Abstract
Background
Hypo/hyperglycemia is a known cause of chorea and hemiballism. The temporallobes, hippocampus, basal ganglia, and substantia nigra are most susceptible to hypoglycemic changes.
Methods
We present a caseof bilateral chorea and bi-ballism accompanied by encephalopathyin the setting of severe hypoglycemia and diabetic ketoacidosis. The patient had brain MRI changes involving both caudate nuclei, temporal lobes, and hippocampi.
Discussion
This case demonstrates the basal ganglia's vulnerability to hypoglycemia and the need for cautious evaluation of involuntary movements when they occur in the setting of encephalopathy.
Keywords: Encephalopathy, hypoglycemia, chorea, ballism, diabetic ketoacidosis, hyperglycemia
Introduction
We present a case of bilateral chorea and ballism (bi-ballism) accompanied by encephalopathy in a patient with severe hypoglycemia and diabetic ketoacidosis. This case was significant because the movements were initially mistaken as simple flailing in the setting of hypoglycemic encephalopathy. Although there have been case reports of chorea and hemiballism associated with hypo/hyperglycemia,1–3 the findings in this case manifested bilaterally and were associated with brain magnetic resonance imaging (MRI) changes involving both right and left caudate nuclei, temporal lobes, and hippocampi. The clinical manifestations of hypoglycemia can be broad and may include neurological manifestations such as amnesia, seizures, and hyperkinetic movement disorders.1 The temporal lobes, hippocampus, basal ganglia, and substantia nigra appear most susceptible.4 This case emphasizes the sensitivity of the basal ganglia to hypoglycemia, and the need for careful and thoughtful evaluation of involuntary movements when they co-occur with encephalopathy.
Case report
A 19-year-old Caucasian male with insulin-dependent type I diabetes developed involuntary movements of all four extremities after several episodes of prolonged hypoglycemia. These movements were initially described as “flailing” movements associated with diabetic encephalopathy. He had no previous history of chorea or kernicterus, and he had no family history of Huntington's disease, benign hereditary chorea, neuroacanthocytosis, or other neurodegenerative disorders. He was admitted with diabetic ketoacidosis with an anion gap of 24. On day 5 of his hospitalization, he suffered two episodes of severe hypoglycemia with blood glucoses recorded in the range of 37–44 mg/dL. The episodes of hypoglycemia lasted approximately 2 hours. Within hours of these episodes, the patient developed encephalopathy and involuntary movements of his arms, legs, and face.
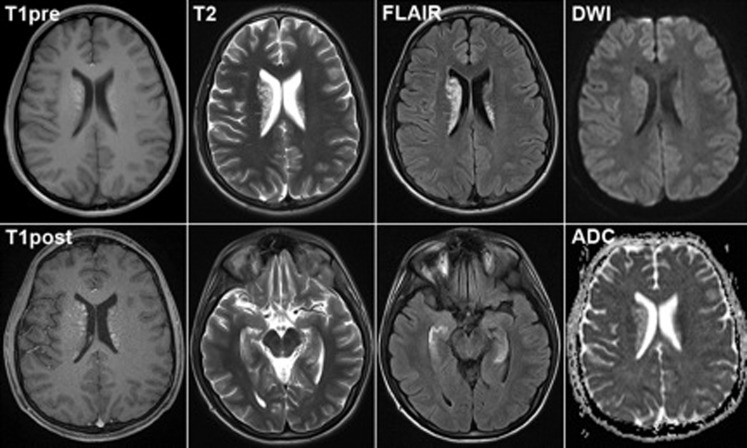
During his examination, he appeared mildly agitated and in some distress due to his hyperkinetic movements. He was alert and oriented to self, time, and place. He had scanning dysarthric speech, but was easily understood. He demonstrated deficits in recall and attention. His language was intact. Cranial nerves were normal. He had full strength and his reflexes were 2+ throughout with plantar flexor responses bilaterally. His involuntary movements included facial chorea and chorea of the fingers, hands, arms, and legs. He also had prominent intermittent ballistic movements of the arms and leg. His electroencephalogram showed evidence of mild background slowing. His ceruloplasmin was normal at 26 mg/dL and his cerebrospinal fluid analysis revealed eight red blood cells and two white blood cells with a normal range protein and negative bacterial cultures. His brain MRI demonstrated abnormal T1 and T2/FLAIR (fluid attenuation inversion recovery) signal in the caudate nucleus and basal ganglia bilaterally, right greater than left (Figure 1). There was also edema in both hippocampal gyri. Diffusion imaging showed no restriction on diffusion.
Figure 1. MRI: T1 images show patchy hyperintense signal in the bilateral caudate nuclei, right greater than left, but no enhancement post contrast. T2 and FLAIR imaging revealed similar heterogenoushyperintense signal within the caudate nuclei and both hippocampal gyri (lower panels). Diffusion weighted imaging and ADC imaging revealed no evidence of associated ischemia.
He was treated with olanazpine 5 mg twice a day for 3 days without change in his hyperkinetic movements. Eventually he was started on clonazepam 1 mg twice a day with a significant decrease in involuntary movements after 24 hours of treatment. In addition, he was placed on an insulin drip for improved blood glucose control. Within 24 hours of these changes his involuntary movements had largely resolved. Coincident with improvement in his involuntary movements his encephalopathy also improved.
Discussion
Repetitive episodes of hypoglycemia can lead to chorea and hyperkinesis.1–2 These symptoms can be a temporary or a permanent sequelae. Although there are several case reports of transient chorea in the setting of hypoglycemia,5–8 to our knowledge there have been no cases in which bilateral chorea and bi-ballism have been reported with hypoglycemia and associated closely with the co-occurrence encephalopathy.
In our patient we observed abnormal T2 and T1 hyperintensities in the basal ganglia and hippocampi bilaterally without evidence of infection or an inflammatory process. At the cellular level, both glucose deprivation and ischemia/anoxia in the brain can result in decreased energy reserve, alterations in membrane potentials, and overall cell stress. However, in cases of hypoglycemia, there is a topographical preference for the hippocampus, temporal lobe, and basal ganglia.9 This topographical preference is well correlated with the T2 changes on brain MRI in hypoglycemic individuals.9,10 In addition, consistent with previous reports, our patient did not demonstrate restricted diffusion on MRI, making ischemia/anoxia less likely.
Although the exact pathophysiology of chorea in hypoglycemia is still not well understood, based on MRI and single photon emission computed tomography (SPECT), it has been postulated that hypoglycemia results in temporary striatal dysfunction.1 SPECT imaging in patients with hypoglycemia and chorea has shown decreased blood flow in the basal ganglia and increased perfusion of the thalamus contralateral to the side of the body manifesting chorea.11 These findings have led to speculation that decreased pallidal inhibitory input to the thalamus, resulting in increased thalamocortical outflow, might explain the chorea and the hyperkinesia, although the exact mechanism remains unknown.11
Interestingly, several case reports of hyperglycemia-induced chorea–ballism associated with striatal lesions on MRI have been reported.12,13 It is thought that depletion of gamma-aminobutyric acid (GABA) and acetylcholine, which are needed as an alternative energy source during non-ketotic hyperglycemia, can lead to a decreased inhibitory signal to the thalamus, resulting in a hyperkinetic movement disorder.14,15 However, this theory has been disputed as chorea–ballism can also manifest in ketotic patients where acetoacetate is abundant and can be utilized as a source of GABA. Furthermore, there have been other cases of chorea–ballism in patients with hypoglycemia.7
Another hypothesis has been that of cerebrovascular insufficiency. SPECT and positron emission tomography suggest the presence of regional hypoperfusion of the striatum of patients with hyperglycemia induced chorea–ballism.16,17 Furthermore, MR spectroscopy in these same patients has shown neuronal loss and damage in the striatum.13,18,19 Abe et al.18 recently reported six patients with hyperglycemia-induced movement disorder who had biopsies of the striatum performed. They found patchy necrotic tissue, severe thickening of all layers of arterioles, and marked narrowing of vessel lumens. Hyaline degeneration of the arteriolar walls, extravasation of erythrocytes, and prominent capillary proliferation were also noticed together with lymphocytic infiltration and macrophage invasion. They coined this finding diabetic striatopathy.18 However, whether the relationship between ischemic insult and changes on MRI is associated with striatal vasculopathy is still to be elucidated.20 To date, there have been no cases of hypoglycemia-induced movement patients with biopsies performed.
Treatment of chorea and ballism with low-dose olanzapine monotherapy was ineffective, but the addition of clonazepam notably resulted in rapid improvement of symptoms; however, this improvement occurred coincident to tighter glucose control. In the current case, tight regulation of blood glucose may have resulted in a rapid improvement of the transient metabolic dysfunction of the basal ganglia, and also improvement of the encephalopathy. Interestingly, the recovery was more rapid than that in previously reported cases. In a previous case of hypoglycemia-induced choreoathetosis, the patient was treated with tiapride for 4 weeks with improvement, but residual hyperkinetic issues still remained. The same patient continued to experience symptoms even after blood glucose normalization.21 Takashai and Ohkawa2 described a patient with hypoglycemia-induced bilateral ballism whose symptoms disappeared after controlling serum glucose with insulin treatment. A recent report of 25 patients from Korea with hyperglycemia induced chorea–ballism found that all patients recovered within 1 month with glycemic control and administration of neuroleptics.22
We propose that correction of the metabolic derangement and rapid glucose control with an insulin drip if necessary should play a critical role in the best management of these cases. Furthermore, this current case suggests that the addition of clonazepam may be useful in some cases for control of hypoglycemia-induced chorea–ballism. Finally, patients with encephalopathy, hypoglycemia and flailing extremities should prompt clinicians to obtain rapid neurological consultation, MRI scanning, and glycemic control, and this syndrome should not be mistaken for a simple mental status change in a patient trying to get out of bed.
Footnotes
Funding: N.R.M. is supported by NIH K08-NS067024.
Financial Disclosures: None.
Conflict of Interests: The authors report no conflict of interest.
References
- 1.Lai SL, Tseng YL, Hsu MC, et al. Magnetic resonance imaging and single-photon emission computed tomography changes in hypoglycemia-induced chorea. Mov Disord. 2004;19:475–478. doi: 10.1002/mds.10676. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi S, Ohkawa S. Paroxysmal bilateral ballism induced by hypoglycemia. Rinsho Shinkeigaku. 2006;4:278–280. [PubMed] [Google Scholar]
- 3.Cheema H, Federman D, Kam A. Hemichorea-hemiballismus in non-ketotic hyperglycaemia. J Clin Neurosci. 2011;2:293–294. doi: 10.1016/j.jocn.2010.04.036. [DOI] [PubMed] [Google Scholar]
- 4.Fujioka M, Okuchi K, Hiramatsu K, Sakaki T, Sakaguchi S, Ishii Y. Specific changes in human brain after hypoglcyemia injury. Stroke. 1997;28:584–587. doi: 10.1161/01.STR.28.3.584. [DOI] [PubMed] [Google Scholar]
- 5.Newman RP, Kinkel WR. Paroxysmal choreoathetosis due to hypoglycemia. Arch Neurol. 1984;41:341–342. doi: 10.1001/archneur.1984.04050150123033. [DOI] [PubMed] [Google Scholar]
- 6.Winer JB, Fish DR, Sawyers D, Marsden CD. A movement disorder as a presenting feature of recurrent hypoglycemia. Mov Disord. 1990;5:176–177. doi: 10.1002/mds.870050217. [DOI] [PubMed] [Google Scholar]
- 7.Hefter H, Mayer P, Benecke R. Persistent chorea after recurrent hypoglycemia. Eur Neurol. 1993;33:244–247. doi: 10.1159/000116946. [DOI] [PubMed] [Google Scholar]
- 8.Shaw C, Haas L, Miller D, Delahunt J. A case report of paroxysmal dystonic choreoathetosis due to hypoglycemia induced by an insulinoma. J Neurol Neurosurg Psychiatry. 1996;61:194–195. doi: 10.1136/jnnp.61.2.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujioka M, Okuchi K, Hiramatsu KI, Sakaki T, Sakaguchi S, Ishii Y. Specific changes in human brain after hypoglycemic injury. Stroke. 1997;28:584–587. doi: 10.1161/01.STR.28.3.584. [DOI] [PubMed] [Google Scholar]
- 10.Finelli PF. Diffusion-weighted MR in hypoglycemic coma. Neurology. 2001;57:933–935. doi: 10.1212/WNL.57.5.933-a. [DOI] [PubMed] [Google Scholar]
- 11.Kim JS, Lee KS, Lee KH, et al. Evidence of thalamic disinhibition in patients with hemichorea: semiquantitative analysis using SPECT. J Neurol Neurosurg Psychiatry. 2002;72:329–333. doi: 10.1136/jnnp.72.3.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takamatsu K, Ohta T, Sato S, et al. Two diabetics with hemichorea-hemiballism and striatal lesions. No To Shinkei. 1995;2:167–172. [PubMed] [Google Scholar]
- 13.Shan DE, Ho DM, Chang C, Pan HC, Teng M. Hemichorea-hemiballism: an explanation for MR signal changes. AJNR Am J Neuroradiol. 1998;19:863–870. [PMC free article] [PubMed] [Google Scholar]
- 14.Guisado R, Arieff AI. Neurological manifestations of diabetic comas: correlation with biochemical alterations in the brain. Metabolism. 1975;24:665–679. doi: 10.1016/0026-0495(75)90146-8. [DOI] [PubMed] [Google Scholar]
- 15.Rector WG, Jr, Herlon HF, Moses H., 3rd Nonketotic hyperglycemia appearing as choreoathetosis or ballis. Arch Intern Med. 1982;142:154–155. doi: 10.1001/archinte.1982.00340140156029. [DOI] [PubMed] [Google Scholar]
- 16.Chang MH, Li JY, Lee SR, Men CY. Non-ketotic hyperglycaemic chorea: a SPECT study. J Neurol Neurosurg Psychiatry. 1996;60:428–430. doi: 10.1136/jnnp.60.4.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsu JL, Wang HC, Hsu WC. Hyperglycemia-induced unilateral basal ganglion lesions with and without hemichorea. A PET study. J Neurol. 2004;251:1486–1490. doi: 10.1007/s00415-004-0571-4. [DOI] [PubMed] [Google Scholar]
- 18.Abe Y, Yamamoto T, Soeda T, et al. Diabetic striatal disease: clinical presentation, neuroimaging, and pathology. Intern Med. 2009;48:1135–1141. doi: 10.2169/internalmedicine.48.1996. [DOI] [PubMed] [Google Scholar]
- 19.Battisti C, Forte F, Rubenni E, et al. Two cases of hemichorea-hemiballism with nonketotic hyperglycemia: a new point of view. Neurol Sci. 2009;30:179–183. doi: 10.1007/s10072-009-0039-5. [DOI] [PubMed] [Google Scholar]
- 20.Fujioka M, Taoka T, Matsuo Y, Hiramatsu KI, Sakaki T. Novel brain ischemic change on MRI. Delayed ischemic hyperintensity on T1-weighted images and selective neuronal death in the caudoputamen of rats after brief focal ischemia. Stroke. 1999;30:1043–1046. doi: 10.1161/01.STR.30.5.1043. [DOI] [PubMed] [Google Scholar]
- 21.Wolz M, Reichmann H, Reuner U, Storch A, Gerber J. Hypoglycemia-induced choreoathetosis associated with hyperintense basal ganglia lesions in T1-weighted brain MRI. Mov Disord. 2010;25:966–968. doi: 10.1002/mds.23112. [DOI] [PubMed] [Google Scholar]
- 22.Lee SH, Shin JA, Kim JH, et al. Chorea-ballism associated with nonketotic hyperglycaemia or diabetic ketoacidosis: characteristics of 25 patients in Korea. Diabetes Res Clin Pract. 2011;2:e80–83. doi: 10.1016/j.diabres.2011.05.003. [DOI] [PubMed] [Google Scholar]