Abstract
The use of the herbicide paraquat (1,1′-dimethyl-4,4′-bipyridylium dichloride; PQ) has been fiercely challenged due to its severe acute toxicity, putative neurotoxicity after long-term exposure and lack of antidotes. Breakthrough research on PQ is therefore required for an effective risk control and to allow a safer use of PQ in the future. The silencing or inhibition of quinone oxidoreductase 2, a NAD(P)H-independent flavoenzyme, was shown to significantly attenuate PQ toxicity in vitro, in primary pneumocytes and astroglial U373 cells, and to strongly antagonize PQ-induced systemic toxicity and animal mortality. The novel results reported in this issue of BJP, added to recent findings using sodium salicylate and lysine acetylsalicylate, in which full survival of PQ-intoxicated rats was also achieved, open the door for new preventative and therapeutic strategies that may lead to safer use of this effective pesticide.
Keywords: paraquat, human poisoning, Parkinson's disease, quinone oxidoreductase 2, survival
Comment
In the present issue of the British Journal of Pharmacology, the research group supervised by Vincenzo Mollace reports a new finding of great interest concerning the antidotal effect against paraquat (PQ)-induced toxicity, attained through inhibition of quinone oxidoreductase 2 (QR2) (Janda et al., 2012). This research may lead to safer paraquat use.
The introduction of PQ (1,1′-dimethyl-4,4′-bipyridylium dichloride) as an herbicide in 1962 has brought to the world one of the most controversial and studied pesticides over the last 50 years. At first, it appeared as a cheap and extremely effective, fast-acting and non-selective foliage-applied contact herbicide, killing a wide range of grasses and dicotyledonous weeds, and its use rapidly became widespread worldwide. However, it soon became notorious due to its toxic effects; human poisonings with this compound were frequently fatal due to its severe acute toxicity and lack of antidotes. For this reason, PQ was held responsible for thousands of deaths from both accidental and voluntary ingestion (the majority of the cases), as well as from dermal exposure (Dinis-Oliveira et al., 2008). In July 2007, the European Union (EU) Court of First Instance annulled the Directive 2003/112 authorizing the use of PQ in the EU. Among other reasons, concerns about potential links between PQ and the Parkinson's disease and the requirement of human protection, which prohibits any exposure higher than the acceptable operator exposure level, as well as the protection of animal health, dictated the ban. In parallel, several non-governmental organizations were involved in fierce campaigns for a global ban of PQ, and some countries are restricting its use or applying a phase-out procedure.
With such a dark shadow over this herbicide, PQ research is becoming a complicated issue, both for scientists and for grant providers. Is it worth investigating or providing grants for the research on a compound that seems to be doomed? Although some research groups, including our own, have already suffered this stigma in the financial support for PQ research, in our opinion, this area of research is more important than ever. It is noteworthy that PQ is still registered and applied in over 100 countries. The reasons for this are obvious and well beyond financial issues, namely its rapid action upon contact with the leaves, its lack of effect on roots and rhizomes, thus holding the soil together and preventing soil erosion, and its rapid deactivation by strong sorption to soil, thus limiting movement by leaching or surface run-off, decreasing possible ecotoxicological effects and allowing rapid reseeding or replanting after the killing of weeds (Bromilow, 2004). It is also worth mentioning that PQ can be applied safely when used according to the manufacturer's guidelines (Hart, 1987). PQ is highly hydrophilic and thus not absorbed through intact skin. Aerosolized PQ particles are large in diameter and thus do not reach the human alveoli when inhaled. Indeed, typical spray equipment generates droplet sizes with a median volume diameter over 100 µm (Dinis-Oliveira et al., 2008).
Looking at the number of PQ publications per year in the National Library of Medicine database PubMed® (Figure 1), one rapidly realizes that, within this century, it doubled from 2000 (134) to 2007 (266) and then stabilized, but curiously the number of publications each year concerning PQ and human poisoning was fairly stable until 2007, after which it showed a sustained increase. Even more interesting is the number of publications per year relating PQ to Parkinson's disease. In 2000, this number was residual (3) and then steadily increased up to 10 times in 2007 (30), to stabilize thereafter, although another publication peak appears in 2011. These data suggest that fatalities due to PQ poisoning continue to be a heavy burden worldwide and that the possible involvement of PQ in the development or aggravation of Parkinson's disease is an area of increasing research interest.
Figure 1.
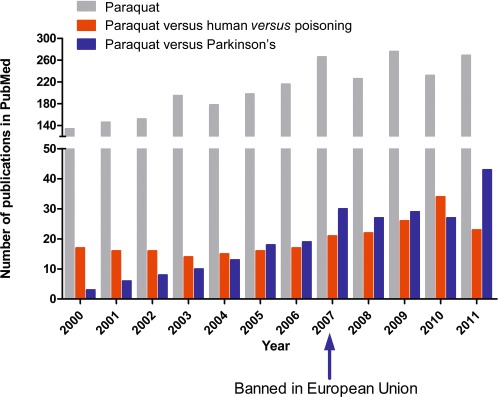
Number of publications related to paraquat per year, since 2000, in the National Library of Medicine database PubMed®.
It has long been postulated that NAD(P)H-dependent diaphorases are responsible for the in vivo reduction of PQ to the free radical monocation PQ.+, which is then rapidly re-oxidized (returning to its original form) in the presence of O2 with subsequent generation of superoxide anion radicals and other reactive oxygen species that are responsible for PQ toxicity (Dinis-Oliveira et al., 2008). On the other hand, as now observed, the inhibition or silencing of the NAD(P)H-independent flavoenzyme QR2 significantly attenuates PQ toxicity in vitro, in primary pneumocytes and astroglial U373 cells, and strongly antagonizes PQ-induced systemic toxicity and animal mortality (Janda et al., 2012). Most importantly, Wistar rats administered a specific QR2 inhibitor 2 h after PQ, which was subsequently maintained by several supporting doses, all survived an otherwise fatal dose of PQ (Janda et al., 2012). The novel results reported in this issue of the BJP add to recent findings, which demonstrated that PQ-intoxicated rats all survived if treated with sodium salicylate or lysine acetylsalicylate (Dinis-Oliveira et al., 2007, 2009), and open the door for new preventative and therapeutic strategies to be applied to PQ-intoxicated patients. Although this may constitute a questione disputate, breakthrough research on PQ, as in the present case, may ultimately lead to an effective risk control and allow the safer use of PQ in the future. Many other pesticides, such as organophosphates, are involved in a high number of voluntary intoxications, but do not pose a problematic treatment scenario and, therefore, continue to serve mankind. Hopefully, this may also happen with PQ, if further research significantly increases its safety.
Glossary
- EU
European Union
- PQ
paraquat
- QR2
quinone oxidoreductase 2
References
- Bromilow RH. Paraquat and sustainable agriculture. Pest Manag Sci. 2004;60:340–349. doi: 10.1002/ps.823. [DOI] [PubMed] [Google Scholar]
- Dinis-Oliveira RJ, Sousa C, Remião F, Duarte JA, Navarro AS, Bastos ML, et al. Full survival of paraquat-exposed rats after treatment with sodium salicylate. Free Radic Biol Med. 2007;42:1017–1028. doi: 10.1016/j.freeradbiomed.2006.12.031. [DOI] [PubMed] [Google Scholar]
- Dinis-Oliveira RJ, Duarte JA, Sánchez-Navarro A, Remião F, Bastos ML, Carvalho F. Paraquat poisonings: mechanisms of lung toxicity, clinical features, and treatment. Crit Rev Toxicol. 2008;38:13–71. doi: 10.1080/10408440701669959. [DOI] [PubMed] [Google Scholar]
- Dinis-Oliveira RJ, Pontes H, Bastos ML, Remião F, Duarte JA, Carvalho F. An effective antidote for paraquat poisonings: the treatment with lysine acetylsalicylate. Toxicology. 2009;255:187–193. doi: 10.1016/j.tox.2008.10.015. [DOI] [PubMed] [Google Scholar]
- Hart TB. Paraquat – a review of safety in agricultural and horticultural use. Hum Toxicol. 1987;6:13–18. doi: 10.1177/096032718700600103. [DOI] [PubMed] [Google Scholar]
- Janda E, Parafati M, Aprigliano S, Carresi C, Visalli V, Sacco I, et al. The antidote effect of quinone oxidoreductase 2 (QR2) inhibitor on paraquat-induced toxicity in vitro and in vivo. Br J Pharmacol. 2012;168:46–59. doi: 10.1111/j.1476-5381.2012.01870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


