Abstract
The aetiology of status asthmaticus (SA), a complication of severe asthma, is unknown. Fungal exposure, as measured by fungal atopy, is a major risk factor for developing asthma, but the relationship of fungi in SA per se has not previously been reported. In this five patient retrospective case series study, lower respiratory tract cultures were performed on bronchoalveolar lavage or tracheal aspirate fluid, comparing standard clinical laboratory cultures with a specialized technique in which respiratory mucus was removed prior to culture. We show mucolytic treatment allows increased detection of fungal growth, especially yeast, from the lower airways of all SA patients. We also demonstrate that the yeast Candida albicans inhalation readily induces asthma-like disease in mice. Our observations suggest, SA may represent a fungal infectious process, and supports additional prospective studies utilizing anti-fungal therapy to supplement conventional therapy, broad-spectrum antibiotics and high-dose glucocorticoids, which can promote fungal overgrowth.
Keywords: status asthmaticus, tracheobronchial, candidiasis, asthma, allergic
1. Introduction
Status Asthmaticus (SA), also referred to as acute severe asthma or severe asthma exacerbation, is a relatively rare, pre-morbid complication of asthma that requires aggressive medical treatment, frequently in an intensive care unit setting and systemic immunosuppression using glucocorticoids. Unlike conventional asthma attacks, the major symptom of SA is profound dyspnea that responds poorly to therapy. As with conventional asthma, SA is thought to be induced through inhalational exposure to common allergens derived from moulds, pollens, and environmental agents such as dust mites and insects. Elevated levels of airborne fungal conidia have previously been linked to increased asthma deaths and emergency room visits, indicating a potentially critical role for fungal allergens in SA [1,2]. Moreover, fungal allergy as determined by sensitization to hyphal and yeast forms is associated with both asthma prevalence and severity [3,4]. Finally, fungal sensitive asthmatics and allergic broncho pulmonary aspergillosis patients have been should to benefit from antifungal therapy [5,6].
In addition to fungal atopy contributing to disease expression, additional evidence suggests that fungal infection may also contribute to the pathogenesis of asthma. We have recently demonstrated that numerous environmental fungi are capable of infecting the mouse airway and inducing allergic disease that resembles asthma [7,8]. However, whereas both filamentous and yeast fungal species are frequently isolated from the airways of patients with allergic bronchopulmonary mycosis, the relationship of fungal infection to SA remains obscure. In this retrospective case-series study, fungal cultures of lower respiratory tract specimens from five consecutive patients with SA were performed to determine if fungal airway infection is observed. We compared standard hospital fungal culture procedures to a procedure utilizing respiratory mucolysis prior to culturing at both room and body temperature. Finally, we exposed mice by inhalation to a clinical isolate of Candida albicans, a yeast isolated from all 5 patients in this study, to determine its allergenic potential.
2. Methods
2.1 Patients
From September 2010 to September 2011, one of the study physicians identified five consecutive patients who were admitted to the Ben Taub General Hospital (BTGH) medical intensive care unit with a primary admission diagnosis of SA (Table 1). Patients included consecutive adult men and women with a prior history of asthma admitted with respiratory failure requiring endotracheal intubation and mechanical ventilation. All patients underwent sampling of the lower airway secretions by bronchoalveolar lavage (BAL) or tracheal aspiration. Patient information was retrospectively obtained by the authors with permission obtained through the Baylor College of Medicine Institutional Review Board.
Table 1.
Patient Characteristics
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | |
|---|---|---|---|---|---|
| Age/Sex | 36/M | 32/F | 66/F | 44/F | 31/M |
| Ethnicity | Caucasian | American Indian | African American | African American | Hispanic |
| Asthma History | Yes | Yes | Yes | Yes | Yes |
| Comorbidities | HIV (CD4 291), DM (unknown HgA1C), obesity (BMI 39·7) | Seasonal allergies, obesity (BMI 30·9) | DM (HgA1C 6·5), MGUS, anemia, depression | Cocaine abuse, MJ abuse, GERD, noncompliance | Tobacco abuse |
| Asthma medications at admission | Albuterol MDI & neb, tiotropium, montelukast | Albuterol MDI | Albuterol MDI & neb, ipratropium neb, fluticasone- salmeterol, montelukast | Albuterol MDI & neb, budesonide- formoterol, loratadine | Albuterol MDI & neb |
| IgE (IU/mL) | 889 | 2702 | 795 | 798 | 76 |
| Blood absolute eosinophils | 0 | 1250 | 430 | 480 | 170 |
| Aspergillus fumigatus IgE (kU/L) | - | <0.05 | 0.14 | 0.17 | - |
2.2 Microbiology of lower respiratory tract specimens
Lower airway samples (by BAL, n=3; by tracheal aspirate, n=2) were processed using standard culture techniques in the BTGH microbiology laboratory which involves inoculating fungal culture tubes with unprocessed specimens and culturing at 30°C for 7 days followed by 25°C for 8 days. In parallel to the hospital cultures, 5 mL of the same samples from patients 2 through 5 underwent specialized processing to remove mucus and cells prior to culture. Prior to mucolysis, approximately 50 μl of liquid samples containing solid debris was applied to a glass slide and pressed under a glass cover slip for light microscopic examination for the presence of fungal elements. Dithiothreitol was added to all samples to a concentration of 50mM followed by vigorous vortexing for five minutes to solubilize mucus. The samples were centrifuged at 10,000 × g for 15 minutes and the entire process repeated until the pellet was free of visible mucus. After removal of the fluid, the pellet was suspended in 1 mL of phosphate buffered saline (PBS) and 0.25 mL of the prepared suspension was cultured onto 2 Sabourand’s fungal media plates, supplemented with 50mg/L ampicillin; one plate was cultured at 25C and one at 37C. Plates were checked every 24 hours for signs of fungal growth and microorganisms were identified by the authors with confirmation by The Fungus Testing Laboratory at The University of Texas Health Science Center (San Antonio, Texas, USA).
2.3 Preparation of C. albicans
A confirmed clone of C. albicans isolated from patient 4 was propagated in Sabourand’s media broth supplemented with 50mg/L ampicillin. Yeast were collected from the broth and washed four times with PBS by centrifugation (4,000 × g 5 min.). The washed yeast were enumerated on a hemocytometer and the concentration of yeast adjusted to 160 × 106 yeast per ml using PBS. Individual aliquots were prepared and snap frozen in liquid nitrogen. Subsequently, viability of the preps were confirmed to be >90% by culturing on Sabourand’s plates. Heat killed preps were prepared by incubating viable cultures at 95°C for 1 h follow by storage in liquid nitrogen. Yeast killing was confirmed by failure to grow on Sabourand’s media.
2.4 Mice and assessment of asthma phenotype
Female C57BL/6 mice were used between four to six weeks of age according to Baylor College of Medicine institutional guidelines. Mice were challenged with PBS alone, 25,000,viable, or 25,000 dead C. albicans cells intranasally three days per week for a total of 8 exposures. Twenty-four hours after the last intranasal administration of yeast, bronchial responsiveness to acetylcholine (AcH) intravenous challenge, BAL fluid cellularity, and the total numbers of IL-4, IFN-γ, and IL-17A secreting cells from lung homogenate were determined as previously described [9,10].
2.4 Statistical Analysis
Data are presented as means ± standard error of the mean. One-way ANOVA with Bonferroni multiple group post test was used to analyze ELISpot and BAL differential count data. Airway physiology was analyzed using two-way ANOVA with repeated measures test for each acetyl-choline dose and Bonferroni multiple group post test.
3. Results
3.1. Status asthmaticus case series data
Table 1 summarizes demographic and prior medication data on the five patients. All patients presented to the emergency room as outpatients with acute respiratory complaints and were self-administering inhaled beta 2 agonist medications for asthma prior to admission. No patient had a history of eosinophilic lung disease other than asthma or atopy. Four patients were either prior or current smokers. Comorbidities varied and included HIV (CD4 count 291), obesity, type 2 diabetes mellitus, noncompliance, and drug abuse. Patients 3 and 4 had been taking inhaled glucocorticoids prior to admission. No patients were taking oral glucocorticoids or had a history of recent or chronic use of such medications at the time of admission. None of the patients were on chronic therapy with immunosuppressive or antibacterial agents. Four of the five patients manifested elevated IgE levels and patients 2 and 4 had elevated serum eosinophilia. Two of three tested patients expressed IgE antibodies to Aspergillus fumigatus.
Table 2 summarizes the therapies and days utilized during the treatment of each patient during their hospitalization including fungal culture observations are also included. Prior to ICU admission, all patients were initiated on intravenous corticosteroids, inhaled short-acting bronchodilators, and oxygen therapy. The patients failed conservative medical therapy as evidenced by persistent symptoms and signs of SA and all underwent endotracheal intubation. In addition to being continued on systemic corticosteroids and inhaled bronchodilators, the patients were placed on empiric antibacterial therapy upon admission to the ICU. The post-intubation chest roentgenograms of all patients demonstrated no infiltrates or other evidence of pulmonary infection. Lower respiratory tract cultures were obtained by protected sampling via tracheal fluid or BAL fluid aspiration during the time of intubation within seven days of the initiation of antibiotics and corticosteroids. Of the two patients who had differential counts performed on BAL fluid, neither had evidence of eosinophilia. Upon positive fungal culture results, four patients received antifungal antibiotic therapy prior to extubation. The exception was patient 4 who received no antifungal therapy during his hospital stay. The duration of mechanical ventilation varied, ranging from 5 to 30 days. Patients 1, 2, 3, and 5 received antifungal treatment, resulting in removal of mechanical ventilation within 17, 3, 1, and 6 days post antifungal initiation, respectively. Patient 1 had a prolonged hospital course secondary to difficulty with weaning from the ventilator and required a tracheostomy. Intravenous corticosteroid therapy was changed to oral therapy by the time of the patients’ discharge. All patients were ultimately weaned off mechanical ventilation.
Table 2.
Inpatient Care
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | |
|---|---|---|---|---|---|
| Hospital Stay | 44 Days | 16 Days | 11 Days | 10 Days | 13 Days |
| ICU Stay | Day 1→37 | Day 1→14 | Day 1→6 | Day 3→8 | Day 1→12 |
| Ventilation | Day 1→30 | Day 1→11 | Day 1→5 | Day 3→7 | Day 1→10 |
| Fungal Culture Started | Hospital: Day 9 | Hospital: Day 7 Special: Day 8 |
Hospital: Day 5 Special: Day 3 |
Hospital: Day 4 Special: Day 4 |
Hospital: Day 2 Special: Day 4 |
| Hospital Culture Results | C. albicans, C. krusei | Asp. fumigatus, Pen. species | No fungal growth | No fungal growth | No fungal growth |
| Special Culture Results | Not done | Asp. terreus, Asp. flavus, Asp. fumigatus, C. albicans | C. albicans, Asp. Flavus | C. albicans | Paeci. lilacinus, C. albicans |
| Fungal elements on microscopy | Not done | lancet-shaped elements | Hyphae | Hyphae | Hyphae; lancet- shaped elements |
| Antifungal | Itraconazole Day 15→20, 24→28 | Micafungin Day 8→9 Voriconazole Day 9→16 |
Itraconazole Day 4→11 | None | Itraconazole Day 4→10 |
| Steroids | Methylpred. Day 1→24 Prednisone Day 1, 9, 25→44 | Methylpred. Day 1→5 Prednisone Day 5→16 | Methylpred. Day 1→7 Prednisone Day 8→11 | Methylpred. Day 1, 3→8 Prednisone Day 1→2, 8→10 | Methylpred. Day 1→8 Prednisone Day 8→13 |
| Antibacterials | Azithromycin Day 1, 11→27 Moxifloxacin Day 1→8 Ivermectin Day 12→17 Albendazole Day 15 Doripenem Day 24→28 Vancomycin Day 4→6, 24→32 |
Azithromycin Day 2→5 Vancomycin Day 7→15 |
Azithromycin Day 2 Ceftriaxone Day 2→9 |
Moxifloxacin Day 1→8 | Cefepime Day 1 Azithromycin Day 1→4, 12→13 Ceftriaxone Day 1→6 Vancomycin Day 1, 3→5 Moxifloxacin Day 1 |
| Other Asthma Medications | Albuterol Day 1→44 Levalbuterol Day 1 Ipratropium Day 1→44 Budesonide Day 2→32, 37→44 |
Albuterol Day 1→16 Ipratropium Day 1→16 Budesonide/form oterol Day 15→16 Budesonide Day 3→11 |
Albuterol Day 1→11 Ipratropium Day 1→11 Budesonide/form oterol Day 8→11 |
Albuterol Day 1→10 Ipratropium Day 1→10 Tiotropium Day 7→10 Budesonid/formo terol Day 3, 7→10 Budesonide Day 5→7 |
Albuterol Day 1→13 Ipratropium Day 1→13 Budesonide Day 2→13 |
“Day” information indicates on what day of the hospitalization a treatment, test, or ICU stay occurred. (i.e. “Day 1, 11→27” indicates utilization on the 1st day and from the 11th through 27th days.
3.2 Microbiological findings from status asthmaticus patients
No bacterial pathogens were identified from either blood or respiratory tract specimens. Hospital laboratory fungal cultures revealed few yeast from patient 1 and Aspergillus fumigatus and a Penicillium species from patient 2, but fungi were not identified from the other three patients. In contrast, removal of respiratory mucus greatly improved culture recovery as Candida albicans was isolated in all patients which profusely grew colonies too numerous to tabulate in 3 of the 4 patients analyzed by post-mucolytic culturing. All patients except patient 4 further grew more than one species of fungus and filamentous fungi were cultured from 3 of the 5 patients. In addition to confirming the presence of A. fumigatus in patient 2, post-mucolytic cultures revealed the presence of two additional Aspergillus species. Patient 5 further grew multiple filamentous fungi, including one fungus that could not be identified by microscopic examination due to a failure to develop distinct reproductive structures. Culture data is summarized in Table 2 with the day initially taken indicated. Microscopic examination of concentrated respiratory tract specimens prior to the removal of respiratory mucus further revealed abundant fungal elements from all specimens including hyphae or lancet-shaped structures, a common spore shape frequently associated with alternaria species or charcot leyden crystals which are common in asthma (4 of 4 patients; Table I and Fig. 1a).
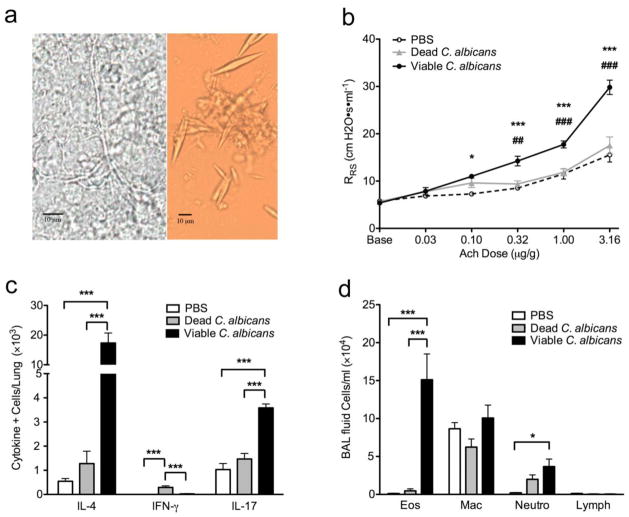
Figure 1.
Airway fungi are linked to human status asthmaticus and experimental asthma. (a) Photomicroscopic images of fungal hyphae (left) and lancet-shaped elements (right) discovered from the lower airway specimens of Patient 2. Original magnification, 400×. C57BL/6 mice were then given intranasal PBS alone, 25,000 dead, or viable C. albicans isolated from patient 4 every other day for 8 exposures after which (b) airway responsiveness to acetylcholine challenge, (c) bronchoalveolar lavage fluid differential cell count including total numbers of eosinophils (Eos), macrophages (Mac), neutrophils (Neu), and lymphocytes (Lymph), and (d) total numbers of lung IΛ-4, IFN-γ, and IL-17A-secreting cells were determined. n=5 mice/group. *P < 0.05, **P<0.01, ***P<0.001 compared to PBS (b) or indicated comparison bars (c,d). # in (a) is comparison to dead C. albicans.
3.3 Experimental infection with Candida albicans
The overwhelming presence of C. albicans in the lower airways of our patients suggested this organism might contribute to disease. To determine experimentally if this is possible, mice were intranasally (i.n.) inoculated with 25,000 viable or heat killed yeast cultured from a clinical isolate of C. albicans and the resulting immune and airway physiological responses were measured. After 8 i.n. exposures over an 18 day period, wild-type C57Black6 mice developed significant increases in airway hyperreactivity, a canonical marker of the asthma phenotype, in response to viable C. albicans as compared to vehicle (phosphate buffer saline: PBS) or heat inactivated yeast challenged animals (Fig. 1b). Viable yeast-challenged mice additionally showed significantly increased allergic inflammation including increased total BAL fluid cellularity composed primarily of eosinophils (Fig. 1c), and predominance of IL-4 secreting cells in the lungs accompanied by increases in IL-17A-secreting cells as compared to controls as analyzed by ELISpot technique (Fig. 1d). Dose-dependant inhalation experiments were also performed and showed an increase in asthma phenotype endpoints with an increase in yeast exposure (Supplementary Fig. 1). Inhalation of dead yeast allergen triggered mild inflammation without Th1 or Th2 dominance (Fig. 1b-d). Thus, the yeast C. albicans potently induces allergic lung disease in normal mice suggesting C. albicans airway infection could cause or exacerbate asthma and status asthmaticus.
4. Discussion
We initiated a retrospective study of patients with SA due to an unexpected increase in prevalence of this disorder at a single site, BTGH. Using specialized culture techniques adopted from Ponikau and Kontoyiannis [11,12], we have observed abundant yeast and moulds from the lower respiratory tract specimens of five consecutive patients with status asthmaticus. We also demonstrated the novel finding that C. albicans inhalation, similar to inhalation of spores from many other moulds [7,8], induces robust allergic airway disease in mice. The limited population size and non-hypothesis driven nature of this observational retrospective case-series reports are incapable of providing definitive conclusions, but can support the development of prospective clinical studies to address the issues discussed herein.
Our findings support prior investigators’ findings that conventional mycologic culture techniques that do not remove respiratory mucus prior to analysis and culture clinically isolated samples only at room temperature may be inadequate to detect fungi embedded within the particularly thick mucus present in the respiratory tract secretions of many asthmatics [11,12]. Comparison of standard hospital fungal culture techniques to the mucolysis with dual temperature fungal culture procedure described indicate an increase in fungal positive cultures from 40 to 100 percent. While a small retrospective case-series investigation is inappropriate to detect a failing in common hospital fungal culturing techniques, our results do support the reevaluation of these techniques presented by other clinical mycology studies [11,12]. Regardless of the culture methodology, the yield of fungi from SA patients seems unusually high given that the positive fungal growth rate for lower respiratory tract specimens at the study hospital using standard protocols is around 6% (21 of 350 consecutive BAL samples analyzed at BTGH). Combined with our microscopic findings, the abundant respiratory fungal growth observed in this case-series report suggests a link between fungal airway infection and status asthmaticus and supports the development of prospective clinical research studies to elucidate the precise relationship.
The role of fungi in the etiology of asthma has long been linked to the elaboration of allergens that perpetuate the atopic state, the most important risk factor for asthma [13]. Unlike most common allergens, fungi are capable of growing within the airway and producing allergen in situ. Filamentous fungi further secrete proteinases that are both potent adjuvant factors mediating allergic inflammation and required for full expression of allergic disease in the setting of fungal airway infection. Like all fungi, Candida spp. produce proteinases [14] and rare case reports have previously suggested the possibility that C. albicans can cause allergic lung disease under some circumstances [15,16]. Herein, we experimentally determined for the first time that inhalation of C. albicans can induce asthma in mice. Similar to our findings using gamma radiation killed Aspergillus niger, the development of Th2 dominate allergic lung disease was dependent on viability, and presumably airway mucosal infection, and not the inhalation of simple allergens because the same dose of dead yeast failed to elicit asthma-like allergic lung disease[7].
Profound immunosuppression related to the taking of immune modulating medications and underlying immunosuppressive illnesses are known risk factors for tracheobronchial candidiasis leading to severe, invasive disease. In contrast, isolation of yeast from the respiratory tract specimens of immunologically competent individuals is generally perceived as representing contamination, innocuous colonization, or a relatively mild disease process such as thrush that does not warrant specific therapy [17]. However, the abundant quantities of yeast present in the lower airways of our patients having no known constitutive immunodeficiency suggested that this organism could also be contributing to or in fact causing the SA. To investigate this possibility, the allergic potential of viable C. albicans was determined directly in mice. Indeed, our studies demonstrate that C. albicans inhalation is as capable of inducing de novo allergic lung disease in normal, naïve mice as filamentous fungi spore inhalation [7,8].
There are several potential explanations for the presence of Candida albicans in the airway specimens in this case series. Contamination from the oropharynx is a possibility, although cultures were obtained by protected sampling via endotracheal tubes, thus bypassing the oropharynx. Organisms originating from the oropharynx may have bypassed the tracheal cuff of the endotracheal tubes to contaminate the lower airway and thus our tracheal samples, but the massive recovery of Candida spp. suggests that this mechanism is sufficient to account for only a relatively small fraction of the organisms found. Use of inhaled corticosteroids (ICS) prior to admission could have led to Candida overgrowth in the oropharynx and conceivably the lower airway. However, only two of five patients were noted to have used ICS prior to admission.
A possible explanation of the abudant yield of Candida spp. in these patients appears to be the nosocomial administration of systemic corticosteroids and broad-spectrum antibiotics. Immunosuppression of many kinds, but especially the use of systemic glucocorticoids, is linked to Candida overgrowth on mucosal surfaces [18–20]. Broad-spectrum antibiotic use has also long been associated with yeast overgrowth in the human gut and oropharynx [21–23]. Antibiotics are theoretically useful in asthma exacerbated by bacterial infection, but studies with sufficient power to detect a beneficial effect are lacking, and for this reason the empiric use of antibiotics in SA is not universally recommended [24]. However, neither the use of antibiotics nor glucocorticoids has been linked to lower respiratory tract candidiasis and actual worsening of disease. In contrast, our findings suggest that the combined use of broad-spectrum antibiotics and high dose glucocorticoids might be particularly hazardous with the potential to cause or exacerbate tracheobronchial mycosis in asthma. Together, our findings suggest that standard therapy for severe asthma might predispose to tracheobronchial mycosis that if sufficiently severe, might lead to or exacerbate SA.
In addition to Candida spp., we also identified from most of our patients filamentous fungi, producing a combined fungal profile that far exceeded that from the clinical mycology laboratory (Table 2). The improved fungal yield was likely due to the removal of respiratory tract mucus and inflammatory cells that presumably inhibit fungal growth. This laborious process is time consuming and is not practically applied to the high-throughput environment of the clinical laboratory. Nonetheless, our findings emphasize the highly insensitive nature of clinical mycology at least as applied to respiratory tract specimens. We suspect that the artificially low yield of fungi from respiratory specimen cultures may contribute to an impression that active fungal infection is uncommon or non-contributory in airway diseases of many kinds [25,26].
Given the non-controlled nature of retrospective case-series studies, our findings remain associative and it is impossible to determine with any certainty if the moulds and yeast cultured from the lower airways of our patients contributed to their disease. Similarly, the study design used precludes determining if the antifungal therapy received by most patients contributed to their eventual recovery, although the relatively rapid response of moribund patients 2 and 3 after initiation of anti-fungals suggested a beneficial response. Controlled multi-site studies of antifungal agents in SA are clearly warranted to more definitively address these issues.
In summary, using unconventional, highly sensitive culture techniques, we have identified in a small cohort of patients with status asthmaticus a robust presence of multiple moulds and Candida spp. within the lower respiratory tract. The consistency with which tracheobronchial mycosis was found in this case-series, the ability of both fungal forms to induce asthma-like disease in mice, and the salutary effect of anti-fungal therapy in asthma by other investigators[4,5] together suggest that airway fungal growth can contribute to disease severity of disease and be, in part, iatrogenic. Pending carefully controlled prospective studies and irrespective of individual clinical mycology data, our findings suggest early initiation of anti-fungal therapy may be useful in those with life-threatening asthma exacerbations who are receiving glucocorticoids and empiric antibiotic administration in asthma.
Supplementary Material
Highlights.
Candida albicans inhalation causes of asthma-like disease in mice.
Mucolysis and incubation at room and body temperature increased fungal recovery.
Glucocorticoid and antibacterial therapy may aid fungal growth and exacerbate disease.
Anti-fungal use in status asthmaticus should be considered in some cases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Targonski PV, Persky VW, Ramekrishnan V. Effect of environmental molds on risk of death from asthma during the pollen season. J Allergy Clin Immunol. 1995;95:955–61. doi: 10.1016/s0091-6749(95)70095-1. [DOI] [PubMed] [Google Scholar]
- 2.Dales RE, Cakmak S, Judek S, et al. The role of fungal spores in thunderstorm asthma. Chest. 2003;123:745–50. doi: 10.1378/chest.123.3.745. [DOI] [PubMed] [Google Scholar]
- 3.O'Driscoll BR, Hopkinson LC, Denning DW. Mold sensitization is common amongst patients with severe asthma requiring multiple hospital admissions. BMC Pulm Med. 2005;5:4. doi: 10.1186/1471-2466-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Black PN, Udy AA, Brodie SM. Sensitivity to fungal allergens is a risk factor for life-threatening asthma. Allergy. 2000;55:501–4. doi: 10.1034/j.1398-9995.2000.00293.x. [DOI] [PubMed] [Google Scholar]
- 5.Denning DW, O'Driscoll BR, Powell G, et al. Randomized controlled trial of oral antifungal treatment for severe asthma with fungal sensitization: The Fungal Asthma Sensitization Trial (FAST) study. Am J Respir Crit Care Med. 2009;179:11–8. doi: 10.1164/rccm.200805-737OC. [DOI] [PubMed] [Google Scholar]
- 6.Wark PA, Hensley MJ, Saltos N, et al. Anti-inflammatory effect of itraconazole in stable allergic bronchopulmonary aspergillosis: a randomized controlled trial. J Allergy Clin Immunol. 2003;111(5):952–7. doi: 10.1067/mai.2003.1388. [DOI] [PubMed] [Google Scholar]
- 7.Porter P, Susarla SC, Polikepahad S, et al. Link between allergic asthma and airway mucosal infection suggested by proteinase-secreting household fungi. Mucosal Immunol. 2009;2:504–17. doi: 10.1038/mi.2009.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Porter PC, Roberts L, Fields A, et al. Necessary and sufficient role for T helper cells to prevent fungal dissemination in allergic lung disease. Infect Immun. 2011;79:4459–71. doi: 10.1128/IAI.05209-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kheradmand F, Kiss A, Xu J, Lee SH, Kolattukudy PE, Corry DB. A protease-activated pathway underlying Th cell type 2 activation and allergic lung disease. J Immunol. 2002;169:5904–11. doi: 10.4049/jimmunol.169.10.5904. [DOI] [PubMed] [Google Scholar]
- 10.Corry DB, Grunig G, Hadeiba H, et al. Requirements for allergen-induced airway hyperreactivity in T and B cell-deficient mice. Mol Med. 1998;4:344–55. [PMC free article] [PubMed] [Google Scholar]
- 11.Ponikau JU, Sherris DA, Kern EB, Homburger HA, Frigas E, Gaffey TA, Roberts GD. The diagnosis and incidence of allergic fungal sinusitis. Mayo Clin Proc. 1999 Sep;74(9):877–84. doi: 10.4065/74.9.877. [DOI] [PubMed] [Google Scholar]
- 12.Kontoyiannis DP, Chamilos G, Hassan SA, Lewis RE, Albert ND, Tarrand JJ. Increased culture recovery of Zygomycetes under physiologic temperature conditions. Am J Clin Pathol. 2007 Feb;127(2):208–12. doi: 10.1309/7KU5XWURYM0151YN. [DOI] [PubMed] [Google Scholar]
- 13.NHLBI. [accessed Dec. 9, 2011];Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. 2007 Aug 28; www.nhlbi.nih.gov/guidelines/asthma/
- 14.Stewart K, Abad-Zapatero C. Candida proteases and their inhibition: prospects for antifungal therapy. Curr Med Chem. 2001;8:941–8. doi: 10.2174/0929867013372698. [DOI] [PubMed] [Google Scholar]
- 15.Kenney EL. Candida asthma. Ann Intern Med. 1951;34:223–6. doi: 10.7326/0003-4819-34-1-223. [DOI] [PubMed] [Google Scholar]
- 16.Gumowski P, Lech B, Chaves I, Girard JP. Chronic asthma and rhinitis due to Candida albicans, epidermophyton, and trichophyton. Ann Allergy. 1987;59:48–51. [PubMed] [Google Scholar]
- 17.Meersseman W, Lagrou K, Spriet I, et al. Significance of the isolation of Candida species from airway samples in critically ill patients: a prospective, autopsy study. Intensive Care Med. 2009;35:1526–31. doi: 10.1007/s00134-009-1482-8. [DOI] [PubMed] [Google Scholar]
- 18.Burton DM, Seid AB, Kearns DB, Pransky SM. Candida laryngotracheitis: a complication of combined steroid and antibiotic usage in croup. Int J Pediatr Otorhinolaryngol. 1992;23:171–5. doi: 10.1016/0165-5876(92)90053-r. [DOI] [PubMed] [Google Scholar]
- 19.Wong KK, Pace-Asciak P, Wu B, Morrison MD. Laryngeal candidiasis in the outpatient setting. J Otolaryngol Head Neck Surg. 2009;38:624–7. [PubMed] [Google Scholar]
- 20.Hardy JR, Rees E, Ling J, et al. A prospective survey of the use of dexamethasone on a palliative care unit. Palliat Med. 2001;15:3–8. doi: 10.1191/026921601673324846. [DOI] [PubMed] [Google Scholar]
- 21.Charles PE, Dalle F, Aube H, et al. Candida spp. colonization significance in critically ill medical patients: a prospective study. Intensive Care Med. 2005;31:393–400. doi: 10.1007/s00134-005-2571-y. [DOI] [PubMed] [Google Scholar]
- 22.Samonis G, Gikas A, Toloudis P, et al. Prospective study of the impact of broad-spectrum antibiotics on the yeast flora of the human gut. Eur J Clin Microbiol Infect Dis. 1994;13:665–7. doi: 10.1007/BF01973996. [DOI] [PubMed] [Google Scholar]
- 23.Akpan A, Morgan R. Oral candidiasis. Postgrad Med J. 2002;78:455–9. doi: 10.1136/pmj.78.922.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lazarus SC. Clinical practice. Emergency treatment of asthma. N Engl J Med. 2010;363:755–64. doi: 10.1056/NEJMcp1003469. [DOI] [PubMed] [Google Scholar]
- 25.Mortensen KL, Johansen HK, Fuursted K, et al. A prospective survey of Aspergillus spp. in respiratory tract samples: prevalence, clinical impact and antifungal susceptibility. Eur J Clin Microbiol Infect Dis. 2011;30:1355–63. doi: 10.1007/s10096-011-1229-7. [DOI] [PubMed] [Google Scholar]
- 26.Greub G, Bille J. Aspergillus species isolated from clinical specimens: suggested clinical and microbiological criteria to determine significance. Clin Microbiol Infect. 1998;4:710–6. doi: 10.1111/j.1469-0691.1998.tb00656.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.