Abstract
Canonical transient receptor potential (TRPC) channels are Ca2+-permeable nonselective cation channels that are widely expressed in numerous cell types. Seven different members of TRPC channels have been isolated. The activity of these channels is regulated by the filling state of intracellular Ca2+ stores and/or diacylglycerol and/or Ca2+/calmodulin. However, no evidence is available as to whether TRPC channels are regulated by direct phosphorylation on the channels. In the present study, TRPC isoform 3 (TRPC3) gene was overexpressed in HEK293 cells that were stably transfected with protein kinase G (PKG). We found that the overexpressed TRPC3 mediated store-operated Ca2+ influx and that this type of Ca2+ influx was inhibited by cGMP. The inhibitory effect of cGMP was abolished by KT5823 or H8. Point mutations at two consensus PKG phosphorylation sites (T11A and S263Q) of TRPC3 channel markedly reduced the inhibitory effect of cGMP. In addition, TRPC3 proteins were purified from HEK293 cells that were transfected with either wild-type or mutant TRPC3 constructs, and in vitro PKG phosphorylation assay was carried out. It was found that wild-type TRPC3 could be directly phosphorylated by PKG in vitro and that the phosphorylation was abolished in the presence of KT5823. The phosphorylation signal was greatly reduced in mutant protein T11A or S263Q. Taken together, TRPC3 channels could be directly phosphorylated by PKG at position T11 and S263, and this phosphorylation abolished the store-operated Ca2+ influx mediated by TRPC3 channels in HEK293 cells.
Transient receptor potential (TRP) channels are Ca2+-permeable channels first discovered in Drosophila (1). More than 20 unique TRP mammalian homologs have been cloned from human, mouse, rat, rabbit, and bovine (1). These mammalian TRP homologs have been divided into three subfamilies: canonical TRP (TRPC), vanilloid TRP (TRPV), and melastatin TRP (TRPM) (1). Within the TRPC subfamily, TRPC isoform 3 (TRPC3) is one of the most extensively characterized channels. Originally cloned by Zhu et al. (2), TRPC3 channels are activated by agonist stimulation on plasma membrane G protein-coupled receptors. Receptor stimulation activates different isoforms of phospholipase C (PLC) (1). Signal transduction pathway subsequent to PLC activation, however, appears to be diverse and sometimes controversial. The proposed mechanisms of TRPC3 activation include Ca2+ store-dependent activation mediated by inositol 1,4,5-trisphosphate receptors (InsP3R) (3–5) and a Ca2+ store-independent mechanism that involves diacylglycerol (6, 7). Calcium ions may also modulate the activity of TRPC3 channels (8). A calmodulin (CaM)-binding site is found in the C terminus of TRPC3 that overlaps with InsP3R-binding domain. CaM suppresses the activity of TRPC3 under resting condition. Activation of InsP3Rs displaces the CaM from the CaM/InsP3R-binding site, resulting in the activation of TRPC3 (8).
Besides diacylglycerol, calcium ions, and store depletion, very little information is available regarding the regulation of TRPC3, and TRPC subfamily as a whole, by other factors. Analysis of TRPC primary sequences reveals multiple potential phosphorylation sites, including those for PKA, PKC, protein kinase G (PKG), myosin light chain kinase, and tyrosine kinase, implying that the activity of TRPC channels might be modulated by protein phosphorylation. However, up to the present, there has been only one report, from Venkatachalam et al. (9), suggesting that the activity of TRPC might be modulated by PKC. Venkatachalam et al. (9) showed that the activation of PKC by phorbol ester inhibited the stimulatory action of diacylglycerol on TRPC3 and that a PKC inhibitor GF 109203X could reverse the action of phorbol ester. The action of PKC on TRPC3 appeared to be indirect because PKC affected only the receptor-activated Ca2+ influx, but it had no effect on the store-operated Ca2+ influx induced by the Ca2+ pump blocker thapsigargin (9).
Works by us and others have showed that store-operated Ca2+ influx is inhibited by cGMP in several cell types (10–12), and this inhibitory effect of cGMP is probably mediated by PKG (10). The inhibition of store-operated Ca2+ influx by the nitric oxide (NO)/cGMP/PKG pathway may serve as a negative feedback mechanism to protect cells from detrimental effect of excessive NO and Ca2+ (10, 13). TRPC3 is one of the channels that participate in store-operated Ca2+ influx (3–5, 14), and analysis of the primary sequences shows the presence of multiple potential PKG phosphorylation sites in TRPC3 sequence. In the present study, we explored the role of PKG phosphorylation in modulating the activity of TRPC3 channels. We found that TRPC3 could be directly phosphorylated by PKG and that this phosphorylation abolished store-operated Ca2+ influx mediated by TRPC3 channels in HEK293 cells.
Materials and Methods
Materials. Human coronary artery endothelial cell line (HCAEC-CCL2585) was from BioWhittaker. Human embryonic kidney (HEK) cell line HEK293 was from American Type Culture Collection (ATCC). There were two primary antibodies against TRPC3: one (anti-TRPC3) was from Alomone Laboratories (Jerusalem), and the other (A15) was from Santa Cruz Biotechnology. Primary antibodies against PKG (KAP-PK005) were from StressGen Biotechnologies (Victoria, Canada), and those against Ser-239 phosphorylated vasodilator-stimulated phosphoprotein (VASP) (16C2) and total VASP (M4) were from Nanotools (Munich, Germany) and ImmunoGlobe (Himmelstadt, Germany), respectively. Protein A-agarose and alkaline phosphatase were from Roche. Fluo3/acetoxymethyl ester (Fluo3/AM) and pluronic F127 were from Molecular Probes. Lipofectamine 2000, blasticidin, soybean trypsin inhibitor, PMSF, FBS, culture media EGM-MV (microvascular endothelial cell growth medium), and DMEM were from Invitrogen. 8-BrcGMP, 8-BrcAMP, KT5823, H8 (N-[2-(ethylamino)ethyl]-isoquinoline sulfonamide), thapsigargin, aprotinin, leupeptin, and active PKG1α were from Calbiochem. Nonidet P-40, EGTA, trypsin, and NiCl2 were from Sigma.
Cell Culture. Human coronary artery endothelial cell line was cultured in EGM-MV supplemented with endothelial cell growth factors supplied by the manufacturer. Other cells (HEK293, EOMA, and A7r5) were cultured in DMEM supplemented with 10% FBS. Cells were grown at 37°C in a 5% CO2 humidified incubator.
Cloning of PKG and TRPC3 and Construction of Mutants. PKG1α gene (2,213 bp) was cloned by patching together an RT-PCR product, which was amplified from total RNA extracted from human coronary artery endothelial cells, with an EST clone that contains the C-terminal region of human PKG1α (dbEST id, 867466; EST name, rz58d02.r1; ATCC). The coding region of TRPC3 gene (2,546 bp) was obtained by RT-PCR from human coronary artery endothelial cells. PCR primers were designed based on a published nucleotide sequence of human TRPC3 (GenBank accession no. NM_003305). Amplified TRPC3 gene was cloned into pALTER-MAX vector (Promega) and then annealed with specially designed mutagenic oligonucleotides, followed by DNA elongation to create point mutations (Altered Sites II Mammalian Mutagenesis System, Promega). Mutagenic oligonucleotides were GCATCACTGCCATGCGTCT for construct T11A, ACAGGGGCTCAGTGCGAGACGCTTG for T134A, and TTGCATTGCATTTGGAGCTTCCGATA for S263Q (the mutated nucleotides are underlined). Wild-type and mutant TRPC3 constructs and PKG1α genes were each cloned into mammalian expression vector pcDNA6 (Invitrogen). All clones were autosequenced by ABI310 autosequencer (Perkin–Elmer–Cetus) to verify the authenticity of the genes or mutants.
Transient and Stable Transfection. HEK293 cells were transfected by using Lipofectamine 2000. The transfected genes were TRPC3 and/or PKG1α in pcDNA6. Briefly, transfection was done with 4 μg of DNA plasmid and 6 μl of Lipofectamine 2000 in 200 μl of Opti-MEM reduced serum medium in six-well plates, which contained ≈6 × 104 cells per well. Approximately 80% of HEK293 cells were successfully transfected under this transfection condition as determined by a control transfection using a β-galactosidase construct. A stable PKG-containing cell line was established under the selection pressure of blasticidin. For transient transfection of TRPC3, functional studies were performed 3 days posttransfection.
[Ca2+]i Measurements. Cell preparation and Ca2+ measurement were performed as described (10). Briefly, cells were loaded with Fluo-3/AM in a normal physiological solution (NPSS) that contained 140 mM NaCl, 1 mM KCl, 1 mM CaCl2, 1 mM MgCl2, 10 mM glucose, and 5 mM Hepes (pH 7.4). For store depletion, cells were pretreated with 4 μM thapsigargin or 100 μM ATP for 15 min in 0Ca2+-PSS which contained 140 mM NaCl, 1 mM KCl, 1 mM MgCl2, 10 mM glucose, 2 mM EGTA and 5 mM Hepes, pH 7.4. Ca2+ influx was initiated by replacing 0 Ca2+-PSS with NPSS or 0.5 Ca2+-PSS, a solution similar to NPSS but contained only 0.5 mM instead of 1 mM CaCl2. Unless stated otherwise, the cells were pretreated with or without 2 mM 8-BrcGMP, 1 μM KT5823, 10 μM H8, 3 mM Ni2+, or 2 mM 8-BrcAMP for 5 min before the experiments. The fluorescence signal was monitored and recorded by an MRC-1000 laser scanning confocal imaging system, and the data were analyzed by using metaflour software. Each experiment had 5–10 cells. Changes in [Ca2+]i were displayed as a ratio of fluorescence relative to the intensity before the application of extracellular Ca2+ (F1/F0).
SDS/PAGE and Immunoblots. Whole-cell lysates were extracted with detergent extraction buffer, which contained 1% (vol/vol) Nonidet P-40, 150 mM NaCl, 20 mM Tris·HCl (pH 8.0), with addition of 1 μg/ml soybean trypsin inhibitor, 5 μg/ml leupeptin, 5 μg/ml aprotinin, and 100 μM PMSF. Eighty micrograms of extracted proteins were separated on 8% SDS/PAGE gel and were blotted onto a poly(vinylidene difluoride) (PVDF) membrane (Millipore). The membrane was incubated at 4°C overnight with appropriate antibodies in TBS buffer containing 0.1% Tween 20 and 5% nonfat dry milk. Immunodetection was accomplished with horseradish-conjugated secondary antibodies, followed by ECL detection system (Amersham Pharmacia). TRPC3 antigen preabsorption control experiments were performed by incubating anti-TRPC3 antibody with a 45-fold molar excess of peptide antigen for 1 h at room temperature, followed by 5-min centrifugation at 10,000 × g. An antibody against β-tubulin was used to show that an equal amount of proteins was loaded onto each lane.
Immunoprecipitation and in Vitro PKG Phosphorylation. TRPC3 was immunoprecipitated from extracted proteins by incubating 800 μg of extracted proteins with 5 μg of Anti-TRPC3 (Alomone Laboratories) on a rocking platform overnight at 4°C. Protein A-agarose was added, followed by 3 h incubation at 4°C. The immunoprecipitates were collected by centrifugation and were washed with saline. For immunoblots, immunoprecipitated proteins were solubilized in Laemmli buffer and then resolved in SDS/PAGE. For in vitro PKG phosphorylation assay, the immunoprecipitates were incubated in phosphorylation buffer at 37°C for 5 min, followed by an addition of active PKG1α (40 ng/reaction) and 0.125 μCi/μl[γ-32P]ATP (1 Ci = 37 GBq). The phosphorylation reactions were carried out at 37°C for 5 min in a total reaction volume of 40 μl. The reactions were then stopped by Laemmli buffer, and phosphorylated products were resolved by SDS/PAGE followed by autoradiography. Phosphorylation buffer contained 30 mM Tris·HCl (pH 7.4), 1 mM MgSO4, 1 mM EDTA, 40 μM ATP, and 10 μM cGMP. KT5720 (1 μM) was included in all phosphorylation reactions to inhibit PKA. When needed, 2 μM of KT5823 was added. For dephosphorylation reactions, phosphorylated immunoprecipitates were washed, and the reactions were carried out at 37°C for 30 min in a dephosphorylation buffer containing 50 mM Tris·HCl (pH 8.5), 0.1 mM EDTA, and 5 units of alkaline phosphatase.
Results
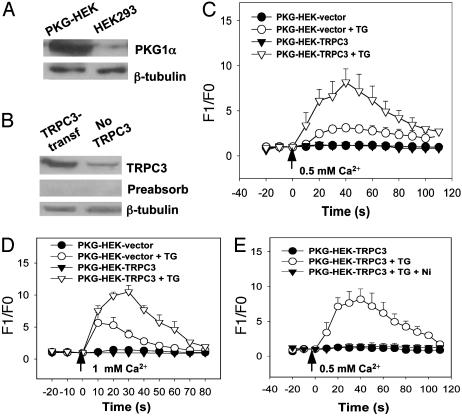
Transfection of HEK293 Cells with PKG and TRPC3. Wild-type HEK293 expressed only TRPC1 and low levels of TRPC3. No obvious signal could be detected in immunoblots with antibodies against TRPC4–6 (Fig. 6, which is published as supporting information on the PNAS web site). Because the expression level of PKG1 proteins in wild-type HEK293 cells was low as determined by immunoblots, we first established a stably PKG1α-transfected HEK293 cell line and named it PKG-HEK. PKG-HEK expressed a much higher level of PKG1α compared with that of wild-type HEK293 cells (Fig. 1A). TRPC3 gene was then transiently transfected into PKG-HEK to study possible regulatory roles of PKG1α on TRPC3 function. As expected, TRPC3-transfected cells expressed a much higher level of TRPC3 proteins than nontransfected cells did (Fig. 1B). An antigen preabsorption control was included, which demonstrated the specificity of anti-TRPC3 antibody (Fig. 1B).
Fig. 1.
Effect of TRPC3 overexpression on store-operated Ca2+ influx in PKG-HEK cells. (A) Immunoblots with an anti-PGK antibody showed that PKG-HEK cells, a HEK293 cell line stably transfected with PKG construct, expressed a much higher level of PKG compared with that of wild-type HEK293 cells. Immunoblots with anti-β-tubulin antibody showed that an equal amount of protein was loaded onto each lane. (B) Immunoblots with an anti-TRPC3 antibody showed that TRPC3-transfected PKG-HEK cells expressed a much higher level of TRPC3 proteins than that of non-TRPC3-transfected cells. The specificity of TRPC3 antibody was demonstrated by antigen preabsorption experiments. (C) Store-operated Ca2+ influx of PKG-HEK cells in 0.5 Ca2+-PSS, which contained 0.5 mM Ca2+. PKG-HEK cells were transiently transfected either with TRPC3 gene (PKG-HEK-TRPC3) or with the empty vector pcDNA6 (PKG-HEK-vector). Cells were treated with or without 4 μM thapsigargin (TG) in 0 Ca2+-PSS for 15 min. At the time indicated by the arrow, the media were changed to 0.5 Ca2+-PSS. Shown is the mean ± SE (n = 12 experiments). (D) Store-operated Ca2+ influx of PKG-HEK cells in 1 mM extracellular Ca2+. The procedures were the same as for C except that 1 mM Ca2+ instead of 0.5 mM Ca2+ was used. Shown is the mean ± SE (n = 12 experiments). (E) The effect of Ni2+ on [Ca2+]i rise induced by store depletion. Ni2+ (3 mM) blocked the store-operated [Ca2+]i rise in TRPC3-transfected cells. Shown is the mean ± SE (n = 3 experiments).
Overexpression of TRPC3 Promotes Store-Operated Ca2+ Influx in PKG-HEK Cells. In this series of experiments, PKG-HEK cells were treated with 4 μM thapsigargin for 15 min to deplete Ca2+ from intracellular Ca2+ stores. Ca2+ influx was then initiated by changing extracellular medium from a Ca2+-free (0 Ca2+-PSS) to a Ca2+-containing solution (0.5 Ca2+-PSS). Control PKG-HEK cells without thapsigargin pretreatment were washed by and then maintained briefly in 0 Ca2+-PSS. For the control cells, there was no change in [Ca2+]i when extracellular Ca2+ was elevated (Fig. 1C). In contrast, for those cells that were pretreated with 4 μM thapsigargin, an elevation in extracellular Ca2+ evoked a drastic rise in [Ca2+]i (Fig. 1C). The magnitude of this store-operated Ca2+ influx was much greater in TRPC3-transfectd cells than that of non-TRPC3-transfected cells (Fig. 1C). We also tested different concentrations of extracellular Ca2+ and found that, compared with 1 mM Ca2+, a relative low concentration of extracellular Ca2+ (0.5 mM) reduced the endogenous store-operated Ca2+ inf lux in non-TRPC3-transfected HEK293 cells so that a larger difference in Ca2+ influx can be resolved between the cells transfected with and without TRPC3 (Fig. 1 C and D). For this reason, 0.5 mM Ca2+ was chosen in later experiments to study the mechanism of TRPC3-mediated Ca2+ influx.
To confirm that the change in [Ca2+]i was indeed caused by Ca2+ influx instead of Ca2+ release from thapsigargin-insensitive intracellular stores, we tested the effect of Ni2+ and found that Ni2+ (3 mM) completely blocked the increase of [Ca2+]i, confirming that the rise in [Ca2+]i was due to Ca2+ influx (Fig. 1E).
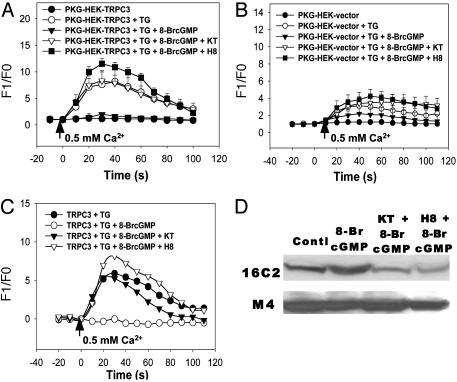
TRPC3-Mediated Store-Operated Ca2+ Influx Is Inhibited by cGMP by Means of a PKG-Dependent Mechanism. 8-BrcGMP, a membrane permeable analog of cGMP, was used to examine the effect of cGMP on the store-operated Ca2+ entry mediated by TRPC3. Application of 8-BrcGMP at 2 mM completely abolished the Ca2+ entry in TRPC3-overexpressing PKG-HEK cells (Fig. 2A), and it also greatly reduced the Ca2+ entry in the PKG-HEK cells that were not transfected with TRPC3 (Fig. 2B). We also tested the effect of cAMP and found that 8-BrcAMP had no effect on Ca2+ entry (data not shown). Because cGMP is an intracellular second messenger that activates PKG, we next examined the possible involvement of PKG. KT5823 (1 μM), a potent and highly specific PKG inhibitor, and H8 (10 μM), another PKG inhibitor, abolished the inhibitory action of 8-BrcGMP and restored the store-operated Ca2+ entry in both TRPC3-overexpressing cells (Fig. 2 A) and non-TRPC3 transfected PKG-HEK cells (Fig. 2B). Subtraction of corresponding data generated in non-TRPC3-transfected cells from those collected in TRPC3-overexpressing cells yields the components that are attributed only to the overexpressed TRPC3 (Fig. 2C). It is very clear that 8-BrcGMP inhibited the store-operated Ca2+ influx due to overexpressed TRPC3 and that the inhibition was abolished in the presence of KT5823 or H8. In addition to thapsigargin, we also used an agonist (ATP 100 μM) to deplete Ca2+ stores and to initiate store-operated Ca2+ influx. 8-BrcGMP (2 mM) also inhibited this type of Ca2+ influx (Fig. 7, which is published as supporting information on the PNAS web site). Taken together, these results suggest that the store-operated Ca2+ entry mediated by TRPC3 is down-regulated by means of a PKG-dependent mechanism.
Fig. 2.
Effect of cGMP, KT5823, and H8 on TRPC3-mediated store-operated Ca2+ influx in PKG-HEK cells. (A) Store-operated Ca2+ influx in TRPC3-transfected PKG-HEK cells. Shown is the mean ± SE (n = 14 experiments). (B) Store-operated Ca2+ influx in PKG-HEK cells that were transfected with empty vector pcDNA6. Shown is the mean ± SE (n = 14 experiments). Cells were depleted with 4 μM thapsigargin in 0 Ca2+-PSS for 15 min. 8-BrcGMP (2 mM) with/without KT5823 (1 μM) or H8 (10 μM) was introduced 5 min before the experiments. At the time indicated by the arrow, the media were changed to 0.5 Ca2+-PSS. (C) Subtraction of corresponding data in B from A yields the components that are attributed only to the overexpressed TRPC3. (D Upper) Immunoblots with antibody against phosphorylated VASP (16C2) in PKG-HEK cells. (Lower) Immunoblots with antibody against total VASP (M4) in PKG-HEK cells. Cells were treated with 8-BrcGMP (2 mM) in the absence or presence of KT5823 (1 μM) or H8 (10 μM) for 5 min. Control had no treatment. Only one main VASP band of 50 kDa was consistently observed. Another VASP band of 46 kDa (not shown) was very weak and was observed only occasionally.
One previous report showed that KT5823 inhibited PKG in vitro but not in intact platelets and mesangial cells (15). We tested the effectiveness of KT5823 in intact HEK293 cells using the VASP Ser-239 phosphorylation assay, a well characterized method for analyzing PKG activity (16). As determined by a monoclonal antibody (16C2) against Ser-239-phosphorylated VASP, 8-BrcGMP (2 mM) treatment for 5 min increased the amount of phosphorylated VASP, as a result of increased PKG activity, in PKG-HEK cells, whereas KT5823 (1 μM) or H8 (10 μM) drastically reduced it (Fig. 2D). These treatments had no effect on the total number of VASP proteins as determined by anti-VASP antibody M4 (Fig. 2D). These results demonstrated that KT5823 could effectively inhibit PKG in intact HEK293 cells.
H8 is not a good inhibitor for PKG because there is only a very small difference separating its IC50 for PKG (≈0.5 μM) and IC50 for PKA (≈1 μM). At the concentration we used (10 μM), H8 could potentially inhibit PKA, and in a less degree PKC. Therefore, data from H8 experiments could be interpreted only together with those derived from KT5823 experiments.
Several previous reports showed that cGMP-PKG might affect InsP3R-mediated Ca2+ release. It inhibited the Ca2+ release in rat megakaryocytes (17), human platelets (18), and PKG-transfected Chinese hamster ovary cells (19), but it stimulated the Ca2+ release in rat hepatocytes (20). Because an inhibition on Ca2+ release would reduce store depletion, resulting in a decreased Ca2+ influx, we examined the effect of cGMP on the Ca2+ release in PKG-transfected HEK293 cells. We found that cGMP had no effect on either thapsigargin (4 μM)- or ATP (100 μM)-induced Ca2+ release in PKG-HEK cells (Fig. 8, which is published as supporting information on the PNAS web site). Therefore, the effect of cGMP on TRPC3-mediated Ca2+ influx was not caused by an inhibition of cGMP on Ca2+ release.
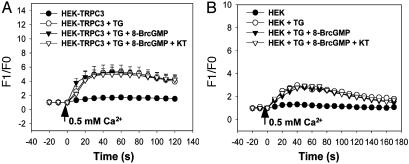
cGMP Has No Effect on the Store-Operated Ca2+ Influx Mediated by TRPC3 in HEK293 Cells That Are Not Transfected with PKG. As shown in Fig. 1 A, wild-type HEK293 cells expressed PKG only at a very low level such that PKG proteins were barely detectable in immunoblot experiments. Fig. 3 shows that application of 8-BrcGMP at 2 mM had no effect on store-operated Ca2+ influx in both the wild-type HEK293 cells and the HEK293 cells that were transfected with TRPC3 but without PKG. These results support the notion that the effect of cGMP on TRPC3 was indeed mediated through PKG and that the residual amount of PKG activity in non-PKG-transfected HEK293 cells was insufficient for TRPC3 inhibition.
Fig. 3.
Effect of cGMP, KT5823 on TRPC3-mediated store-operated Ca2+ influx in HEK293 cells without PKG transfection. 8-BrcGMP (2 mM) and KT5823 (1 μM) had no effect on the store-operated Ca2+ influx in the HEK293 cells that were transiently transfected with TRPC3 (A) or in wild-type HEK293 cells (B). Cells were not transfected with the PKG gene. Cells were depleted with 4 μM thapsigargin in 0 Ca2+-PSS for 15 min. Control had no thapsigargin treatment. 8-BrcGMP with or without KT5823 was introduced 5 min before the experiments. At the time indicated by the arrow, the media were changed to 0.5 Ca2+-PSS. Shown is the mean ± SE (n = 8 experiments).
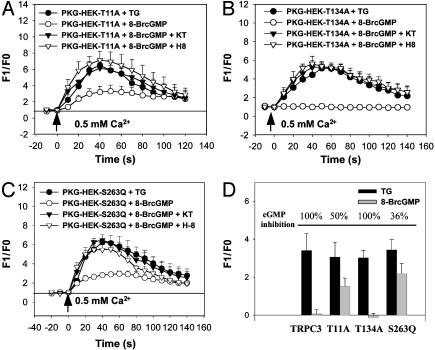
Point Mutations at Potential PKG Phosphorylation Sites Greatly Reduce cGMP Inhibition. A PhosphoBase sequence search found that TRPC3 contains three potential PKG phosphorylation sites with a consensus sequence of (R/K)2–3-X1–2-S/T, all of which are located near its N-terminal regions (Thr-11, Thr-134, and Ser-263). Point mutations were made at each of the three potential PKG sites. We found that conversion of threonine residue at position 11 to alanine (T11A) or serine residue at position 263 to glutamine (S263Q) reduced the inhibitory effect of cGMP on TRPC3-mediated Ca2+ influx (Fig. 4 A, C, and D). The percentage of the inhibition by cGMP was 50 ± 9% (n = 3) for T11A and 36 ± 10% (n = 4) for S263Q (Fig. 4 A, C, and D). On the other hand, mutation at position 134 (T134A) had no effect, and the inhibition by cGMP was 97 ± 10% (n = 4) (Fig. 4 B and D).
Fig. 4.
Effect of point mutations at potential PKG phosphorylation sites on inhibitory action of cGMP and PKG. (A) Store-operated Ca2+ influx in PKG-HEK cells that were transfected with mutant construct T11A. (B) Store-operated Ca2+ influx in PKG-HEK cells that were transfected with mutant construct T134A. (C) Store-operated Ca2+ influx in PKG-HEK cells that were transfected with mutant construct S263Q. A reference line was added in A and C to better illustrate the reduction in cGMP action in cells that were transfected with T11A or S263Q. (D) Summary of cGMP inhibition on TRPC3-mediated Ca2+ influx in HEK293 cells transfected with different TRPC3 constructs. A–C display the raw data collected from the cells transfected with different constructs whereas D shows only the components attributed to TRPC3. In D, similar to Fig. 2C, endogenous store-operated Ca2+ influx in non-TRPC3-transfected cells was subtracted away. Furthermore, only peak Ca2+ response was compared in D. The percentage of cGMP inhibition on the Ca2+ influx is illustrated on top of bar charts in D. All other experimental protocols for A–D were the same as in Fig. 2 A–C. Shown is the mean ± SE (n = 3–4 experiments).
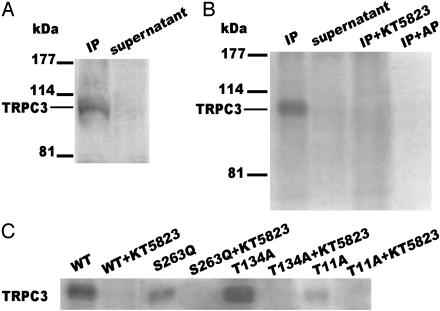
PKG Directly Phosphorylates TRPC3 Proteins. To further substantiate that TRPC3 is the direct target of PKG phosphorylation, in vitro PKG assays were carried out. TRPC3 proteins were purified by immunoprecipitation from TRPC3-transfected HEK293 cells. Fig. 5A shows that an anti-TRPC3 antibody from Alomone Laboratories could successfully purify TRPC3 because there was little TRPC3 protein left in the supernatant fraction after immunoprecipitation reactions. Fig. 5B shows that TRPC3 proteins purified by immunoprecipitation could be phosphorylated by PKG and that this phosphorylation was abolished in the presence of PKG inhibitor KT5823 (2 μM). Fig. 5B also shows that the phosphorylation could be removed by alkaline phosphatase treatment (5 units). Alternatively, TRPC3 proteins were also synthesized in vitro by using the rabbit reticulocyte transcription/translation system, followed by immunoprecipitation and in vitro PKG phosphorylation. TRPC3 proteins synthesized by this method could also be phosphorylated by PKG in vitro (Fig. 9, which is published as supporting information on the PNAS web site). Taken together, these results clearly demonstrated a PKG-specific phosphorylation on TRPC3 proteins.
Fig. 5.
Direct PKG phosphorylation on TRPC3 proteins. (A) Immunoblot of TRPC3 proteins immunoprecipitated by an anti-TRPC3 (Alomone Laboratories). The antibody used for immunoblot was a second anti-TRPC3 antibody (A-15, Santa Cruz Biotechnology). (B) In vitro PKG phosphorylation of wild-type TRPC3 proteins purified by immunoprecipitation from TRPC3-transfected HEK293 cells. Both immunoprecipitated (IP) (lanes 1, 3, and 4) and supernatant fraction (lane 2) were used for in vitro phosphorylation assays. Lane 3, PKG phosphorylation was carried out in the presence of 2 μM KT5823. Lane 4, PKG phosphorylation was followed by treatment with 5 units of alkaline phosphatase. (C) In vitro PKG phosphorylation of TRPC3 proteins purified by immunoprecipitation from HEK293 cells that were transfected with wild-type or different mutant constructs. The experiments in the presence of 2 μM KT5823 are also displayed.
In vitro PKG assays were also performed with mutated TRPC3 proteins purified by immunoprecipitation from HEK293 cells that were transfected with different mutant constructs. Strong PKG phosphorylation was observed in wild-type TRPC3 and mutant T134A, but the phosphorylation signal was greatly reduced in mutant T11A or S263Q (Fig. 5C). These data are consistent with those from Ca2+ influx studies, suggesting that T11 and S263 are the functionally important PKG phosphorylation sites.
Discussion
TRP channels are Ca2+-permeable nonselective cation channels with diverse permeability to other cation ions. Among the three mammalian TRP subfamilies, melastatin TRP (TRPM) contains an atypical α-kinase domain at the COOH-terminal region (21–23). While still being controversial, the phosphorylation of TRPM7 has been suggested to modulate the activity of the channel (22). Recent reports showed that TRPV4, a member of the vanilloid TRP (TRPV) subfamily, is regulated by tyrosine phosphorylation and PKC (24, 25). TRPC is the most intensively studied TRP subfamily. Seven different members of TRPC channels have been isolated, and, on the basis of sequence homology, they can be divided into four subgroups: TRPC1; TRPC2; TRPC4,5; and TRPC3,6,7 (26). Functionally, TRPC channels are suggested to be responsible for store-operated Ca2+ entry as well as receptor-operated but store-independent Ca2+ influx (1, 21, 26–28). Several consensus PKA and PKC phosphorylation sites are located at both the NH2 and the COOH terminus (21), suggesting that the activity of TRPC channels may be modulated by protein phosphorylation. However, no evidence is available as to whether phosphorylation can modulate the activity of the channels. A recent report suggested that PKC might influence TRPC3 activity. However, the action of PKC on TRPC3 is likely to be indirect because PKC affected only the receptor-activated Ca2+ influx whereas it had no effect on the store-operated Ca2+ influx (9). In the present study, we studied human TRPC3 channels and made several findings: (i) Analysis of the primary amino acid sequence of TRPC3 revealed three potential phosphorylation sites for PKG. (ii) Activation of PKG by cGMP inhibited TRPC3-mediated store-operated Ca2+ influx in PKG-HEK cells. The inhibitory effect of cGMP was abolished in the presence of PKG inhibitor KT5823 or H8. (iii) Point mutations at two consensus PKG phosphorylation sites (T11A and S263Q) markedly reduced the inhibitory effect of cGMP. (iv) Wild-type TRPC3 proteins purified by immunoprecipitation from transfected HEK293 cells could be phosphorylated by PKG in vitro, and the phosphorylation signal was greatly reduced in mutant T11A and S263Q. These results strongly support an important role of PKG phosphorylation in modulating TRPC3 channel activity. To our knowledge, there have been no previous reports of direct phosphorylation of TRPC channels as a regulatory mechanism to influence the channel function. Protein sequence alignment also shows that two functionally important PKG phosphorylation sites, Thr-11 and Ser-263, are conserved in two other TRPC isoforms, TRPC6 and TRPC7, suggesting that PKG phosphorylation may also play a role in other TRPC isoforms.
There is controversy regarding the gating mechanisms of TRPC channels. Several studies demonstrated that TRPC3 was activated by store depletion and that this process involved a functional coupling of TRPC3 with InsP3R (3–5). However, many other reports showed that TRPC3 behaved as a receptor-operated channel that could not be further activated by Ca2+ store depletion (6, 29, 30). In another study, Hofmann et al. (7) showed that TRPC3 was activated by 1-oleoyl-2-acetyl-sn-glycerol (OAG), a diacylglycerol analogue, providing a possible activation mechanism of the channels by phospholipase C-linked receptors, independent of InsP3 and store depletion (7). Our results showed that human TRPC3 expressed in HEK293 formed a store-operated Ca2+ influx pathway in HEK293. This finding is in agreement with the results from several studies (3, 4), but it is in contrast with those from others (6, 7, 29, 30). These apparent conflicts in results could be, at least partly, explained by variations in TRPC3 expression levels in different experimental systems. A recent study by Vazquez et al. (14) found that the expression level of TRPC3 determined the gating mechanism of whether the channels were store-operated or not. At low levels of expression, TRPC3 channels were activated by store depletion whereas, at high expression levels, TRPC3 channels were no longer store-operated but could be activated through receptor-coupled phospholipase C (14). It is difficult to compare the relative expression level of TRPC3 in TRPC3-transfected HEK293 cells used in different laboratories because some important experimental procedures such as transfection methods were very different. However, we did find that other experimental conditions such as extracellular Ca2+, which was used to initiate store-operated Ca2+ influx, was also important for us to evaluate whether cells express store-operated Ca2+ influx. The store-operated Ca2+ influx mediated by TRPC3 could be much better resolved if a relative low concentration of external Ca2+ (0.5 mM), instead of ≈2 mM as in most other reports, was used to initiate Ca2+ influx. Low concentration of Ca2+ reduced the endogenous store-operated Ca2+ influx in HEK293 cells such that the Ca2+ influx due to TRPC3 could be identified easily.
The conclusion that PKG phosphorylation down-regulates TRPC3-mediated store-operated Ca2+ influx has important physiological implications. Store-operated Ca2+ influx is the predominant Ca2+ influx pathway in nonexcitable cells. The mechanism of how a decreased store-filling status leads to the opening of store-operated channels is still debatable. Three major hypotheses have been put forward, each of which has substantial experimental supports. The first hypothesis proposes the release of a putative calcium influx factor from endoplasmic reticulum (28, 31). The second hypothesis suggests direct conformational coupling of InsP3R in the endoplasmic reticulum with store-operated channels in the plasma membrane (3, 28). The third hypothesis proposes an addition of new channels onto the plasma membrane via secretion-like vesicle fusion (28). Recent studies suggest that store-operated Ca2+ influx is subjected to feedback regulation, in which a rise in cytosolic Ca2+ level serves to limit store-operated Ca2+ influx, thus protecting the cells from the detrimental effects of excessive Ca2+ (13, 32). At least in certain cell types, such as vascular endothelial cells, this feedback involves the inhibition of store-operated Ca2+ influx channels by the cGMP/PKG pathway (10, 11, 13, 33). An elevated cytosolic Ca2+ level increases the activity of NO synthase. The resultant rise in NO level stimulates the cGMP and PKG pathway, eventually reducing the store-operated Ca2+ influx in a negative feedback manner. This present study provides strong evidence for TRPC3 as a target of cGMP/PKG-mediated negative feedback inhibition.
A previous study by Thyagarajan et al. (34) showed that NO donor DEANO (2-(N,N-diethylamino)diazenolate-2-oxide) had no effect on store-operated Ca2+ influx in TRPC3-transfected HEK293 cells. This finding is inconsistent with our present results in which 8-BrcGMP was found to inhibit TRPC3-mediated store-operated Ca2+ influx. One possibility for this discrepancy is a low activity of guanylyl cyclase in HEK293 cells, which is the enzyme required for NO-stimulated cGMP production. A more likely explanation, however, is the difference in PKG activity in two experimental systems. Indeed, in our experiments (Fig. 3), 8-BrcGMP had no effect on the store-operated Ca2+ influx due to TRPC3 in cells that were not transfected with PKG genes. In the experiments by Thyagarajan et al., TRPC3-transfected HEK293 cells were not cotransfected with PKG, and the level of PKG was not examined. A point to note is that PKG is a relatively unstable enzyme. Even for cells originally expressing PKG, the expression level tends to decrease during cell culture condition. After several cell passages, PKG activity may diminish (35). For this reason, primarily isolated cells or the cells that are transfected with PKG genes may be a necessity for PKG-related studies.
In conclusion, the present study demonstrates that human TRPC3 expressed in HEK293 cells forms store-operated Ca2+ influx channels, the activity of which is inhibited by PKG. The inhibition is due to a direct phosphorylation of PKG on TRPC3 channels at position T11 and S263. It is likely that TRPC3 channels are the targets of NO/cGMP/PKG-mediated negative feedback inhibition on store-operated Ca2+ influx.
Supplementary Material
Acknowledgments
We thank Miss M. W. Leung for technical help. This study was supported by the Hong Kong Research Grant Council (CUHK4174/02M).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: PKG, protein kinase G; InsP3R, inositol 1,4,5-trisphosphate receptor; TRPC, canonical transient receptor potential; TRPC3, TRPC isoform 3; HEK, human embryonic kidney; VASP, vasodilator-stimulated phosphoprotein.
References
- 1.Minke, B. & Cook, B. (2002) Physiol. Rev. 82, 429-472. [DOI] [PubMed] [Google Scholar]
- 2.Zhu, X., Jiang, M., Peyton, M., Boulay, G., Hurst, R., Stefani, E. & Birnbaumer, L. (1996) Cell 85, 661-671. [DOI] [PubMed] [Google Scholar]
- 3.Kiselyov, K., Xu, X., Mozhayeva, G., Kuo, T., Pessah, I., Mignery, G., Zhu, X., Birnbaumer, L. & Muallem, S. (1998) Nature 396, 478-482. [DOI] [PubMed] [Google Scholar]
- 4.Boulay, G., Brown, D. M., Qin, N., Jiang, M., Dietrich, A., Zhu, M. X., Chen, Z., Birnbaumer, M., Mikoshiba, K. & Birnbaumer, L. (1999) Proc. Natl. Acad. Sci. USA 96, 14955-14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vazquez, G., Lievremont, J. P., Bird, G. S. & Putney, J. W., Jr. (2001) Proc. Natl. Acad. Sci. USA 98, 11777-11782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McKay, R. R., Szymechzek-Seay, C. L., Lievremont, J. P., Bird, G. S., Zitt, C., Jingling, E., Luckhoff, A. & Putney, J. W., Jr. (2000) Biochem. J. 351, 735-746. [PMC free article] [PubMed] [Google Scholar]
- 7.Hofmann, T., Obukhov, A. G., Schaefer, M., Harteneck, C., Gudermann, T. & Schultz, G. (1999) Nature 397, 259-263. [DOI] [PubMed] [Google Scholar]
- 8.Zhang, Z., Tang, J., Tikunova, S., Johnson, J. D., Chen, Z., Qin, N., Dietrich, A., Stefani, E., Birnbaumer, L. & Zhu, M. X. (2001) Proc. Natl. Acad. Sci. USA 98, 3168-3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Venkatachalam, K., Zheng, F. & Gill, D. L. (2003) J. Biol. Chem. 278, 29031-29040. [DOI] [PubMed] [Google Scholar]
- 10.Kwan, H. Y., Huang, Y. & Yao, X. (2000) J. Biol. Chem. 275, 6758-6763. [DOI] [PubMed] [Google Scholar]
- 11.Delkova, E. & Blatter, L. A. (2002) J. Physiol. 539, 77-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu, X., Star, R. A., Tortorici, G. & Muallem, S. (1994) J. Biol. Chem. 269, 12645-12653. [PubMed] [Google Scholar]
- 13.Yao, X. & Huang, Y. (2003) Trends Pharmacol. Sci. 24, 263-266. [DOI] [PubMed] [Google Scholar]
- 14.Vazquez, G., Wedel, B. J., Trebak, M., Bird, G. S. & Putney, J. W., Jr. (2003) J. Biol. Chem. 278, 21649-21654. [DOI] [PubMed] [Google Scholar]
- 15.Burkhardt, M., Glazova, M., Gambaryan, S., Vollkommer, T., Butt, E., Bader, B., Heermeier, K., Lincoln, TM., Walter, U. & Palmetshofer, A. (2000) J. Biol. Chem. 275, 33536-33541. [DOI] [PubMed] [Google Scholar]
- 16.Smolenski, A., Bachmann, C., Reinhard, K., Honig-Liedl, P., Jarchau. T., Hoschuetzky, H. & Walter, U. (1998) J. Biol. Chem. 273, 20029-20035. [DOI] [PubMed] [Google Scholar]
- 17.Tertyshnikova, S., Yan, X. W. & Fein, A. (1998) J. Physiol. 512, 89-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cavallini, L., Coassin, M., Borean, A. & Alexandre, A. (1996) J. Biol. Chem. 271, 5545-5551. [DOI] [PubMed] [Google Scholar]
- 19.Ruth, P., Wang, G. X., Boekhoff, I., May, B., Pfeifer, A., Penner, R., Korth, M., Breer, H. & Hofmann, F. (1993) Proc. Natl. Acad. Sci. USA 90, 2623-2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rooney, T. A., Joseph, S. K., Queen, C. & Thomas, A. P. (1996) J. Biol. Chem. 271, 19817-19825. [DOI] [PubMed] [Google Scholar]
- 21.Nilius, B. & Droogman, G. (2003) Endothelium 10, 5-15. [DOI] [PubMed] [Google Scholar]
- 22.Montell, C., Birnbaumer, L. & Flockerzi, V. (2002) Cell 108, 595-598. [DOI] [PubMed] [Google Scholar]
- 23.Yamaguchi, H., Matsushita, M., Nairn, A. C. & Kuriyan, J. (2001) Mol. Cell 7, 1047-1057. [DOI] [PubMed] [Google Scholar]
- 24.Xu, H., Zhao, H., Tian, W., Yoshida, K., Roullet, J. B. & Cohen, D. M. (2003) J. Biol. Chem. 278, 11520-11527. [DOI] [PubMed] [Google Scholar]
- 25.Xu, F., Satoh, E. & Iijima, T. (2003) Br. J. Pharmacol. 140, 413-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clapham, D. E., Runnels, L. W. & Strubing, C. (2001) Nat. Rev. Neurosci. 2, 387-396. [DOI] [PubMed] [Google Scholar]
- 27.Venneken, R., Voets, T., Bindels, R. J. M., Droogmans, G. & Nilius, B. (2002) Cell Calcium 31, 253-264. [DOI] [PubMed] [Google Scholar]
- 28.Zitt, C., Halaszovich, C. R. & Luckhoff, A. (2002) Prog. Neurobiol. 66, 243-264. [DOI] [PubMed] [Google Scholar]
- 29.Ma, H. T., Patterson, R. L., Rossum D. B., Birnbaumer, L., Mikoshiba, K. & Gill, D. L. (2000) Science 287, 1647-1651. [DOI] [PubMed] [Google Scholar]
- 30.Zhu, X., Jiang, M. & Birnbaumer, L. (1998) J. Biol. Chem. 273, 133-142. [DOI] [PubMed] [Google Scholar]
- 31.Kim, H. Y. & Hanley M. R. (1999) Mol. Cell 9, 326-332. [PubMed] [Google Scholar]
- 32.Choy, J. C., Granville, D. J., Hunt, D. W. & McManus, B. M. (2001) J. Mol. Cell. Cardiol. 33, 1673-1690. [DOI] [PubMed] [Google Scholar]
- 33.Kwan, H. Y., Leung, P. C., Huang, Y. & Yao, X. (2003) Circ. Res. 92, 286-292. [DOI] [PubMed] [Google Scholar]
- 34.Thyagarajan, B., Poteser, M., Romanin, C., Kahr, H., Zhu, M. X. & Groschner, K. (2001) J. Biol. Chem. 276, 48149-48158. [DOI] [PubMed] [Google Scholar]
- 35.Draijer, R., Vaandrager, A. B., Nolte, C., Jonge, H. R., Walter, U. & van Hinsbergh, V. M. M. (1995) Circ. Res. 77, 897-905. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.