Abstract
BACKGROUND AND PURPOSE
Escitalopram, the S(+)-enantiomer of citalopram is the most selective 5-HT reuptake inhibitor approved. Although all 5-HT selective reuptake inhibitors (SSRIs) increase extracellular levels of 5-HT ([5-HT]ext). some also enhance, to a lesser extent, extracellular levels of noradrenaline ([NA]ext). However, the mechanisms by which SSRIs activate noradrenergic transmission in the brain remain to be determined.
EXPERIMENTAL APPROACH
This study examined the effects of escitalopram, on both [5-HT]ext and [NA]ext in the frontal cortex (FCx) of freely moving wild-type (WT) and mutant mice lacking the 5-HT transporter (SERT−/−) by using intracerebral microdialysis. We explored the possibilities that escitalopram enhances [NA]ext, either by a direct mechanism involving the inhibition of the low- or high-affinity noradrenaline transporters, or by an indirect mechanism promoted by [5-HT]ext elevation. The forced swim test (FST) was used to investigate whether enhancing cortical [5-HT]ext and/or [NA]ext affected the antidepressant-like activity of escitalopram.
KEY RESULTS
In WT mice, a single systemic administration of escitalopram produced a significant increase in cortical [5-HT]ext and [NA]ext. As expected, escitalopram failed to increase cortical [5-HT]ext in SERT−/− mice, whereas its neurochemical effects on [NA]ext persisted in these mutants. In WT mice subjected to the FST, escitalopram increased swimming parameters without affecting climbing behaviour. Finally, escitalopram, at relevant concentrations, failed to inhibit cortical noradrenaline and 5-HT uptake mediated by low-affinity monoamine transporters.
CONCLUSIONS AND IMPLICATIONS
These experiments suggest that escitalopram enhances, although moderately, cortical [NA]extin vivo by a direct mechanism involving the inhibition of the high-affinity noradrenaline transporter (NET).
Keywords: antidepressant, behaviour, escitalopram, intracerebral microdialysis, frontal cortex, 5-HT selective reuptake inhibitor, 5-HT transporter
Introduction
Selective 5-HT reuptake inhibitors (SSRIs) have proved to be effective in the treatment of depression. This class of antidepressant drugs exerts their therapeutic effects by inhibiting the reuptake of 5-HT, thereby prolonging its duration of action at the postsynaptic level (Frazer, 2001). Although many patients benefit from SSRIs, approximately 50% of depressed individuals do not respond adequately to these agents (Berton and Nestler, 2006). The SSRI escitalopram is the active S(+)-enantiomer of the racemic molecule citalopram. (Sanchez et al., 2003a; Jacquot et al., 2007). In vitro studies on embryonic kidney cells heterologously expressing the human monoaminergic transporters have demonstrated that the affinity of escitalopram for the 5-HT transporter (SERT) is much greater than for the noradrenaline transporter (NET) or the dopamine transporter (DAT) (Ki values: 1.1; 7841 and 27 410 nM respectively) (Owens et al., 2001; transporter and receptor nomenclature follow Alexander et al., 2011). These data were confirmed in functional studies from rat brain synaptosomes showing that escitalopram blocked the NET and DAT with marginal potency (Sanchez et al., 2003a). Consistent with its potent inhibitory action on the SERT, in vivo studies have reported that an acute administration of escitalopram suppressed the firing rate of dorsal raphe (DR) 5-HT neurons in rats with an ED50 of 60 µg·kg−1 (El Mansari et al., 2005). Escitalopram was also shown to enhance extracellular 5-HT levels in the rat frontal cortex (FCx) (Mork et al., 2003) and produce antidepressant/anxiolytic-like effects in various animal models (Sanchez et al., 2003a,b). Interestingly, these electrophysiological, neurochemical and behavioural responses are partially inhibited by R(–)-citalopram (Mork et al., 2003; Sanchez et al., 2003a,b; El Mansari et al., 2005). After sustained administration, escitalopram produces a faster desensitization of somatodendritic 5-HT1A autoreceptors in the DR than citalopram (El Mansari et al., 2005), an effect that probably accounts for the robust increase in cortical extracellular 5-HT levels ([5-HT]ext) observed after only 2 weeks of treatment (Ceglia et al., 2004). In humans, escitalopram demonstrates a rapid onset of antidepressant action, and recent data suggest that it may be more effective than other SSRIs and at least as effective as dual 5-HT/noradrenaline reuptake inhibitors in the treatment of major depression (Kennedy et al., 2009; Kornstein et al., 2009; Garnock-Jones and McCormack, 2010). Interestingly, SSRIs, such as paroxetine, fluoxetine and citalopram can also inhibit uptake of [3H]noradrenaline in rat cortical synaptosomes in vitro (Hughes and Stanford, 1996) and consequently enhance extracellular noradrenaline levels ([NA]ext) in the FCx and hippocampus after acute administration in rodents (Jordan et al., 1994; Shachar et al., 1997; Millan et al., 2001; Beyer et al., 2002; Bymaster et al., 2002; Koch et al., 2002; David et al., 2003; Kobayashi et al., 2008). Although this property seems to be a common feature of SSRIs in vivo in rodents, it is still unknown whether SSRIs and more particularly escitalopram enhance the level of [NA]ext by a direct mechanism involving the inhibition of the high-affinity noradrenaline transporter (NET), or by an indirect mechanism in response to increases in [5-HT]ext.
Anatomical and functional studies have demonstrated that 5-HT and noradrenaline have reciprocal interactions at both somatodendritic and nerve terminal levels. The locus coeruleus (LC), the major noradrenergic brainstem nucleus, sends projections into the DR, while the DR projects into the LC, creating ample opportunity for cross-modulation (Pudovkina et al., 2002; Guiard et al., 2008a). The physiological importance of such connections is demonstrated, for example, by the observation that SSRIs modulate the activity of noradrenergic neurons. Escitalopram, but also the other SSRIs, can decrease the spontaneous neuronal activity of LC noradrenergic neurons through the local activation of postsynaptic 5-HT2A/C receptors (Szabo and Blier, 2001a,b; Dremencov et al., 2007; Miguelez et al., 2009). Since it is difficult to reconcile these electrophysiological data with the fact that SSRIs increase [NA]ext at nerve terminals, the present study was aimed to evaluate the effects of an acute administration of escitalopram on cortical extracellular levels of both 5-HT and noradrenaline by using intracerebral microdialysis in awake, freely- moving wild-type (WT) and also in knockout mice lacking the 5-HT transporter (SERT−/−).
In addition to the high-affinity NET and SERT, other categories of transporters have recently been implicated in 5-HT and noradrenaline clearance in the brain. Organic cation transporters (OCTs; Breidert et al., 1998; Amphoux et al., 2006; Koepsell et al., 2007) and the plasma membrane monoamine transporter (PMAT; Engel et al., 2004; Engel and Wang, 2005) have been shown in vitro to transport these monoamines. OCT2, OCT3 and PMAT, in particular, are expressed in various brain areas including the cortex (Engel et al., 2004; Vialou et al., 2004; 2008; Dahlin et al., 2007), and a direct role in 5-HT and noradrenaline clearance in vivo in the mouse brain was recently demonstrated for OCT2 (Bacq et al., 2011). Interestingly, some of these transporters were shown to be moderately inhibited by antidepressants in vitro (Kekuda et al., 1998; Wu et al., 2000; Haenisch and Bönisch, 2010), raising the possibility that they could be secondary targets of these drugs. Indeed, it was recently suggested that a functionally up-regulated alternative transporter for 5-HT may prevent extracellular 5-HT from attaining levels sufficiently high enough to trigger the adaptive neurochemical events necessary for the antidepressant activity of SSRIs (Baganz et al., 2008). In support of this hypothesis, systemic administration of the OCT blocker decynium 22 (D22) was found to decrease 5-HT clearance and exhibit antidepressant-like activity in 5-HT transporter knockout (SERT−/−) mice (Baganz et al., 2008).
The main results presented in this present study show that the acute systemic administration, or local injection of escitalopram in the FCx, significantly increased both the extracellular levels of 5-HT and noradrenaline in the FCx of WT mice. Unexpectedly, the ability of escitalopram to increase cortical noradrenaline levels remained effective in SERT−/− mice, but not in SERT+/+ mice administered with desipramine.In addition, escitalopram, at physiological concentrations, failed to inhibit cortical noradrenaline and 5-HT ex vivo uptake by the low-affinity monoamine transporters. These data suggest that this SSRI, previously considered highly selective for the SERT, may also non-selectively block the NET, when administered at high doses.
Methods
Animals
All animal care and experimental procedures were conducted in conformity with the institutional guidelines in compliance with national policy (Council directive #87–848, October 19, 1987, Ministère de l'Agriculture et de la Forêt, Service Vétérinaire de la Santé et de la Protection Animale, permissions #005037 to AM Gardier). Male WT and SERT−/− mice, 4–6 months old, weighing 25–35 g, were used in this study. Mutant mice originally produced by homologous recombination (Bengel et al., 1998) were housed in our animal care facility in groups of three to six and kept under standard conditions (room temperature of 22–23°C, 12:12 light–dark cycle, free access to food and water). Mice were tested between 9:00 a.m. and 5:00 p.m. during the light phase.
Drugs and administration
Escitalopram oxalate (H. Lundbeck, Paramus, NJ, USA) was administered by the i.p. route at a dose of 4, 8 or 16 mg·kg−1. In reverse microdialysis experiments, escitalopram (0.5 µM) or desipramine (50 or 100 µM) was dissolved in artificial CSF (aCSF; for composition see below) and then perfused locally into the FCx. The concentration of escitalopram was chosen on the basis of previous microdialysis experiments showing that the perfusion of 1 µM of the racemic compound (i.e. citalopram) into the FCx produced a significant increase in local extracellular 5-HT levels in mice (Guilloux et al., 2006). The concentration of desipramine was selected on the basis of its previously reported enhancing effect on extracellular noradrenaline levels in mice (Fisher et al., 2007).
Microdialysis procedure
Mice anaesthetized with chloral hydrate (400 mg·kg−1, i.p.) were implanted with probes (CMA7 model, Carnegie Medicin, Stockholm, Sweden) located in the FCx (stereotaxic coordinates in mm from bregma) (Hof et al., 2000): A =+1.6, L =+1.3, V =−1.6; A, anterior; L, lateral; and V, ventral (Guilloux et al., 2006). Animals were allowed to recover from the surgery overnight. The next day, ≍20 h after surgery, the probes were continuously perfused with aCSF (composition in mM: NaCl 147, KCl 3.5, CaCl2 1.26, MgCl2 1.2, NaH2PO4 1.0, pH 7.4 ± 0.2) at a flow rate of 1.0 µL·min−1 in the cortex using a CMA/100 pump (Carnegie Medicin). The animals were awake and freely moving in their cage during the perfusion procedures. The noradrenaline and 5-HT microdialysis samples were collected from independent animals. One hour after the start of aCSF perfusion stabilization period, four fractions were collected (one every 20 min) to measure the basal monoamine values (mean ± SEM corresponding to B0 at t0) calculated for each mouse before systemic administration or local perfusion of vehicle, escitalopram or desipramine. Subsequent dialysate samples were then collected for a 0–120 or 0–240 min post-treatment period and analysed for 5-HT and noradrenaline by a HPLC system (XL-ODS, 4.6 × 75 mm, particle size 3 µm; Beckman, Fullerton, CA, USA) coupled to an amperometric detector (1049 A, Hewlett-Packard, Les Ulis, France). The mobile phase for 5-HT and noradrenaline contained 107 or 100 mM NaH2PO4, 140 or 151 µM disodium EDTA; 0.77 or 3 mM I-octanesulphonic acid, respectively, and 20% (v/v) methanol (pH adjusted between 4.1 and 4.3 with phosphoric acid). Its flow rate through the HPLC column was set at 0.7 mL·min−1 using a 118 pump (Beckman). The limit of sensitivity for 5-HT or noradrenaline was ≍0.5 fmol per sample (signal-to-noise ratio = 2). At the end of the experiments, localization of microdialysis probes was verified histologically (Bert et al., 2004; Guilloux et al., 2006).
Forced swimming test (FST) procedure
FST was performed in groups of mice, separate from those used in the microdialysis experiments. The FST procedure was modified to enhance the sensitivity for detecting the putative antidepressant-like activity of drugs (Porsolt et al., 1977; Holick et al., 2008). Mice were briefly placed into clear plastic buckets (20 cm in diameter and 23 cm deep), filled two-thirds with water at 23–25°C. Automated scoring in the FST was done using automated software X'PERT FST developed by Bioseb (Vitrolles, France) to assess antidepressant-like activity in mice (Rainer et al., 2011). Each bucket was instrumented with a sensor recording the vibrations due to movements of the mice, and a video was recorded from above. The system synchronizes mouse position, data calculated from the video recording and the vibration data. This information allows the system to compute characteristic values (based on speed of the animal, as well as different frequencies and power of the vibrations (via a fast Fourier transform calculation), describing the animal behaviour every second. Dependent variables were mobility, swimming and climbing duration. This test was performed 30 min after drug administration. The mobility duration is an index of antidepressant-like activity. Swimming behaviour relies on the 5-hydroxytryptaminergic system and climbing behaviour on the noradrenergic system in mice (Dulawa et al., 2004; Holick et al., 2008).
Monoamine uptake
Male WT mice (Janvier, St. Berthevin, France) were killed by decapitation, and the FCx was dissected, minced on ice and resuspended in 10 volumes (w/v) of ice-cold sucrose (0.32 M). The cells were dissociated by filtering through nylon mesh of decreasing pore size (180–60 µm; Small Parts, Miramar, FL) and resuspended in ice-cold sucrose. The cellular suspension was preincubated 10 min at 37°C in 3 vol of Krebs Ringer HEPES (KRH) LiCl buffer (25 mM HEPES–KOH, pH 7.4, 125 mM LiCl, 4.8 mM KCl, 5.6 mM D(+)-glucose, 1.2 mM CaCl2, 1.2 mM KH2PO4 and 1.2 mM MgSO4). To determine specifically the potential role played by low-affinity monoamine transporters in the effects of escitalopram on 5-HT and noradrenaline, we examined the uptake of radiolabelled 5-HT and noradrenaline in cellular suspensions treated with inhibitors of the high-affinity monoamine transporters such as 10 µM of venlafaxine (Tocris Bioscience, Bristol, UK), 5 µM of desipramine, 100 µM of GBR12935 or 10 µM of pargyline (Sigma-Aldrich, St. Louis, MO). Reserpine (10 µM was used to block the vesicular transporter VMAT2 (Sigma-Aldrich). These cellular suspensions were incubated for 15 min at 37°C in the same buffer supplemented with 10 µM [3H]noradrenaline, [3H]5-HT (Perkin Elmer, Boston, MA. The effect of escitalopram on endogenous low-affinity transporter activity was evaluated in the presence of increasing concentrations of this antidepressant (0.1–10 mM) during preincubation and uptake. The reaction was terminated by rapid filtration through Unifilter-96 GF/C filters (Perkin Elmer). The cells were washed four times with 3 vol of uptake buffer, and the radioactivity retained on the filters was assessed by liquid scintillation counting. The protein concentration in tissue extracts was measured by the method of Bradford standardized with BSA. Low-affinity transporter-mediated uptake was quantified by inhibition with the specific inhibitor decynium 22 (D22: 1,1′-diethyl-2,2′-cyanine iodide) (500 µM; Sigma-Aldrich). Data are expressed as mean ± SEM of low-affinity uptake from one experiment performed in triplicate. D22-sensitive uptake rates were defined by subtracting uptake in the presence of D22 from the uptake observed in its absence (D22 uptake fraction ± escitalopram was subtracted from the control fraction ± escitalopram) and were expressed as mean ± SEM of D22-sensitive uptake from three to four independent experiments.
Data analysis
Statistical analyses were performed using the computer software StatView 5.0. (Abacus Concepts, Inc., Berkley, CA). Statistical comparisons between basal [5-HT]ext and [NA]ext in WT and SERT−/− mice were made using the nonparametric Newman–Keuls test. All others values for microdialysis studies were calculated as percentage change at each time point relative to the average of four baseline values. Significant differences were determined on AUC values using Student's t-test or a one- or two-way anova followed by Fisher's protected last significance difference (PLSD) post hoc test when appropriate. For behavioural studies, values were calculated on swimming or climbing duration, and significant differences were determined on this parameter using a one-way anova followed by Fisher's PLSD post hoc test when appropriate. With respect to the uptake experiments, one-way anova followed by Fisher's PLSD post hoc was applied. The level of statistical significance was set at P < 0.05.
Results
Basal extracellular levels of 5-HT ([5-HT]ext) and of noradrenaline ([NA]ext) in the frontal cortex of WT and SERT−/− mice
The effects of systemic administration of escitalopram on [5-HT]ext and [NA]ext in the FCx were evaluated by conventional microdialysis. Table 1 shows the mean ± SEM of basal cortical [5-HT]ext and [NA]ext levels [in fmol·(20 µL)−1] in WT and SERT−/− mice. 5-HT levels were sixfold higher in SERT−/− than in WT mice (P < 0.001), whilst noradrenaline levels were twofold lower (P < 0.001).
Table 1.
Basal [5-HT]ext and [NA]ext values in the FCx of WT and SERT−/− mice
| Basal/mice | WT | SERT−/− |
|---|---|---|
| [5-HT]ext | 2.16 ± 0.19 (n= 32) | 13.29 ± 3.02*** (n = 16) |
| [NA]ext | 6.77 ± 0.57 (n= 35) | 3.08 ± 0.78*** (n = 16) |
Significantly different from WT mice.
Effects of systemic administration of escitalopram on cortical [5-HT]ext and swimming time in the FST in WT mice
A dose–response experiment was undertaken to determine the effects of systemic administration of escitalopram on [5-HT]ext and [NA]ext in the FCx (Figures 1 and 2). One-way anova on 5-HT outflow in the FCx measured from AUC values calculated during the 120 min post-treatment period revealed significant treatment-related effects (F[3,31]= 12.19; P < 0.001). Escitalopram increased cortical [5-HT]ext at the doses of 8 and 16 mg·kg−1, i.p., compared with vehicle-treated mice (P < 0.001 and P < 0.001, respectively) (time course, Figure 1A). The maximal increase in AUC values was of 378 ± 80% of baseline for the dose of 16 mg·kg−1 (Figure 1B).
Figure 1.
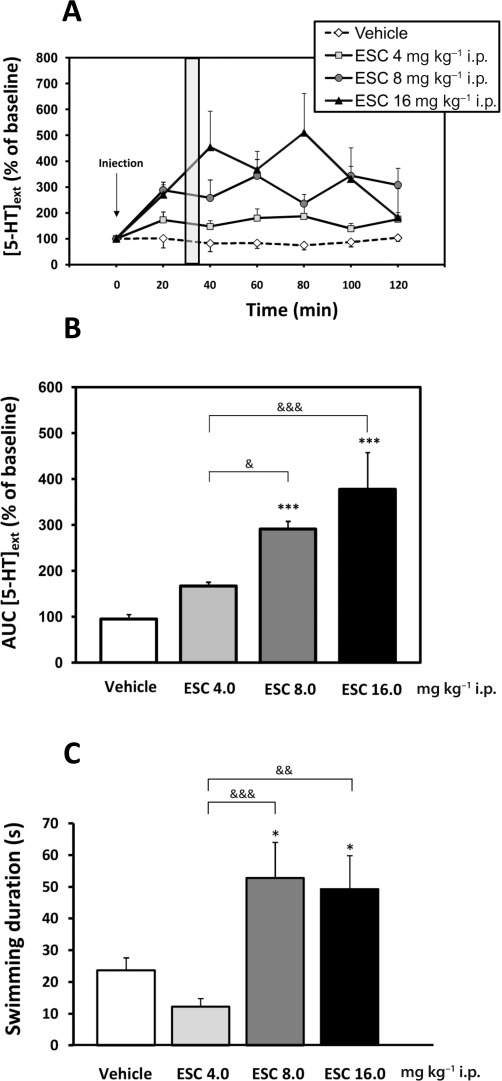
Effects of acute escitalopram administration on extracellular 5-HT levels in the FCx and on swimming behaviour in the FST in WT mice. Mice received either vehicle or escitalopram (ESC; 4, 8, 16 mg·kg−1, i.p.). (A) Time course. Data are mean± SEM. Values of [5-HT]ext in the FCx are expressed as percentages of baseline (B0) following exposure to vehicle or escitalopram (n= 7–8 mice per group). Baseline 5-HT in vehicle, escitalopam 4, 8 and 16 mg·kg−1, i.p., were 2.65 ± 0.21, 2.21 ± 0.18, 2.11 ± 0.09 and 1,6 ± 0.29 fmol·(20 µL)−1 respectively. The grey area indicates the duration time of the FST (i.e. 6 min). Microdialysis and behavioural experiments were carried out with the same administration protocol, and the swimming parameter in the FST was measured, in a separate group of mice, at the maximum effect of escitalopram 8 mg·kg−1. (B) AUC values (mean ± SEM) were calculated for the amount of 5-HT outflow collected during 0–120 min post treatment for escitalopram. (C) Antidepressant-like effect of the escitalopram on the swimming time in the FST in WT mice (n= 9–10 mice per group). *P < 0.05; ***P < 0.001: significantly different from vehicle-injected mice. &P < 0.05; &&P < 0.01; &&&P < 0.001 compared with the escitalopram (8; 16 mg·kg−1, i.p.) group and escitalopram 4 mg·kg−1, i.p. (one-way anova, Fisher's PLSD post hoc test).
FST was carried out in an independent group of mice during the peak of the escitalopram effect (30 min after the drug; see grey area in Figure 1A). The mobility and swimming times were measured during the last 4 min of the 6 min test in mice. One-way anova revealed a significant effect of escitalopram on mobility (F[3,35]= 6.26; P= 0.01) and swimming times (F[3,35]= 6.26; P= 0.01; Figure 1C), suggesting an antidepressant-like activity in this test. Escitalopram induced a statistically significant increase in the mobility duration at the doses of 8 and 16 mg·kg−1, i.p. (52.9 ± 11.2 s and 49.4 ± 10.5 s, respectively), when compared with vehicle-treated group (24.0 ± 4.0 s).
Effects of systemic administration of escitalopram on cortical [NA]ext levels and climbing time in the FST in WT mice
One-way anova on noradrenaline outflow in the FCx revealed significant treatment-related effects (F[2,34]= 11.87; P < 0.001). Escitalopram increased cortical [NA]ext at the doses of 4 and 8 mg·kg−1, i.p., compared with vehicle-treated mice (P < 0.01 and P < 0.001, respectively) (time course, Figure 2A). The maximal increase in AUC values was 148 ± 14% of baseline at an escitalopram dose of 8 mg·kg−1, i.p. (Figure 2B).
Figure 2.

Effects of acute escitalopram administration on [NA]ext in the FCx and on the climbing time in WT mice in the FST. Mice received either the vehicle or escitalopram (4 and 8 mg·kg−1, i.p.). (A) Time course. Data are mean ± SEM values of [NA]ext in the FCx expressed as percentages of baseline (B0) following exposure to vehicle or escitalopram (ESC; n= 7–8 mice per group). Baseline [NA]ext in vehicle, escitalopam 4 and 8 mg·kg−1, i.p., were 6.23 ± 0.29, 8.01 ± 0.91 and 6.01 ± 0.76 fmol·(20 µL)−1 respectively. The grey area indicates the duration time of the FST (i.e. 6 min). Microdialysis and behavioural experiments were carried out with the same protocol, and the climbing parameter in the FST was measured, in a separate group of mice, at the maximum effect of escitalopram 8 mg·kg−1. (B) AUC values (means ± SEM) were calculated for the amount of noradrenaline outflow collected during 0–120 min post treatment for escitalopram. (C) Antidepressant-like effect of escitalopram on climbing time in the FST in WT mice (n= 9–10 mice per group). **P < 0.01; ***P < 0.001: significantly different from vehicle-injected mice (one-way anova, Fisher's PLSD post hoc test).
In contrast, one-way anova revealed no effect of escitalopram on climbing behaviour in the FST (F[2,27]= 3.1; P= 0.06) (Figure 2C).
Effects of systemic administration of escitalopram on cortical [5-HT]ext and [NA]extin SERT−/− mice and WT littermates
To test the hypothesis that the enhancement of noradrenergic transmission induced by escitalopram resulted from the blockade of the NET at nerve terminals, the neurochemical effects of escitalopram were studied by using intracerebral microdialysis in mice lacking the SERT (SERT−/− mice).
Figure 3 compares the effects of an acute administration of escitalopram (8 mg·kg−1, i.p.) on cortical [5-HT]ext and [NA]ext in WT and SERT−/− mice. A two-way anova (treatment × genotype) on 5-HT outflow, measured from AUC calculated during a 120 min post-treatment period, revealed not only a significant effect of treatment (F[1,29]= 76.26; P= 0.001) and genotype factors (F[1,29]= 55,19; P= 0.001) but also a significant treatment × genotype interaction (F[1,29]= 61.97; P= 0.001). As expected, when the main target of the SSRIs, the SERT, was genetically inactivated, escitalopram failed to increase cortical [5-HT]ext in SERT−/− mice (Figure 3A,C).
Figure 3.
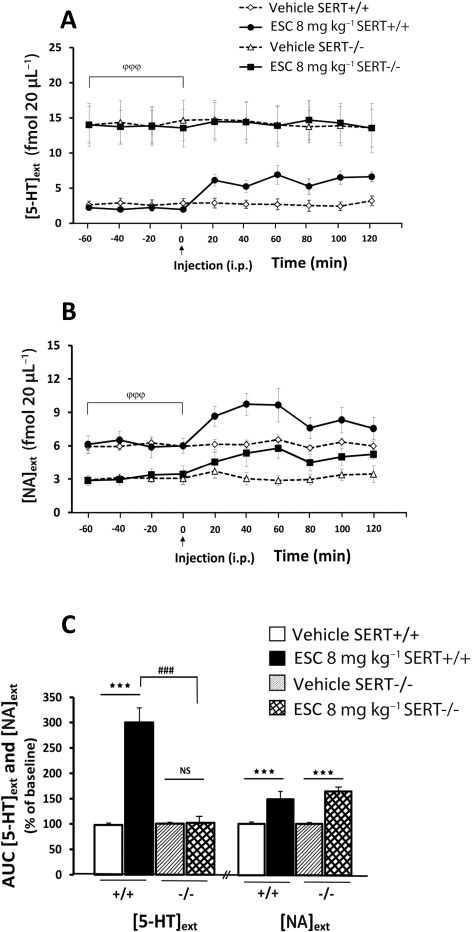
Effect of systemic administration of escitalopram on [5-HT]ext and [NA]extin the FCx in WT and SERT−/− mice. (A, B) Time course. Data are mean ± SEM values of [5-HT]ext and [NA]ext expressed as percentages of baseline (B0) following exposure to vehicle or escitalopram (ESC; n= 5–7 mice per group). Arrow indicates the time at which the injection of either vehicle in WT or SERT−/− mice, or escitalopram 8 mg·kg−1, i.p., in WT or SERT−/− mice was performed. Baseline [5-HT]ext values were 2.65 ± 0.21 and 2.11 ± 0.09 fmol·(20 µL)−1 in WT mice administered vehicle and escitalopam 8 mg·kg−1, i.p., respectively, and 13.01 ± 3.28 and 13.69 ± 2.04 fmol·(20 µL)−1 in SERT−/− mice. Baseline [NA]ext values were 6.35 ± 0.81 and 6.62 ± 1.13 fmol·(20 µL)−1 in WT mice administered vehicle and escitalopram 8 mg·kg−1, i.p., respectively, and 2.93 ± 0.87 and 3.15 ± 0.97 fmol·(20 µL)−1 in SERT−/− mice. (C) AUC values (means ± SEM) were calculated for the amount of 5-HT and noradrenaline outflow collected during 0–120 min post treatment for ESC. ***P < 0.001: significantly different between controls and escitalopram-treated mice. &&&P < 0.001: significantly different from SERT−/− mice.
Similarly, a two-way anova (treatment × genotype) on noradrenaline outflow indicated a significant effect of treatment (F[1,40]= 30.25; P= 0.001), but not of genotype (F[1,40]= 0.13; P > 0.05) (Figure 3B,C). Escitalopram increased [NA]ext in both WT and SERT−/− mice (P < 0.001 and P < 0.001 respectively).
As escitalopram was administered systemically in these experiments, it was not possible to determine the brain region where it exerted its neurochemical effect. To test the hypothesis that escitalopram acted preferentially at noradrenergic nerve terminals, without involving an action in the LC, its effects were studied after local perfusion of escitalopram in the FCx via reverse dialysis.
Effects of continuous local perfusion of escitalopram on cortical [5-HT]ext and noradrenaline levels in SERT−/− mice and WT littermates
Figure 4 compares the effects of local perfusion of escitalopram (0.5 µM) in both WT and SERT−/− mice. Under basal conditions (i.e. before treatment), 5-HT outflow in FCx of WT mice was stable. Local perfusion of escitalopram significantly increased cortical 5-HT levels in WT mice (AUC values = 569% of baseline; P < 0.001) but had no significant effect on 5-HT outflow in SERT−/− mice (P > 0.05; Figure 4A).
Figure 4.
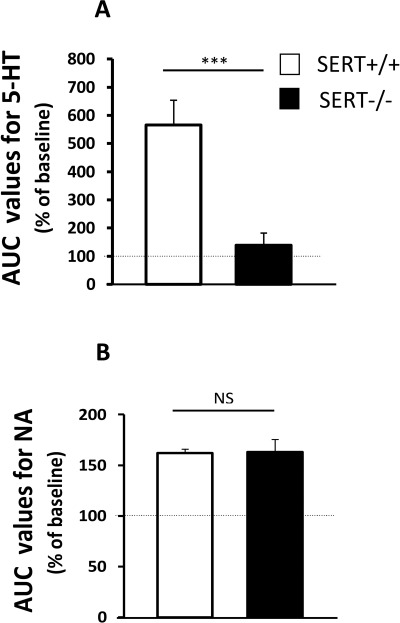
Effect of local perfusion of escitalopram on [5-HT]ext and [NA]ext in the FCx in WT and SERT−/− mice. (A) AUC values (mean ± SEM) were calculated for the amount of 5-HT outflow collected during the cortical 120 min perfusion of escitalopram (ESC;0.5 µM; n= 4–6 mice per group). ***P < 0.001: significantly different from SERT+/+ mice in the same drug condition. (B) AUC values (mean ± SEM) were calculated for the amount of noradrenaline outflow collected during the cortical 120 min perfusion of escitalopram 0.5 µM (n= 4–6 mice per group). NS, not statistically significant.
With respect to noradrenaline, local perfusion of escitalopram (0.5 µM) significantly increased cortical noradrenaline levels in both WT and SERT−/− mice (P < 0.001 and P < 0.001 respectively). These increases reached a maximum of approximately 163% of baseline in both genotypes (Figure 4B). No differences were detected in [NA]ext between WT and SERT−/− mice after local perfusion of escitalopram (P > 0.05).
Effects of pharmacological inactivation of the noradrenaline transporter on escitalopram-induced changes in cortical [NA]ext in WT mice
Figure 5 shows the effects of an acute administration of escitalopram (8 mg·kg−1, i.p.) on cortical [NA]ext in the presence of locally applied desipramine (50 µM) in the perfusion medium in WT mice. Student's t-test on AUC0–120 values for noradrenaline outflow in the FCx revealed no differences in the effects of desipramine between groups (P= 0.9). Similarly, analysis on AUC120–240 values indicated no significant effect of escitalopram compared with vehicle-treated mice (P= 0.16), demonstrating that the blockade of the NET by desipramine prevented escitalopram-induced increase in cortical [NA]ext in WT mice.
Figure 5.
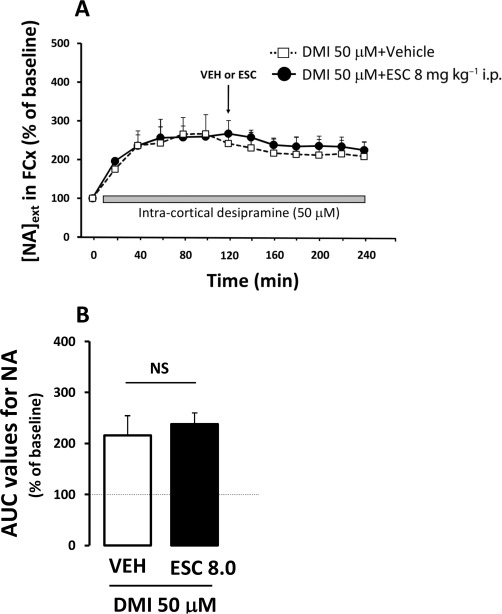
Effects of pharmacological inactivation of the NET on escitalopram-induced changes in cortical [NA]ext in WT mice. (A) Time course. Data are means ± SEM values of [NA]ext expressed as percentages of baseline (B0) following exposure to vehicle or escitalopram (n= 5–7 mice per group). Arrow indicates the time at which the injection of vehicle or escitalopram (ESC; 8 mg·kg−1, i.p.), was performed. Grey bar represent the local perfusion of desipramine (DMI, 50 µM) into the FCx. (B) AUC values (mean ± SEM) were calculated for the amount of noradrenaline outflow collected during 120–240 min post injection.
Effects of escitalopram on noradrenaline and 5-HT uptake mediated by low-affinity monoamine transporters in ex vivo cell extracts from WT mice
The atypical transporters OCT2, OCT3 and PMAT have recently been proposed to participate in low-affinity monoamine clearance in the brain, in complement to the high-affinity transporters. To investigate whether escitalopram could inhibit noradrenaline or 5-HT transport mediated by these low-affinity transporters, the effects of this SSRI on [3H]noradrenaline and [3H]5-HT uptake were evaluated in cell suspensions from WT mouse cortex, in the presence of inhibitors of the high-affinity monoamine transporters, venlafaxine, desipramine and GBR12935, and of an inhibitor of vesicular transporter VMAT2, reserpine. The specific contribution of low-affinity transporters was determined in the presence and absence of an inhibitor of low-affinity transporters, decynium 22 (D22). At the concentration used in the present study (500 µM), D22 is a potent inhibitor of OCTs (Hayer-Zillgen et al., 2002) and PMAT (Engel and Wang, 2005), with affinities within the submicromolar range. In these ex vivo experiments, D22 alone decreased the uptake of 5-HT (Figure 6A) in agreement with previous in vivo microdialysis studies (Feng et al., 2005; 2010), but also that of noradrenaline (Figure 6C). One-way anova revealed a significant effect of escitalopram upon D22-sensitive 5-HT uptake (F[3,10]= 7.04; P < 0.01; Figure 6A,B), but no significant effect upon D22-sensitive noradrenaline uptake (F[3,12]= 1.08, P > 0.05), with an inhibition of noradrenaline uptake at 1 and 10 mM escitalopram (Figure 6C,D).
Figure 6.

Effects of escitalopram on ex vivo noradrenaline and 5-HT uptake by low-affinity transporter in the frontal cortex in WT mice. (A–C) Representative [3H]noradrenaline and 5-HT uptake in cortex extracts in the presence of increasing concentrations of escitalopram (ESC; 1 µM to 10 mM) with or without the inhibitor D22 is expressed as mean fmol ± SEM, normalized by protein concentration (n= 3–5 experiments). (B–D) D22-sensitive [3H]noradrenaline and 5-HT uptake in the presence of increasing concentrations of escitalopram (100 µM to 10 mM) is expressed as percentage of D22-sensitive uptake in absence of citalopram (Control; n= 3–5). D22-sensitive uptake rates were defined by subtracting uptake in the presence of D22 from the uptake observed in its absence. *P < 0.05; **P < 0.01: significantly different from control (one-way anova followed by Fisher's post hoc test).
Discussion
Antidepressants, such as paroxetine, fluoxetine, citalopram and escitalopram, used for the treatment of major depressive disorders, exert their therapeutic effects mainly by inhibiting 5-HT reuptake into presynaptic nerve terminals, thereby enhancing 5-HT neurotransmission which might be diminished in depressed patients (Invernizzi et al., 1995; Sanchez et al., 2003a). Although SSRIs share a common target (i.e. the SERT), the affinity, selectivity and potency to block monoaminergic transporters vary substantially. In vitro, the six SSRIs approved in Europe are all potent 5-HT reuptake inhibitors (Richelson and Pfenning, 1984; Sanchez and Hyttel, 1999). In agreement with this inhibitory action, intracerebral in vivo microdialysis studies in mice revealed that an acute systemic administration of an SSRI significantly increases cortical extracellular 5-HT levels ([5-HT]ext) (David et al., 2003; Guiard et al., 2004; Guilloux et al., 2006; Richardson-Jones et al., 2010) in a strain-dependent manner (Calcagno et al., 2007). The present study extends these neurochemical observations to escitalopram by showing that in WT mice, this SSRI induced a dose-dependent increase in cortical [5-HT]ext. In addition, consistent with a previous study (Zomkowski et al., 2010), similar doses of escitalopram produced a robust antidepressant-like effect as shown by a significant increase in swimming duration (Figure 1C). This parameter is believed to reflect the activation of the brain 5-hydroxytryptaminergic system in rodents (Detke et al., 1995; Reneric and Lucki, 1998; Cryan and Lucki, 2000) since pretreatment with the tryptophan hydroxylase inhibitor p-chlorophenylalanine, prevented SSRI-induced increase in swimming behaviour (Page et al., 1999).
Considerable variations in the 5-HT/noradrenaline selectivity ratios of SSRIs have been reported in vitro. Based on their inhibition constants, citalopram and escitalopram emerge as the most selective compounds amongst the SSRIs, whilst fluoxetine is the least selective (Sanchez and Hyttel, 1999; Owens et al., 2001). Despite this apparent selectivity for SERT, the present data indicate that the systemic or local injection of escitalopram also significantly increases [NA]ext in the FCx of WT mice. These results are consistent with previous in vivo microdialysis experiments, which revealed that the acute administration of fluoxetine, paroxetine or citalopram enhanced noradrenaline levels in the FCx and/or hippocampus of rodents (Jordan et al., 1994; Hughes and Stanford, 1996; 1998; Shachar et al., 1997; Millan et al., 2001; Beyer et al., 2002; Bymaster et al., 2002; Koch et al., 2002; David et al., 2003; Kobayashi et al., 2008). In the present study, despite the neurochemical data, escitalopram failed to increase climbing behaviour (Figure 2C), a parameter reflecting the activation of noradrenergic neurons arising from cells located in the lateral tegmentum as shown in rats (Cryan et al., 2002) or mice (Dulawa et al., 2004). This observation suggests that the 50% increase in cortical [NA]ext induced by escitalopram was not sufficient to affect this behavioural response. When comparing the relative ability of SSRIs in our microdialysis studies, escitalopram (8 mg·kg−1, i.p.) was more potent in increasing cortical [5-HT]ext than citalopram and paroxetine, but surprisingly less potent than the SNRI venlafaxine (8 mg·kg−1, i.p.) (Table 2). The fact that the most selective 5-HT reuptake inhibitor escitalopram also demonstrated a neurochemical noradrenergic-enhancing property was particularly intriguing. Our results suggest that noradrenaline may play an important role in mediating the acute neurochemical actions of this SSRI. This inference is in agreement with previous findings showing that the neurochemical effects of SSRIs are attenuated in noradrenaline-deficient mice (Cryan et al., 2004). Several possibilities may explain these neurochemical effects on cortical [NA]ext in WT mice, such possibilities include direct or indirect actions at the SERT, the NET or an interaction with other transporters.
Table 2.
In vivo selectivity of antidepressant drugs in mice measured by intracerebral microdialysis
| Ratio of AUC values in WT mice | Escitalopram (8 mg·kg−1, i.p.) | Citalopram (8 mg·kg−1, i.p.) | Paroxetine (8 mg·kg−1, i.p.) | Venlafaxine (8 mg·kg−1,i.p.) |
|---|---|---|---|---|
| Present study | David et al., 2003 | |||
| 5-HT/NE | 2.03 | 1.68 | 0.91 | 2.91 |
Potential heterologous reuptake of noradrenaline through the SERT or the DAT
The cloning and sequencing of monoamine transporters revealed that this family shows a high degree of structural homology (Gether et al., 2006). This observation may explain, at least in part, the fact that SSRIs, despite their supposed selectivity, can enhance not only 5-hydroxytryptaminergic but also noradrenergic neurotransmission. Vizi et al. (2004) showed previously that the noradrenaline uptake was substantially reduced, but not completely abolished, in the hippocampus and FCx of NET−/− mice. Interestingly, the neuronal component of this residual uptake was markedly decreased in the presence of citalopram, suggesting that noradrenaline can be taken up by 5-hydroxytryptaminergic varicosities through the SERT. This heterologous uptake could explain the increase in noradrenaline outflow induced by escitalopram in the present study. However, the fact that basal cortical levels of [NA]ext are markedly attenuated in SERT−/− mice suggests that the SERT does not contribute significantly to noradrenaline uptake in this region.
In addition, since the NET and DAT cooperate, to a certain degree, in the clearance of catecholamines (i.e. dopamine and noradrenaline) in the prefrontal cortex (Carboni et al., 1990; Morón et al., 2002; Guiard et al., 2008b; Borgkvist et al., 2011), the possibility that escitalopram blocked the DAT, and thereby enhancing [NA]ext, cannot be excluded. However, the lack of effect of escitalopram on cortical [DA]ext (see Figure S1) strongly argues against this hypothesis.
Potential action of escitalopram at low-affinity monoamine transporters
Beside the high-affinity transporters, other transporters such as OCTs and PMAT have been proposed to participate in monoamine clearance in the brain, a role recently confirmed in vivo for OCT2 (Bacq et al., 2011). The affinities of various monoamines for these transporters, as defined in vitro and tested in the millimolar range, have been shown to vary depending on transporter type and species. Specifically, rat OCT2 show affinities of 0.8–3.6 and 2–4.4 mM and rat OCT3 affinities of 0.5–1 and 0.4–1.9 mM for 5-HT and NE respectively (Grundemann et al., 1998a,b; Wu et al., 2000; Amphoux et al., 2006), whilst human PMAT show affinities of approximately 0.1 and 2.6 mM for 5-HT and NE respectively (Engel et al., 2004; Engel and Wang, 2005). Both OCTs and PMAT show a high transport capacity (Wu et al., 2000; Engel et al., 2004), which may compensate for their low substrate affinity and hence, improve transport efficacy. In addition, OCT3 from rodent species was shown to be inhibited by antidepressants such as desipramine and imipramine (Kekuda et al., 1998; Wu et al., 2000). Human PMAT has been shown to be inhibited by a large array of antidepressants (Haenisch and Bönisch, 2010), albeit with affinities in the 5–200 µM range. These findings raise the possibility that escitalopram could interact with some of these low-affinity transporters in vivo, thereby contributing to the increase in [NA]ext .
To explore whether the escitalopram-mediated increase in cortical [NA]ext could result from an action at low-affinity monoamine transporters, we evaluated the action of this SSRI on noradrenaline and 5-HT uptake in brain cortex cell suspensions in the presence of the high-affinity monoamine transporter inhibitors venlafaxine, desipramine and GBR12935 and of the vesicular transporter VMAT2 inhibitor reserpine. Specific uptake by low-affinity transporters was evaluated using a selective inhibitor of OCTs and PMAT, D22 (Hayer-Zillgen et al., 2002; Engel and Wang, 2005). Under these conditions, most of the 5-HT and noradrenaline uptake could be inhibited by D22, indicating the presence in the cortex of the low-affinity transporters, insensitive to the high-affinity transporter blockers, as previously published (Bacq et al., (2011). In these cortical extracts, escitalopram, even at high concentrations, had no significant action upon D22-sensitive noradrenaline uptake, whilst concentrations as high as 1 mM were required to inhibit D22-sensitive 5-HT uptake. This differential effect of high concentrations of escitalopram suggests that several transporters with distinct specificities contributed to D22-sensitive uptake in the cortex. PMAT, has been shown to transport 5-HT with a higher efficacy than noradrenaline and may be a more significant transport mechanism than OCT3 in certain heterologous systems (Duan and Wang, 2010). Hence PMAT could contribute notably to 5-HT clearance in certain brain areas and it might represent the component sensitive to 1–10 mM escitalopram. In any case, these inhibitory concentrations are far higher than the therapeutically active concentrations of escitalopram found in the brain of rodents after acute administration. Recent acute dosing studies in mice have shown that a 30 min escitalopram pretreatment (0.3 mg·kg−1, s.c.), produces a SERT occupancy of 77% and mean plasma and brain levels of approximately 15.9 and 10.6 ng·mL−1 (38 and 26 nM) respectively. At the dose of 5 mg·kg−1 of escitalopram, SERT occupancy reaches 99%, whereas plasma and brain levels were of 247 and 401 ng·mL−1 (600 and 970 nM), respectively (unpubl. obs.). Importantly, in rodents, steady-state plasma levels have been evaluated in the range of 6–21 ng·mL−1 for escitalopram (Kreilgaard et al., 2008). Similarly, for citalopram, the concentrations found in rodent brains after chronic treatment are 1000-fold above the concentrations of citalopram required to inhibit uptake in this ex vivo model (Uhr and Grauer, 2003; Cervo et al., 2005; Bacq et al., 2011). Taken together, these data suggest that at therapeutically relevant concentrations, this SSRI does not interact directly with the low-affinity monoamine transporters present in the brain, OCT2, OCT3 or PMAT.
Potential effect of escitalopram on the noradrenergic system through 5-HT or blockade of the NET
The present study explored the possibility that the effects of escitalopram on noradrenaline outflow could be attributable to an excitatory effect of 5-HT on noradrenergic neurons. Close anatomical and functional interactions exist between the serotonergic and noradrenergic systems in the brain (Mongeau et al., 1997; Nutt, 2002). In particular, it is well established that the increase in 5-HT transmission induced by SSRIs inhibits noradrenergic neuronal activity (Dremencov et al., 2007), an effect believed to be mediated through activation of 5-HT2A and/or 5-HT2C receptors expressed on GABA neurons (Szabo and Blier, 2001b; Bymaster et al., 2002; Miguelez et al., 2011). Accordingly, in the present study, basal cortical [5-HT]ext was increased in knock-out SERT (SERT−/−) mice, whilst cortical [NA]ext was significantly attenuated compared with WT littermates. Thus, a putative excitatory effect of 5-HT on noradrenaline neurons would most likely involve a local action at nerve terminals. Consistent with this hypothesis, initial microdialysis studies reported that the local perfusion of the SSRIs fluoxetine or citalopram can enhance cortical [NA]ext (Hughes and Stanford, 1996; 1998). Interestingly, increases in the synaptic availability of 5-HT in the hippocampus have also been shown to activate the noradrenergic transmission through 5-HT1A (Hajos-Korcsok et al., 1999) and/or 5-HT3 receptors (Mongeau et al., 1994). To clarify this point, we hypothesized that, if the escitalopram-induced increase in cortical [NA]ext was due to its local action at 5-HT terminals, it should be attenuated in mice lacking the SERT, which display no detectable 5-HT reuptake in the FCx (Perez et al., 2006). As expected, escitalopram did not increase cortical [5-HT]ext in SERT−/− mice. However, the ability of the systemic administration or local perfusion of escitalopram to increase cortical [NA]ext remained unchanged in SERT−/− as compared with WT mice (164% vs. 148% and 162% vs. 163%, respectively), indicating that elevation of 5-HT was not required to stimulate cortical noradrenaline release. These data strongly suggest that escitalopram can increase cortical [NA]ext by a SERT-independent mechanism, involving a potentially direct inhibition of NET.
To explore this possibility further, we anticipated that, if the escitalopram-induced increase in cortical [NA]ext resulted from the blockade of NET, it would be attenuated if this transporter had been previously inactivated. We thus evaluated the effect of escitalopram on cortical [NA]ext in the presence of locally applied desipramine (50 µM) which inhibits only noradrenaline reuptake in the FCx (Figure S2A,B). Significantly, with respect to the occupancy of the NET, we can legitimately assume that 50 µM desipramine optimally inactivated this transporter, since higher concentrations (100 µM) failed to enhance further [NA]ext (Figure S2B). As previously demonstrated in anaesthetized mice (Fisher et al., 2007), our results show that desipramine alone increased cortical [NA]ext. in awake WT mice. However, escitalopram when administered i.p. 2 h after the beginning of the intra-cortical desipramine perfusion, failed to enhance further cortical [NA]ext. These findings strengthen the possibility that escitalopram can inhibit the NET. Importantly, apart from NET, desipramine can bind to various receptors including 5-HT1A/2A and α1/2-adrenoceptors, which are known to modulate both the 5-hydroxytryptaminergic and noradrenergic systems (Guiard et al., 2008a). Indeed, a significant inhibition of specific radioligand binding by desipramine was shown in vitro on rat brain synaptosomes (Thomas et al., 1987). On the other hand, paroxetine and citalopram have little affinity for these receptor subtypes (Sanchez and Hyttel, 1999). It is thus conceivable that a direct interaction of desipramine with these receptors could account for its preventive effects. In addition, desipramine was also shown to block PMAT (Haenisch and Bönisch, 2010), which may consequently favour cortical [NA]ext accumulation. This effect could induce an eventual ‘ceiling effect’, which could hamper the detection of a further enhancement of [NA]ext by escitalopram. Finally, if desipramine enhanced [5-HT]ext by blocking either high- and/or low-affinity transporters, high levels of 5-HT could exert a negative feedback on noradrenergic systems, thereby masking an elevation of [NA]ext in response to escitalopram. Nevertheless, despite these conflicting hypotheses, the combination of our in vivo results with the uptake data strongly suggests that escitalopram acts primarily on the NET.
In conclusion, the present study suggests that in vivo, escitalopram is not as selective for transporters as it has been reported in vitro and is likely to interact with the NET locally in the FCx. So far, the explanation for this drastic difference between in vivo and in vitro observations remains unclear. Pharmacokinetic parameters may provide an interesting explanation since desmethylcitalopram, the major metabolite of escitalopram, displays a twofold higher affinity for the NET than citalopram (Tatsumi et al., 1997). In addition, increased action of escitalopram at NET in vivo could be due to environment-based post-translational modifications of the transporter modulating its affinity, such as phosphorylation or interaction with regulatory proteins (Blakely and De Felice, 2007). Nevertheless, the increased noradrenaline efflux in the FCx appears to play a minor role in the antidepressant-like activity of escitalopram administered acutely, since climbing activity in the FST did not change in WT mice. On the other hand, it is possible that escitalopram increases dialysate noradrenaline levels also in other brain regions more directly involved in behavioural activity (the hippocampus, amygdala), than the brain region studied here. These effects of escitalopram on cortical [NA]ext are probably dependent on the doses given to mice. It is likely that the exposures produced by the doses used in the present study exceed those that will be achieved in clinical use. It will thus be interesting in future investigations to complete the present study by evaluating the antidepressant-like activity of escitalopram in rodents and explore the neurochemical and behavioural consequences of a repeated administration.
Acknowledgments
This study was supported by H. Lundbeck A/S. The Department of Neuropharmacology is an ‘Equipe d'accueil’ (EA 3544) from the ‘Ministère de la Jeunesse, de l'Education Nationale et de la Recherche’ (MJENR-France). AB is a recipient of a fellowship from the French Ministry for Research and the Société Française de Pharmacologie et Thérapeutique. This work has been supported by the technical assistance of Valerie Dupont-Domergue and all her staff from the animal care facility of the Institut Federatif de recherche-IFR141 of the Paris XI University. We thank Dr I Seif for providing the SERT knock-out mice used in this study.
Glossary
- aCSF
artificial CSF
- D22
1,1′-diethyl-2,2′-cyanine iodide
- DR
dorsal raphe
- FCx
frontal cortex
- LC
locus coeruleus
- MDD
major depressive disorder
- NET
noradrenaline transporter
- SERT
5-HT transporter
- SSRIs
selective 5-HT reuptake inhibitors
Conflict of interest
None.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Figure S1 Effects of acute escitalopramadministration on extracellular dopamine ([DA]ext)levels in the FCx in WT mice. Mice received either vehicle orescitalopram (8 mg·kg−1, i.p.). (A) Timecourse. Data are mean ± SEM. Values of [DA]ext inthe FCx are expressed as percentages of baseline (B0)following exposure to vehicle or escitalopram (n = 7–8mice per group). Baseline [DA]ext in vehicle andescitalopam 8 mg·kg−1, i.p., were 0.46± 0.11 and 0.68 ± 0.21 fmol·(20µL)−1 respectively. (B) AUC values (mean± SEM) were calculated for the amount of dopamine outflowcollected during 0–120 min post treatment forescitalopram.
Figure S2 Effects of intra-cortical perfusionof desipramine on extracellular 5-HT and noradrenaline levels in WTmice. Mice received an intra-cortical perfusion of desipramine (50and 100 µM). (A, B) Time course. Data are mean ± SEM.Values of [5-HT]ext (A) or of [NA]ext (B) inthe FCx are expressed as percentages of baseline (B0)perfusion of desipramine (n = 4–5 mice per group). Thegrey area indicates the duration of desipramine perfusion byreverse dialysis.
References
- Alexander S, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 5th edition. Br J Pharmacol. 2011;164(Suppl. 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amphoux A, Vialou V, Drescher E, Brüss M, Mannoury La Cour C, Rochat C, et al. Differential pharmacological in vitro properties of organic cation transporters and regional distribution in rat brain. Neuropharmacology. 2006;50:941–952. doi: 10.1016/j.neuropharm.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Bacq A, Balasse L, Biala G, Guiard B, Gardier AM, Schinkel A, et al. Organic cation transporter 2 controls brain norepinephrine and serotonin clearance and antidepressant response. Mol Psychiatry. 2011;17:926–939. doi: 10.1038/mp.2011.87. [DOI] [PubMed] [Google Scholar]
- Baganz NL, Horton RE, Calderon AS, Owens WA, Munn JL, Watts LT, et al. Organic cation transporter 3: keeping the brake on extracellular serotonin in serotonin-transporter-deficient mice. Proc Natl Acad Sci U S A. 2008;105:18976–18981. doi: 10.1073/pnas.0800466105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bengel D, Murphy DL, Andrews AM, Wichems CH, Feltner D, Heils A, et al. Altered brain serotonin homeostasis and locomotor insensitivity to 3, 4-methylenedioxymethamphetamine (‘Ecstasy’) in serotonin transporter-deficient mice. Mol Pharmacol. 1998;53:649–655. doi: 10.1124/mol.53.4.649. [DOI] [PubMed] [Google Scholar]
- Bert L, Favale D, Jego G, Greve P, Guilloux JP, Guiard BP, et al. Rapid and precise method to locate microdialysis probe implantation in the rodent brain. J Neurosci Methods. 2004;140:53–57. doi: 10.1016/j.jneumeth.2004.04.042. [DOI] [PubMed] [Google Scholar]
- Berton O, Nestler EJ. New approaches to antidepressant drug discovery: beyond monoamines. Nat Rev Neurosci. 2006;7:137–151. doi: 10.1038/nrn1846. [DOI] [PubMed] [Google Scholar]
- Beyer CE, Boikess S, Luo B, Dawson LA. Comparison of the effects of antidepressants on norepinephrine and serotonin concentrations in the rat frontal cortex: an in-vivo microdialysis study. J Psychopharmacol. 2002;16:297–304. doi: 10.1177/026988110201600403. [DOI] [PubMed] [Google Scholar]
- Blakely RD, De Felice LJ. All aglow about presynaptic receptor regulation of neurotransmitter transporters. Mol Pharmacol. 2007;71:1206–1208. doi: 10.1124/mol.107.035493. [DOI] [PubMed] [Google Scholar]
- Borgkvist A, Malmlöf T, Feltmann K, Lindskog M, Schilström B. Dopamine in the hippocampus is cleared by the norepinephrine transporter. Int J Neuropsychopharmacol. 2011;15:531–540. doi: 10.1017/S1461145711000812. [DOI] [PubMed] [Google Scholar]
- Breidert T, Spitzenberger F, Gründemann D, Schömig E. Catecholamine transport by the organic cation transporter type 1 (OCT1) Br J Pharmacol. 1998;125:218–224. doi: 10.1038/sj.bjp.0702065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bymaster FP, Zhang W, Carter PA, Shaw J, Chernet E, Phebus L, et al. Fluoxetine, but not other selective serotonin uptake inhibitors, increases norepinephrine and dopamine extracellular levels in prefrontal cortex. Psychopharmacology (Berl) 2002;160:353–361. doi: 10.1007/s00213-001-0986-x. [DOI] [PubMed] [Google Scholar]
- Calcagno E, Canetta A, Guzzetti S, Cervo L, Invernizzi RW. Strain differences in basal and post-citalopram extracellular 5-HT in the mouse medial prefrontal cortex and dorsal hippocampus: relation with tryptophan hydroxylase-2 activity. J Neurochem. 2007;103:1111–1120. doi: 10.1111/j.1471-4159.2007.04806.x. [DOI] [PubMed] [Google Scholar]
- Carboni E, Tanda GL, Frau R, Di Chiara G. Blockade of the noradrenaline carrier increases extracellular dopamine concentrations in the prefrontal cortex: evidence that dopamine is taken up in vivo by noradrenergic terminals. J Neurochem. 1990;55:1067–1070. doi: 10.1111/j.1471-4159.1990.tb04599.x. [DOI] [PubMed] [Google Scholar]
- Ceglia I, Acconcia S, Fracasso C, Colovic M, Caccia S, Invernizzi RW. Effects of chronic treatment with escitalopram or citalopram on extracellular 5-HT in the prefrontal cortex of rats: role of 5-HT1A receptors. Br J Pharmacol. 2004;142:469–478. doi: 10.1038/sj.bjp.0705800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervo L, Canetta A, Calcagno E, Burbassi S, Sacchetti G, Caccia S, et al. Genotype-dependent activity of tryptophan hydroxylase-2 determines the response to citalopram in a mouse model of depression. J Neurosci. 2005;25:8165–8172. doi: 10.1523/JNEUROSCI.1816-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Lucki I. Antidepressant-like behavioral effects mediated by 5-Hydroxytryptamine(2C) receptors. J Pharmacol Exp Ther. 2000;295:1120–1126. [PubMed] [Google Scholar]
- Cryan JF, Page ME, Lucki I. Noradrenergic lesions differentially alter the antidepressant-like effects of reboxetine in a modified forced swim test. Eur J Pharmacol. 2002;436:197–205. doi: 10.1016/s0014-2999(01)01628-4. [DOI] [PubMed] [Google Scholar]
- Cryan JF, O'Leary OF, Jin SH, Frieldland JC, Ouyang M, Hirsch BR, et al. Norepinephrine-deficient mice lack responses to antidepressant drugs, including selective serotonin reuptake inhibitors. Proc Natl Acad Sci U S A. 2004;101:8186–8191. doi: 10.1073/pnas.0401080101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlin A, Xia L, Kong W, Hevner R, Wang J. Expression and immunolocalization of the plasma membrane monoamine transporter in the brain. Neuroscience. 2007;146:1193–1211. doi: 10.1016/j.neuroscience.2007.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David DJ, Bourin M, Jego G, Przybylski C, Jolliet P, Gardier AM. Effects of acute treatment with paroxetine, citalopram and venlafaxine in vivo on noradrenaline and serotonin outflow: a microdialysis study in Swiss mice. Br J Pharmacol. 2003;140:1128–1136. doi: 10.1038/sj.bjp.0705538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detke MJ, Rickels M, Lucki I. Active behaviours in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology (Berl) 1995;121:66–72. doi: 10.1007/BF02245592. [DOI] [PubMed] [Google Scholar]
- Dremencov E, El Mansari M, Blier P. Noradrenergic augmentation of escitalopram response by risperidone: electrophysiologic studies in the rat brain. Biol Psychiatry. 2007;61:671–678. doi: 10.1016/j.biopsych.2006.05.015. [DOI] [PubMed] [Google Scholar]
- Duan H, Wang J. Selective transport of monoamine neurotransmitters by human plasma membrane monoamine transporter and organic cation transporter 3. J Pharmacol Exp Ther. 2010;335:743–753. doi: 10.1124/jpet.110.170142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulawa SC, Holick KA, Gundersen B, Hen R. Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharmacology. 2004;29:1321–1330. doi: 10.1038/sj.npp.1300433. [DOI] [PubMed] [Google Scholar]
- El Mansari M, Sanchez C, Chouvet G, Renaud B, Haddjeri N. Effects of acute and long-term administration of escitalopram and citalopram on serotonin neurotransmission: an in vivo electrophysiological study in rat brain. Neuropsychopharmacology. 2005;30:1269–1277. doi: 10.1038/sj.npp.1300686. [DOI] [PubMed] [Google Scholar]
- Engel K, Wang J. Interaction of organic cations with a newly identified plasma membrane monoamine transporter. Mol Pharmacol. 2005;68:1397–1407. doi: 10.1124/mol.105.016832. [DOI] [PubMed] [Google Scholar]
- Engel K, Zhou M, Wang J. Identification and characterization of a novel monoamine transporter in the human brain. J Biol Chem. 2004;279:50042–50049. doi: 10.1074/jbc.M407913200. [DOI] [PubMed] [Google Scholar]
- Feng N, Mo B, Johnson PL, Orchinik M, Lowry CA, Renner KJ. Local inhibition of organic cation transporters increases extracellular serotonin in the medial hypothalamus. Brain Res. 2005;1063:69–76. doi: 10.1016/j.brainres.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Feng N, Lowry CA, Lukkes JL, Orchinik M, Forster GL, Renner KJ. Organic cation transporter inhibition increases medial hypothalamic serotonin under basal conditions and during mild restraint. Brain Res. 2010;1326:105–113. doi: 10.1016/j.brainres.2010.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher AS, Stewart RJ, Yan T, Hunt SP, Stanford SC. Disruption of noradrenergic transmission and behavioural response to a novel environment NK1R−/− mice. Eur J Neurosci. 2007;28:1195–1204. doi: 10.1111/j.1460-9568.2007.05369.x. [DOI] [PubMed] [Google Scholar]
- Frazer A. Serotonergic and noradrenergic reuptake inhibitors: prediction of clinical effects from in vitro potencies. J Clin Psychiatry. 2001;62(Suppl. 12):16–23. [PubMed] [Google Scholar]
- Garnock-Jones KP, McCormack PL. Escitalopram: a review of its use in the management of major depressive disorder in adults. CNS Drugs. 2010;24:769–796. doi: 10.2165/11204760-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Gether U, Andersen PH, Larsson OM, Schousboe A. Neurotransmitter transporters: molecular function of important drug targets. Trends Pharmacol Sci. 2006;27:375–383. doi: 10.1016/j.tips.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Grundemann D, Koster S, Kiefer N, Breidert T, Engelhardt M, Spitzenberger F, et al. Transport of monoamine transmitters by the organic cation transporter type 2, OCT2. J Biol Chem. 1998a;273:30915–30920. doi: 10.1074/jbc.273.47.30915. [DOI] [PubMed] [Google Scholar]
- Grundemann D, Schechinger B, Rappold GA, Schomig E. Molecular identification of the corticosterone-sensitive extraneuronal catecholamine transporter. Nat Neurosci. 1998b;1:349–351. doi: 10.1038/1557. [DOI] [PubMed] [Google Scholar]
- Guiard BP, Przybylski C, Guilloux JP, Seif I, Froger N, De Felipe C, et al. Blockade of substance P (neurokinin 1) receptors enhances extracellular serotonin when combined with a selective serotonin reuptake inhibitor: an in vivo microdialysis study in mice. J Neurochem. 2004;89:54–63. doi: 10.1046/j.1471-4159.2003.02304.x. [DOI] [PubMed] [Google Scholar]
- Guiard BP, El Mansari M, Blier P. Cross-talk between dopaminergic and noradrenergic systems in the rat ventral tegmental area, locus ceruleus, and dorsal hippocampus. Mol Pharmacol. 2008a;74:1463–1475. doi: 10.1124/mol.108.048033. [DOI] [PubMed] [Google Scholar]
- Guiard BP, El Mansari M, Merali Z, Blier P. Functional interactions between dopamine, serotonin and norepinephrine neurons: an in-vivo electrophysiological study in rats with monoaminergic lesions. Int J Neuropsychopharmacol. 2008b;11:625–639. doi: 10.1017/S1461145707008383. [DOI] [PubMed] [Google Scholar]
- Guilloux JP, David DJ, Guiard BP, Chenu F, Reperant C, Toth M, et al. Blockade of 5-HT1A receptors by (+/-)-pindolol potentiates cortical 5-HT outflow, but not antidepressant-like activity of paroxetine: microdialysis and behavioral approaches in 5-HT1A receptor knockout mice. Neuropsychopharmacology. 2006;31:2162–2172. doi: 10.1038/sj.npp.1301019. [DOI] [PubMed] [Google Scholar]
- Haenisch B, Bönisch H. Interaction of the human plasma membrane monoamine transporter (hPMAT) with antidepressants and antipsychotics. Naunyn Schmiedebergs Arch Pharmacol. 2010;381:33–39. doi: 10.1007/s00210-009-0479-8. [DOI] [PubMed] [Google Scholar]
- Hajos-Korcsok E, McQuade R, Sharp T. Influence of 5-HT1A receptors on central noradrenergic activity: microdialysis studies using (+/-)-MDL 73005EF and its enantiomers. Neuropharmacology. 1999;38:299–306. doi: 10.1016/s0028-3908(98)00175-0. [DOI] [PubMed] [Google Scholar]
- Hayer-Zillgen M, Brüss M, Bönisch H. Expression and pharmacological profile of the human organic cation transporters hOCT1, hOCT2 and hOCT3. Br J Pharmacol. 2002;136:829–836. doi: 10.1038/sj.bjp.0704785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hof PR, Young WG, Bloom FE, Belichenko PV, Celio MR. Comparative Cytoarchitectonic Atlas of the C57BL/6 and 129/Sv Mouse Brains with CD ROM. San Diego, CA: Elsevier; 2000. [Google Scholar]
- Holick KA, Lee DC, Hen R, Dulawa SC. Behavioural effects of chronic fluoxetine in BALB/cJ mice do not require adult hippocampal neurogenesis or the serotonin 1A receptor. Neuropsychopharmacology. 2008;33:406–417. doi: 10.1038/sj.npp.1301399. [DOI] [PubMed] [Google Scholar]
- Hughes ZA, Stanford SC. Increased noradrenaline efflux induced by local infusion of fluoxetine in the rat frontal cortex. Eur J Pharmacol. 1996;317:83–90. doi: 10.1016/s0014-2999(96)00715-7. [DOI] [PubMed] [Google Scholar]
- Hughes ZA, Stanford SC. Evidence from microdialysis and synaptosomal studies of rat cortex for noradrenaline uptake sites with different sensitivities to SSRIs. Br J Pharmacol. 1998;124:1141–1148. doi: 10.1038/sj.bjp.0701947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Invernizzi R, Bramante M, Samanin R. Extracellular concentrations of serotonin in the dorsal hippocampus after acute and chronic treatment with citalopram. Brain Res. 1995;696:62–66. doi: 10.1016/0006-8993(95)00730-e. [DOI] [PubMed] [Google Scholar]
- Jacquot C, David DJ, Gardier AM, Sanchez C. Escitalopram and citalopram: the unexpected role of the R-enantiomer. Encephale. 2007;33:179–187. doi: 10.1016/s0013-7006(07)91548-1. [DOI] [PubMed] [Google Scholar]
- Jordan S, Kramer GL, Zukas PK, Moeller M, Petty F. In vivo biogenic amine efflux in medial prefrontal cortex with imipramine, fluoxetine, and fluvoxamine. Synapse. 1994;18:294–297. doi: 10.1002/syn.890180404. [DOI] [PubMed] [Google Scholar]
- Kekuda R, Prasad PD, Wu X, Wang H, Fei YJ, Leibach FH, et al. Cloning and functional characterization of a potential-sensitive, polyspecific organic cation transporter (OCT3) most abundantly expressed in placenta. J Biol Chem. 1998;273:15971–15979. doi: 10.1074/jbc.273.26.15971. [DOI] [PubMed] [Google Scholar]
- Kennedy SH, Andersen HF, Thase ME. Escitalopram in the treatment of major depressive disorder: a meta-analysis. Curr Med Res Opin. 2009;25:161–175. doi: 10.1185/03007990802622726. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Ikeda Y, Haneda E, Suzuki H. Chronic fluoxetine bidirectionally modulates potentiating effects of serotonin on the hippocampal mossy fiber synaptic transmission. J Neurosci. 2008;28:6272–6280. doi: 10.1523/JNEUROSCI.1656-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch S, Perry KW, Nelson DL, Conway RG, Threlkeld PG, Bymaster FP. R-fluoxetine increases extracellular DA, NE, as well as 5-HT in rat prefrontal cortex and hypothalamus: an in vivo microdialysis and receptor binding study. Neuropsychopharmacology. 2002;27:949–959. doi: 10.1016/S0893-133X(02)00377-9. [DOI] [PubMed] [Google Scholar]
- Koepsell H, Lips K, Volk C. Polyspecific organic cation transporters: structure, function, physiological roles, and biopharmaceutical implications. Pharm Res. 2007;24:1227–1251. doi: 10.1007/s11095-007-9254-z. [DOI] [PubMed] [Google Scholar]
- Kornstein SG, Li D, Mao Y, Larsson S, Andersen HF, Papakostas GI. Escitalopram versus SNRI antidepressants in the acute treatment of major depressive disorder: integrative analysis of four double-blind, randomized clinical trials. CNS Spectr. 2009;14:326–333. doi: 10.1017/s1092852900020320. [DOI] [PubMed] [Google Scholar]
- Kreilgaard M, Smith DG, Brennum LT, Sanchez C. Prediction of clinical response based on pharmacokinetic/pharmacodynamic models of 5-hydroxytryptamine reuptake inhibitors in mice. Br J Pharmacol. 2008;155:276–284. doi: 10.1038/bjp.2008.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguelez C, Fernandez-Aedo I, Torrecilla M, Grandoso L, Ugedo L. alpha(2)-Adrenoceptors mediate the acute inhibitory effect of fluoxetine on locus coeruleus noradrenergic neurons. Neuropharmacology. 2009;56:1068–1073. doi: 10.1016/j.neuropharm.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Miguelez C, Grandoso L, Ugedo L. Locus coeruleus and dorsal raphe neuron activity and response to acute antidepressant administration in a rat model of Parkinson's disease. Int J Neuropsychopharmacol. 2011;14:187–200. doi: 10.1017/S146114571000043X. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Gobert A, Lejeune F, Newman-Tancredi A, Rivet JM, Auclair A, et al. S33005, a novel ligand at both serotonin and norepinephrine transporters: I. Receptor binding, electrophysiological, and neurochemical profile in comparison with venlafaxine, reboxetine, citalopram, and clomipramine. J Pharmacol Exp Ther. 2001;298:565–580. [PubMed] [Google Scholar]
- Mongeau R, De Montigny C, Blier P. Effect of long-term administration of antidepressant drugs on the 5-HT3 receptors that enhance the electrically evoked release of [3H]noradrenaline in the rat hippocampus. Eur J Pharmacol. 1994;271:121–129. doi: 10.1016/0014-2999(94)90272-0. [DOI] [PubMed] [Google Scholar]
- Mongeau R, Blier P, de Montigny C. The serotonergic and noradrenergic systems of the hippocampus: their interactions and the effects of antidepressant treatments. Brain Res Brain Res Rev. 1997;23:145–195. doi: 10.1016/s0165-0173(96)00017-3. [DOI] [PubMed] [Google Scholar]
- Mork A, Kreilgaard M, Sanchez C. The R-enantiomer of citalopram counteracts escitalopram-induced increase in extracellular 5-HT in the frontal cortex of freely moving rats. Neuropharmacology. 2003;45:167–173. doi: 10.1016/s0028-3908(03)00138-2. [DOI] [PubMed] [Google Scholar]
- Morón JA, Brockington A, Wise RA, Rocha BA, Hope BT. Dopamine uptake through the norepinephrine transporter in brain regions with low levels of the dopamine transporter: evidence from knock-out mouse lines. J Neurosci. 2002;22:389–395. doi: 10.1523/JNEUROSCI.22-02-00389.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt DJ. The neuropharmacology of serotonin and noradrenaline in depression. Int Clin Psychopharmacol. 2002;17(Suppl. 1):S1–12. doi: 10.1097/00004850-200206001-00002. [DOI] [PubMed] [Google Scholar]
- Owens MJ, Knight DL, Nemeroff CB. Second-generation SSRIs: human monoamine transporter binding profile of escitalopram and R-fluoxetine. Biol Psychiatry. 2001;50:345–350. doi: 10.1016/s0006-3223(01)01145-3. [DOI] [PubMed] [Google Scholar]
- Page ME, Detke MJ, Dalvi A, Kirby LG, Lucki I. Serotonergic mediation of the effects of fluoxetine, but not desipramine, in the rat forced swimming test. Psychopharmacology (Berl) 1999;147:162–167. doi: 10.1007/s002130051156. [DOI] [PubMed] [Google Scholar]
- Perez XA, Bianco LE, Andrews AM. Filtration disrupts synaptosomes during radiochemical analysis of serotonin uptake: comparison with chronoamperometry in SERT knockout mice. J Neurosci Methods. 2006;154:245–255. doi: 10.1016/j.jneumeth.2005.12.017. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Pudovkina OL, Cremers TI, Westerink BH. The interaction between the locus coeruleus and dorsal raphe nucleus studied with dual-probe microdialysis. Eur J Pharmacol. 2002;445:37–42. doi: 10.1016/s0014-2999(02)01663-1. [DOI] [PubMed] [Google Scholar]
- Rainer Q, Xia L, Guilloux JP, Gabriel C, Mocaër E, Hen R, et al. Beneficial behavioural and neurogenic effects of agomelatine in a model of depression/anxiety. Int J Neuropsychopharmacol. 2011;81:106–112. doi: 10.1017/S1461145711000356. [DOI] [PubMed] [Google Scholar]
- Reneric JP, Lucki I. Antidepressant behavioral effects by dual inhibition of monoamine reuptake in the rat forced swimming test. Psychopharmacology (Berl) 1998;136:190–197. doi: 10.1007/s002130050555. [DOI] [PubMed] [Google Scholar]
- Richardson-Jones JW, Craige CP, Guiard BP, Stephen A, Metzger KL, Kung HF, et al. 5-HT1A autoreceptor levels determine vulnerability to stress and response to antidepressants. Neuron. 2010;65:40–52. doi: 10.1016/j.neuron.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richelson E, Pfenning M. Blockade by antidepressants and related compounds of biogenic amine uptake into rat brain synaptosomes: most antidepressants selectively block norepinephrine uptake. Eur J Pharmacol. 1984;104:277–286. doi: 10.1016/0014-2999(84)90403-5. [DOI] [PubMed] [Google Scholar]
- Sanchez C, Hyttel J. Comparison of the effects of antidepressants and their metabolites on reuptake of biogenic amines and on receptor binding. Cell Mol Neurobiol. 1999;19:467–489. doi: 10.1023/A:1006986824213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez C, Bergqvist PB, Brennum LT, Gupta S, Hogg S, Larsen A, et al. Escitalopram, the S-(+)-enantiomer of citalopram, is a selective serotonin reuptake inhibitor with potent effects in animal models predictive of antidepressant and anxiolytic activities. Psychopharmacology (Berl) 2003a;167:353–362. doi: 10.1007/s00213-002-1364-z. [DOI] [PubMed] [Google Scholar]
- Sanchez C, Gruca P, Papp M. R-citalopram counteracts the antidepressant-like effect of escitalopram in a rat chronic mild stress model. Behav Pharmacol. 2003b;14:465–470. doi: 10.1097/01.fbp.0000087733.21047.60. [DOI] [PubMed] [Google Scholar]
- Shachar D, Klein E, Tabak A, Finberg JP. Effect of single and repeated administration of fluvoxamine on noradrenaline release in rat brain. Eur J Pharmacol. 1997;332:237–243. doi: 10.1016/s0014-2999(97)01084-4. [DOI] [PubMed] [Google Scholar]
- Szabo ST, Blier P. Functional and pharmacological characterization of the modulatory role of serotonin on the firing activity of locus coeruleus norepinephrine neurons. Brain Res. 2001a;922:9–20. doi: 10.1016/s0006-8993(01)03121-3. [DOI] [PubMed] [Google Scholar]
- Szabo ST, Blier P. Serotonin (1A) receptor ligands act on norepinephrine neuron firing through excitatory amino acid and GABA(A) receptors: a microiontophoretic study in the rat locus coeruleus. Synapse. 2001b;42:203–212. doi: 10.1002/syn.10009. [DOI] [PubMed] [Google Scholar]
- Tatsumi M, Groshan K, Blakely RD, Richelson E. Pharmacological profile of antidepressants and related compounds at human monoamine transporters. Eur J Pharmacol. 1997;340:249–258. doi: 10.1016/s0014-2999(97)01393-9. [DOI] [PubMed] [Google Scholar]
- Thomas DR, Nelson DR, Johnson AM. Biochemical effects of the antidepressant paroxetine, a specific 5-hydroxytryptamine uptake inhibitor. Psychopharmacology (Berl) 1987;93:193–200. doi: 10.1007/BF00179933. [DOI] [PubMed] [Google Scholar]
- Uhr M, Grauer MT. abcb1ab P-glycoprotein is involved in the uptake of citalopram and trimipramine into the brain of mice. J Psychiatr Res. 2003;37:179–185. doi: 10.1016/s0022-3956(03)00022-0. [DOI] [PubMed] [Google Scholar]
- Vialou V, Amphoux A, Zwart R, Giros B, Gautron S. Organic cation transporter 3 (Slc22a3) is implicated in salt-intake regulation. J Neurosci. 2004;24:2846–2851. doi: 10.1523/JNEUROSCI.5147-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vialou V, Balasse L, Callebert J, Launay JM, Giros B, Gautron S. Altered aminergic neurotransmission in the brain of organic cation transporter 3-deficient mice. J Neurochem. 2008;106:1471–1482. doi: 10.1111/j.1471-4159.2008.05506.x. [DOI] [PubMed] [Google Scholar]
- Vizi ES, Zsilla G, Caron MG, Kiss JP. Uptake and release of norepinephrine by serotonergic terminals in norepinephrine transporter knock-out mice: implications for the action of selective serotonin reuptake inhibitors. J Neurosci. 2004;24:7888–7894. doi: 10.1523/JNEUROSCI.1506-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X, Huang W, Ganapathy ME, Wang H, Kekuda R, Conway SJ, et al. Structure, function, and regional distribution of the organic cation transporter OCT3 in the kidney. Am J Physiol Renal Physiol. 2000;279:F449–F458. doi: 10.1152/ajprenal.2000.279.3.F449. [DOI] [PubMed] [Google Scholar]
- Zomkowski AD, Engel D, Gabilan NH, Rodrigues AL. Involvement of NMDA receptors and l-arginine-nitric oxide-cyclic guanosine monophosphate pathway in the antidepressant-like effects of escitalopram in the forced swimming test. Eur Neuropsychopharmacol. 2010;20:793–801. doi: 10.1016/j.euroneuro.2010.07.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


