Abstract
Carbon monoxide (CO) synthesized by heme oxygenase 2 (HO2) and nitric oxide (NO) produced by neuronal NO synthase (nNOS) mediate nonadrenergic/noncholinergic (NANC) intestinal relaxation. In many areas of the gastrointestinal tract, NO and CO function as coneurotransmitters. In the internal anal sphincter (IAS), NANC relaxation is mediated primarily by CO. Vasoactive intestinal polypeptide (VIP) has also been shown to participate in NANC relaxation throughout the intestine, including the IAS. By using a combination of pharmacology and genetic knockout of the biosynthetic enzymes for CO and NO, we show that the physiologic effects of exogenous and endogenous VIP in the IAS are mediated by HO2-synthesized CO.
Nonadrenergic/noncholinergic (NANC) neurotransmission is prominent in the peripheral autonomic nervous system, especially the gastrointestinal pathway, where it is a mediator of physiologic peristalsis (1). Initial efforts to identify NANC neurotransmitters focused on peptides, especially vasoactive intestinal polypeptide (VIP). Thus, immunoantagonism of VIP blocks NANC transmission in the lower esophageal sphincter of the opossum (2, 3), exogenous VIP mimics NANC transmission (4–6), and VIP is localized to myenteric plexus neurons that mediate NANC transmission (4, 7).
In recent years, abundant evidence has established nitric oxide (NO) and carbon monoxide (CO) as NANC transmitters (8). Neuronal NO synthase (nNOS) and heme oxygenase 2 (HO2), the biosynthetic enzyme for CO, are localized to the myenteric plexus of gastrointestinal neurons (9, 10). Moreover, in many instances, nNOS and HO2 are colocalized, suggesting that they may function as cotransmitters (11–14). Inhibitors of nNOS and HO2 block NANC transmission (15–17). Targeted deletion of nNOS and HO2 have provided compelling evidence for NO and CO as NANC transmitters. Thus, NANC transmission in mouse intestine, monitored biochemically or electrophysiologically, is partially reduced in nNOS-/- and HO2-/- mice (13) and abolished in double knockouts (DKOs) (18).
How can VIP be a NANC neurotransmitter if NANC transmission is abolished by the elimination of biosynthetic enzymes for CO and NO? In the present article, we demonstrate that VIP exerts its NANC transmitter role by acting through HO2 to augment CO transmission.
Materials and Methods
Reagents. Atropine, propanolol, indomethacin, sodium nitroprusside (SNP), l-nitroarginine methyl ester (l-NAME), 1H-[1,2,4]-oxadiazolo-[4,3-a]-quinoxaline-1-one, 2′,5′-dideoxyadenosine, and VIP were obtained from Sigma. Tin protoporphyrin IX (SnPPIX) was obtained from Porphyrin Products (Logan, UT).
Experimental Animals. WT, nNOS-/-, and HO2-/- mice were allowed free access to food and water except when they were fasted, as indicated, for experiments. DKO mice with deletion of nNOS and HO2 were created by using standard breeding techniques with nNOS-/- mice and HO2-/- mice, as described (18). Age-matched adult mice were used as controls. Genotypes of all animals were confirmed by PCR of tail tissue and Western blotting.
Organ-Bath Physiology. Animals were killed by cervical dislocation, and an anal probe was inserted into the anal canal as a dissection guide. The abdomen was opened, and the descending colon and anus were visualized. Incisions were made bilaterally 1–2 cm from midline and distally to the external sphincter and the anus. The internal and external sphincter were excised and placed in 37°C oxygenated Krebs buffer without Ca2+. The internal anal sphincter (IAS) was carefully freed of the striated muscle fibers of the external anal sphincter and other extraneous tissues, such as large blood vessels. The mucosal layer was removed by sharp dissection with the assistance of a binocular microscope, with the anal canal remaining intact. The IAS was subsequently hung luminally between two hooks and placed in an organ bath containing continuously oxygenated Krebs solution with Ca2+. Only tissues that generated spontaneous tone were used in experiments. Induction of NANC, measurement of relaxation, and data acquisition and analysis were performed exactly as described (19).
Immunohistochemistry. Animals were killed by cervical dislocation, and the IAS was dissected and immediately imbedded with Tissue-Tek OCT 4583 (Sakura Finetek, Torrance, CA), placed in dry ice, and allowed to freeze. Sections (10 μm) were fixed for 5 minutes in 4.0% paraformaldehyde, washed in PBS, and permeabilized with 0.1% Triton X-100 in PBS. For simultaneous detection of two antigens (20), sections were incubated overnight in primary antibodies of either mouse antineurofilament (1:500 dilution; Zymed) or rabbit anti-HO2 (1:500) (21). Sections were then sequentially incubated overnight with sheep anti-VIP antibody (1:500 dilution; Chemicon) after rinsing in PBS. Visualization of neurofilament and HO2 was achieved by incubation for 30 minutes with FITC-conjugated donkey anti-mouse or Texas red-conjugated donkey anti-rabbit Igs (1:150 dilution; Jackson ImmunoResearch), respectively. Sections were then washed, and VIP was visualized by incubation with FITC- or Texas Red-conjugated donkey anti-sheep Igs (1:150 dilution; The Binding Site, Birmingham, England). Fluorescent images were acquired and analyzed by using an Ultraview (Perkin–Elmer) confocal microscope (×60 objective).
IAS HO2 Activity. Tissues were homogenized in 10 mM potassium phosphate buffer containing 50 mM phenylmethylsulfonylfluoride by using a Polytron tissue homogenizer (Beckman Coulter). The tissue homogenate was cleared of debris and nuclei by sequential centrifugation at 5,000 × g and 12,000 × g for 30 minutes. The resultant 12,000 × g supernatant was centrifuged at 100,000 × g to obtain the microsomal fraction. HO activity in the microsomal fraction was performed exactly as described in ref. 22.
cGMP Assay. IAS tissues were equilibrated and stimulated in the organ bath as described above and snap-frozen in liquid nitrogen at the point of maximal relaxation, as described in ref. 23. cGMP measurements on snap-frozen tissue were performed according to the manufacturer's protocols (Amersham Biosciences).
Statistical Analysis. Graphing and statistical analysis were performed by using sigmaplot (SPSS, Chicago). Statistical significance was determined by using unpaired two-tailed t tests. Where tested, Ki values were determined by fitting the data to the four-parameter logistic equation y = min + (max - min)/[1 + (x/EC50)Hillslope] by using sigmaplot. All data points are the pooled average of at least three, and in most cases five or more, separate determinations.
Results
Colocalization of HO2 and VIP. Inhibitors of HO have been useful tools for investigating physiologic roles for CO, although the principal HO inhibitors used (noniron metalloprotoporphyrins) exert other actions, such as inhibiting guanylyl cyclase (24), inhibiting NOS (25, 26), and blocking VIP receptors (VIPRs) (27). Rattan and Chakder (16, 28) reported that SnPPIX inhibited VIP-dependent NANC transmission in the IAS of the opossum, suggesting that VIP might act through HO. Recently, we showed in the mouse that NANC transmission in IAS is mediated predominantly by CO, rather than NO, because it is greatly reduced in HO2-/- mice but not in nNOS-/- animals (19). Accordingly, we have explored a relationship of VIP and HO2 in mouse IAS.
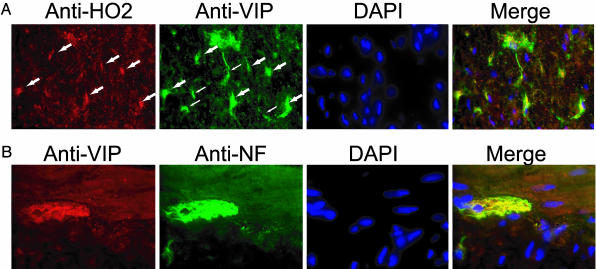
To determine whether VIP and HO2 reside within the same neurons within the IAS, we performed immunohistochemistry of HO2, VIP, and the neuronal marker neurofilament (Fig. 1). We detect strong immunoreactivity of HO2 and VIP in neurons of the myenteric plexus innervating the IAS, similar to what has been shown in studies (11, 29). In ≈50% of these neurons, we detect colocalization of VIP and HO2, although they also occur in distinct neurons (Fig. 1 A). Colabeling with VIP and neurofilament indicate, as expected, that the majority of VIP immunoreactivity is restricted to the neuronal ganglia (Fig. 1B). The colocalizations of VIP and HO2 within the same neurons of the IAS suggest that the relaxant effects of VIP may be mediated by CO.
Fig. 1.
Colocalization of HO2 and VIP. (A) Anal sphincter tissue from WT mice was dissected, sectioned, and stained with anti-HO2 (red), anti-VIP (green), and the nuclear stain 4′,6-diamidino-2-phenylindole (DAPI). Neurons of the myenteric plexus in which both HO2 and VIP are present are indicated by large arrows and appear yellow in the merged image. VIP-positive neurons that are not strongly stained with HO2 are indicated by small arrows. (B) VIP (red) is present exclusively in the neuronal innervation of the IAS, as demonstrated by colocalization with the neuronal marker neurofilament (NF; green). The results were obtained in two separate experiments with three mice per group.
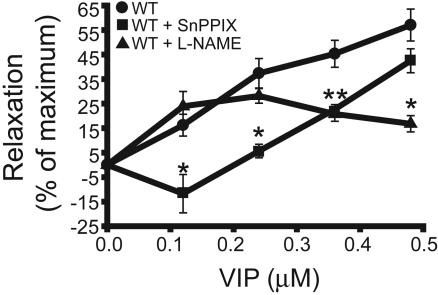
VIP Actions on IAS Relaxation and cGMP Levels in WT, HO2-/-, and nNOS-/- Mice. To investigate the possibility that VIP acts through CO and/or NO, we initially used enzyme inhibitors SnPPIX (to inhibit HO) and l-NAME (to inhibit NOS) (Fig. 2). Exogenously added VIP potently relaxes IAS smooth muscle, with a sharp concentration–response relationship between 0.1 and 0.5 μM. SnPPIX blocks the effects of VIP most prominently at 0.1 μM VIP. l-NAME fails to influence actions of 0.1 μM VIP but partially reverses the effects at higher doses of VIP. These observations suggest that CO mediates VIP actions, especially at lower, more physiologic concentrations. At higher VIP concentrations, NO may also contribute to NANC relaxation.
Fig. 2.
The HO inhibitor SnPPIX inhibits NANC relaxation induced by physiologic concentrations of VIP. NANC conditions were induced in ex vivo organ bath preparations of the IAS, and the relaxant effects of VIP (0.1–0.5 μM) were examined. Where indicated, IAS preparations were pretreated for 30 minutes with the HO inhibitor SnPPIX (1 μM) or the nNOS inhibitor l-NAME (1 mM) before VIP stimulation. Results are expressed as the percentage of SNP-induced relaxation. Data are expressed as mean ± SEM of five separate experiments. *, P < 0.02, relative to no drug pretreatment.
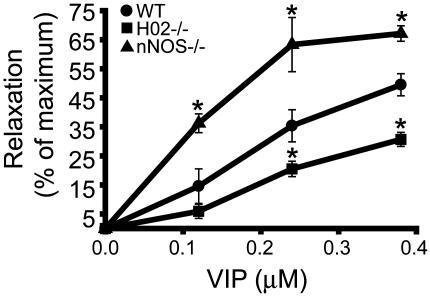
Of the various metalloprotoporphyrins, SnPPIX is the most selective inhibitor of HO (9, 28), but it still may exert nonspecific effects. Accordingly, we explored the effect of exogenously added VIP in HO2-/- and nNOS-/- animals (Fig. 3). VIP responses are reduced in HO2-/- IAS preparations at all concentrations tested. The residual relaxation in HO2-/- mice may be due to the direct action of exogenously added VIP on smooth muscle because it is not inhibited by the addition of tetrodotoxin (data not shown). Surprisingly, in nNOS-/- animals, the response to VIP is augmented significantly at all concentrations.
Fig. 3.
VIP-mediated relaxation is diminished in HO2-/- mice. NANC relaxation in WT, HO2-/-, and nNOS-/- mice in response to exogenous VIP was examined, as shown in Fig. 2. VIP-induced relaxation is reduced significantly in HO2-/- mice and augmented in nNOS-/- mice. Results are expressed as a percentage of SNP-induced relaxation. Data are expressed as mean ± SEM of five separate experiments. *, P < 0.02, compared with WT.
NO and CO act by stimulating soluble guanylyl cyclase (sGC) to augment tissue levels of cGMP (19). We demonstrated (13) in mouse ileum that decreases in NANC transmission in HO2-/- and nNOS-/- animals are paralleled by comparable decreases in tissue levels of cGMP. Accordingly, we monitored effects of VIP on cGMP levels in the mutant mice (Table 1). In WT IAS, VIP almost doubles cGMP levels. The augmentation in cGMP is mediated by sGC because no effect is seen in the presence of the sGC inhibitor 1H-[1,2,4]-oxadiazolo-[4,3-a]-quinoxaline-1-one. The adenylyl cyclase inhibitor, 2′,5′-dideoxyadenosine, has no effect on the response to VIP. In HO2-/- preparations, basal levels of cGMP are reduced ≈30%, indicating a role for endogenous CO in the maintenance of basal levels. VIP stimulation of cGMP is abolished in HO2-/- tissues, with levels substantially lower than in the unstimulated preparations. VIP stimulation of cGMP levels is essentially the same in nNOS-/- as it is in WT preparations. Levels of cGMP in tissues from DKO mice are similar to HO2-/- mice. In summary, the cGMP tissue levels parallel the NANC relaxation responses to exogenous VIP. The only difference between the two studies is that the augmented relaxation response to VIP in the nNOS-/- preparations is not paralleled by increased cGMP levels.
Table 1. VIP increases cGMP levels in WT and nNOS-/-, but not HO2-/- or DKO, animals.
| cGMP, fmol/mg
|
||||
|---|---|---|---|---|
| Treatment | WT | nNOS-/- | HO2-/- | DKO |
| Unstimulated | 960 ± 45 | 802 ± 61 | 652 ± 18* | 724 ± 33* |
| VIP-stimulated | 1,643 ± 102† | 1,731 ± 97† | 747 ± 54* | 805 ± 27* |
| ODQ + VIP | 489 ± 23 | 393 ± 32 | 415 ± 29 | 450 ± 36 |
| DDA + VIP | 1,509 ± 51† | 1,617 ± 125† | 633 ± 44* | 761 ± 48* |
Anal sphincter muscle strips equilibrated in the organ bath were treated with VIP (250 nm) for 30 s and instantaneously frozen. cGMP content was determined by radioimmunoassay and expressed as fmol/mg. Where indicated, tissues were preincubated for 30 minutes with the cGMP inhibitor ODQ or the cAMP inhibitor DDA prior to simulation with 250 μM VIP. Data represent mean ± SEM. ODQ, 1H-[1,2,4]-oxadiazolo-[4,3-a]-quinoxaline-1-one; DDA, 2′,5′-dideoxyadenosine. *, P < 0.05, compared with WT. †, Significant increase by VIP, P < 0.05.
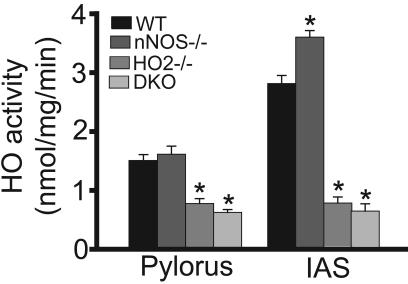
The abolition of VIP responses in HO2-/- mice strongly implies that VIP acts through HO2, indicating that NANC relaxation and stimulation of cGMP levels are mediated by CO. We wondered what might account for the enhanced response to VIP in nNOS-/- mice. Both NO and CO can elicit NANC transmission. One possibility is that they act in a coordinated fashion, indicating that a loss of NO transmission might be countered by augmented CO transmission. Accordingly, we monitored HO2 activity in IAS and the pylorus of the stomach in the various mutant mice (Fig. 4). As anticipated, HO activity is reduced substantially in HO2-/- animals. However, HO2 activity is increased in the IAS of nNOS-/- animals, although there is no alteration in the pylorus. Thus, the augmented response to VIP in nNOS-/- IAS may be caused by increased HO2 activity.
Fig. 4.
HO activity is elevated in IAS of nNOS-/- mice. HO activity in WT, HO2-/-, nNOS-/-, and DKO mice was measured from pylorus and IAS tissues. Note the increase in IAS HO activity in nNOS-/- mice. *, P < 0.01, compared with WT.
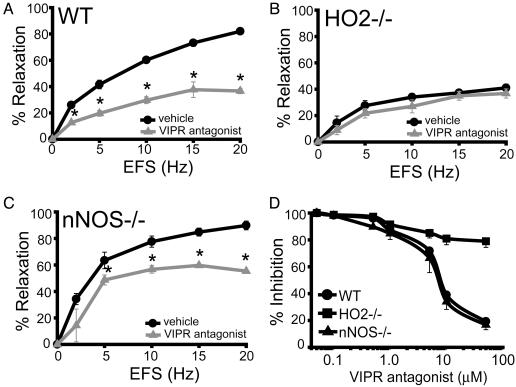
HO2-/- Mice Have Reduced Electrical-Field Stimulation (EFS)-Induced NANC Relaxation and Are Insensitive to VIP Antagonists. The experiments already described establish that relaxant responses to exogenous VIP are mediated by HO2. To establish that physiologic NANC neurotransmission acts by means of VIP stimulation of CO production, we monitored NANC neurotransmission elicited by EFS (Fig. 5). We used a partial sequence fragment of VIP, VIP10–28, which functions as a VIPR antagonist (30, 31). VIP10–28 blocks NANC transmission >50% in WT preparations, demonstrating the critical role of endogenous VIP released from neurons in mediating NANC relaxation (Fig. 5A). In HO2-/- IAS, EFS-induced relaxation in the absence of VIPR antagonist is reduced to levels similar to WT in the presence of VIP10–28 (Fig. 5B). Importantly, VIP10–28 has no effect on the residual EFS-induced relaxation of the IAS in HO2-/- mice. In contrast, nNOS-/- mice have an EFS response similar to WT and are sensitive to VIP10–28 (Fig. 5C). Concentration–response relationships for VIP10–28 reveal a Ki of 7 μM for both WT and nNOS-/- preparations, resembling the potency of this antagonist in other systems (32). NANC relaxation in HO2-/- IAS is unaffected by VIP10–28 concentrations as high as 50 μM, establishing that the relaxant effects of VIP, released endogenously by neurons, depend on HO2 activity (Fig. 5D). We observe similar effects with another peptide antagonist of VIPR, VIP6–28 (data not shown). These experiments establish that, in IAS, physiologic NANC transmission requires VIP-dependent activation of CO synthesis by HO2.
Fig. 5.
Endogenous VIP-induced NANC relaxation is blocked in HO2-/- mice. EFS of IAS preparations in the presence or absence of the VIP (10–28), a VIPR antagonist, was used to assess endogenous VIP-induced NANC relaxation. (A) EFS-induced relaxation in WT mice with (gray) or without (black) VIPR antagonist. *, P < 0.001, compared with no antagonist. (B) EFS response in HO2-/- mice. There is a pronounced reduction in relaxation compared with WT mice (A), which is not affected by the addition of VIPR antagonist. (C) EFS response in nNOS-/- mice. *, P < 0.02 relative to no antagonist. (D) Concentration–response curve for the inhibition of EFS induced relaxation by VIP (10–28) in WT, HO2-/-, and nNOS-/- mice. VIP (10–28) inhibited WT and nNOS-/- mice responses with a Ki of 7 μM, a value that is consistent with previous studies (32). VIP (10–28) did not have a statistically significant effect on HO2-/- mice. DKO mice had EFS responses indistinguishable from HO2-/- (data not shown). EFS frequency was 10 Hz. All data represent the mean ± SEM of at least three determinations. In some cases, error bars are smaller than the symbols.
Discussion
The major finding of this study is that VIP is a NANC neurotransmitter but acts indirectly by stimulating HO2 to form CO. Evidence includes the antagonism of VIP relaxation by the HO inhibitor SnPPIX and the abolition of VIP effects on relaxation and cGMP levels in HO2-/- animals. Moreover, physiologic NANC transmission is blocked by VIP antagonists in WT and nNOS-/- preparations but not in HO2-/- or DKO mice. The response to HO2 deletion is quite specific because there is no decline in VIP responses in nNOS-/- mice but rather a marked augmentation of the relaxant response to exogenous VIP (Fig. 3). In contrast, VIP can stimulate nNOS activity in mouse gastric fundus (33, 34, 41) and airway smooth muscle (35) to mediate NANC relaxation. Why would NANC relaxation in the IAS be mediated primarily by CO, whereas NO and CO are coneurotransmitters in other intestinal segments? CO is distinguished from NO by its stability in biological tissues, which would presumably result in longer-lasting relaxation. This property would make CO ideal as a NANC neurotransmitter in the IAS, which exhibits slow relaxation and contraction relative to other sphincters of the gastrointestinal tract.
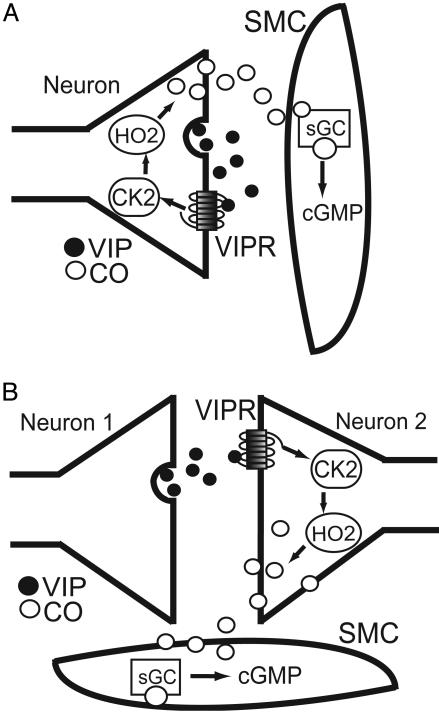
How might various neurons of the myenteric plexus interact with each other and smooth muscle in mediating these responses? Studies have indicated that VIP can relax smooth muscle directly (36, 37). Our findings suggest that, at least for NANC neurotransmission in IAS, VIP acts on neurons, rather than influencing muscle directly. VIP antagonists block the EFS-induced relaxation in WT, but not HO2-/-, mice (Fig. 5). Because HO2 has a predominantly neuronal localization, these effects cannot be explained by VIP actions on smooth muscle. HO2-/- mice display no rise in cGMP in response to VIP, demonstrating that neuronally derived CO is required for sGC activation in response to this agonist. Furthermore, tetrodotoxin partially blocks the response of WT mice to exogenous VIP but has no effect on HO2-/- mice (data not shown). Similarly, VIP-dependent NANC relaxation of the gastric fundus is blocked by ω-conotoxin (34). Our immunohistochemical studies reveal predominant localizations of VIP and HO2 to the myenteric plexus of IAS, with some colocalizations (Fig. 1). In other studies, we have observed HO2 and nNOS predominantly in the myenteric plexus throughout the gastrointestinal pathway (13, 41). The similarity of VIP, nNOS, and HO2 localizations in mouse IAS to localizations throughout the gastrointestinal tract (11–13) implies that VIP-NANC transmission at other sites involves similar mechanisms as are involved in IAS. Our findings can be explained by VIP acting on HO2 in neurons that contain both of these substances, i.e., in an autocrine fashion (Fig. 6A). Alternatively, VIP that is released from one neuron might be acting on HO in another neuron (Fig. 6B).
Fig. 6.
Mechanism of VIP-induced IAS relaxation. VIP is released from neurons within the IAS, where it can act on neurons and possibly smooth muscle cells (SMC). Neuronally localized HO2 participates either by means of autocrine (A) or paracrine (B) actions of VIP on neurons. Studies have shown that EFS-induced relaxation of IAS requires both HO2 and protein kinase CK2 activity (19). Therefore, we hypothesize that VIPR activation is linked to increases in CK2 activity (arrows), resulting in HO2 activation. Supporting this hypothesis, specific CK2 inhibitors block relaxant responses to exogenous VIP (C.C.W., D.B., C.D.F., A.I.K., and S.H.S., unpublished data). CO produced by HO2 diffuses to SMC, where it activates sGC and subsequent cGMP production, leading to SMC relaxation. •, VIP; ○, CO.
What interactions would account for the augmented responses to VIP in nNOS-/- mice? Both NO and CO relax intestinal smooth muscle. Because nNOS and HO2 are often colocalized in the same neurons, their biosynthesis can be regulated in a coordinate fashion. Thus, by means of an unspecified feedback mechanism, a loss of nNOS would be compensated by increased HO2 activity, as we have observed in IAS of nNOS-/- mice (Fig. 4). We have not characterized whether HO2 protein levels are altered, whether the Km or Vmax of HO2 are changed, or whether another regulatory mechanism is involved. NO can inhibit the catalytic activity of both HO1 (38) and HO2 (39, 40) directly. Therefore, the enhanced NANC transmission in the IAS of nNOS-/- mice could reflect decreased inhibition of HO2 activity by NO.
At least two HO2 regulatory mechanisms have been identified recently. HO2 is selectively phosphorylated by CK2 (formerly casein kinase 2), which markedly augments its catalytic activity (19). CK2 physiologically regulates CO neurotransmission because selective CK2 inhibitors prevent NANC neurotransmission in mouse IAS in proportion to their potency as inhibitors of the enzyme. Activation of HO2 by CK2 appears to be involved in VIP actions because VIP-induced relaxation of IAS is blocked by the specific CK2 inhibitor 4,5,6,7-tetrobromobenzotriazole (C.C.W., D.B., C.D.F., A.I.K., and S.H.S., unpublished data). Rapid and transient activation of HO2 is achieved also by calcium–calmodulin binding to HO2 after cytosolic calcium elevation, augmenting HO2 catalysis (D.B. and S.H.S., unpublished data).
Acknowledgments
We thank Michael Delannoy and The Johns Hopkins University Microscope Facility for assistance with confocal microscopy. This work was supported by U.S. Public Health Service Grant DA-000266 and Research Scientist Award DA-00074 (to S.H.S.) and by National Research Service Awards MH-12545 (to C.C.W.) and NS-043850 (to D.B.).
Abbreviations: VIP, vasoactive intestinal polypeptide; NANC, nonadrenergic/noncholinergic; HO2, heme oxygenase 2; nNOS, neuronal NO synthase; SnPPIX, tin protoporphyrin IX; IAS, internal anal sphincter; SNP, sodium nitroprusside; l-NAME, l-nitroarginine methyl ester; DKO, double knockout; EFS, electrical-field stimulation; VIPR, VIP receptor; sGC, soluble guanylyl cyclase.
References
- 1.Bennett, M. R. (1997) Prog. Neurobiol. 52, 159-195. [DOI] [PubMed] [Google Scholar]
- 2.Goyal, R. K., Rattan, S. & Said, S. I. (1980) Nature 288, 378-380. [DOI] [PubMed] [Google Scholar]
- 3.De Beurme, F. A. & Lefebvre, R. A. (1988) J. Pharm. Pharmacol. 40, 711-715. [DOI] [PubMed] [Google Scholar]
- 4.Uddman, R., Alumets, J., Edvinsson, L., Hakanson, R. & Sundler, F. (1978) Gastroenterology 75, 5-8. [PubMed] [Google Scholar]
- 5.Rattan, S., Said, S. I. & Goyal, R. K. (1977) Proc. Soc. Exp. Biol. Med. 155, 40-43. [PubMed] [Google Scholar]
- 6.Behar, J., Field, S. & Marin, C. (1979) Gastroenterology 77, 1001-1007. [PubMed] [Google Scholar]
- 7.Bryant, M. G., Polak, M. M., Modlin, I., Bloom, S. R., Albuquerque, R. H. & Pearse, A. G. (1976) Lancet 1, 991-993. [DOI] [PubMed] [Google Scholar]
- 8.Boehning, D. & Snyder, S. H. (2003) Annu. Rev. Neurosci. 26, 105-131. [DOI] [PubMed] [Google Scholar]
- 9.Zakhary, R., Gaine, S. P., Dinerman, J. L., Ruat, M., Flavahan, N. A. & Snyder, S. H. (1996) Proc. Natl. Acad. Sci. USA 93, 795-798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ward, S. M., Xue, C., Shuttleworth, C. W., Bredt, D. S., Snyder, S. H. & Sanders, K. M. (1992) Am. J. Physiol. 263, G277-G284. [DOI] [PubMed] [Google Scholar]
- 11.Battish, R., Cao, G. Y., Lynn, R. B., Chakder, S. & Rattan, S. (2000) Am. J. Physiol. 278, G148-G155. [DOI] [PubMed] [Google Scholar]
- 12.Ny, L., Alm, P., Larsson, B. & Andersson, K. E. (1997) J. Auton. Nerv. Syst. 65, 49-56. [DOI] [PubMed] [Google Scholar]
- 13.Zakhary, R., Poss, K. D., Jaffrey, S. R., Ferris, C. D., Tonegawa, S. & Snyder, S. H. (1997) Proc. Natl. Acad. Sci. USA 94, 14848-14853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller, S. M., Reed, D., Sarr, M. G., Farrugia, G. & Szurszewski, J. H. (2001) Neurogastroenterol. Motil. 13, 121-131. [DOI] [PubMed] [Google Scholar]
- 15.Ny, L., Alm, P., Ekstrom, P., Larsson, B., Grundemar, L. & Andersson, K. E. (1996) Br. J. Pharmacol. 118, 392-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rattan, S. & Chakder, S. (1993) Am. J. Physiol. 265, G799-G804. [DOI] [PubMed] [Google Scholar]
- 17.Werkstrom, V., Ny, L., Persson, K. & Andersson, K. E. (1997) Br. J. Pharmacol. 120, 312-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xue, L., Farrugia, G., Miller, S. M., Ferris, C. D., Snyder, S. H. & Szurszewski, J. H. (2000) Proc. Natl. Acad. Sci. USA 97, 1851-1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boehning, D., Moon, C., Sharma, S., Hurt, K. J., Hester, L. D., Ronnett, G. V., Shugar, D. & Snyder, S. H. (2003) Neuron 40, 129-137. [DOI] [PubMed] [Google Scholar]
- 20.Wessendorf, M. W. & Elde, R. P. (1985) J. Histochem. Cytochem. 33, 984-994. [DOI] [PubMed] [Google Scholar]
- 21.Baranano, D. E., Wolosker, H., Bae, B. I., Barrow, R. K., Snyder, S. H. & Ferris, C. D. (2000) J. Biol. Chem. 275, 15166-15173. [DOI] [PubMed] [Google Scholar]
- 22.Ferris, C. D., Jaffrey, S. R., Sawa, A., Takahashi, M., Brady, S. D., Barrow, R. K., Tysoe, S. A., Wolosker, H., Baranano, D. E., Dore, S., et al. (1999) Nat. Cell Biol. 1, 152-157. [DOI] [PubMed] [Google Scholar]
- 23.Rattan, S., Moummi, C. & Chakder, S. (1991) Am. J. Physiol. 260, G764-G769. [DOI] [PubMed] [Google Scholar]
- 24.Grundemar, L. & Ny, L. (1997) Trends Pharmacol. Sci. 18, 193-195. [DOI] [PubMed] [Google Scholar]
- 25.Meffert, M. K., Haley, J. E., Schuman, E. M., Schulman, H. & Madison, D. V. (1994) Neuron 13, 1225-1233. [DOI] [PubMed] [Google Scholar]
- 26.Luo, D. & Vincent, S. R. (1994) Eur. J. Pharmacol. 267, 263-267. [DOI] [PubMed] [Google Scholar]
- 27.Rattan, S., Fan, Y. P. & Chakder, S. (1999) Am. J. Physiol. 276, G138-G145. [DOI] [PubMed] [Google Scholar]
- 28.Rattan, S. & Chakder, S. (2000) J. Pharmacol. Exp. Ther. 294, 1009-1016. [PubMed] [Google Scholar]
- 29.Chakder, S., Cao, G. Y., Lynn, R. B. & Rattan, S. (2000) Gastroenterology 118, 477-486. [DOI] [PubMed] [Google Scholar]
- 30.Gozes, I., Lilling, G., Davidson, A., Bardea, A., Reshef, A., Glazer, R., Zamostiano, R., Ashur-Fabian, O., Ticher, A., Ashkenazi, I. E., et al. (1996) Ann. N. Y. Acad. Sci. 805, 159-171. [DOI] [PubMed] [Google Scholar]
- 31.Turner, J. T., Jones, S. B. & Bylund, D. B. (1986) Peptides (Tarrytown, NY) 7, 849-854. [DOI] [PubMed] [Google Scholar]
- 32.Bissonnette, B. M., Collen, M. J., Adachi, H., Jensen, R. T. & Gardner, J. D. (1984) Am. J. Physiol. 246, G710-G717. [DOI] [PubMed] [Google Scholar]
- 33.Ergun, Y. & Ogulener, N. (2001) J. Pharmacol. Exp. Ther. 299, 945-950. [PubMed] [Google Scholar]
- 34.Mashimo, H., He, X. D., Huang, P. L., Fishman, M. C. & Goyal, R. K. (1996) J. Clin. Invest. 98, 8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hasaneen, N. A., Foda, H. D. & Said, S. I. (2003) Chest 124, 1067-1072. [DOI] [PubMed] [Google Scholar]
- 36.Nurko, S. & Rattan, S. (1988) J. Clin. Invest. 81, 1146-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Milenov, K., Kalfin, R. & Mandrek, K. (1991) Acta Physiol. Pharmacol. Bulg. 17, 13-18. [PubMed] [Google Scholar]
- 38.Wang, J., Lu, S., Moenne-Loccoz, P. & Ortiz de Montellano, P. R. (2003) J. Biol. Chem. 278, 2341-2347. [DOI] [PubMed] [Google Scholar]
- 39.Willis, D., Tomlinson, A., Frederick, R., Paul-Clark, M. J. & Willoughby, D. A. (1995) Biochem. Biophys. Res. Commun. 214, 1152-1156. [DOI] [PubMed] [Google Scholar]
- 40.Ding, Y., McCoubrey, W. K., Jr., & Maines, M. D. (1999) Eur. J. Biochem. 264, 854-861. [DOI] [PubMed] [Google Scholar]
- 41.Mule, F. & Serio, R. (2003) Br. J. Pharmacol. 140, 431-437. [DOI] [PMC free article] [PubMed] [Google Scholar]