Abstract
Background and Purpose
Recombinant human erythropoietin (rHuEPO) is currently the mainstay of renal anaemia treatment. Recently, rHuEPO has been shown to provide pleiotrophic tissue protection in various pathological conditions. However, the benefits of rHuEPO beyond anaemia treatment are limited because it increases red blood cell mass. Carbamylated erythropoietin (CEPO) is the first rHuEPO derivative that lacks erythropoietic activity but retains tissue protection properties. Since carbamylation targets lysine residues on rHuEPo, we hypothesized that targeted lysine modifications of rHuEPO may result in a novel non-erythropoietic erythropoietin.
Experimental Approach
rHuEPO was subjected to various targeted lysine modifications. In vitro cytoprotection and apoptosis were evaluated using P19 and HEK293 cells. In vivo erythropoiesis was performed by administering the derivatives to animals for 2 weeks. Renoprotection was tested on an ischaemia/reperfusion (I/R) model.
Key Results
We synthesized a novel derivative, a glutaraldehyde erythropoietin (GEPO). This construct abolished in vivo erythropoiesis. Biochemical characterization showed that GEPO was more electrostatically negative than rHuEPO. Immunoprecipitation experiments revealed that GEPO bound to the IL3RB/EPOR heterotrimeric receptor and ameliorated cellular apoptosis via the activation of Bcl-2. Notably, Bcl-2 activation was suppressed by the JAK2 inhibitor, tyrphostin AG490. In vivo experiments showed that GEPO also ameliorated kidney damage due to I/R injury both functionally and histologically.
Conclusions and Implications
Herein, we describe a novel lysine-modified rHuEPO, glutaradehyde-EPO (GEPO), obtained from a simple reaction. This derivative has no erythropoietic properties but retains cell-protective characteristics both in vitro and in vivo, with promise for future use as an adjunctive treatment of kidney disease.
Keywords: erythropoietin, non-erythropoietic, ischaemia/reperfusion, EPO, carbamylated, kidney injury, apoptosis, Bcl-2, JAK, glutaraldehyde erythropoietin
Introduction
Erythropoietin (EPO), a 165-amino-acid glycoprotein, regulates red blood cell maturation by inhibiting apoptosis of erythrocyte progenitor cells in bone marrow. In the adult, circulating EPO functions as a hormone and is produced by peritubular fibroblast-like cells in the kidneys (Lacombe and Mayeux, 1998; 1999; Fisher, 2003; Brines and Cerami, 2006). It is now clear that EPO is also produced locally by many other tissues as a defensive paracrine signal in response to multiple insults (Marti et al., 1996; Morishita et al., 1997; Maiese et al., 2005). Moreover, administration of recombinant human EPO (rHuEPO) has been reported to protect various organs, including the kidney, from injuries (Brines et al., 2000; Gorio et al., 2002; Junk et al., 2002; Calvillo et al., 2003; Yang et al., 2003; Abdelrahman et al., 2004). With this evidence, it is clear that EPO is a pleiotropic cytokine for tissue protection, and the use of rHuEPO as an adjunctive treatment in various diseases seems promising (Coleman and Brines, 2004; Jelkmann and Wagner, 2004). However, to elicit tissue protection, one has to administer exogenous rHuEPO at a relatively high dose that, in turn, will be complicated by pathological increases in red blood cell mass (i.e. polycythaemia). This drawback limits clinical use of rHuEPO for tissue protection; therefore, a non-erythropoietic EPO that provides tissue protection is strongly required.
Recently, carbamylated EPO (CEPO) has been created by subjecting rHuEPO to cyanide carbamylation (in strict terminology; carbamoylation) (Jelkmann, 2008). This molecule is the first modified rHuEPO to provide tissue protection without significantly increasing red blood cell production (Leist et al., 2004; Fiordaliso et al., 2005; Coleman et al., 2006; Mennini et al., 2006; Moon et al., 2006; Imamura et al., 2007; King et al., 2007). Further studies suggest that CEPO may not mediate its actions by the conventional homodimerization of EPO receptors (EPOR) but by heterotrimerization between one EPOR and two IL-3 common β-receptors (IL3RB) (Brines et al., 2004). The carbamylation reacts on free amino acids; thus, eight lysine residues and one terminal amino acid on EPO are potential targets. Previous data also hinted that the electrical charge on lysine residues of EPO molecule was critical in mediating the erythropoiesis effect (Satake et al., 1990). Neutralizing the lysine positive charge, for example, by acetylation, carbamylation or by making the lysine charge negative through succinylation, caused significantly decreased erythropioesis. However, whether these lysine-modified EPOs retain tissue protective activities like CEPO remains to be elucidated. In addition, it should be possible to obtain novel non-erythropiotic EPOs with therapeutic potential by targeting lysine modification with other methods as well.
It should be noted that rHuEPO is a complex glycoprotein with many post-translational modifications. Therefore, rHuEPO from different sources do not have identical structures or share biosimilarity (Combe et al., 2005; Wiecek and Mikhail, 2006; Jelkmann, 2007). Moreover, pharmaceutical formulations are different from the pro-drug due to the addition of various stabilizing agents to prevent the degradation that, in turn, prohibits molecular modifications. Amidst the complexity and variation of the commercial rHuEPOs, the modified conditions of each formulation diverge drastically. To elucidate the relevant reactions, we attempted to modify lysine residues on a marketed rHuEPO, Eprex®, which is distributed worldwide; its formulation was extensively researched (Schellekens, 2005; Villalobos et al., 2005; Deechongkit et al., 2006). Herein, a novel lysine-modified rHuEPO, glutaradehyde-EPO (GEPO), obtained from a simple reaction, is reported. This derivative evinced no erythropoietic properties but retained cell-protective characteristics both in vitro and in vivo.
Methods
The pharmaceutical preparation rHuEPO (Eprex®), in prefilled syringe, was purchased from Janssen-Cilag, Bangkok, Thailand. According to the company's declaration to Thailand FDA, this preparation consists of rHuEPO alfa 2000 IU, NaCl 4.38 mg mL−1, glycine 5.0 mg mL−1 and polysorbate 80 0.3 mg mL−1. The amount of protein concentration in each syringe was 0.03 mg mL−1. All other reagents (unless otherwise indicated) were obtained from Sigma Chemical Co. (St. Louis, MO, USA).
Chemical modification and characterization of lysine residues on rHuEPO
Carbamylation
rHuEPO was mixed with 2 M cyanate solution to a final concentration of 1 M, and the reaction mixture was incubated for 1 day at 37°C (Rimon and Perlmann, 1968; Horkko et al., 1992; Park et al., 2004).
Glutaraldehyde modification
rHuEPO in 10 mM sodium acetate and 5 mM calcium acetate buffer (pH 7.5) was mixed with 25% glutaraldehyde solution to make a final concentration of 0.25% glutaraldehyde in the mixed solution. The mixed solution was gently stirred for 2 h at 4°C (Carla et al., 2004).
Guanidination
rHuEPO was mixed with 200 μL Tris–HCl, pH 8.4, containing 1 M NaCl; 1000 μL of 1.0 M O-methylisourea was then added to the mixture. The pH was adjusted to 10.5 with NaOH. The mixture was stirred for 4 days at 4°C (Cupo et al., 1980).
Phosphopyridoxylation
rHuEPO was mixed with a 100-fold molar excess of pyridoxal-5-phosphate. Reactions were carried out for 15 min at 25°C. Subsequently, a freshly prepared solution of sodium borohydride (30 mg mL−1) was added to the mixture to achieve a final concentration of 0.6 mg mL−1 (Lundblad and Noyes, 1984).
Acetylation
rHuEPO in 100 mM phosphate buffer was mixed with N-acetylbenzotriazole in 30 s to make a final concentration of 9.6 mM (Michèle and Charis, 1976).
Succinylation
rHuEPO in 0.5 M NaHCO3 (pH 8.0) containing 0.2 M NaCl was incubated with a 15-fold molar excess of succinic anhydride for 1 h at 25°C (Habeeb et al., 1958; Dixon and Perham, 1968).
Excess reagents were removed by extensive dialysis against water at 4°C, followed by centrifugal–ultrafiltration with Microcon Centrifugal Filter (Millipore, Bedford, MA). The modified EPOs from the above reactions were carbamylated EPO (CEPO), glutaraldehyde EPO (GEPO), guanidinated EPO (GuEPO), phosphopyridoxylated EPO (PEPO), acetylated EPO (AEPO) and succinylated EPO (SEPO). The loss of free lysine residues was determined by using the trinitrobenzenesulfonic (TNBS) acid method as previously described (Fields, 1971).
ζ-potential analysis
The magnitude of electrostatic charges on the protein surface was determined by measuring the ζ-potential of protein dispersion as a function of pH. ζ-potential values were obtained by using Zetasizer (NanoZS 4700, Malvern Instruments, Worcestershire, UK) at room temperature. All measurements were conducted at a wavelength of 633 nm at 25°C with a scattering angle of 90°. The reported results were the mean of three determinations.
PAGE of GEPO
Twenty micrograms of rHuEPO and GEPO were subjected to electrophoresis in either 7% native or SDS polyacrylamide gel. To visualize the protein, gels were stained with Coomassie Brilliant Blue.
Oxidative stress and serum-deprivation induced cell damage
HEK-293 and unstimulated murine P19 cells (Cell Line Service, Eppelheim, Germany) were cultured in 10% FBS DMEM (Gibco, Grand Island, NY, USA) supplemented with 2 mM L-glutamine, 100 units mL−1 penicillin G-streptomycin. For oxidation-induced cell damage, HEK-293 cells were cultured overnight with serum-free medium or pretreated with 0.03μg mL−1 of rHuEPO derivatives in serum-free medium. To induce apoptosis, the culture media was replaced with freshly prepared culture media supplement with 2 mM H2O2 for 2 h.
For serum-deprivation-induced cell damage, P19 cells were cultured overnight in media either with or without EPO derivatives. To induce apoptosis, culture media was replaced with serum free media for 2 h (Sirén et al., 2001).
LDH concentration in culture media was measured in triplicate using the Dimension RXL automated analyser (Dade Behring, Newark, DE, USA).
Immunoblotting and immunoprecipitation
Cultured cells were lysed in ice-cold RIPA buffer containing 10 mmol L−1 PMSF. The supernatant was collected, and the protein concentration was measured using the Bradford protein assay (Biorad, Hercules, CA, USA). For immunoblotting, 50 μg of protein were loaded on acrylamide gel for electrophoresis under reducing conditions. Separated proteins were then transferred to PVDF membrane and incubated with 1:200 IL3RB antibody (K17, Santa Cruz Biotechnology, Santa Cruz, CA, USA), EPOR (M20 and H194, Santa Cruz Biotechnology) at final dilution 1:500 or EPO 1:500 for 1 h. After the blots were extensively washed and incubated with suitable peroxidase secondary antibodies, the specific protein bands were visualized using the ECL system.
For immunoprecipitation, P19 cells were incubated with culture media supplemented with 0.03 μg mL−1 GEPO for 20 min. A complex of GEPO-associated receptors were subjected to protein A immunoprecipitation with a mixture of two anti-EPOR antibodies (M20 and H194) at final dilution 1:200 as previously described (Brines et al., 2004). The precipitation proteins were then electrophoresed and immunoblotted with specific antibodies.
UT-7 colony forming and proliferation assay
For the colony-forming unit (CFU) assay, UT-7 cells (gift from Nicole Casadevall, Hospital Hotel Dieu, Paris, France.) were cultured in semisolid gel consisting of 10% FBS DMEM, 3% methylcellulose with or without erythropoietin derivative (either 0.03 μg mL−1 of rHuEPO or GEPO). The forming colonies were counted at day 7 and day 14. For MTT cell proliferation assay, UT-7 cells were cultured in 10% FBS DMEM supplemented with 2 mM L-glutamine, 100 units mL−1 penicillin G -streptomycin with and without erythropoietin derivative (either rHuEPO or GEPO) supplementation for 2 days. Cell proliferation was measured by using the Quick Cell Proliferation Assay Kit (Biovision, Milpitas, CA, USA) according to the manufacturer's instructions. In short, the assay was started by adding 100 μL mL−1 of WST-1/ECS solution to the each well. The cells were then incubated for 1 h in culture conditions. The measurement of cell proliferation was determined by measuring the absorbance at 440 nm with spectrometer (Biowave II, Biochrom, Cambridge, UK).
Apoptosis study
Apoptosis on a cell monolayer and formalin-fixed renal tissues was detected by in situ DNA fragmentation, using ApopTag (Chemicon, Temecula, CA, USA), according to the manufacturer's instruction.
In vivo erythropoiesis and ischaemia reperfusion (I/R) induced kidney injury
ICR female mice (30–35 g) were purchased from the National Laboratory Animal Center (Nakhon Pathom, Thailand); 35 mice were used for testing the erythropoietic activity of modified molecule and 22 mice in the I/R experiment (2 mice die due to bleeding during the operation (one control, one CEPO). The mice were housed in a temperature-controlled facility with a 12 h light on–off schedule and free access to food and water. All studies involving animals are reported in accordance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath et al., 2010). All procedures involving animals were carried out under the approval of the animal ethics committees of Lerdsin general hospital and conformed to the guidelines of the National Research Council of Thailand.
In vivo erythropoiesis
Five mice were injected with 0.3 mg kg−1 of each EPO derivative on days 0, 3, 7, 10 and 12. Haematocrit was measured from tail blood on days 0, 7 and 14.
I/R induced kidney injury
ICR mice were s.c. injected with 0.3 mg kg−1 of rHuEPO or GEPO, CEPO or an equal volume of 0.9%NaCl (NSS) (n = 5 per group). Thirty minutes later, under diethyl ether anaesthesia (1–3%), animals were subjected to bilateral I/R injury by simultaneous clamping of both renal pedicles for 40 min. We routinely monitor respiratory rate and depth, capillary refill, full muscle relaxation and loss of pedal reflex, limb withdraw and tail pinch throughout the surgical procedure. During the ischaemic phase, the abdomen was partially closed and the surgical table temperature was set at 39°C as previously described (Manotham et al., 2005). After 24 h of reperfusion, the animals were killed by an overdose of anaesthetic (diethyl ether) and the kidneys were removed.
Creatinine measurement
Blood samples were collected by heart puncture just before the animals were killed. SCr was measured using the Dimension RXL automated analyser (Dade Behring, Deerfield, IL, USA) (Ortega et al., 2006).
RT-PCR
Total RNA was isolated from snap-frozen tissue in liquid nitrogen with Purezol (Biorad, Hercules, CA, USA) according to the manufacturer's protocol. cDNA synthesis was carried out with 1 μg of isolated RNA using the Protoscript® first strand cDNA synthesis kit (New England Biolabs, Ipswich, MA, USA). One microgram of cDNA was used for PCR with the corresponding primers Bcl-2: 5′-TGCACCTGACGCCCTTCAC-3′, 5′-AGACAGCCAGGAGAAATCAAACAG-3′ and β-actin: 5′-CTTTCTACAATGAGCTGCGTG-3′, 5′-TCATGAGGTAGTCTGTCAGG-3′ with Stratagene Mx 3000p (Stratagene, La Jolla, CA, USA) and EXTaq DNA polymerase (Takara, Shiga, Japan). The PCR products were electrophoresed and visualized under UV light.
Histological examination and semiquantitative scoring system
All the scoring was determined in a blinded manner. Histological damage, including tubular epithelial injury (TI), proteinacious cast (TC) or tubular dilatation (TD), were scored on 15 randomly selected non-overlapping 200 × fields or entire specific area per mouse, according to the following scoring methods: 0, no damage; 1, mild damage; 2, moderate damage; 3, severe damage (Manotham et al., 2005). Apoptotic cells were counted as TUNEL-positive cells on 15 randomly selected 200 × fields or an entire specific area.
Immunohistochemistry of Bcl-2 was performed on 3 μm of paraffin-embedded Methyl Carnoy's fixed renal tissues. Endogenous peroxidase activity was quenched by incubation with 3% H2O2 in PBS for 10 min. Non-specific binding was blocked with 4% skimmed milk and 1%BSA in PBS, 0.1%Tween 20 for 30 min. Slides were incubated with 1:500 monoclonal mouse anti Bcl-2 in 1%BSA in PBS, 0.1%Tween 20 for 1 h at room temperature, followed by biotinylated secondary antibodies for 30 min. Avidin peroxidase was applied at the final step, and colour was developed with 3,3′-diaminobenzidine tetrahydrochloride (Dako, Glostrup, Denmark). Finally, tissues were counterstained with haematoxylin.
All histological slides were examined by light microscopy (Olympus BX51, Tokyo, Japan), and pictures were taken by Olympus DP71 system.
Statistical analysis
Data are expressed as mean ± SD. Multiple comparisons among groups were performed by one-way anova using the post hoc LSD test (SPSS version 13, SPSS Inc, Chicago, IL, USA). P < 0.05 was considered statistically significant.
Results
Successive lysine modification and cytoprotection of modified derivatives
As depicted in Figure 1A, more than 90% of free amino acids were lost by gluteraldehyde modification, resulting in GEPO, or by guanidinate reaction, resulting in GuEPO. This was considered highly efficient. The CEPO resulted in approximately 80% free amino acid loss; while phosphorylated, acetylated and succinilated reactions reduced free amino acid by only 45.6%, 42.7% and 39.4% respectively.
Figure 1.
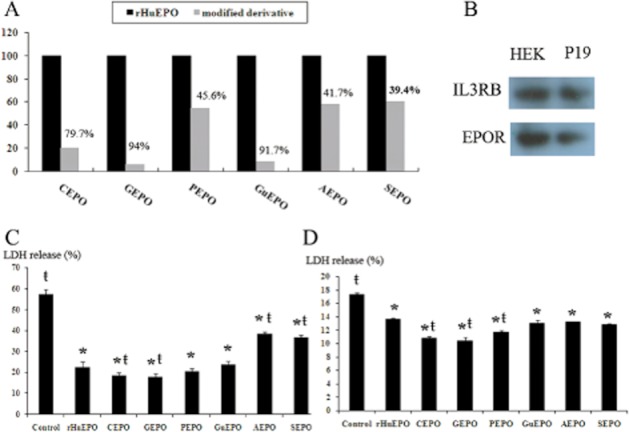
Successive lysine modifications of rHuEPO. (A) Analysis of free amino acid loss by TNBS assay showed diverse yields of rHuEPO modification in various reactions. Based on these findings, the glutaraldehyde modification and guanidination were considered as the most efficient reactions. (B) Immunoblot of HEK-293 and P19 cells showed that both cell lines expressed EPOR and IL3RB. (C) LDH release from HEK-293 cells after induced oxidative cell injuries demonstrated that each modified EPO has different cytoprotective activities. The LDH release of H2O2-treated HEK-293 cells was 57.23%, which was significantly increased as compared with the non-treated cells and blank media (data not shown). Pretreatment with rHuEPO and modified EPOs significantly reduced LDH. (*P < 0.05 vs. control, ŧP < 0.05 vs. rHuEPO). (D) LDH release from P19 cells subjected to serum deprivation is an index of cell damage. The LDH release was highest in the control cells. Pretreatment with EPO derivatives significantly reduced LDH release, suggesting the cytoprotective properties of those molecules. (*P < 0.05 vs. control, ŧP < 0.05 vs. rHuEPO).
Cytoprotection of modified EPOs was assessed by determining the ratio of LDH released from HEK-293 cells subjected to oxidative damage and of P19 cells following 2 h of serum-free culture. RT-PCR revealed that both cell lines expressed EPOR and IL3RB (data not shown), which was confirmed by immunoblotting (Figure 1B). As shown in Figure 1C and D, treatment with EPO and modified derivatives significantly reduced LDH release in both cell lines, suggesting that these derivatives retain the cytoprotective activities of EPO.
GEPO completely abolishes in vivo erythropoiesis
Next, in vivo erythropoiesis was performed to test the erythropoietic effect of modified EPOs. Mice were s.c. injected with 0.3 mg kg−1 of rHuEPO or modified-EPOs at days 0, 3, 7, 10 and 12. There were no differences in haematocrit at the beginning, but haematocrit levels were significantly raised in mice receiving all EPOs except for GEPO, by the end of the first week (Figure 2). At day 14, haematocrit values were unchanged and comparable with those of control animals only in mice treated with GEPO. Interestingly, the haematocrit of the GuEPO-treated group was increased in a similar fashion to that of rHuEPO-treated mice, although most of the free amino acids were drastically modified. Since we were unable to completely modify lysine residues on CEPO, PEPO and SEPO, elevated haematocrit levels in mice treated with these derivatives may be confounded by unmodified EPO and do not necessarily indicate that these derivatives are erythropoietic.
Figure 2.
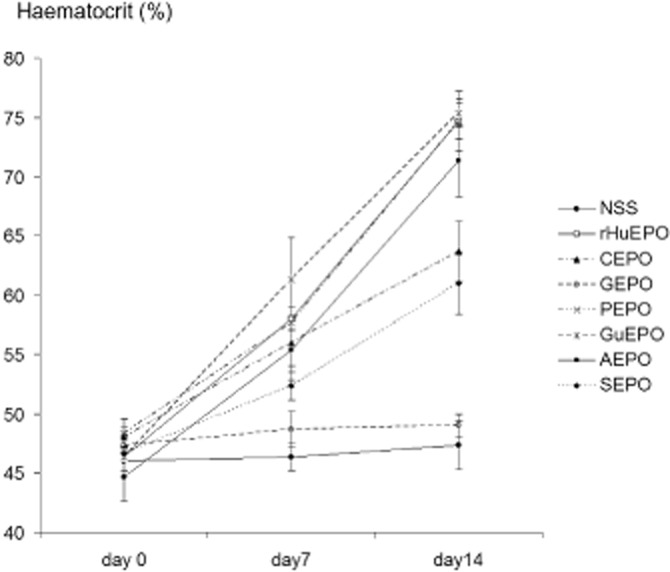
Effect of rHuEPO and modified EPOs on in vivo erythropoiesis. rHuEPO and modified EPOs at the doses of 0.3 mg kg−1 were s.c. injected in mice (n = 5 each) at days 0, 3, 7, 10 and 12 (about 6000-fold the starting dosage for the treatment of renal anaemia in humans). The results showed that GEPO was completely devoid of erythropoietic activities. Substantial activity loss was also observed with CEPO and SEPO.
GEPO was more negatively charged than rHuEPO and bound to heterotrimertic receptors
Since GEPO did not increase red blood cell mass and exhibited cytoprotective effects in vitro, we reasoned that GEPO is a novel non-erythopoietic EPO that provides tissue protection. To provide further insights into the basic properties of the GEPO molecule, the surface charge of modified EPO proteins was studied through a ζ-potential measurement (Figure 3A). Isoelectric points (ISP), the pH values where the surface electric charge is zero, were also determined. The ISP of rHuEPO was 6.84. GEPO was slightly negative as compared with the pro-drug, with an ISP of 6.00. This finding was ascertained by native PAGE, which showed that GEPO was moved towards the cathode faster than rHuEPO (Figure 3B).
Figure 3.
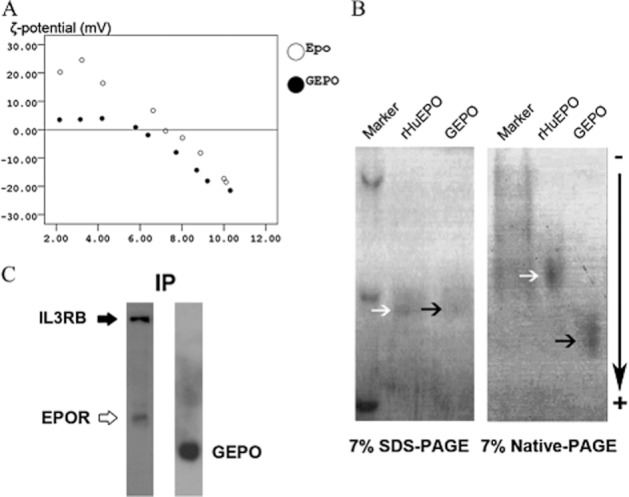
Characterization of GEPO and receptor immunoprecipitation. (A) ζ-potential analysis of rHuEPO and GEPO. (B) SDS-PAGE electrophoresis suggested that the molecular weight of GEPO was not different from that of rHuEPO. Native PAGE electrophoresis demonstrated that GEPO, which was almost completely modified, formed a homogeneous band that moved towards the cathode faster than rHuEPO, indicating that this molecule has more negative charge. The single band of GEPO on native-PAGE also suggested that the glutaraldehyde reaction resulted in an intra-molecule crosslink derivative rather than an inter-molecule crosslink derivative. (C) Lysate protein from GEPO-treated P19 cells was precipitated with anti-EPOR. Left panel: the precipitate protein was reacted with IL3RB antibody and EPOR antibodies. Right panel: the precipitated protein was also detected with anti-EPO antibody with cross-reactivity for GEPO.
To investigate the binding of GEPO with IL3RB/EPOR heterotrimeric receptors, immunoprecipitation of cell lysate was performed with EPOR antibodies. Immunoblots of precipitate protein stained for both IL3RB and GEPO (Figure 3C) suggested that GEPO bond to the heterotrimeric receptor was composed of EPOR and IL3RB.
Pretreatment with GEPO had no affect on rHuEPO-induced UT-7 cell proliferation
To confirm that GEPO had no erythropoietic activities, the colony forming of erythropoietic cell lines UT-7 was performed. The colony count of rHuEPO treated UT-7 cells at day 7 and day 14 was 21.3 ± 0.6 and 86.0 ± 4.0. This was significantly higher than that of untreated cells. In contrast, the colony count of GEPO-treated UT-7 cells was not different from the untreated cells (Figure 4A). MTT proliferation assay also supported this result (Figure 4B) and indicated that GEPO completely prevented in vitro erythropoiesis. Notably, the proliferation rate of UT-7 cells, pre-saturated with GEPO, was increased by adding rHuEPO to culture media. Since UT-7 proliferation was mediated by homodimeric EPOR, this result suggests that GEPO is not a competitive antagonist of rHuEPO on classical EPO receptors.
Figure 4.
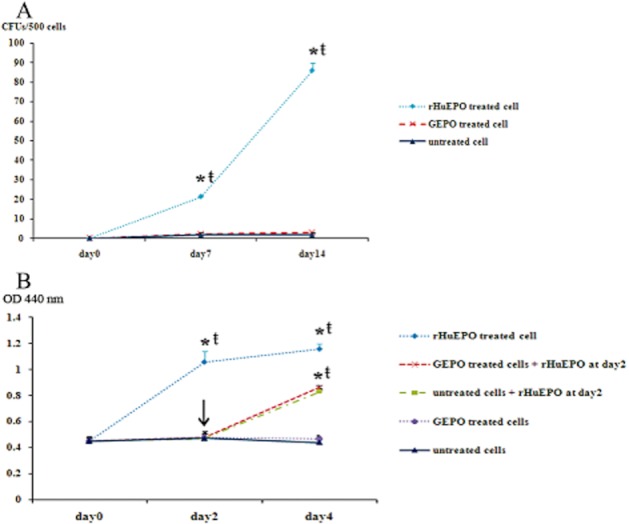
GEPO exhibits no effect on rHuEPO-induced proliferation of UT-7 cells. (A) UT-7 cells were cultured in semisolid medium with or without either 0.03 μg mL−1 of rHuEPO or GEPO. The colony count of rHuEPO-treated UT-7 cells at day 7 and day 14 was significantly higher than that of GEPO treated and untreated cells. In contrast, the colony count of GEPO treated UT-7 was not different from the untreated cells. (*P < 0.05 vs. untreated cells, ŧP < 0.05 vs. GEPO treated cells). (B) MTT proliferation assay results after culturing UT-7 cells in 10% FBS DMEM supplemented with and without either rHuEPO or GEPO also showed that the proliferation rate of rHuEPO-treated UT-7 cells was markedly higher than that of GEPO-treated and untreated cells. Notably, the proliferation rate of UT-7 cells that were pretreated with GEPO was increased by adding rHuEPO to culture media. (*P < 0.05 vs. untreated cells, ŧP < 0.05 vs. GEPO-treated cells)
GEPO reduced apoptosis of HEK-293 and P19 cells
The anti-apoptotic effect of rHuEPO and the non-erythropoietic derivatives was tested on both HEK-293 and P19 cell lines. As shown in Figure 5A, the number of TUNEL-positive cells in HEK-293 cells subjected to H2O2 (data pooled from three independent experiments) was 60.3 ± 6.1 for 5000 cells. Pretreatment with rHuEPO and GEPO significantly decreased this number to 45.3 ± 3.5 and 27 ± 4.3 cells for 5000 cells, respectively. Treatment with CEPO also reduced the number of apoptotic cells, but this trend did not reach a statistically significant level. P19 cells subjected to serum-free media also displayed increased apoptosis (Figure 5B). Pretreatment with rHuEPO and non-erythropoietic derivatives significantly reduced P19 cell apoptosis. Notably, the number of apoptotic cells was lowest after GEPO treatment. Taken together, these results confirm that GEPO had anti-apoptotic activities greater than those of the parent drug.
Figure 5.
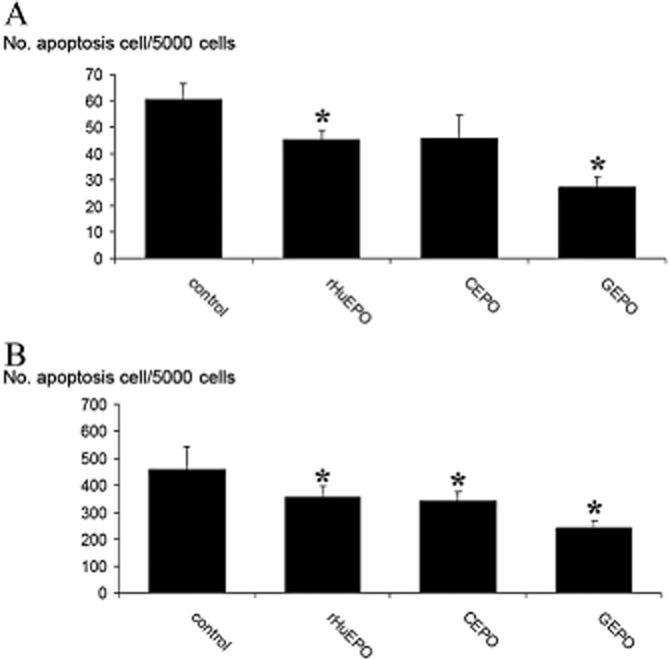
GEPO protects HEK-293 cells and P19 cells from apoptosis. (A) The number of TUNEL-positive cells in cultured HEK-293 cells that were pretreated with modified EPOs demonstrates levels of induced apoptosis. Data from three independent experiments show markedly decreased apoptosis after GEPO pretreatment. (*P < 0.05 vs. control). (B) The number of TUNEL-positive P19 cells after 2 h serum-free culture of untreated (control) cells and cells treated with modified EPOs. (*P < 0.05 vs. control).
GEPO induces JAK-2 dependent Bcl-2 activation
Immunoblotting showed very small amounts of the anti-apoptotic molecule, Bcl-2, in P-19 cells (Figure 6). After 2 h of incubation with rHuEPO and GEPO, Bcl-2 expression was increased. Notably, GEPO induced Bcl-2 activation higher than rHuEPO, despite slightly lower phosphorylated JAK-2. In the presence of a JAK-2 inhibitor, AG490 (50 μmol L−1), rHuEPO- and GEPO-induced Bcl-2 activation were completely and partially abolished respectively (Figure 6). This suggested that GEPO mediated its action on Bcl-2 by both a JAK-2-dependent and -independent pathway. In contrast, CEPO only slightly increased the activation of Bcl-2, which was not affected by the JAK-2 inhibitor. Taken together, these results suggest that GEPO induced Bcl-2 activation by a similar mechanism to rHuEPO, but not to CEPO.
Figure 6.
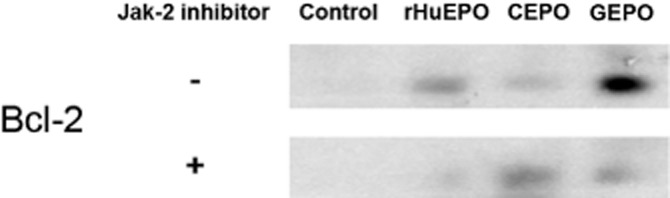
Activation of Bcl-2 by rHuEPO and non-erythropoietic derivatives. Representative immunoblot of Bcl-2 from three independent experiments, indicating that Bcl-2 was markedly lower in unstimulated P19 cells. rHuEPO and GEPO treatments markedly increased Bcl-2 levels. In addition, Bcl-2 activation by rHuEPO and GEPO was inhibited by a JAK-2 inhibitor. In contrast, activation of Bcl-2 by CEPO was less impressive and independent of JAK-2 inhibition.
GEPO protects the kidney from I/R renal damage
Next, I/R-induced kidney damage was employed to clarify whether GEPO has retained protective properties when compared with rHuEPO and CEPO. Renal function was assessed by measuring serum creatinine (SCr) at 24 h of reperfusion. In the saline-treated control animals (NSS), SCr was 67.47 ± 7.69 μmol L−1. Treatment with GEPO markedly decreased SCr to 26.40 ± 1.52 μmol L−1 (P < 0.01 vs. control). rHuEPO and CEPO also significantly lessened SCr but to a lesser extent. Levels were 57.79 ± 11.95 and 43.12 ± 6.16 μmol L−1 (P < 0.05 vs. control) respectively.
Histological examination revealed severe kidney injuries in the saline-treated control animals (Figure 7A). Renal damage was significantly ameliorated by GEPO and rHuEPO treatment and to a lesser degree by CEPO (Figure 7B–D). Semiquantitative scores confirmed this observation (Figure 7E). Additionally, this suggested that renal damage was appreciably lower in the GEPO treated animals. A TUNEL assay also demonstrated severe tubular cell apoptosis in the kidneys of the control animals. rHuEPO, GEPO and CEPO were able to inhibit tubular apoptosis induced by I/R (Figure 7F–I). The mean number of TUNEL-positive cells in the control animals was 86.8 ± 15.8 cells per field. This number decreased to 57.3 ± 26.7, 52.3 ± 28.06 and 23.8 ± 13.9 cells per field for rHuEPO, CEPO and GEPO respectively (Figure 7J). Immunohistochemistry analysis showed that intra-renal transcription of Bcl-2 increased in mice treated with rHuEPO and GEPO (Figure 7K–N). This result was also confirmed by RT-PCR of Bcl-2. (Figure 7O)
Figure 7.
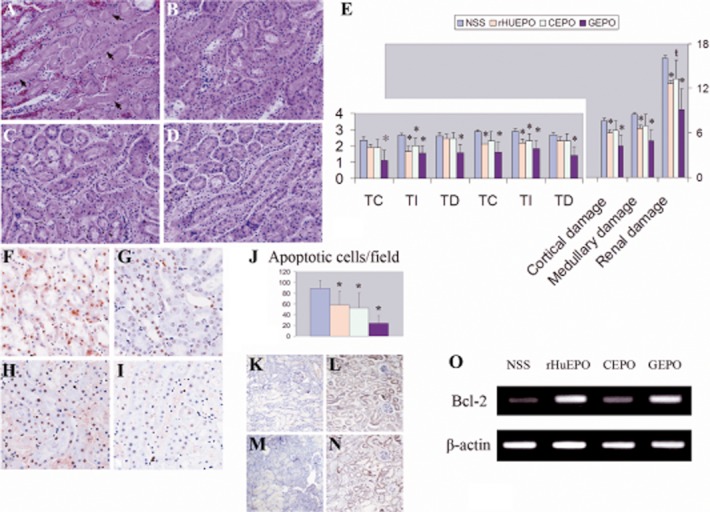
GEPO ameliorates I/R injuries. Renal histomorphology obtained from NSS (A). rHuEPO (B), CEPO (C) and GEPO (D) groups at 24 h after reperfusion. Severe tubular cell damage was observed in I/R mice receiving NSS treatment (A). Clear necrosis in accompanied by misshapen nuclei in many areas. Improvement of renal histology was remarkably conspicuous after GEPO treatment (D) and also in rHuEPO (B) and CEPO (C) treatment. A semiquantitative scoring system clarified that renal protection resulted from EPO and modified EPO treatment (E) (TC = tubular cast, TI = tubular injuries and TD = tubular dilatation). Notably, GEPO effects were more pronounced for renoprotection, while the histological evidence of CEPO protection was less impressive than that for GEPO and rHuEPO. (*P < 0.05 vs. control), (ŧP = 0.05). A TUNEL assay showed prominent apoptosis of cells in the kidneys of saline-treated animals (F). The numbers of apoptotic cells were significantly decreased in GEPO- (I), rHuEPO- (G) and CEPO-treated (H) mice, as shown by the semiquantitative score in (J). (*P < 0.05 vs. control). Immunohistochemistry analysis demonstrated rHuEPO- (L) and GEPO-treated animals (N) expressed higher Bcl-2 protein in renal tissues as compare with the control (K) and CEPO-treated animal (M). RT-PCR also showed markedly increased intra-renal Bcl-2 transcription in rHuEPO-and GEPO -treated animals as compared with saline-treated animals. Bcl-2 transcription was slightly increased in CEPO-treated mice (O).
Discussion and conclusions
This current work reports simple reactions to modify lysine residues on the marketed formulation of rHuEPO in order to obtain non-erythropoietic EPO. The formulation that we employed contained polysorbate 80 and glycine as stabilizers in a fixed ratio that was stoichiometric with EPO (Schellekens, 2005). Previous works indicated that this preparation formed polysorbate 80-EPO-associated micelles (Villalobos et al., 2005). On account of the complexity of the formulation, the effectiveness of modifications was low and different from those found in previous studies especially in carbamylated (Satake et al., 1990; Leist et al., 2004), phosphorylated and succinated reactions (Satake et al., 1990). In contrast, the glutaraldehyde modification and guanidination were considerably more effective on this rHuEPO formulation. Cytotoxicity screening and in vivo administration revealed that GEPO lost its erythropoietic effect but still had cytoprotective activity; it was therefore considered to be a non-erythropoietic EPO. Notably, SEPO also shared some properties of the non-erythropoietic EPO. However, because of the incomplete modifications, we were unable to demonstrate this conclusively and further elucidate this molecule's actions.
Satake et al. (1990) speculated that lysine residues on the erythropoietin molecule, specifically their positive charge, were crucial in mediating the erythropoietic effect. In their work, decreasing lysine's positive charge reduced erythropoietic activities, while chemical reactions that did not change lysine's positive charge, such as guanidination resulted in retained in vitro erythropoietic activities. Our in vivo experiment clearly showed that GuEPO induced erythropoiesis. The results of native PAGE and ζ potential analysis suggested that guanidination caused a slight increase in the positive charge on the erythropoietin molecule (data not shown). Satake previously reported that GuEPO increases red blood cell production more than the pro-drug. Therefore, our results confirmed Satake's in vitro finding.
Gluteraldehyde modification is a simple reaction that reduces the lysine positive charge by inducing a crosslink at lysine residues. This may also change protein conformation and function (Carla et al., 2004; Kawaguchi et al., 2006). Theoretically, the gluteraldehyde reaction may be able to induce lysine crosslinks between two or more molecules of rHuEPO. However, a single band of GEPO on native-PAGE suggested that the reaction induced intra-molecular crosslinks rather than inter-molecular crosslinks. The native PAGE and ζ potential analysis confirmed that GEPO was more negative than rHuEPO, supporting the previous notion that the positive lysine charge on EPO is essential for erythropoiesis.
Previous studies indicated that cytoprotection of rHuEPO and CEPO were mediated, at least in part, by their anti-apoptotic activity (Fiordaliso et al., 2005; Coleman et al., 2006; Moon et al., 2006). In this work, we test the anti-apoptotic activity of GEPO with two unrelated apoptotic models, H2O2 in HEK cells and serum deprivation in P19 cells. H2O2-induced apoptosis is a representative model of the apoptosis induced by reactive oxygen species (ROS) (Dumont, 1999; Kim et al., 2000; Liu et al., 2007). The serum deprivation of P19 cells is also a standard apoptogenic model and represents apoptosis induced by the loss of surviving signals (Galli and Fratelli, 1993; Ninomiya et al., 1997; Sirén et al., 2001). Although we clearly showed the anti-apoptotic effect of GEPO in both independent models, it should be noted that the results relied on the TUNEL assay, which has its own limitations.
Studies from Brines and Cerami (2005) suggest that CEPO binds to an IL3RB/EPOR heterotrimeric receptor, unlike EPO, which binds to homodimeric EPOR. Therefore, differential receptor binding was postulated to be the underlying mechanism that dissociates the cytoprotective actions of EPO from its erythropoietic actions. Nonetheless, this postulate is difficult to verify as a finding that is universally true; it may be a CEPO-specific observation if the only available non-erythropoietic EPO was CEPO. Recently, evidence has been obtained indicating that EPO might directly interact with IL3RB. This work demonstrated that GEPO, the other non-erythropoietic EPO, also interacted with heterotrimeric receptors. Moreover, the data on UT-7 suggested that GEPO was not a competitive antagonist of EPO on erythropoietic proliferation, thereby supporting the hypothesis that differential receptor binding controls EPO actions. However, the action of GEPO was not identical to CEPO, at least with regard to Bcl-2 activation. Other studies have suggested that the protective effects of EPO on tissues is also related to Bcl-2 activation (Yang et al., 2003; Brines and Cerami, 2005). However, CEPO did not activate Bcl-2 in a previous study (Leist et al., 2004). In the present study, it was found that GEPO, similar to rHuEPO, was able to activate Bcl-2 in a JAK-2-dependent manner but also by another mechanism. Phosphorylation of JAK-2 is pivotal for class I cytokine action, including EPOR and IL3RB signalling pathways (Quelle et al., 1994; Digicaylioglu and Lipton, 2001; Brines and Cerami, 2005). Taken together, these results suggest that the effects of GEPO are similar to those of EPO, although this molecule is devoid of erythropoietic activity.
Previous studies have demonstrated a renoprotective action of rHuEPO in I/R-induced kidney damage, via multiple mechanisms (Yang et al., 2003; Abdelrahman et al., 2004; Gong et al., 2004; Patel et al., 2004; Sharples et al., 2004; Vesey et al., 2004; Spandou et al., 2006). Yang et al.'s (2003) showed that the kidney protection of EPO is associated with HSP70 activation. A study by Patel provided evidence that rHuEPO reduced intra-renal inflammation, as shown by decreased renal malondialdehyde (Patel et al., 2004). Gong et al. (2004) showed that rHuEPO prevented the down-regulation of sodium channels and aquaporin, the proper functioning of which is essential for maintaining renal functions. Recently, the renoprotection of CEPO was demonstrated in the context of I/R, with decreased apoptosis as an indicator of protective effect (Imamura et al., 2007). In the present study, we clearly demonstrated that the novel non-erythropoietic EPO, GEPO, also provided substantial renoprotection. Pretreatment with GEPO significantly decreased histological damage and preserved renal function, accompanied by increased intra-renal Bcl-2 transcription. GEPO may exert a renoprotective action in the context of I/R-induced renal damage by inhibiting apoptosis via the Bcl-2 pathway. In addition to I/R-induced kidney damage, renoprotection without erythropoietic activity of GEPO may have a therapeutic advantage for long-term use in chronic evolving renal diseases, for example, diabetic renal disease (Menne et al., 2007) and secondary renal progression (Bahlmann et al., 2004).
In conclusion, the novel non-erythropoietic EPO described here was efficiently obtained from a simple reaction of commercial rHuEPO. This series of experiments showed that this agent possesses potent cytoprotective properties both in vitro and in vivo, with promise for future use as an adjunctive treatment for kidney disease.
Acknowledgments
We are grateful to Dr Masaomi Nangaku (University of Tokyo, Tokyo) for critically reading the manuscript and Dr Ryoji Sassa (Okasaki, Nagoya) for his generous support. A part of this work was supported by a grant from Thailand Research Fund (TRF), the Department of Medical Services of Thailand and the Japan International Cooperation Agency.
Glossary
- AEPO
acetylated erythropoietin
- CEPO
carbamylated erythropoietin
- CFU
colony-forming unit
- EPO
erythropoietin
- EPOR
erythropoietin receptor
- GEPO
glutaraldehyde erythropoietin
- GuEPO
guanidinated EPO
- I/R
ischaemia/reperfusion
- ISP
isoelectric points
- NSS
normal saline
- PEPO
phosphopyridoxylated EPO
- rHuEPO
recombinant human erythropoietin
- SCr
serum creatinine
- SEPO
succinylated EPO
- TC
proteinacious cast
- TD
tubular dilatation
- TI
tubular epithelial injury
- TNBS
trinitrobenzenesulfonic
- IL3RB
IL-3 common β-receptor
Conflicts of interest
The authors state no conflict of interest.
References
- Abdelrahman M, Sharples EJ, McDonald MC, Collin M, Patel NS, Yaqoob MM, et al. Erythropoietin attenuates the tissue injury associated with hemorrhagic shock and myocardial ischemia. Shock. 2004;22:63–69. doi: 10.1097/01.shk.00001276869.21260.9d. [DOI] [PubMed] [Google Scholar]
- Bahlmann FH, Song R, Boehm SM, Mengel M, von Waseljewski R, Lindschau C, et al. Low-dose therapy with the long-acting erythropoietin analogue darbepoetin alpha persistently activates endothelial Akt and attenuates progressive organ failure. Circulation. 2004;110:1006–1012. doi: 10.1161/01.CIR.0000139335.04152.F3. [DOI] [PubMed] [Google Scholar]
- Brines M, Cerami A. Emerging biological roles for erythropoietin in the nervous system. Nat Rev Neurosci. 2005;6:484–494. doi: 10.1038/nrn1687. [DOI] [PubMed] [Google Scholar]
- Brines M, Cerami A. Discovering erythropoietin's extra-hematopoietic functions: biological and clinical promise. Kidney Int. 2006;70:246–250. doi: 10.1038/sj.ki.5001546. [DOI] [PubMed] [Google Scholar]
- Brines M, Ghezzi P, Keenan S, Agnello D, de Lanerolle NC, Cerami C, et al. Erythropoietin crosses the blood–brain barrier to protect against experimental brain injury. Proc Natl Acad Sci U S A. 2000;97:10526–10531. doi: 10.1073/pnas.97.19.10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brines M, Grasso G, Fiordaliso F, Sfacteria A, Ghezzi P, Fratelli M, et al. Erythropoietin mediates tissue protection through an erythropoietin and common β–subunit heteroreceptor. Proc Natl Acad Sci U S A. 2004;10:14907–14912. doi: 10.1073/pnas.0406491101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvillo L, Latini R, Kajstura J, Leri A, Anversa P, Ghezzi P, et al. Recombinant human erythropoietin protects the myocardium from ischemia-reperfusion injury and promotes beneficial remodeling. Proc Natl Acad Sci U S A. 2003;100:4802–4806. doi: 10.1073/pnas.0630444100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carla JSM, Fernanda S, Georg G, Artur C-P. Chemical modifications on proteins using glutaraldehyde. Food Technol Biotechnol. 2004;42:51–56. [Google Scholar]
- Coleman T, Brines M. Science review: recombinant human erythropoietin in critical illness: a role beyond anemia? Crit Care. 2004;8:337–341. doi: 10.1186/cc2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman TR, Westenfelder C, Tögel FE, Yang Y, Hu Z, Swenson L, et al. Cytoprotective doses of erythropoietin or carbamylated erythropoietin have markedly different pro-coagulant and vasoactive activities. Proc Natl Acad Sci U S A. 2006;103:5965–5970. doi: 10.1073/pnas.0601377103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combe C, Tredree RL, Schellekens H. Biosimilar epoetins: an analysis based on recently implemented European medicines evaluation agency guidelines on comparability of biopharmaceutical proteins. Pharmacotherapy. 2005;25:954–962. doi: 10.1592/phco.2005.25.7.954. [DOI] [PubMed] [Google Scholar]
- Cupo P, EI-Deiry W, Whitney PL, Awad WM. Stabilization of protein by guanidination. J Biol Chem. 1980;255:10828–10833. [PubMed] [Google Scholar]
- Deechongkit S, Aoki KH, Park SS, Kerwin BA. Biophysical comparability of the same protein from different manufacturers: a case study using epoetin alfa from epogen and eprex. J Pharm Sci. 2006;95:1931–1943. doi: 10.1002/jps.20649. [DOI] [PubMed] [Google Scholar]
- Digicaylioglu M, Lipton SA. Erythropoietin-mediated neuroprotection involves cross-talk between Jak2 and NF-kB signaling cascades. Nature. 2001;412:641–647. doi: 10.1038/35088074. [DOI] [PubMed] [Google Scholar]
- Dixon HBF, Perham RN. Reversible blocking of amino groups with citraconic anhydride. Biochem J. 1968;109:312–314. doi: 10.1042/bj1090312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont A. Hydrogen peroxide-induced apoptosis is CD 95-independent, requires the release of mitochondria-derived reactive oxygen species and the activation of NF-kappa B. Oncogene. 1999;18:747–757. doi: 10.1038/sj.onc.1202325. [DOI] [PubMed] [Google Scholar]
- Fields R. The measurement of amino groups in proteins and peptides. Biochem J. 1971;124:581–590. doi: 10.1042/bj1240581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiordaliso F, Chimenti S, Staszewsky L, Bai A, Carlo E, Cuccovillo I, et al. A nonerythropoietic derivative of erythropoietin protects the myocardium from ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2005;102:2046–2051. doi: 10.1073/pnas.0409329102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher JW. Erythropoietin: physiology and pharmacology update. Exp Biol Med. 2003;228:1–14. doi: 10.1177/153537020322800101. [DOI] [PubMed] [Google Scholar]
- Galli G, Fratelli M. Activation of apoptosis by serum deprivation in a teratocarcinoma cell line: inhibition by L-acetylcarnitine. Exp Cell Res. 1993;204:54–60. doi: 10.1006/excr.1993.1008. [DOI] [PubMed] [Google Scholar]
- Gong H, Wang W, Kwon TH, Jonassen T, Li C, Ring T, et al. EPO and alpha-MSH prevent ischemia/reperfusion-induced down-regulation of AQPs and sodium transporters in rat kidney. Kidney Int. 2004;66:683–695. doi: 10.1111/j.1523-1755.2004.00791.x. [DOI] [PubMed] [Google Scholar]
- Gorio A, Gokmen N, Erbayraktar S, Yilmaz O, Madaschi L, Cichetti C, et al. Recombinant human erythropoietin counteracts secondary injury and markedly enhances neurological recovery from experimental spinal cord trauma. Proc Natl Acad Sci U S A. 2002;99:9450–9455. doi: 10.1073/pnas.142287899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habeeb AF, Cassidy HG, Singer SG. Molecular structural effects produced in proteins by reaction with succinic anhydride. Biochem Biophys Acta. 1958;29:587–593. doi: 10.1016/0006-3002(58)90016-7. [DOI] [PubMed] [Google Scholar]
- Horkko S, Savolainen MJ, Kervinen K, Kesaniemi YA. Carbamylation-induced alterations in low-density lipoprotein mechanism. Kidney Int. 1992;4:1175–1181. doi: 10.1038/ki.1992.179. [DOI] [PubMed] [Google Scholar]
- Imamura R, Isaka Y, Ichimaru N, Takahara S, Okuyama A. Carbamylated erythropoietin protects the kidney from ischemia-reperfusion injury without stimulating erythropoiesis. Biochem Biophys Res Commun. 2007;353:786–792. doi: 10.1016/j.bbrc.2006.12.099. [DOI] [PubMed] [Google Scholar]
- Jelkmann W. Recombinant EPO production points the nephrologist should know. Nephrol Dial Transplant. 2007;22:2749–2753. doi: 10.1093/ndt/gfm392. [DOI] [PubMed] [Google Scholar]
- Jelkmann W. ‘O’, erythropoietin carbamoylation versus carbamylation. Nephrol Dial Transplant. 2008;23:3033. doi: 10.1093/ndt/gfn342. [DOI] [PubMed] [Google Scholar]
- Jelkmann W, Wagner K. Beneficial and ominous aspects of the pleiotropic action of erythropoietin. Ann Hematol. 2004;83:673–686. doi: 10.1007/s00277-004-0911-6. [DOI] [PubMed] [Google Scholar]
- Junk AK, Mammis A, Savitz SI, Singh M, Roth S, Malhotra S, et al. Erythropoietin administration protects retinal neurons from acute ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2002;99:10659–10664. doi: 10.1073/pnas.152321399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Ito A, Appella E, Yao T. Charge modification at multiple C-terminal lysine residues regulates p53 oligomerization and its nucleus-cytoplasm trafficking. J Biol Chem. 2006;281:1394–1400. doi: 10.1074/jbc.M505772200. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. NC3Rs Reporting Guidelines Working Group. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Lee TH, Park ES, Suh JM, Park SJ, Chung HK, et al. Role of peroxiredoxins in regulating intracellular hydrogen peroxide and hydrogen peroxide-induced apoptosis in thyroid cells. J Biol Chem. 2000;275:18266–18270. doi: 10.1074/jbc.275.24.18266. [DOI] [PubMed] [Google Scholar]
- King VR, Averill SA, Hewazy D, Priestley JV, Torup L, Michael-Titus AT. Erythropoietin and carbamylated erythropoietin are neuroprotective following spinal cord hemisection in the rat. Eur J Neurosci. 2007;26:90–100. doi: 10.1111/j.1460-9568.2007.05635.x. [DOI] [PubMed] [Google Scholar]
- Lacombe C, Mayeux P. Biological of erythropoietin. Haematologica. 1998;83:724–732. [PubMed] [Google Scholar]
- Lacombe C, Mayeux P. The molecular biological of erythropoietin. Nephrol Dial Transplant. 1999;14(Suppl 2):22–28. doi: 10.1093/ndt/14.suppl_2.22. [DOI] [PubMed] [Google Scholar]
- Leist M, Ghezzi P, Grasso G, Bianchi R, Villa P, Fratelli M, et al. Derivatives of erythropoietin that are tissue protective but not erythropoietic. Science. 2004;305:239–242. doi: 10.1126/science.1098313. [DOI] [PubMed] [Google Scholar]
- Liu CL, Xie LX, Li M, Durairajan SSK, Goto S, Huan JD. Salvianolic Acid B Inhibits Hydrogen Peroxide-Induced Endothelial Cell Apoptosis through Regulating PI3K/Akt Signaling. Plos ONE. 2007;19:e1321. doi: 10.1371/journal.pone.0001321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundblad RL, Noyes CM. The modification of lysine. In: Lundblad RL, Noyes CM, editors. Chemical Reagent for Protein Modification. Boca Raton, FL: CRC Press; 1984. pp. 127–170. [Google Scholar]
- Maiese K, Li F, Chong ZZ. New avenues of exploration for erythropoietin. JAMA. 2005;293:90–95. doi: 10.1001/jama.293.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manotham K, Tanaka T, Ohse T, Kojima I, Miyata T, Inagi R, et al. A biologic role of HIF-1 in the renal medulla. Kidney Int. 2005;67:1428–1439. doi: 10.1111/j.1523-1755.2005.00220.x. [DOI] [PubMed] [Google Scholar]
- Marti HH, Wenger RH, Rivas LA, Straumann U, Digicaylioglu M, Henn V, et al. Erythropoietin gene expression in human, monkey and murine brain. Eur J Neurosci. 1996;8:666–676. doi: 10.1111/j.1460-9568.1996.tb01252.x. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reportingexperiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menne J, Park JK, Shushakova N, Mengel M, Meier M, Fliser D. The continuous erythropoietin receptor activator affects different pathways of diabetic renal injury. J Am Soc Nephrol. 2007;18:2046–2053. doi: 10.1681/ASN.2006070699. [DOI] [PubMed] [Google Scholar]
- Mennini T, De Paola M, Bigini P, Mastrotto C, Fumagalli E, Barbera S, et al. Nonhematopoietic erythropoietin derivatives prevent motoneuron degeneration in vitro and in vivo. Mol Med. 2006;12:153–160. doi: 10.2119/2006-00045.Mennini. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michèle RR, Charis G. N-acetylbenzotriazole as a protein reagent. Eur J Biochem. 1976;65:25–33. doi: 10.1111/j.1432-1033.1976.tb10385.x. [DOI] [PubMed] [Google Scholar]
- Moon C, Krawczyk M, Paik D, Coleman T, Brines M, Juhaszova M, et al. Erythropoietin, modified to not stimulate red blood cell production, retains its cardioprotective properties. J Pharmacol Exp Ther. 2006;316:999–1005. doi: 10.1124/jpet.105.094854. [DOI] [PubMed] [Google Scholar]
- Morishita E, Masuda S, Nagao M, Yasuda Y, Sasaki R. Erythropoietin receptor is expressed in rat hippocampal and cerebral cortical neurons, and erythropoietin prevents in vitro glutamate-induced neuronal death. Neuroscience. 1997;76:105–116. doi: 10.1016/s0306-4522(96)00306-5. [DOI] [PubMed] [Google Scholar]
- Ninomiya Y, Adams R, Morriss-Kay GM, Eto K. Apoptotic cell death in neuronal differentiation of P19 EC cells: cell death follows reentry into S phase. J Cell Physiol. 1997;172:25–35. doi: 10.1002/(SICI)1097-4652(199707)172:1<25::AID-JCP3>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Ortega A, Rámila D, Ardura JA, Esteban V, Ruiz-Ortega M, Barat A, et al. Role of parathyroid hormone-related protein in tubulointerstitial apoptosis and fibrosis after folic acid-induced nephrotoxicity. J Am Soc Nephrol. 2006;17:1594–1603. doi: 10.1681/ASN.2005070690. [DOI] [PubMed] [Google Scholar]
- Park KD, Mun KC, Chang EJ, Park SB, Kim HC. Inhibition of erythropoietin activity by cyanate. Scand J Urol Nephrol. 2004;38:69–72. doi: 10.1080/00365590310006291. [DOI] [PubMed] [Google Scholar]
- Patel NS, Sharples EJ, Cuzzocrea S, Chatterjee PK, Britti D, Yaqoob MM, et al. Pretreatment with EPO reduces the injury and dysfunction caused by ischemia/reperfusion in the mouse kidney in vivo. Kidney Int. 2004;66:983–989. doi: 10.1111/j.1523-1755.2004.00847.x. [DOI] [PubMed] [Google Scholar]
- Quelle FW, Sato N, Witthuhn BA, Inhorn RC, Eder M, Miyajima A, et al. JAK2 associates with the beta c chain of the receptor for granulocyte-macrophage colony-stimulating factor, and its activation requires the membrane-proximal region. Mol Cell Biol. 1994;14:4335–4341. doi: 10.1128/mcb.14.7.4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimon S, Perlmann G. Carbamylation of pepsinogen and pepsin. J Biol Chem. 1968;243:3566–3572. [PubMed] [Google Scholar]
- Satake R, Kozutsumi H, Takeuchi M, Asano K. Chemical modification of erythropoietin: an increase in in vivo activity by guanidination. Biochim Biophys Acta. 1990;1038:125–129. doi: 10.1016/0167-4838(90)90020-g. [DOI] [PubMed] [Google Scholar]
- Schellekens H. Factors influencing the immunogenicity of therapeutic proteins. Nephrol Dial Transplant. 2005;20(Suppl 6):3–9. doi: 10.1093/ndt/gfh1092. [DOI] [PubMed] [Google Scholar]
- Sharples EJ, Patel N, Brown P, Stewart K, Mota-Philipe H, Sheaff M, et al. Erythropoietin protects the kidney against the injury and dysfunction caused by ischemia-reperfusion. J Am Soc Nephrol. 2004;15:2115–2124. doi: 10.1097/01.ASN.0000135059.67385.5D. [DOI] [PubMed] [Google Scholar]
- Sirén AL, Fratelli M, Brines M, Goemans C, Casagrande S, Lewczuk P, et al. Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress. Proc Natl Acad Sci U S A. 2001;98:4044–4049. doi: 10.1073/pnas.051606598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spandou E, Tsouchnikas I, Karkavelas G, Dounousi E, Simeonidou C, Guiba-Tziampiri O, et al. Erythropoietin attenuates renal injury in experimental acute renal failure ischaemic/reperfusion model. Nephrol Dial Transplant. 2006;21:330–336. doi: 10.1093/ndt/gfi177. [DOI] [PubMed] [Google Scholar]
- Vesey DA, Cheung C, Pat B, Endre Z, Gobé G, Johnson DW. Erythropoietin protects against ischemic acute renal injury. Nephrol Dial Transplant. 2004;19:348–355. doi: 10.1093/ndt/gfg547. [DOI] [PubMed] [Google Scholar]
- Villalobos AP, Gunturi SR, Heavner GA. Interaction of polysorbate 80 with erythropoietin: a case study in protein–surfactant interactions. Pharm Res. 2005;22:1186–1194. doi: 10.1007/s11095-005-5356-7. [DOI] [PubMed] [Google Scholar]
- Wiecek A, Mikhail A. European regulatory guidelines for biosimilars. Nephrol Dial Transplant. 2006;21(Suppl 5):17–20. doi: 10.1093/ndt/gfl477. [DOI] [PubMed] [Google Scholar]
- Yang CW, Li C, Jung JY, Shin SJ, Choi BS, Lim SW, et al. Preconditioning with erythropoietin protects against subsequent ischemia-reperfusion injury in rat kidney. FASEB J. 2003;17:1754–1755. doi: 10.1096/fj.02-1191fje. [DOI] [PubMed] [Google Scholar]


