas.
Introduction
MicroRNAs (miRNAs) are ∼22 nucleotide (nt) small, non-coding RNAs that post-transcriptionally regulate gene expression. An miRNA molecule is usually transcribed by RNA polymerase 2 as a primary miRNA transcript (Lee et al., 2004), which is sequentially cleaved by the Drosha–Pasha complex to generate a ∼70-nt stem-loop precursor miRNA (pre-miRNA) (Lee et al., 2003). Pre-miRNAs are exported to the cytoplasm by Exportin-5 and processed by Dicer to liberate mature miRNAs (Yi et al., 2003; Lund et al., 2004). Mature miRNA associates with Argonaute proteins to form a RNA-induced silencing complex and directs binding of this complex to the 3′UTR of target messenger RNAs (mRNAs), resulting in either the degradation of target mRNAs or their translational inhibition (BARTEL, 2004; Gregory et al., 2005).
MiRNAs play important roles in carcinogenesis and have been the focus of much research in this area. First, miRNA expression varies in different tumor types, and the specificity of miRNA expression has led to their use as biomarkers for cancer diagnosis and prognosis (Chang et al., 2012; Liu et al., 2012). Second, miRNAs are involved in many important cellular processes such as proliferation (Zhang et al., 2012), apoptosis (Frank et al., 2012), differentiation (Dill et al., 2012), metabolism (Zhu et al., 2011), and tumor metastasis (Li et al., 2011), and therefore inhibition of oncogenic miRNAs or over-expression of tumor suppressor miRNAs may provide novel anti-cancer therapies. Third, tumorigenesis is a complex process involving the deregulation of many genes, and therefore miRNA research will advance our knowledge of the molecular mechanisms that control tumorigenesis and cancer development.
Endometrial cancer is the most common gynecological malignancy, with an estimated 46,470 new cases being reported in the United States alone in 2011 (Siegel et al., 2011). The development of endometrial cancer is a complex process involving many oncogenes and tumor suppressor genes, although the molecular mechanisms are not clear. In recent years, the expression and function of miRNAs in endometrial cancer have been widely investigated. Boren et al. measured miRNA expression in endometrial cancer, normal endometrium, and atypical hyperplasia samples, and identified 13 miRNAs (p<0.02) associated with endometrial cancer development (Boren et al., 2008). Of these, 5 miRNAs were upregulated (let-7i, miR-221, miR-193, miR-152, and miR-30c) and 8 were downregulated (miR-185, miR-106a, miR-181a, miR-210, miR-423, miR-103, miR-107, and let-7c). Other miRNAs, such as miR-152 (Tsuruta et al., 2011), miR-125b (Jiang et al., 2011), and miR-34b (Hiroki et al., 2011), have also been reported to play important roles in endometrial cancer development.
We previously used microarrays to analyze differences in miRNA expression between endometrial adenocarcinomas and normal tissue (Xia et al., 2009), and found that of the 111 miRNAs exhibiting changes in expression, 69 of them were upregulated, while 43 of them were downregulated. Among these changed miRNAs, miR-200b exhibited the most significant difference. Other miRNAs identified in our microarray analysis included the previously identified miR-221, miR-193, miR-106a, miR-103/107, and miR-199a. In the present study, we continued our investigation into the function of miR-200b in endometrial adenocarcinoma development by comparing miR-200b expression in cancer and normal adjacent tissues and observed specific miR-200b overexpression in cancer tissues. We transfected a miR-200a and miR-200b mimics into the human endometrial cancer cell line, HEC-1A, and used a range of assays, including reporter assays, quantitative real-time PCR (qRT-PCR), western blotting, enzyme-linked immunosorbent assay (ELISA), and reverse gelatin zymography, to demonstrate that miR-200b reduces tissue inhibitor of metalloproteinase 2 (TIMP2) expression and enhances matrix metallopeptidase 2 (MMP2) activity.
Materials and Methods
Patients and samples
All samples were provided by Beijing Obstetrics and Gynecology Hospital. Patients received no treatment before surgery and signed informed consent for sample collection. Clinicopathologic background information was available for all paients/samples (Table 1).
Table 1.
Clinicopathologic Background of the Patients Used in This Study
| Number | Cancer tissues | Normal adjacent tissues | |
|---|---|---|---|
| Age (years) | |||
| <60 | 4 | 2 | 2 |
| ≥60 | 7 | 3 | 4 |
| Stage | |||
| 1 | 0 | 0 | 0 |
| 2 | 2 | 1 | 1 |
| 3 | 9 | 4 | 5 |
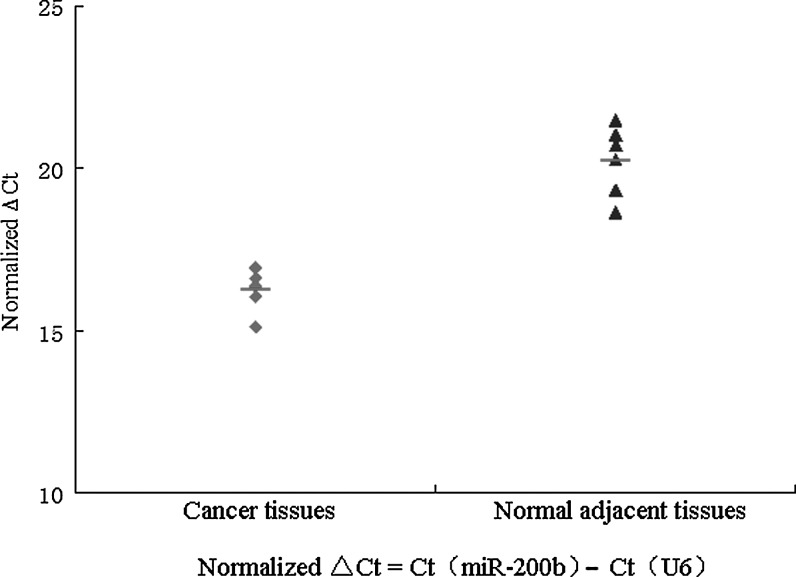
| Average ΔCt | 16.214 | 20.232 | |
ΔCt=Ct (miR-200b) – Ct (U6).
Plasmid construction
The wild-type 3′ untranslated region (3′UTR) of the TIMP2 gene containing predicted miR-200b target sites was amplified by PCR from HEC-1A cell genomic DNA and cloned into a modified pGL3-control plasmid (Promega) downstream of the firefly luciferase coding region between the PstI and EcoRI sites, as previously described (Cui et al., 2007).
Cell culture and transfection
HEC-1A cells purchased from Peking Union Medical College were grown in Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal bovine serum (FBS) and 100 μg/mL penicillin and streptomycin (Gibco). Synthetic miRNA mimics and negative control (NC) siRNAs were synthesized by Ribobio and miRNA transfection was performed using Lipofectamine 2000 (Invitrogen). Cells were seeded into 6-well plates at a density of 2×105 cells per well. For each well, 5 μL miRNA or NC (100 pmol) was added to a 250-μL aliquot of DMEM, and 5 μL of Lipofectamine 2000 was added to a second aliquot of 250 μL DMEM. The miRNA and lipofectamine solutions were mixed together and incubated for 20 minutes. The mixture was then added to the cells and incubated for 4 hours before replacing the medium with fresh DMEM. Total RNA and protein were prepared after 48 hours and used for qRT-PCR or western blotting analysis respectively. The miRNA mimic and NC sequences were (miR-200a was anthor control sequence): miR-200a sense, 5′-UAACACUGUCUGGUAACGAUGU −3′; miR-200a anti-sense, 5′-AUCGUUACCAGACAGUGUAAAU −3′; miR-200b sense, 5′-UAAUACUGCCUGGUAAUGAUGA-3′; miR-200b anti-sense, 5′-AUCAUUACCAGGCAGUAUAAAU-3′; NC sense, 5’-UUCUCCGAACGUGUCACGUTT-3’ and NC anti-sense, 5’-ACGUGACACGUUCGGAGAATT-3’
Reporter assays
HEC-1A cells were plated into 24-well plates and transfected with 100 ng pGL-3 control plasmid, 10 ng pRL-CMV plasmid (Promega), and 1 μL (20 pmol) miR-200a or miR-200b mimic or NC siRNA using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. Cells were harvested 48 hours after transfection and reporter activity was measured using a dual-luciferase reporter assay (Promega).
RNA extraction and qRT-PCR
Total RNA was extracted from HEC-1A cells using TRI Reagent (Sigma-Aldrich), and complementary DNA was made using M-MLV reverse transcriptase (Promega), according to the manufacturer's protocol. qRT-PCR was performed with an Mx3000p (Stratagene) and a SYBR Green I kit (Toyobo Life Science), using the method described in Fu et al., 2006. Beta-actin was used for normalization. Primers were: β-actin forward, 5′-TGAAGTGTGACGTGGACATCCGC-3′; β-actin reverse, 5′-GCCAATCTCATCTTGTTTTCTGCGC-3′; TIMP2 forward, 5′- ATTTGACCCAGAGTGGAACG-3′; TIMP2 reverse, 5′- AGACCAACGTGTGTGGATCA-3′; miR-200b forward, 5′-TAATACTGCCTGGTAATGATGA-3′; miR-200b reverse, 5′- GCGAGCACAGAATTAATACGAC-3′; U6 forward, 5′- CGCTTCGGCAGCACATATACTA-3′; U6 reverse, 5′- CGCTTCACGAATTTGCGTGTCA-3′.
Western blotting
Cells were washed, harvested in ice-cold phosphate-buffered saline, centrifuged at 2,000 rpm at 4°C, and then lysed for 30 minutes on ice in radio immunoprecipitation assay buffer in the presence of protease inhibitor cocktail (Sigma-Aldrich). Soluble proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to Hybond-electrochemiluminescence membrane (GE Healthcare). Specific proteins were detected using standard techniques with β-actin (Santa Cruz Biotechnology) and TIMP2 (Santa Cruz Biotechnology) antibodies and visualized with a SuperSignal West Femto Maximum Sensitivity Substrate Trial Kit (Thermo Fisher scientific).
ELISA
After transfection (24 hours), culture medium was replaced with fresh DMEM and cells were incubated for a further 24 hours. After this time, the culture medium was removed and centrifuged at 2,000 rpm at 4°C for 5 minutes, and clarified medium was analyzed using a TIMP2 ELISA kit (Uscn Life Science Inc.) according to the manufacturer's protocol.
Reverse gelatin zymography
HEC-1A cells were seeded into 6-well plates at a density of 2×105 cells per well in DMEM containing 10% FBS, incubated for 16 hours, and then transfected with miR-200a, miR-200b, or NC. After 24 hours, the medium was replaced with fresh DMEM lacking FBS. After a further 24 hours, the culture medium was collected and clarified at 12,000 rpm at 4°C, and stored at −70°C. TIMP2 activity was measured using a TIMP reverse gelatin-zymography kit (Genmed Scientifics Inc.) according to the manufacturer's instructions.
Wound healing assay
HEC-1A cells were treated with miR-200a, miR-200b, and NC for 24hours, then a sterile pipette tip was dragged across the plate to create a cell-free area and photographed using an inverted microscope fitted with a CCD camera (Olympus IX71). At 24 hours and 48 hours after wounding, the plate was photographed again to document cell migration across the wound line.
Transwell assay
The cells were serum starved for 24 hours and then seeded in serum-free medium placed into the upper chamber of the insert (Corning). After 12-hour incubation, cells remaining in the upper chamber were carefully removed. Cells adhering to the lower membrane were fixed with methanol for 20 minutes at −20 °C, stained with Giemsa or crystal violet, and evaluated.
MicroRNA target prediction software
TargetScan Release 5.1 (www.targetscan.org) was used for target prediction.
Results
MiRNA-200b is over-expressed in endometrial adenocarcinomas
We previously reported that out of 111 miRNAs with differential expression, miR-200b exhibited the greatest difference in expression between endometrial adenocarcinoma and normal adjacent tissues. Here we examined miR-200b expression in 5 cancer tissues and 6 normal adjacent tissues and found that miR-200b expression was higher in cancer tissues than in normal adjacent tissues (Fig. 1).
FIG. 1.
miR-200b was detected by quantitative real-time polymerase chain reaction (qRT-PCR) in cancer tissues and paracancer tissues. Cancer tissues showed a higher level of miR-200b than paracancer tissues.
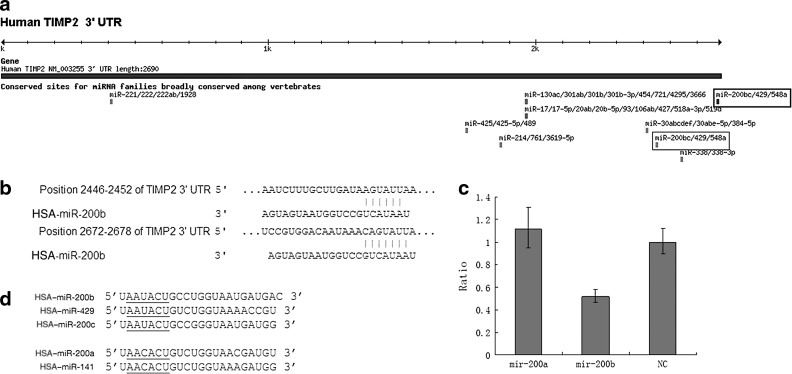
TIMP2 is a novel miR-200b target
Although miR-200a and miR-200b both belong to the miR-200 family, they have different target genes because of sequence differences in their seed regions (Fig. 2d). The 3′UTR of TIMP2 mRNA contains 2 elements with complementarity to the miR-200b seed region (Fig. 2a, b). To investigate whether TIMP2 mRNA is regulated by miR-200b, the 3′UTR of TIMP2 was cloned into a modified pGL-3 control vector, downstream of the luciferase coding sequence, and the activity of this reporter plasmid was assayed in HEC-1A cells in the presence of miR-200a or miR-200b mimics or NC siRNA. We observed that only miR-200b overexpression significantly reduced the reporter activity (Fig. 2c).
FIG. 2.
(a, b) There are 2 elements with complementarity to the miR-200b seed region. (c) Luciferase reporter assays indicated that miR-200b regulates tissue inhibitor of metalloproteinase 2 (TIMP2) expression by targeting the 3’UTR, while miR-200a and negative (NC) could not. (d) The miR-200 family can be grouped into two subfamilies according to their function: mir-200b, mir-200c and mir-429/mir-200a and mir-141.
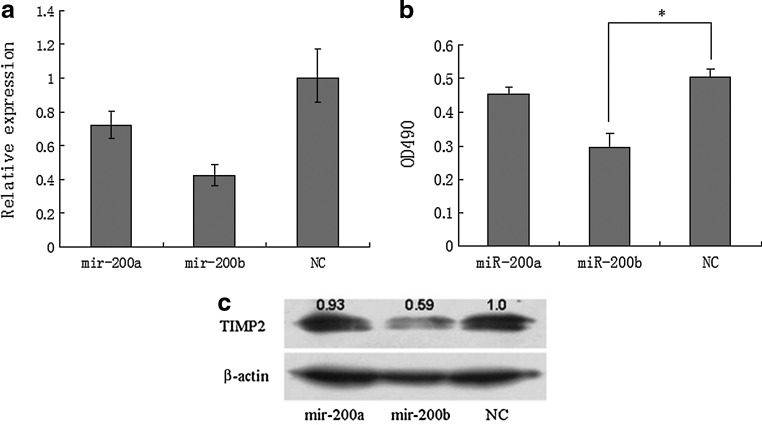
MiRNA-200b downregulates TIMP2 expression
Having confirmed that miR-200b interacts with the TIMP2 3′UTR, we next assessed the capability of miR-200b to regulate endogenous TIMP2 expression. miR-200a or miR-200b mimics were transfected into HEC-1A cells, and qRT-PCR and western blotting were used to measure the levels of TIMP2 mRNA and protein respectively. Since TIMP2 is a secreted protein, we analyzed its expression in culture medium. We observed that TIMP2 mRNA expression was significantly reduced only in cells transfected with the miR-200b mimic (Fig. 3a). Similarly, TIMP2 protein expression (Fig. 3b, c) was specifically reduced following transfection of the miR-200b mimic.
FIG. 3.
miR-200a or miR-200b mimics were transfected into human endometrial cancer cell line HEC-1A cells. (a) qRT-PCR assay showed TIMP2 mRNA expression was significantly reduced only in cells transfected with the miR-200b mimic. (b, c) Enzyme-linked immunosorbent assay and western blot assay showed TIMP2 protein expression was specifically reduced following transfection of the miR-200b mimic. *P=0.05.
MiR-200b enhanced MMP2 activity
Having confirmed that TIMP2 protein and mRNA are downregulated by miR-200b, we next performed reverse gelatin-zymography to detect changes in TIMP2-mediated inhibition of MMP2. In cells transfected with miR-200b, MMP2 was active, whereas MMP2 was inhibited in cells transfected by miR-200a or NC (Fig. 4). We conclude that miR-200b enhances MMP2 activity by downregulating TIMP2 expression in HEC-1A cells.
FIG. 4.
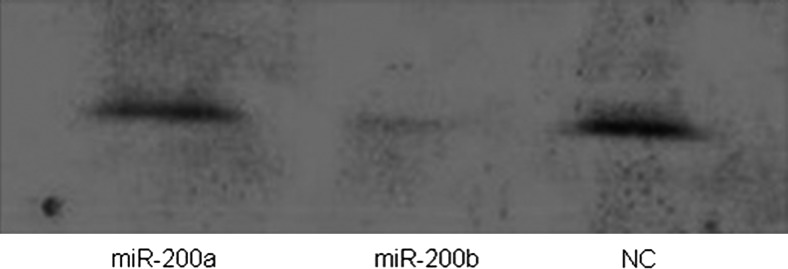
Reverse gelatin-zymography assay was performed to detect TIMP2-mediated inhibition of matrix metallopeptidase 2 (MMP2). The MMP2 was active only in cells transfected with miR-200b.
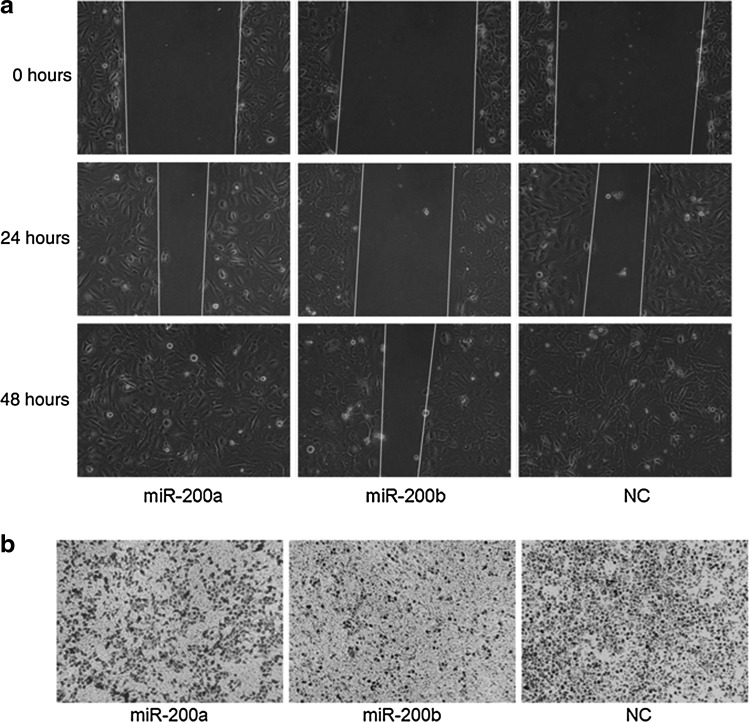
miR-200b inhibited migration in HEC-1A cells
It has been reported that miR-200b could inhibit migration in many kinds of cancer cells in vitro (Sossey-alaoui et al., 2009), thus prompting us to determine whether miR-200b could also inhibit migration in HEC-1A cells. As shown in Fig. 5a, in cells transfected with miR-200b displayed a significant reduction in migration ability compared with cells transfected with miR-200a or NC. The transwell assay also demonstrated that overexpressing miR-200b could inhibit migration in HEC-1A cells (Fig. 5b).
FIG. 5.
miR-200a, miR-200b, and NC were transfected into HEC-1A cells; wound healing assay (a) and transwell assay (b) showed that cells transfected with miR-200b displayed a significant reduction in migration ability compared with cells transfected with miR-200a or NC.
Discussion
MiRNAs are involved in cancer initiation and development through transcriptional or post-transcriptional regulation of target genes mediated by the imperfect paring of miRNA seed sequences with target mRNAs. Depending on the function of their targets, miRNAs can act as either tumor suppressors or oncogenes. The number of human miRNAs reported to date (miRBase release 17) is in excess of 1,400, and many are located in cancer-associated genome regions or in fragile sites (Calin et al., 2004).
MMP2 is an enzyme that degrades type 4 collagen, the major structural component of basement membranes. This enzyme plays a role in endometrial menstrual breakdown (Brun et al., 2009), regulation of vascularization (Chau et al., 2007), and tumor metastasis (Kawamate et al., 1998). As a natural inhibitor of matrix metalloproteinases, especially of MMP2, TIMP2 is thought to be a suppressor of metastasis. Active MMP2 is the main form expressed in endometrial cancer, and its expression in adenocarcinoma tissue correlates with histologic tumor grade and invasion or metastasis (Guo et al., 2002). Graesslin et al. reported that high MMP2 expression and low TIMP-2 expression are the most potent markers for endometrial malignancies with a high risk of local and distant metastasis (Graesslin et al., 2006).
Recently, the mir-200 family has been reported as a powerful marker and determining factor of the epithelial phenotype of cancer cells (Park et al., 2008). By targeting the E-cadherin transcriptional repressors ZEB1 and ZEB2, the mir-200 family could regulate the epithelial to mesenchymal transition and protect tumor cells from apoptosis (Gregory et al., 2008). Numerous studies have demonstrated that members of the mir-200 family are abnormally expressed in hepatocellular tumors (Ladeiro et al., 2008), ovarian cancer (Nam et al., 2008), and gastric cancer (Du et al., 2009), and the mir-200 family inhibits migration and invasion by targeting ZEB1, ZEB2, and WASF3 in many kinds of cancer cells (Sossey-alaoui et al., 2009).
In this study, we investigated miR-200b expression in endometrial adenocarcinomas and normal adjacent tissues. We found that miR-200b expression is higher in cancer tissues than in normal adjacent tissues. We identified TIMP2 as a novel miR-200b target and used a range of assays to show that miR-200b specifically represses TIMP2 expression at both the mRNA and protein levels. In contrast, overexpression of the family member, miR-200a, which has the different seed region, had no effect on TIMP2 mRNA and protein expression. Furthermore, miR-200b specifically enhances MMP2 activity in HEC-1A cells by downregulating TIMP2 expression.
In conclusion, we have demonstrated that miR-200b is overexpressed in endometrial adenocarcinomas and that this miRNA specifically inhibits TIMP2 expression and enhances MMP2 activity in HEC-1A cells. MiR-200b, together with TIMP2 and MMP2, may therefore play an important role in the initiation and development of endometrial adenocarcinomas. Our future research will explore the causes of abnormal miR-200b expression and additional miR-200b functions in endometrial adenocarcinoma.
Acknowledgments
We thank the staff of our group for their assistance. This work was supported by grants from the Major State Basic Research Development Program of China (973 program) (nos. 2011CB811300, 2010CB912801) and the Natural Science Foundation of China (project nos. 30772322, 31100569, 30971630).
Author Disclosure Statement
No competing financial interests exist.
References
- BARTEL D.P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:287–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- BOREN T. XIONG Y. HAKAM A., et al. MicroRNAs and their target messenger RNAs associated with endometrial carcinogenesis. Gynecol. Oncol. 2008;110:206–215. doi: 10.1016/j.ygyno.2008.03.023. [DOI] [PubMed] [Google Scholar]
- BRUN J.L. GALANT C. DELVAUX D., et al. Menstrual activity of matrix metalloproteinases is decreased in endometrium regenerating after thermal ablation. Hum. Reprod. 2009;24:333–340. doi: 10.1093/humrep/den392. [DOI] [PubMed] [Google Scholar]
- CALIN G.A. SEVIGNANI C. DUMITRU C.D., et al. Human microRNA genes are frequently located at fragile sites and genomic regions involved in cancers. Proc. Natl. Acad. Sci. U. S. A. 2004;101:2999–3004. doi: 10.1073/pnas.0307323101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANG H. ZHOU X. WANG Z.N., et al. Increased expression of miR-148b in ovarian carcinoma and its clinical significance. Mol. Med. Report. 2012;5:1277–1280. doi: 10.3892/mmr.2012.794. [DOI] [PubMed] [Google Scholar]
- CHAU K.Y. SIVAPRASAD S. PATEL N., et al. Plasma levels of matrix metalloproteinase-2 and -9 (MMP-2 and MMP-9) in age-related macular degeneration. Eye. (Lond.) 2007;21:1511–1515. doi: 10.1038/sj.eye.6702722. [DOI] [PubMed] [Google Scholar]
- CUI J. FU H. FENG J., et al. The construction of miRNA expression library for human. Prog. Biochem. Biophys. 2007;34:389–394. [Google Scholar]
- DILL H. LINDER B. FEHR A., et al. Intronic miR-26b controls neuronal differentiation by repressing its host transcript, ctdsp2. Genes Dev. 2012;26:25–30. doi: 10.1101/gad.177774.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DU Y. XU Y. DING L., et al. Down-regulation of miR-141 in gastric cancer and its involvement in cell growth. J. Gastroenterol. 2009;44:556–561. doi: 10.1007/s00535-009-0037-7. [DOI] [PubMed] [Google Scholar]
- FRANK D. GANTENBERG J. BOOMGAARDEN I., et al. MicroRNA-20a inhibits stress-induced cardiomyocyte apoptosis involving its novel target Egln3/PHD3. J. Mol. Cell Cardiol. 2012;52:711–717. doi: 10.1016/j.yjmcc.2011.12.001. [DOI] [PubMed] [Google Scholar]
- FU H. ZHU J. YANG M., et al. A novel method to monitor the expression of microRNAs. Mol. Biotechnol. 2006;32:197–204. doi: 10.1385/MB:32:3:197. [DOI] [PubMed] [Google Scholar]
- GRAESSLIN O. CORTEZ A. FAUVET R., et al. Metalloproteinase-2, -7 and -9 and tissue inhibitor of metalloproteinase-1 and -2 expression in normal, hyperplastic and neoplastic endometrium: a clinical-pathological correlation study. Ann. Oncol. 2006;17:637–645. doi: 10.1093/annonc/mdj129. [DOI] [PubMed] [Google Scholar]
- GREGORY P.A. BERT A.G. PATERSON E.L., et al. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat. Cell Biol. 2008;10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- GREGORY R. CHENDRIMADA T. COOCH N., et al. Human RISC couples microRNA biogenesis and posttranscriptional gene silencing. Cell. 2005;123:631–640. doi: 10.1016/j.cell.2005.10.022. [DOI] [PubMed] [Google Scholar]
- GUO W. CHEN G. ZHU C., et al. Expression of matrix metalloproteinase-2, 9 and it's tissue inhibitor-1, 2 in endometrial carcinoma. Zhonghua Fu Chan Ke Za Zhi. 2002;37:604–607. [PubMed] [Google Scholar]
- HIROKI E. SUZUKI F. AKAHIRA J.I., et al. MicroRNA-34b functions as a potential tumor suppressor in endometrial serous adenocarcinoma. J. Cancer. 2011 doi: 10.1002/ijc.27345. [DOI] [PubMed] [Google Scholar]
- JIANG F. LIU T. HE Y., et al. MiR-125b promotes proliferation and migration of type II endometrial carcinoma cells through targeting TP53INP1 tumor suppressor in vitro and in vivo. BMC Cancer. 2011;11:425. doi: 10.1186/1471-2407-11-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAWAMATA H. UCHIDA D. HAMANO H., et al. Active-MMP2 in cancer cell nests of oral cancer patients: correlation with lymph node metastasis. Int. J. Oncol. 1998;13:699–704. [PubMed] [Google Scholar]
- LADEIRO Y. COUCHY G. BALABAUD C., et al. MicroRNA profiling in hepatocellular tumors is associated with clinical features and oncogene/tumor suppressor gene mutations. Hepatology. 2008;47:1955–1963. doi: 10.1002/hep.22256. [DOI] [PubMed] [Google Scholar]
- LEE Y. AHN C. HAN J., et al. The nuclear RNase III Drosha initiates microRNA processing. Nature. 2003;425:415–419. doi: 10.1038/nature01957. [DOI] [PubMed] [Google Scholar]
- LEE Y. KIM M. HAN J., et al. MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 2004;23:4051–4060. doi: 10.1038/sj.emboj.7600385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI X. ZHANG Y. ZHANG H., et al. miRNA-223 promotes gastric cancer invasion and metastasis by targeting tumor suppressor EPB41L3. Mol. Cancer Res. 2011;9:824–833. doi: 10.1158/1541-7786.MCR-10-0529. [DOI] [PubMed] [Google Scholar]
- LIU C.J. LIN S.C. YANG C.C., et al. Exploiting salivary miR-31 as a clinical biomarker of oral squamous cell carcinoma. Head Neck. 2012;34:219–224. doi: 10.1002/hed.21713. [DOI] [PubMed] [Google Scholar]
- LUND E. GUTTINGER S. CALADO A., et al. Nuclear export of microRNA precursors. Science. 2004;303:95–98. doi: 10.1126/science.1090599. [DOI] [PubMed] [Google Scholar]
- NAM E.J. YOON H. KIM S.W., et al. MicroRNA expression profiles in serous ovarian carcinoma. Clin. Cancer Res. 2008;14:2690–2695. doi: 10.1158/1078-0432.CCR-07-1731. [DOI] [PubMed] [Google Scholar]
- PARK S.M. GAUR A.B. LENGYEL E., et al. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev. 2008;22:894–907. doi: 10.1101/gad.1640608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIEGEL R. NAISHADHAM D. JEMAL A. Cancer statistics, 2011. CA Cancer J. Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- SOSSEY-ALAOUI K. BIALKOWSKA K. PLOW E.F., et al. The miR200 family of microRNAs regulates WAVE3-dependent cancer cell invasion. J. Biol. Chem. 2009;284:33019–33029. doi: 10.1074/jbc.M109.034553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSURUTA T. KOZAKI K. UESUGI A., et al. miR-152 is a tumor suppressor microRNA that is silenced by DNA hypermethylation in endometrial cancer. Cancer Res. 2011;71:6450–6462. doi: 10.1158/0008-5472.CAN-11-0364. [DOI] [PubMed] [Google Scholar]
- XIA L. WEI X. YINMEI D., et al. Expression of microRNAs in endometrioid adenocarcinoma. Natl. Med. J. China. 2009;89:1365–1367. [PubMed] [Google Scholar]
- YI R. QIN Y. MACARA I.G., et al. Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes Dev. 2003;17:3011–3016. doi: 10.1101/gad.1158803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG B.G. LI J.F. YU B.Q., et al. microRNA-21 promotes tumor proliferation and invasion in gastric cancer by targeting PTEN. Oncol. Rep. 2012;27:1019–1026. doi: 10.3892/or.2012.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHU H. SHYH-CHANG N. SEGRÈ A.V., et al. The Lin28/let-7 axis regulates glucose metabolism. Cell. 2011;147:81–94. doi: 10.1016/j.cell.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]