Abstract
Background
Both hypothyroidism and Hashimoto's thyroiditis (HT) can rarely be associated with cerebellar ataxia. Severe essential tremor (ET) as well as bilateral thalamic deep brain stimulation (DBS) may lead to subtle cerebellar signs.
Case Report
We report a 74-year-old male with hypothyroidism and a 20-year history of ET who developed cerebellar ataxia after bilateral thalamic DBS. Extensive workup revealed elevated thyroid stimulating hormone and thyroperoxidase antibody titers confirming the diagnosis of HT.
Discussion
Our case demonstrates multiple possible causes of cerebellar ataxia in a patient, including hypothyroidism, HT, chronic ET, and bilateral thalamic DBS. Counseling of patients may be appropriate when multiple risk factors for cerebellar ataxia coexist in one individual.
Keywords: Ataxia, tremor, Hashimoto's thyroiditis, thalamic stimulation, deep brain stimulation, hypothyroidism
Introduction
Cerebellar ataxia (CA) can rarely arise from autoimmune disorders, including Sjogren's syndrome, type I diabetes mellitus (DM), Stiff-person syndrome, and celiac disease.1–7 The progression of ataxia is usually slow in autoimmune-mediated CA, usually occurring over several years. In some cases, the disease may respond to immunomodulatory treatment. Untreated hypothyroidism may also be associated with CA in 5–52% of cases, although the onset is usually more rapid, occurring over several months, and the ataxia can be reversible with thyroid hormone replacement.8,9 Some cases of hypothyroidism-associated CA do not reverse with normalization of their thyroid state with thyroid hormone replacement and may be harboring undiagnosed Hashimoto's thyroiditis (HT).10
Some essential tremor (ET) patients, especially those with severe disease, present with limb and gait ataxia. Shill and colleagues11 reported that the neuropathology in ET patients is heterogeneous, although the most consistent finding was that of cerebellar gliosis. Erickson-Davis and colleagues12 reported that aside from increased torpedoes and Purkinje cell loss in the brains of ET patients, the density of “hairy basket cells” correlated directly with the number of torpedoes and inversely with the number of Purkinje cells. Such findings may be the underlying reason for the occasional ataxia seen in ET patients.
Thalamic deep brain stimulation (DBS) effectively treats tremor, although may lead to subtle limb and gait ataxia in up to 56% of patients.13,14 In patients with multiple sclerosis, thalamic DBS may improve tremor but does not improve limb ataxia.15 We present a patient with severe ET who developed progressive CA after bilateral thalamic DBS and subsequent diagnosis of HT.
Case report
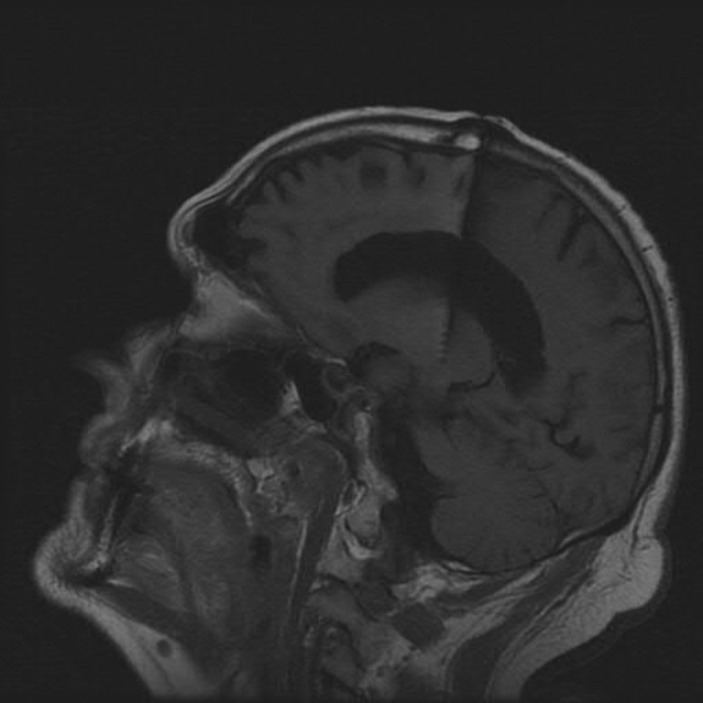
A 74-year-old left-handed male with a past medical history of type 2 diabetes mellitus and hypothyroidism first developed a left hand action tremor at age 54, which eventually spread to his right hand and head. His tremor was partially relieved by alcoholic beverages. He had no family history of ET, Parkinson's disease, or cerebellar ataxia. He was diagnosed as ET by a neurologist, and was prescribed primidone with initial good response. His tremor, however, became disabling and severe by age 66. He eventually underwent bilateral thalamic ventral intermediate nucleus DBS surgery at age 69, with excellent control of his severe bilateral hand tremor (Figure 1). Three years after DBS, he started to experience imbalance, incoordination, slurring of speech, and dysphagia. Although initially thought to be related to DBS, the ataxia did not improve with reprogramming.
Figure 1. Cranial magnetic resonance imaging sagittal T1 image showing the tip of the deep brain stimulation electrode in the ventral intermediate nucleus of the thalamus.
The patient was seen in our institution 5 years after bilateral thalamic DBS. His medications at that time consisted of levothyroxine 125 μg per day, glyburide 2.5 mg per day, metformin 500 mg per day, vitamin E 400 units per day, and multivitamins. The stimulation parameters were as follows: for the right implanted pulse generator (IPG), settings were case positive, contact 1 negative, amplitude of 2.8 volts, pulse width of 60 microseconds, and rate of 185 Hz. For the left IPG, settings were case positive, contact 1 negative, amplitude of 3.6 volts, pulse width of 90 microseconds, and rate of 145 Hz.
Physical examination with the stimulators on revealed no nystagmus, normal ocular saccades and visual pursuits, and moderate reduction in upgaze. On finger-to-nose, he had moderate dysmetria in the right upper extremity and mild dysmetria in the left upper extremity. Dysdiadochokinesia of both hands was noted, moderate in the right and mild in the left. He had severe rebound of both upper extremities. Speech was moderately ataxic. His gait was mildly wide-based and ataxic. The patient was unable to perform tandem gait. On examination immediately after switching the stimulators off, there was no change in his speech or his limb or gait ataxia, although his severe bilateral upper limb tremor and moderate head tremor returned. The patient could not tolerate being switched off more than a few minutes due to his severe tremor. He also keeps the stimulators on at night, as his tremors tend to interfere with sleep, or wake him up in the middle of the night.
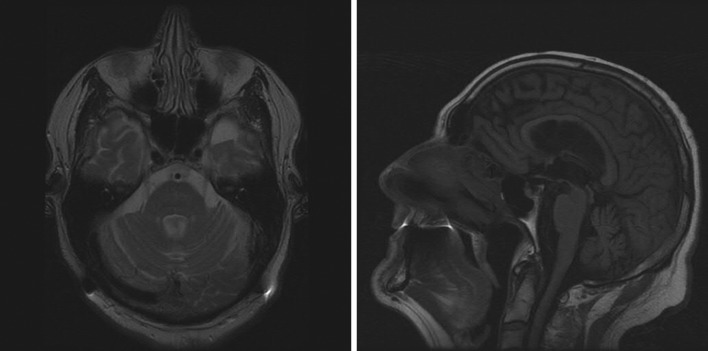
Brain magnetic resonance imaging (MRI) demonstrated mild generalized atrophy and cerebellar atrophy proportional to the cerebral changes (Figure 2 A,B ). Extensive work up for autoimmune, endocrine, infectious, metabolic, and paraneoplastic causes of ataxia was unremarkable. The following studies were negative: urine heavy metal screen, 72 hours stool fat content, Tropheryma whipplei, Lyme serology, human T-lymphotropic virus (HTLV) I and II, antireticulin antibodies (Ab), glutamic acid decarboxylase (GAD) 65 Ab, N-type calcium channel Ab, P/Q-type calcium channel Ab, acetylcholine receptor muscle binding Ab, collapsin response-mediator protein (CRMP) 5 immunoglobulin (Ig)G, anti-neuronal nuclear antibodies (ANNA) 1-3Ab, purkinje cell cytoplasmic antibody (PCA) 1 and 2 Ab, extractable nuclear antigen (ENA), anti-endomysial Ab, striatal muscle Ab, myeloperoxidase Ab, B12, folate, methymalonic acid, homocysteine, and antinuclear antibodies (ANA). The only abnormalities were an elevated thyroid stimulating hormone (TSH) of 8.6 mIU/l (normal <5), elevated thyroperoxidase antibody level of 88.4 IU/ml (normal <9), and low serum copper of 0.69 μg/ml (normal 0.75–1.45). Thyroid ultrasound showed a heterogeneous small and lobulated gland consistent with chronic thyroiditis. Genetic testing for Fragile-X tremor-ataxia syndrome was negative.
Figure 2. Cranial magnetic resonance imaging of our patient showing mild cerebral and cerebellar atrophy. The degree of atrophy of the cerebellum is proportional to the degree of cerebral atrophy. (A) An axial T2 image. (B) A sagittal T1 image.
The patient's levothyroxine dose was increased to 150 μg per day, and a repeat TSH 6 months later was normal. His ataxia, however, did not change with normalization of his TSH. Given reports of copper deficiency-related myeloneuropathy leading to ataxia, the patient was also started on copper supplementation, which did not improve his ataxia.
Discussion
Hashimoto's thyroiditis may rarely be associated with a progressive cerebellar syndrome, with the ataxia typically progressing very slowly. Such patients eventually develop cranial imaging evidence of midline and hemispheric cerebellar atrophy, and even of the brainstem.10 The cerebellar symptoms develop despite a euthyroid state. Rather, the ataxia in HT is thought to be a result of autoimmune attack of cerebellar neurons, leading to neuronal loss and gliosis.
Limb ataxia can be observed in patients with severe ET.16,17 The ataxia is often difficult to appreciate because of the severe tremor. In ET patients who undergo thalamic DBS, although the limb tremor may be significantly improved by DBS, subtle limb ataxia may be apparent.13 Aggressive stimulation parameters may also result in limb, gait, and speech ataxia in ET patients who have undergone thalamic DBS, although adjustments of the programming parameters often alleviates such side effects.
Functional imaging studies have shown that the cerebellum is involved in patients with ET as part of an oscillatory network (primary motor cortex, premotor cortex, thalamus, cerebellum, brainstem).18 The neuropathology of ET was recently characterized by Shill and colleagues11 to be heteregeneous. In their autopsy series, the most common finding was cerebellar gliosis. This may help explain the subtle cerebellar signs that are seen in ET patients, especially those with more severe disease and also the heightened tendency for ET patients to develop ataxia post DBS despite improved tremor.
Cerebellar ataxia from hypothyroidism or from HT is rare. Given that both severe ET and thalamic DBS may lead to some subtle cerebellar signs, we postulate that the tendency to develop CA may be heightened if other factors that can lead to ataxia are present (like hypothyroidism and HT). Our patient did not have any improvement of his speech, gait, or limb ataxia upon switching off the stimulators for a few minutes. However, we are uncertain if his ataxia would have improved if the stimulators were switched off for a more prolonged period. Unfortunately, he could not tolerate having the stimulators off more than a few minutes because of the return of severe upper limb and head tremors. He could not tolerate keeping the stimulators off at night either as the tremors tend to awaken him from sleep or prevent him from sleeping.
In summary, we present a case of cerebellar ataxia in a patient with multiple risk factors including chronic ET, bilateral thalamic DBS, hypothyroidism, and Hashimoto's thyroiditis. Although no definitive conclusions may be derived from a single case, it may be prudent to be vigilant of factors that predispose to cerebellar ataxia, and to counsel patients with ET undergoing bilateral thalamic DBS accordingly.
Footnotes
Funding: Erika Driver-Dunckley received research funding from Ipsen, Chelsea, Merck Serono, and UCB-Schwarz. Virgilio Gerald H. Evidente received research funding from Allergan, UCB-Schwarz, Merz, Ipsen, and Allon.
Competing Interests: Virgilio Gerald H. Evidente MD reports consulting and/or advisory board activities for Merz, Teva, Ipsen, and GSK.
References
- 1.Bayreuther C, Hieronimus S, Ferrari P, et al. Auto-immune cerebellar ataxia with anti-GAD antibodies accompanied by de novo late-onset type 1 diabetes mellitus. Diabetes Metab. 2008;34:386–388. doi: 10.1016/j.diabet.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 2.Bürk K, Farecki ML, Lamprecht G, et al. Neurological symptoms in patients with biopsy proven celiac disease. Mov Disord. 2009;24:2358–2362. doi: 10.1002/mds.22660. [DOI] [PubMed] [Google Scholar]
- 3.Collison K, Rees J. Asymmetric cerebellar ataxia and limbic encephalitis as a presenting feature of primary Sjögren's syndrome. J Neurol. 2007;254:1609–1611. doi: 10.1007/s00415-007-0596-6. [DOI] [PubMed] [Google Scholar]
- 4.Honnorat J, Trouillas P, Thivolet C, et al. Autoantibodies to glutamate decarboxylase in a patient with cerebellar cortical atrophy, peripheral neuropathy, and slow eye movements. Arch Neurol. 1995;52:462–468. doi: 10.1001/archneur.1995.00540290050017. [DOI] [PubMed] [Google Scholar]
- 5.Saiz A, Arpa J, Sagasta A, et al. Autoantibodies to glutamic acid decarboxylase in three patients with cerebellar ataxia, late-onset insulin-dependent diabetes mellitus, and polyendocrine autoimmunity. Neurology. 1997;49:1026–1030. doi: 10.1212/wnl.49.4.1026. [DOI] [PubMed] [Google Scholar]
- 6.Saiz A, Blanco Y, Sabater L, et al. Spectrum of neurological syndromes associated with glutamic acid decarboxylase antibodies: diagnostic clues for this association. Brain. 2008;131:2553–2563. doi: 10.1093/brain/awn183. [DOI] [PubMed] [Google Scholar]
- 7.Wong S, Pollock AN, Burnham JM, et al. Acute cerebellar ataxia due to Sjögren syndrome. Neurology. 2004;62:2332–2333. doi: 10.1212/01.wnl.0000130347.69790.e8. [DOI] [PubMed] [Google Scholar]
- 8.Edvardsson B, Persson S. Subclinical hypothyroidism presenting with gait abnormality. Neurologist. 2010;16:115–116. doi: 10.1097/NRL.0b013e3181be6fdb. [DOI] [PubMed] [Google Scholar]
- 9.Kudrjavcev T. Neurologic complications of thyroid dysfunction. Adv Neurol. 1978;19:619–636. [PubMed] [Google Scholar]
- 10.Selim M, Drachman DA. Ataxia associated with Hashimoto's disease: progressive non-familial adult onset cerebellar degeneration with autoimmune thyroiditis. J Neurol Neurosurg Psych. 2001;71:81–87. doi: 10.1136/jnnp.71.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shill HA, Adler CH, Sabbagh MN, et al. Pathologic findings in prospectively ascertained essential tremor subjects. Neurology. 2008;70:1452–1455. doi: 10.1212/01.wnl.0000310425.76205.02. [DOI] [PubMed] [Google Scholar]
- 12.Erickson-Davis CR, Faust PL, Vonsattel JP, et al. “Hairy baskets” associated with degenerative Purkinje cell changes in essential tremor. J Neuropathol Exp Neurol. 2010;69:262–271. doi: 10.1097/NEN.0b013e3181d1ad04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Earhart GM, Clark BR, Tabbal SD, Perlmutter JS. Gait and balance in essential tremor: variable effects of bilateral thalamic stimulation. Mov Disord. 2009;24:386–391. doi: 10.1002/mds.22356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pahwa R, Lyons KE, Wilkinson SB, et al. Long-term evaluation of deep brain stimulation of the thalamus. J Neurosurg. 2006;104:506–512. doi: 10.3171/jns.2006.104.4.506. [DOI] [PubMed] [Google Scholar]
- 15.Yap L, Kouyialis A, Varma T. Stereotactic neurosurgery for disabling tremor in multiple sclerosis: thalamotomy or deep brain stimulation. Br J Neurosurg. 2007;21:349–354. doi: 10.1080/02688690701544002. [DOI] [PubMed] [Google Scholar]
- 16.Eui-Seong Lim, Man-Wook Seo, Seong-Ryong Woo, et al. Relationship between essential tremor and cerebellar dysfunction according to age. J Clin Neurol. 2005;1:76–80. doi: 10.3988/jcn.2005.1.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stolze H, Petersen G, Raethjen J, et al. The gait disorder of advanced essential tremor. Brain. 2001;124:2278–2286. doi: 10.1093/brain/124.11.2278. [DOI] [PubMed] [Google Scholar]
- 18.Schnitzler A, Münks C, Butz M, et al. Synchronized brain network associated with essential tremor as revealed by magnetoencephalography. Mov Disord. 2009;11:1629–1635. doi: 10.1002/mds.22633. [DOI] [PubMed] [Google Scholar]