Abstract
Background And Purpose
Ca2+-dependent Cl− secretion (CaCC) in airways and other tissues is due to activation of the Cl− channel TMEM16A (anoctamin 1). Earlier studies suggested that Ca2+-activated Cl− channels are regulated by membrane lipid inositol phosphates, and that 1-O-octyl-2-O-butyryl-myo-inositol 3,4,5,6-tetrakisphosphate octakis(propionoxymethyl) ester (INO-4995) augments CaCC. Here we examined whether TMEM16A is the target for INO-4995 and if the channel is regulated by inositol phosphates.
Experimental Approach
The effects of INO-4995 on CaCC were examined in overexpressing HEK293, colonic and primary airway epithelial cells as well as Xenopus oocytes. We used patch clamping, double electrode voltage clamp and Ussing chamber techniques.
Key Results
We found that INO-4995 directly activates a TMEM16A whole cell conductance of 6.1 ± 0.9 nS pF–1 in overexpressing cells. The tetrakisphosphates Ins(3,4,5,6)P4 or Ins(1,3,4,5)P4 and enzymes controlling levels of InsP4 or PIP2 and PIP3 had no effects on the magnitude or kinetics of TMEM16A currents. In contrast in Xenopus oocytes, human airways and colonic cells, which all express TMEM16A endogenously, Cl− currents were not acutely activated by INO-4995. However incubation with INO-4995 augmented 1.6- to 4-fold TMEM16A-dependent Cl− currents activated by ionomycin or ATP, while intracellular Ca2+ signals were not affected. The potentiating effect of INO-4995 on transient ATP-activated TMEM16A-currents in cystic fibrosis (CF) airways was twice of that observed in non-CF airways.
Conclusions And Implications
These data indicate that TMEM16A is the target for INO-4995, although the mode of action appears different for overexpressed and endogenous channels. INO-4995 may be useful for the treatment of CF lung disease.
Keywords: INO-4995; INO4913; anoctamin 1; TMEM16A; inositol phosphates; Ins(3,4,5,6)P4; inositol 3,4,5,6-tetrakisphosphate; Ins(1,3,4,5)P4; inositol 1,3,4,5-tetrakisphosphate; Ca2+-activated Cl− channels; CaCC
Introduction
Ca2+-activated Cl− channels (CaCC) are highly relevant for secretion of electrolytes in epithelial tissues. Activation of CaCC in cystic fibrosis (CF) airways may ameliorate mucociliary clearance and compensate for defective cystic fibrosis transmembrane conductance regulator (CFTR) Cl− channels (Namkung et al., 2011). However, only recently the molecular counterpart of CaCC was identified as TMEM16A (anoctamin 1) (Caputo et al., 2008; Schroeder et al., 2008; Yang et al., 2008). TMEM16A is the major component of Ca2+-dependent Cl− secretion in airways and other epithelial tissues (Ousingsawat et al., 2009; Rock et al., 2009). In contrast to CFTR, activation of CaCC/TMEM16A is transient and does not strictly follow intracellular Ca2+ levels. Cl− currents relax faster than intracellular Ca2+ levels decline upon stimulation of PLC-coupled receptors. However, local compartmentalized Ca2+ in close proximity of the channel may recover quicker than global [Ca2+]i (Morris et al., 1990). Alternatively downstream products of InsP3 such as the inositol tetrakisphosphate Ins(3,4,5,6)P4 may inhibit CaCC, as shown for different types of epithelial cells (Kachintorn et al., 1993; DeLisle et al., 1994; Xie et al., 1996; Ho et al., 1997; Carew et al., 2000). Thus long-term uncoupling of chloride secretion from intracellular calcium levels by Ins(3,4,5,6)P4 has been proposed as a mechanism for the transient Ca2+-dependent Cl− secretion (Vajanaphanich et al., 1994). Following this, a novel therapeutic concept for treatment of CF lung disease had been develop, proposing cell permeant antagonists of endogenous Ins(3,4,5,6)P4 to allow for continuing Ca2+-dependent chloride secretion (Rudolf et al., 1998; 2003). The membrane permeable compound 1-O-octyl-2-O-butyryl-myo-inositol 3,4,5,6-tetrakisphosphate octakis(propionoxymethyl) ester (INO-4995; Inologic, Seattle, WA) was demonstrated both to inhibit the epithelial Na+ channel ENaC and to activate Ca2+-dependent Cl− secretion (Rudolf et al., 1998; Moody et al., 2005; Traynor-Kaplan et al., 2010). Therefore, the primary objective of the present study was to examine whether the novel Ca2+-dependent Cl− channel TMEM16A is the target for INO-4995.
Studies demonstrated that intracellular Ins(3,4,5,6)P4 levels are a result of inositol 1,3,4-trisphosphate 5/6-kinase (ITPK1) dephosphorylation of Ins(1,3,4,5,6)P5 and are regulated by Ins(1,3,4)P3 levels (Menniti et al., 1990; Chamberlain et al. #b1001; Saiardi and Cockcroft, 2008). Enzymes that generate inositolphosphate intermediates may therefore determine the magnitude and duration of CaCC (Vajanaphanich et al., 1994; Traynor-Kaplan et al., 2010). In fact, Yang et al. found that expression of ITPK1 is reduced in murine airway cells lacking functional CFTR, thus causing enhanced Ca2+-activated secretion. ITPK1 was therefore suggested as a novel modifier gene in CF (Shears, 2005; Yang et al., 2006). How Ins(3,4,5,6)P4 inhibits Ca2+-activated Cl− channels is unknown. Effects of Ins(3,4,5,6)P4 on accessory proteins such as protein phosphatases have been proposed, although precise mechanisms remain elusive (Xie et al., 1998; Ho et al., 2001). In order to guide future drug development programs, a more detailed understanding of the regulation by phosphatidylinositols (PI(4,5)P2, PI(3,4,5)P3) and Ins(3,4,5,6)P4 is essential (Shears, 2005). The secondary objective of this study was therefore to examine whether TMEM16A is regulated by phosphoinositides and/or Ins(3,4,5,6)P4 (Moody et al., 2005; Traynor-Kaplan et al., 2010). The results indicate that TMEM16A is activated by INO-4995 but otherwise not affected by phosphoinositides or InsP4.
Methods
Cell culture, cDNAs, siRNAs and transfection
HEK 293, HT29 and CFPAC cells were grown in DMEM and DMEM-F12 (Gibco, Karlsruhe, Germany), respectively, supplemented with 10% FBS at 37°C in a humidified atmosphere with 5% CO2. Cells were plated on fibronectin- and collagen-coated 18 cm diameter coverslips and co-transfected with cDNA encoding either human TMEM16A (a,b,c-Ano1; NM_018043), or empty pcDNA3.1 vector (mock) along with P2Y2 receptor (NM_176072) and CD8. Expression of TMEM16A and PTEN was suppressed by RNAi, and suppression of mRNA was monitored by real-time RT-PCR. For each type of siRNA-knockout, real-time RT-PCR was performed at least in triplicates. It showed siRNA-knockdown of Ano1-mRNA of 82 ± 4% (HT29), 68 ± 5% (CFPAC) and 45 ± 6% for PTEN (HEK293). Duplexes of 25-nucleotide of RNAi were designed and synthesized by Invitrogen (Paisley, UK) and Ambion (Darmstadt, Germany). RNAi was transfected using Lipofectamin (1 μg·μL−1). Cells were examined 48 h or 72 h after transfection. Transfections were carried out using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol.
Primary human bronchial epithelial cells monolayers
Human lung tissues (non-CF and CF) were obtained from the Cardio-Thoracic Surgery Department (Hospital de Santa Marta, Lisbon, Portugal) after receiving patient's written consent and approval by the Ethics Committee. Primary HBE cells were isolated as described previously (Fulcher et al., 2005) and expanded on collagen I–fibronectin-coated plastic dishes before passage to the porous membrane inserts. The primary HBE monolayers (passage 1) were grown on collagen IV-coated porous membranes (Snapwell, Corning-Costar®, Tewksbury, MA, USA) in an air–liquid interface for 3 weeks. Cells were incubated repetitively for 2 h in 5 μM INO-4995 on four subsequent days.
Patch clamping and double electrode voltage clamp
Two to 3 days after transfection, transfected HEK293, HT29 or CFPAC cells were identified by 1–2 min incubation with Dynabeads CD8 (Invitrogen), which permanently bind to transfected cells. Cover slips were mounted on the stage of an inverted microscope (IM35, Zeiss, Göttingen, Germany) and kept at 37°C. The bath was perfused continuously with Ringer solution (mmol·l−1: NaCl 145, KH2PO4 0.4, K2HPO4 1.6, d-glucose 6, MgCl2 1, Ca-gluconate 1.3, pH 7.4) at a rate of 5 mL·min−1. For fast whole-cell and cell-excised patch clamping, pipettes were filled with intracellular like solution containing (mM) KCl 30, potassium gluconate 95, NaH2PO4 1.2, Na2HPO4 4.8, EGTA 1, calcium gluconate 0.758, MgCl2 1.034, d-glucose 5, ATP 3, pH was 7.2 and had an input resistance of 2–4 MΩ. The free Ca2+ concentration in the pipette solution was 0.1 μM. For measurement of excised membranes, patches were excised into a bath solution containing 0.1 μM [Ca2+]. Experiments were conducted as described earlier (Ousingsawat et al., 2009). Typically, the cells were kept in current clamp for most of the time and the membrane voltage was recorded. From time to time the cells were voltage clamped (± 50 mV in steps of 10 mV for 1 s). All data were recorded using an EPC7, and the programme PULSE (HEKA, Lambrecht, Germany). All data were recorded continuously on a hard disc and analysed using PULSE and Chart (AD Instruments GmbH, Spechbach, Germany).
Isolation and microinjection of oocytes have been described in details elsewhere (Bachhuber et al., 2005). Oocytes were pre-incubated with 5 μM INO-4995 for 2 or 24 h. Oocytes were impaled with two electrodes (Clark Instruments Ltd, Salisbury, UK), which had a resistances of <1 MΩ when filled with 2.7 mol·l−1 KCI. Using two bath electrodes and a virtual-ground head stage, the voltage drop across Rserial was effectively zero. Membrane currents were measured by voltage clamping (oocyte clamp amplifier, Warner Instruments LLC, Hamden, CT) in intervals from −60 to +40 mV, in steps of 10 mV, each 1 s. Data were collected and analysed using Powerlab and Chart (AD instruments, Berlin, Germany). The bath was continuously perfused at a rate of 5 mL·min−1. All experiments were conducted at room temperature (22°C).
Measurement of intracellular Ca2+ concentration
For single cell fluorescence measurements, HEK293 cells and HT-29 cells were grown on glass coverslips mounted in a cell chamber and perfused with Ringer solution at 5 mL·min−1 at 37°C. Cell fluorescence measurements was measured continuously on an inverted microscope Axiovert S100 (Zeiss) using a Fluar 20×/0.75 objective (Zeiss) and a high-speed polychromator system (VisiChrome, Visitron Systems, Germany). Cells were loaded (1 h at 37°C) with 2 μM Fura2-AM (Molecular Probes) in Ringer solution or Opti-MEM containing 0.2% pluronic (Molecular Probes, Life Technologies GmbH, Darmstadt, Germany ). Fura-2 was excited at 340/380 nm, and the emission was recorded between 470 and 550 nm using a CCD-camera (CoolSnap HQ, Visitron Systems, Puchheim, Germany). Acquisition and data analysis were done using Meta-Fluor (Universal Imaging, Molecular Devices, Biberach an der Riss, Germany) and Origin (OriginLab Corporation, Northampton, MA, USA). For calibration of intracellular Ca2+ concentration, cells were perfused with Ringer solution and Ca2+ free ringer with 1 μM ionomycin, 10 μM monensin and 5 μM nigericin.
Immunocytochemistry and Western blot
HT29 cells were fixed for 10 min with 4% (w/v) paraformaldehyde at room temperature and permeabilized after blocking with 2% (w/v, PBS) BSA and 0.04% (v/v, PBS) Triton X-100. Cells were incubated for 1 h with primary anti-β-catenin antibody (1:1000; Qiagen, Hilden, Germany) at 37°C. Binding of the primary antibody was visualized by an Alexa 568-labelled secondary antibody (1:1.000; Molecular Probes, Invitrogen). Nuclei were stained with Hoe33342. Cells were examined with an ApoTome Axiovert 200 M fluorescence microscope (Zeiss). For Western blotting, cells were lysed with an appropriate buffer (150 mM NaCl, 50 mM Tris–HCl, 1 mM EDTA, 1% NP-40, protease inhibitor, 100 mM DTT; pH 7.4), and DNA was sheared by sonication. Samples were quantified using a Bio-Rad protein assay (Bio-Rad, Munich, Germany) and the same amount of protein (50 μg) was separated using PAGE (7.5%). Protein were transferred to PVDF membranes (Millipore, Merck Millipore, Billerica, MA, USA), and probed overnight at 4°C with a rabbit monoclonal anti-ANO1 antibody (Novus Biologicals, Littleton, CO, USA). Blots were visualized using a secondary HRP-conjugated anti-rabbit antibody (Acris, Herford, Germany) and Super Signal® West Pico Chemiluminescent Substrate (Pierce, Rockford, IL, USA).
Micro-Ussing chamber recordings
Access to human tissues used in this study received approval from the local Ethics Committee of the Faculty of Medical Sciences, and informed consent was obtained from all patients. HBE monolayers (resistance ≥600 Ωcm2) were mounted in a modified micro-Ussing chamber. Apical and basolateral surfaces were perfused continuously at a rate of 5–10 mL·min−1 (chamber volume 2 mL). The bath solution contained (mmol·L−1): NaCl, 145; KH2PO4, 0.4; K2HPO4, 1.6; d-glucose, 5; MgCl2, 1; Hepes 5; and Ca-gluconate, 1.3. pH was adjusted to 7.4). Experiments were carried out under open-circuit conditions at 37°C. Transepithelial resistance (Rte) was determined by applying short (1 s) current pulses (ΔI = 0.5 μA) and recording of the corresponding voltage deflections (ΔVte). Values for transepithelial voltage (Vte) were referred to the luminal side of the monolayers and equivalent short-circuit currents (Isc) were calculated according to Ohm's law.
Iodide quenching
Quenching of the intracellular fluorescence generated by the iodide-sensitive enhanced yellow fluorescent protein (YFP) was used to measure anion conductance. YFP fluorescence was excited at 490 nm using a semi-automatic Novostar plate reader (BMG-Labtech, Offenburg, Germany). I− influx was induced by replacing 20 mM extracellular Cl− by I−. Background fluorescence was subtracted and auto-fluorescence was negligible. Changes in fluorescence induced by I− are expressed as initial rates of fluorescence decrease (arbitrary units·s−1). For the assays, we used HT29 cells stably expressing YFP. Ca2+ stimulus and iodide were applied together because of the transient nature of the Ca2+ stimulation.
Materials
All compounds used (ionomycin, carbachol, ATP, DAG kinase inhibitor II, U73122) were of highest available grade of purity and were from Sigma (Taufkirchen, Germany) or Merck (Darmstadt, Germany). All cell culture reagents were from Gibco/Invitrogen. The IPMK inhibitor chlorogenic acid (C3878) was from Sigma. The inositol-1,4,5-trisphosphate 3-kinase inhibitor N2-(m-Trifluorobenzyl), N6-(p-nitrobenzyl)purine and d-myo-inositol 1,3,4,5-tetrakisphosphate octapotassium salt were from Merck. d-myo-Inositol 3,4,5,6-tetrakisphosphate from Echelon (Salt Lake City, UT, USA) was from Santa Cruz (Heidelberg, Germany). AO1 was from Sygnature Chemical (Nottingham, UK). INO-4995 and INO-4913 were from Inologic.
Statistical analysis
Student's t-test was used for paired or unpaired samples. P ≤ 0.05 was accepted as significant. Where appropriate, anova was used to test for statistical significance.
Results
INO-4995 and INO-4913 activate human TMEM16A
INO-4995 has been suggested to activate from the cytosolic side a non-CFTR Cl− conductance that is Ca2+-dependent (CaCC) (Traynor-Kaplan et al., 2010). Its non-esterified counterpart INO-4913 was shown to be less membrane permeable and thus less potent in activating CaCC. CaCC in airways and other epithelial and non-epithelial tissues is now known as TMEM16A (Ousingsawat et al., 2009; Rock et al., 2009). It can be readily activated by increase in intracellular Ca2+ due to Ca2+ ionophores such as ionomycin (Figure 1A,B). Notably, cells overexpressing TMEM16A have an increased baseline Cl− conductance, indicating a considerable leakage activity of the overexpressed channel (Kunzelmann et al., 2011). We examined if esterified membrane permeable INO-4995 and the less membrane permeable, non-esterified INO-4913 (Vajanaphanich et al., 1994) are able to activate TMEM16A. Stable baseline currents were measured under control whole cell conditions (Figure S1A). However, when patch pipettes were filled with 1 μM INO-4995 and INO-4913, respectively, whole cell currents were activated in HEK293 cells, expressing human TMEM16A (Figure 1C–E) upon forming a whole cell recording (rupture of the cell attached membrane). Similar to the activation by ionomycin, INO-4995- and INO-4913-activated currents showed little time dependence (Figure 1C,D; right panels) and were completely blocked by the TMEM16A-inhibitor CaCCinh-A01 (AO1; 20 μM) (de la Fuente et al., 2007; Almaca et al., 2009). Activation of TMEM16A by INO-4995 was concentration dependent with a maximal activation at 5 μM (Figure 1G). While 20 μM AO1 showed complete inhibition, 10 μM blocked 95 ± 6% (n = 5) of the current. Moreover, INO-4995 induced conductances were inhibited significantly from 8 ± 1 to 2.7 ± 0.9 nS pF–1. When measured under current clamp, the membrane voltages were depolarized by 11.4 ± 2 mV (n = 6) due to replacement of 115 mM extracellular Cl− by impermeable gluconate (not shown). As HEK293 cells do not have endogenous Ca2+-activated (SK) K+ channels, there is no contribution of K+ currents to Ca2+-activated whole cell currents. Thus, no currents were activated in mock-transfected cells. These experiments suggest that TMEM16A is the target for INO-4995 and INO-4913, and suggest that TMEM16A may be controlled by endogenous tetrakisphosphates.
Figure 1.
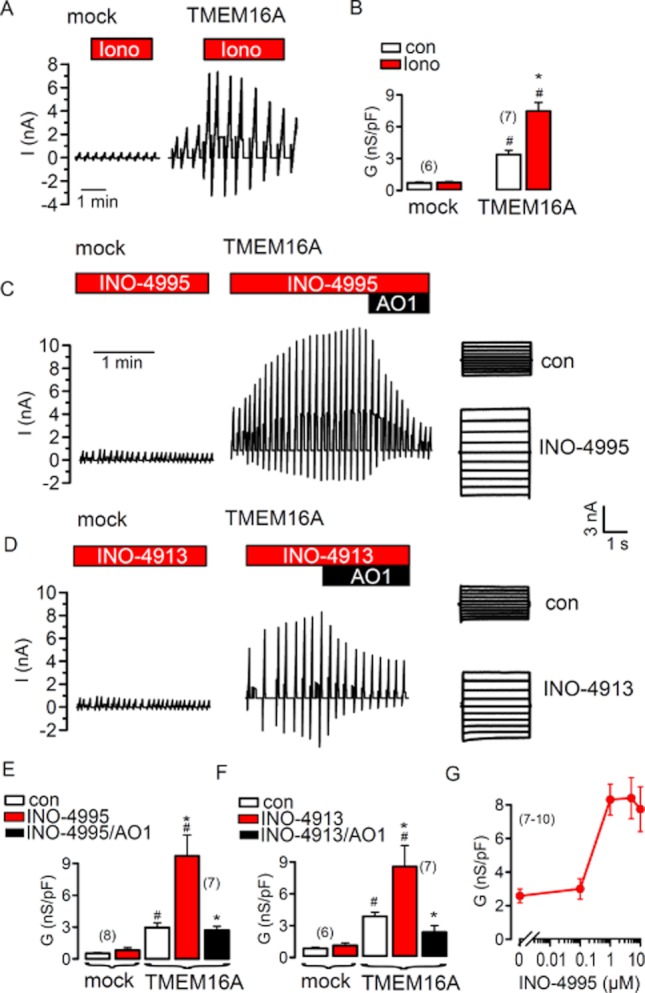
INO-4995 and INO-4913 activate human TMEM16A. (A) Original recordings of ionomycin- (1 μM)-activated whole cell currents measured in mock-transfected HEK293 cells or cells overexpressing TMEM16A. The cells were voltage clamped to ±50 mV. (B) Summary of the calculated whole cell conductances (related to the cell size; conductance density) before and after stimulation with ionomycin. (C) Whole cell currents in mock-transfected and TMEM16A-expressing HEK293 cells. Patch pipettes were filled with 1 μM INO-4995. Upon forming a whole cell configuration, TMEM16A was activated by INO-4995 in TMEM16A-expressing cells. The CaCC blocker AO1 (20 μM) inhibited TMEM16A currents activated by INO-4995. Right panels show overlay currents. (D) Whole cell currents in mock-transfected and TMEM16A expressing HEK293 cells. Patch pipettes were filled with 1 μM INO-4913. The CaCC blocker AO1 (20 μM) inhibited TMEM16A currents activated by INO-4913. Right panels show overlay currents. (E,F) Summary of the effects of INO-4995, INO-4913 and AO1 on whole cell conductances of mock-transfected and TMEM16A expressing cells. (G) Concentration dependence for the effect of INO-4995 on whole cell conductance in TMEM16A-expressing cells. Mean ± SEM (number of cells). *Significant difference from control (paired t-test). #Significant difference from mock (unpaired t-test).
TMEM16A is not controlled by IP4 and phosphatidylinositols
We examined whether TMEM16A is inhibited by Ins(3,4,5,6)P4, since uncoupling of CaCC from intracellular Ca2+ levels by Ins(3,4,5,6)P4 had been observed earlier, leading to transient Cl− currents (Vajanaphanich et al., 1994). The baseline (con) and ionomycin-activated Cl− conductance in TMEM16A-expressing cells were not affected by the presence of either 10 μM Ins(3,4,5,6)P4 or Ins(1,3,4,5)P4 in the patch pipette (Figure 2A). Although baseline currents somewhat varied, ionomycin-activated whole cell currents were quite similar in TMEM16A-overexpressing cells (Figure 2A). We also examined whether the magnitude or time course of Ca2+-activated (1 μM ionomycin) TMEM16A currents was affected by IP4. However, we were unable to detect any significant effects of Ins(3,4,5,6)P4 or Ins(1,3,4,5)P4 on TMEM16A currents activated by ionomycin (Figure 2B). Notably, ionomycin-induced TMEM16A Cl− currents were inactivated in the continuous presence of ionomycin, even below the initial current level that was measured before stimulation with ionomycin (red line). In preliminary studies, we found that this is likely due to Ca2+/CAMKII-dependent phosphorylation of Ano1(unpublished data). Activation of TMEM16A through stimulation of purinergic receptors with 100 μM ATP was also not affected by Ins(3,4,5,6)P4 or Ins(1,3,4,5)P4 (Figure 2C). Moreover, although INO-4913 itself activated TMEM16A (Figure 1D), it did not further augment TMEM16A currents activated by 100 μM ATP (Figure 2D).
Figure 2.
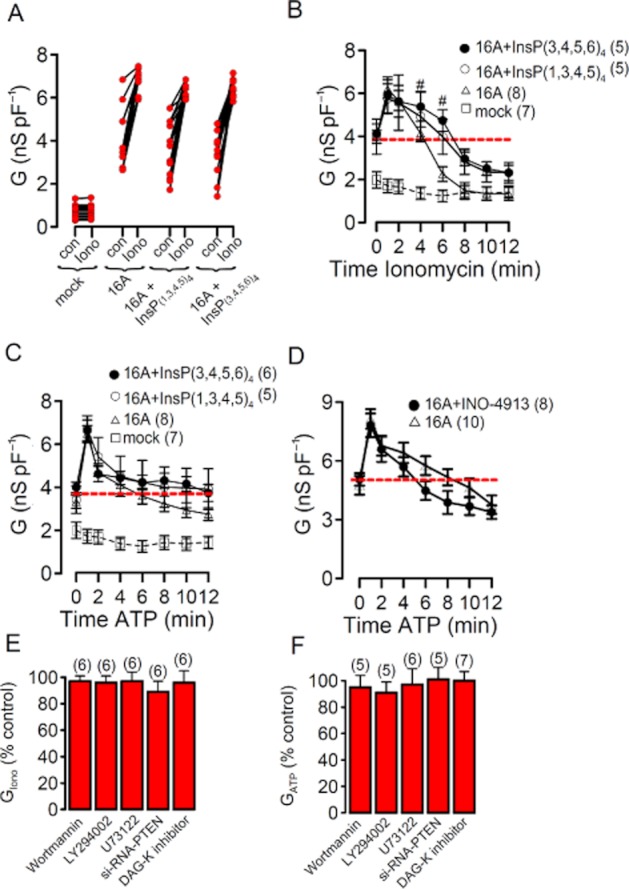
TMEM16A is not controlled by IP4 and phosphatidylinositols. (A) Individual whole cell baseline (no agonist) and ionomycin-activated conductances in mock-transfected HEK293 cells and cells overexpressing TMEM16A (16A). Addition of Ins(3,4,5,6)P4 or Ins(1,3,4,5)P4 to the patch pipette filling solution did not change baseline or ionomycin-activated Cl− conductance in TMEM16A expressing cells. (B) Time course of whole cell Cl− conductances activated by 1 μM ionomycin in mock-transfected and TMEM16A-overexpressing HEK293 cells. Ins(3,4,5,6)P4 or Ins(1,3,4,5)P4 in the pipette filling solution did not change the time course for activation and spontaneous recovery in the presence of ionomycin. (C,D) Time course of whole cell Cl− conductances activated by 100 μM ATP in mock-transfected and TMEM16A-overexpressing HEK293 cells. Neither Ins(3,4,5,6)P4, Ins(1,3,4,5)P4 nor INO-4913 changed ATP-dependent (100 μM) activation of overexpressed TMEM16A. (E,F) Summary of ionomycin- and ATP-induced whole cell Cl− conductances measured in TMEM16A-overexpressing HEK293 cells. Cells were treated with various compounds known to interfere with inositolphosphates and phosphatidylinositols. Activated conductances are shown relative to conductances in non-treated cells. Mean ± SEM (number of cells). *Significant difference from control (paired t-test). #Significant difference from 16A (unpaired t-test).
These data suggest that TMEM16A is not directly controlled by IP4. A number of enzymes are crucial for the production of inositol tetrakisphosphates, such as inositol-1,4,5-trisphosphate 3-Kinase (IP3-3-K), Inositol polyphosphate multikinase (IMPK), and inositol 1,3,4-triphosphate 5/6 kinase (ITPK-1). We incubated TMEM16A expressing cells 2–20 h with inhibitors of IP3-3-K (20 μM N2-(m-Trifluorobenzyl),N6-(p-nitrobenzyl)purine), IMPK (2 μM chlorogenic acid) or knocked down expression of ITPK-1 by siRNA (Aruoma, 1999; Chang et al., 2002; Belkaid et al., 2006). However, none of these treatments inhibited activation of TMEM16A by ionomycin (data not shown). Taken together these data suggest that INO-4913 (and INO-4995) activate overexpressed TMEM16A channels directly, which does not seem to involve endogenous tetrakisphosphates. Moreover, intracellular Ca2+ does not seem to be essential for activation of overexpressed TMEM16A channels: When we applied INO-4995 in a Ca2+ free pipette filling solution (containing 5 mM EGTA), we found a baseline Cl− conductance that was reduced by about 50%. However, whole cell currents were still activated by INO-4995, but were reduced by about 40% when compared with currents activated in the presence of 0.1 μM [Ca2+]i (Figure S1B,C). We performed additional experiments in excised inside-out membrane patches of TMEM16A-expressing HEK293 cells. When we applied 1 μM INO-4913 to the cytosolic side, we observed an increase in channel noise and an increase in the conductance of cell-excised membrane patches (Figure 3A–C). In contrast, no current was activated in membrane patches from mock-transfected cells (Figure 3D–F). These results suggest that INO-4913 is able to activate TMEM16A directly or by influencing regulatory proteins or the membrane lipid composition in close proximity of the channel.
Figure 3.
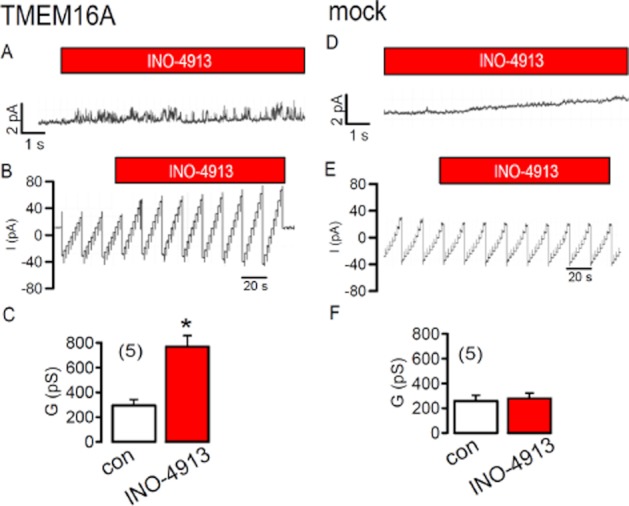
TMEM16A may be directly activated by INO-4913: Current recordings from excised inside out membrane patches of HEK293 cells overexpressing TMEM16A. (A) Current noise was increased by application of 1 μM INO-4913 to the cytosolic site of the excised membrane patch. Clamp voltage was +80 mV. (B) Continuous recording of the patch current during voltage clamp between ± 50 mV. INO-4913 increased the patch current. (C) Summary of the conductance of the excised membrane patch and effect of INO-4913. (D,E,F) Comparable experiments obtained from mock-transfected cells. Mean ± SEM (number of cells). *Significant effect of INO-4913 (paired t-test).
Cytosolic inositolphosphates are closely linked to the membrane phosphatidylinositols phosphatidylinositol 4,5-bisphosphate (PIP2) and phosphatidylinositol 3,4,5-trisphosphate (PIP3). Other ion channels such as Ca2+-activated K+ channels (KCNN4), epithelial Na+ channels (ENaC) and renal K+ channel (ROMK) are controlled by these membrane lipids (Huang et al., 1998; Gerlach et al., 2001; Kunzelmann et al., 2005; Srivastava et al., 2006). We therefore examined the effects of the PI3 kinase inhibitors wortmannin (1 μM/4 h) and LY294002 (100 nM/3 h), which are known to reduce PIP3 levels in the plasma membrane. Moreover, we tested the effects of PLC inhibitor U73122 (10 μM/5 h; increasing PIP2 levels), siRNA knockdown of the enzyme PTEN (decreasing PIP2 levels), as well as the diacylglycerol inhibitor 3-[2-[4-(bis(4-fluorophenyl)methylene)-1-piperidinyl]ethyl]-2,3-dihydro-2-thioxo-4(1H)-quinazolinone (25 μM/5 h; decreasing all phosphatidylinositols). However, we found no significant effects of any of these treatments on the activity of TMEM16A, activated directly by Ca2+ (1 μM ionomycin) or through stimulation of P2Y2 receptors (100 μM ATP) (Figure 2E,F). It is therefore unlikely that INO-4913 and INO-4995 act through any of these pathways. In contrast, these treatments strongly interfered with the activity of the epithelial Na+ channel ENaC, which is known to be regulated by phospholipids (data not shown).
INO-4995 enhances endogenous Ca2+-activated chloride currents
We examined whether INO-4913 and INO-4995 are able to activate endogenous (i.e. non-overexpressed TMEM16A currents). Oocytes from Xenopus laevis are known for their pronounced endogenous Ca2+-activated Cl− current, which is now known to be due to expression of TMEM16A (Schroeder et al., 2008). We found that acute application for up to 2 h, of 5 μM INO-4995 or INO-4913 did not augment baseline Cl− conductance or TMEM16A currents activated by 1 μM ionomycin. In contrast, incubation for 24 h with either INO-4995 or INO-4913 induced an enhanced baseline Cl− current and augmented ionomycin-activated currents (Figure 4). Very similar results were obtained when INO-4995 was directly injected into the oocytes and when the oocytes were measured the following day (Supplement D). Obviously, longer incubation times are necessary to activate endogenous TMEM16A, as already suggested from previous results, obtained for INO-4995-dependent activation of endogenous TMEM16A in human CF airway epithelia and CF mouse airways (Traynor-Kaplan et al., 2010).
Figure 4.
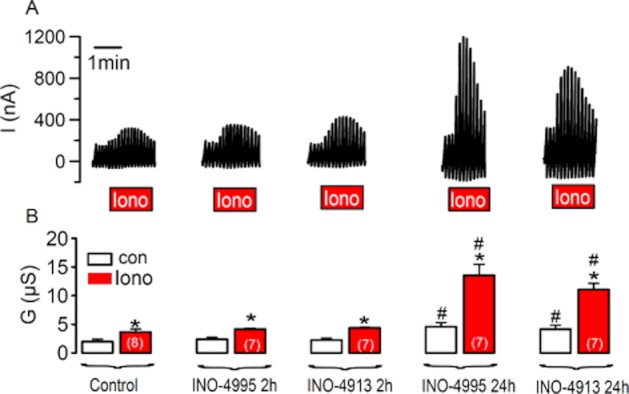
Endogenous TMEM16A expressed in Xenopus oocytes is activated by INO-4995 and INO-4913. (A) Original recordings of whole cell currents measured in Xenopus oocytes. The cells were voltage clamped from −60 to + 40 mV. Ionomycin (1 μM)-activated a whole cell Cl− current, which was enhanced when cells were incubated for 24 h with 5 μM INO-4995 and INO-4913 respectively. (B) Summary of whole cell conductances measured under control conditions and after stimulation with 1 μM ionomycin. Mean ± SEM (number of cells). *Significant difference from control (paired t-test). #Significant difference from control (anova).
We further examined whether INO-4913 controls endogenous TMEM16A expressed in different types of epithelial cells. To that end, we first examined whether TMEM16A is responsible for endogenous Ca2+-activated Cl− currents in epithelial cells of human colon (HT29), human pancreas (CFPAC), and mouse collecting duct (M1). To that end, we knocked down TMEM16A-expression using siRNA and stimulated Ca2+-activated Cl− currents with 100 μM ATP (Almaca et al., 2009; Kunzelmann et al., 2009). In all three cell lines, knockdown of TMEM16A largely reduced ATP-activated whole cell currents, suggesting that TMEM16A is the major component of endogenous CaCC (Figure 5A). Then we went on to examine activation of whole cell Cl− currents by ATP in HT29 control cells, and cells that had been treated with 1 μM INO-4913. We found that ATP-induced whole cell currents were augmented by treatment with INO-4913 (Figure 5B). When cells were incubated with different concentrations of INO-4995 for 2 h at three consecutive days (cf. Traynor-Kaplan et al., 2010), we found a concentrations-dependent increase of the ATP-activated anion permeability, as measured in iodide quenching assays (Figure 5C,D). Similar results were found in (n = 5) whole cell patch clamp experiments (data not shown). Importantly, these positive effects of INO-4913 and INO-4995 on Ca2+-activated TMEM16A currents are not due to effects on [Ca2+]i, since ATP-induced rise in [Ca2+]i in HT29 cells was basically unaffected by INO-4995 up to a concentration of 5 μM (Figure 5E). These results indicate activation of TMEM16A by INO-4913 and INO-4995 in mammalian epithelial cells. We could not detected a change of overall expression of TMEM16A by INO-4995 (Figure S1E); however, immunocytochemistry suggested an increase in membrane expression of TMEM16A in HT29 cells, by treatment with INO-4995 (Figure 5F).
Figure 5.
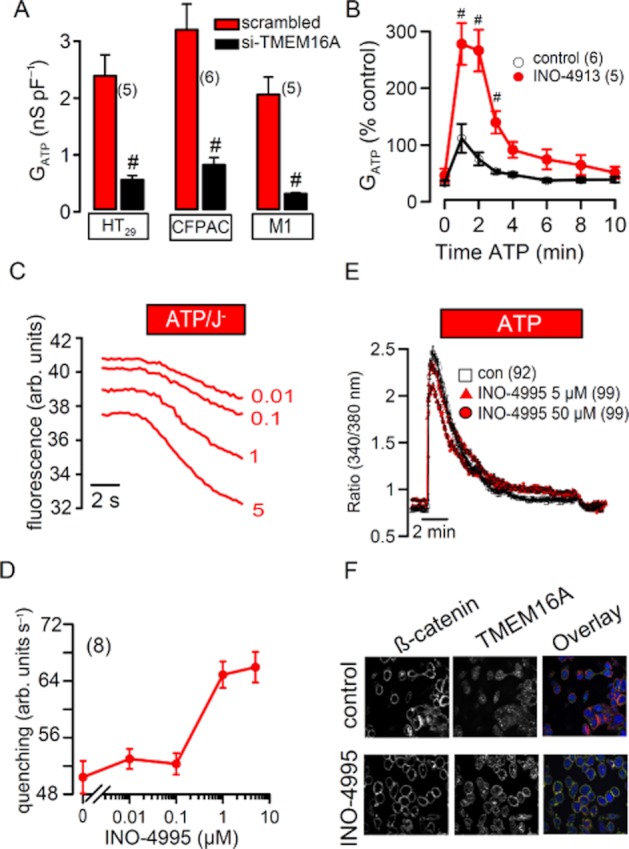
INO-4995 and INO-4913 augment endogenous ATP-activated Cl− currents in epithelial cells. (A) Summary of ATP (100 μM) induced whole cell Cl− currents in HT29, CFPAC and M1 cells. In all three cell lines, Cl− currents were largely reduced after siRNA-knockdown of TMEM16A. (B) Time course of the whole cell conductances relative to control. ATP induced a whole cell Cl− conductance that was about three times larger when INO-4913 was present in the patch pipette solution. (C) Original tracings from I− quenching of fluorescent YFP in HT29 cells after stimulation with 1 μM ATP. Cells were treated with different concentrations of INO-4995 (0.01–5 μM. (D) Concentration-dependent effect of INO-4995 on I− quenching of fluorescent YFP in HT29 cells, upon stimulation with 1 μM ATP. ATP was applied to cells, which had been incubated for 2 h at three consecutive days, with different concentrations of INO-4995. (E) Effect of ATP on intracellular Ca2+ concentrations (measured as Fura-2340/380 nm fluorescence ratio in HT29 cells). Ca2+ increase by ATP was essentially unaffected by 5 μM INO-4995. (F) Immunocytochemistry of TMEM16A and membrane marker β-catenin under control conditions and after treatment with INO-4995. Mean ± SEM (number of cells). #Significant difference from control cells (without INO-4913 or without siRNA-TMEM16A, respectively) (unpaired t-test).
INO-4995 activates TMEM16A in primary cultures of airways
The essential question is, whether INO-4995 is able to increase Ca2+-activated Cl− currents in human airways. Using RT-PCR, we analysed we found expression of TMEM16A in all parts of the human respiratory tract including alveoli. Expression of TMEM16A was compared with that of TMEM16F, which is expressed abundantly, but is not activated through stimulation of purinergic P2Y receptors (Figure 6A) (Martins et al., 2011). The ratio for TMEM16A/TMEM16F expression was 0.2 (large bronchus), 0.7 (small bronchus), 0.8 (bronchiolus) and 0.3 (alveolus). We further conducted experiments in primary cultures of human bronchial epithelial cells obtained from patients undergoing lung transplants. Transepithelial voltages (Vte) were measured under open-circuit conditions in polarized human bronchial epithelial cell monolayers mounted in micro-Ussing chambers. Human airway cultures were treated repetitively (for 2 h in four subsequent days) with INO-4913 and INO-4995, respectively, similar to a previous study (Traynor-Kaplan et al., 2010). Luminal application of 100 μM ATP activated Ca2+-dependent Cl− secretion, which had a transient and a steady-state component (Figure 6B). ATP is known to act primarily on luminal purinergic P2Y2 receptors in human airways (Ousingsawat et al., 2009; Rock et al., 2009). Both components were potently blocked by the CaCC inhibitors niflumic acid (10 μM) (not shown) or CaCCinhAO1 (20 μM) (de la Fuente et al., 2007) (Figure 6C). After treatment with INO-4995 but not INO-4913, ATP-induced voltage deflections were augmented and calculated equivalent short circuit currents (Isc-ATP) were enhanced (Figure 6B,C). In some experiments with INO-4995-treated cells, we applied ATP for up to 20 min and found that the steady-state Isc was substantially larger than in control cells. In additional YFP-quenching experiments, we exposed INO-4995-treated and non-treated HT29 cells to 100 μM ATP for 15 min and re-added iodide every 5 min. The results suggest a prolonged activation of Ca2+-dependent Cl− secretion after treatment with INO-4995 (Figure S1F).
Figure 6.
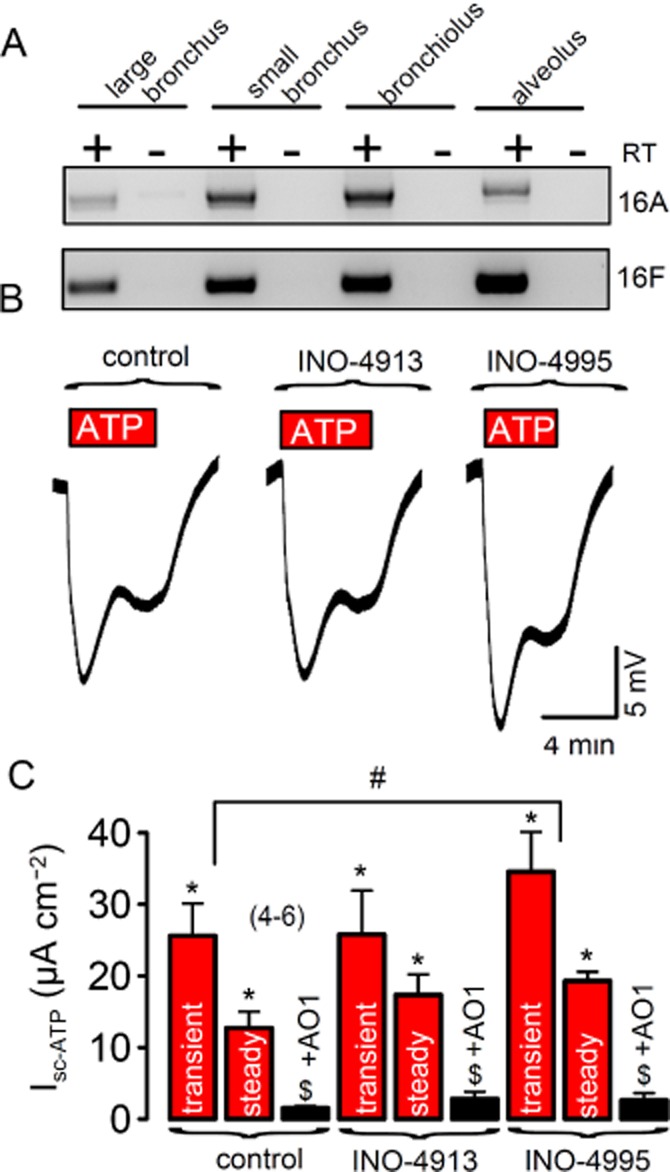
INO-4995 activates endogenous TMEM16A in polarized human non-CF airway epithelial cells: (A) RT-PCR analysis indicates expression of TMEM16A throughout the native human respiratory tract. (B) Original Ussing chamber recordings (open circuit measurements) obtained from human primary airway epithelial cells grown on permeable supports with an air–liquid interface. ATP (100 μM) induced transient and steady-state voltage deflections of the transepithelial voltage, indicating activation of Cl− secretion. ATP-induced voltage deflections were augmented after repetitive treatment (2 h at four consecutive days) with 5 μM INO-4995. (C) Summary of ATP-induced transient and steady-state equivalent short circuit currents (calculated from voltage deflections) in control airway cells and cells treated with INO-4913 and INO-4995 respectively. The inhibitor AO1 (20 μM) completely blocked ATP-induced Isc. Mean ± SEM (number of cells). #Significant difference from control cells (anova). $Significant inhibition by AO1 (paired t-test). Significant activation by ATP (paired t-test).
We also performed the same treatment by INO-4995 with primary cultures from CF airways. As demonstrated in Figure 7, ATP induces a voltage deflection that was clearly enhanced after treatment with INO-4995. Residual CFTR Cl− secretion by genistein in these CF lung monolayers was not affected by INO-4995 (–INO: ΔIsc = 0.18 ± 0.5 μA·cm−2, n = 3 vs. +INO: ΔIsc = 0.20 ± 0.4 μA·cm−2, n = 3). We compared the effects of treatment with INO-4995 in normal and CF airways and found an 50% enhanced effect of INO-4995 on the transient Ca2+-activated Cl− secretion in CF airways. Taken together, the present data indicate that INO-4995 augments Ca2+-activated Cl− secretion by activation of TMEM16A. The results suggest INO-4995 as a useful tool to augment a compensatory Ca2+-dependent Cl− secretion in dehydrated CF lungs.
Figure 7.
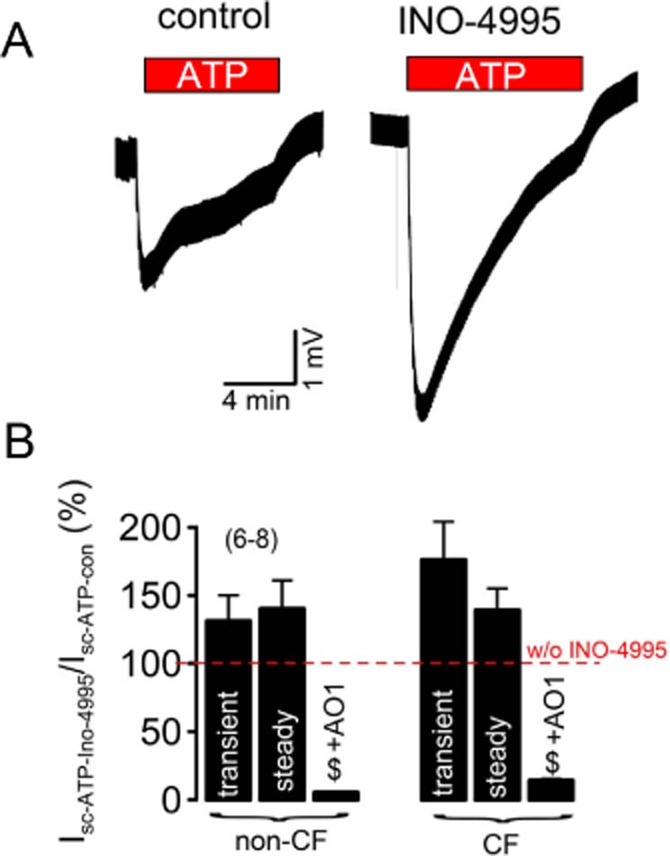
INO-4995 activates endogenous TMEM16A in polarized human CF airway epithelial cells: (A) Original Ussing chamber recordings (open circuit) obtained from CF primary airway epithelial cells grown on permeable supports with an air–liquid interface. ATP (100 μM) induced transient and steady-state transepithelial voltage deflections, indicating activation of Ca2+-dependent Cl− secretion. Effect of treatment by INO-4995 (5 μM; 2 h at four consecutive days). (B) Summary showing activation of transient and steady-state equivalent short circuit currents (calculated from voltage deflections) by ATP after treatment with INO-4995 relative to non-treated cells. The data demonstrate a slightly enhanced effect of INO-4995 on CF-primary. Effect of ATP in non-treated tissues is set to 100%. The inhibitor AO1 (20 μM) completely blocked ATP-induced Isc. Mean ± SEM (number of cells). #Significant difference from control cells (unpaired t-test). $Significant inhibition by 20 μM AO1 (paired t-test).
Discussion
Activation of TMEM16A by INO-4995
The aim of the present study was to examine whether the recently identified Ca2+-activated Cl− channel TMEM16A is the target for two synthetic inositol tetrakisphosphates. INO-4995 and INO-4913 that have been demonstrated earlier to enhance Ca2+-activated Cl− currents and therefore to improve electrolyte balance in airways of CF mice (Rudolf et al., 2003; Traynor-Kaplan et al., 2010). The present results clearly show that both INO-4995 and INO-4913 activate TMEM16A that was overexpressed in HEK293 cells. Interestingly activation of TMEM16A by INO-4995 and the less membrane permeable but active compound INO-4913 occurred even in the absence of cytosolic Ca2+ (Figure S1C). Moreover, the INO-compounds activated overexpressed TMEM16A without increasing intracellular Ca2+ (data not shown) and did not affect intracellular Ca2+ signalling (Figure 5E). Notably, TMEM16A channels, when overexpressed in HEK293 cells and other cell types, are partly active even at resting Ca2+ concentrations (Kunzelmann et al., 2011). The reasons for this is currently unclear; however, we speculate that additional proteins are required to regulate TMEM16A (Tian et al., 2012).
The effects of INO-4995 and INO-4913 were remarkably different on TMEM16A, expressed endogenously in Xenopus oocytes or mammalian cells from colon and airways. Inhibition of TMEM16A by siRNA indicated that TMEM16A is a main component of endogenous CaCC in these cells. Here we could not detect a direct and fast activation of CaCC (i.e. endogenous TMEM16A by INO-4995). However, INO-4995 increased TMEM16A currents, which were stimulated by agonists such as ATP. To see this effect, cells needed to be incubated repetitively with INO-4995, or for a longer duration. The results obtained in these experiments are remarkably similar to those obtained previously on airways of CF mice (Traynor-Kaplan et al., 2010). We have currently no explanation why the effects of INO-4995 is different on overexpressed and endogenous TMEM16A channels.
Notably, we obtained similar results with benzimidazolone compounds, which are known to activate Ca2+-activated (KCNN4) K+ channels. Overexpressed TMEM16A but not endogenous CaCC was directly activated by these compounds (Tian et al., 2011; 2012). From those and the present results, we may speculate that endogenous TMEM16A are inhibited by unknown auxiliary proteins. INO-4995 and INO-4913 may augment endogenous TMEM16A, once released from inhibitory proteins due to increase in [Ca2+]i. Alternatively, prolonged and repetitive incubation with INO-4995 may up-regulate membrane expression of TMEM16A (Figure 5F). Notwithstanding, the data indicate that TMEM16A is the target of INO-4995.
Overexpressed TMEM16A is not controlled by IP4 and phosphatidylinositols
Although the results suggest that INO-4995 and INO-4913 activate TMEM16A by interfering with endogenous inositolphosphates, we found no evidence for a regulation of TMEM16A by IP4 or phosphatidylinositols. Ins(3,4,5,6)P4 has been proposed earlier to control the time course of endogenous CaCC activity (Vajanaphanich et al., 1994; Ho et al., 2001). Evidence has been provided that the Ca2+ peak induced by activation of PLC-coupled receptors switches on CaCC, while prolonged activation is due to CaMKII-dependent phosphorylation, which is modulated by inositolphosphates (for review see (Hartzell et al., 2005). In CFPAC cells, low concentrations of ATP (2 μM) were used to elicit rather small endogenous Cl− currents (Ho et al., 1997). It may be that at higher agonist concentrations of ATP, like those used in the present study, regulation by inositol phosphates is overrun. Alternatively, the endogenous CaCC of CFPAC cells that is inhibited by Ins(3,4,5,6)P4 is not TMEM16A. This, however, is unlikely since endogenous CaCC in CFPAC cells was inhibited by siRNA for TMEM16A in this and in a previous study (Almaca et al., 2009). Moreover, IP4 protein phosphatases seem to have role for the inhibitory effect of IP4 on CaCC (Xie et al., 1998). Taken together, Ins(3,4,5,6)P4-dependent regulation of CaCC is poorly examined and could not be detected in any cases (Worrell and Frizzell, 1991; Chan et al., 1994; Kaetzel et al., 1994; Xie et al., 1996; 1996; Carew et al., 2000). It is likely that Ca2+-activated Cl− channels are composed of several, still unknown proteins, and that TMEM16A is only one, however, essential subunit of a channel complex (Caputo et al., 2008). Data have been provided suggesting that different TMEM16 paralogs can heterooligomerize (Kunzelmann et al., 2009; Schreiber et al., 2010), and two recent papers show that TMEM16A exists as a homodimer (Fallah et al., 2010; Sheridan et al., 2010).
INO-4995 and INO-4913 activate endogenous TMEM16A in various cells types and in human airway cells
As mentioned above, the present results demonstrate that endogenous TMEM16A in Xenopus oocytes, colonic HT29 and human airway epithelial cells is activated by INO-4995. It appears that prolonged or repetitive incubation with INO-4995 increases CaCC, as described in Traynor-Kaplan et al. (2010). This is probably not due to an up-regulation of TMEM16A expression, as we could not detect enhanced TMEM16A protein after repetitive incubation INO-4995 (5 μM/2 h) for up to 4 days (Figure S1E). However, prolonged exposure to INO-4995 appears to increase membrane expression of TMEM16A, as demonstrated in HT29 cells (Figure 5F). Taken together, the present results suggest that INO-4995 and INO-4913 may be useful tools to augment Ca2+-dependent Cl− secretion in the airways of CF patients.
Acknowledgments
Supported by Mukoviszidose e.V. (Projekt-Nr.: S02/10), DFG SFB699A7, FCT-PIC/IC/83103/2007 (Portugal) and BioFig. FD and MS are recipients of fellowships by FCT/FEDER (Portugal/EU) SFRH/BD/35936/2007 PhD fellowship (FCT, Portugal).
Conflict of interest
The authors declare no conflicts of interest.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Figure S1 (A) Original recordings of stable resting whole cell currents in mock-transfected and TMEM16A-expressing HEK293 cells. The cells were voltage clamped to ±50 mV. (B) Activation of whole cell currents by 1 μM INO-4995 in the patch pipette filling solution and in the absence of cytosolic Ca2+ (Ca2+-free pipette solution containing 5 mM EGTA). (C) Summary of the effects of INO-4995 on whole cell conductances (conductance density) in mock-transfected and TMEM16A expressing cells, in the absence of cytosolic Ca2+. (D) Summary of the effects of 1 μM ionomycin on whole cell conductances measured in Xenopus oocytes 24 h after injection of water (control) or INO-4995 (5 μM). (E) Western blot analysis of TMEM16A expression in CFBE airway epithelial cells, with and without treatment by INO-4995 (1 μM for 2 h at three consecutive days). (F) Summary from YFP fluorescence quenching by I− influx, indicating inactivation of Ca2+-activated Cl− conductance in the continuous presence of 100 μM ATP. I− was re-added every 5 min to measure remaining halide conductance. Treatment with INO-4995 (2 h for three consecutive days) prolonged activation. Mean ± SEM (number of cells). #Significant difference from control cells (unpaired t-test). *Significant effects of INO-4995 and AO1 (paired t-test). §Significant difference from non-treated cells (unpaired t-test).
References
- Almaca J, Tian Y, AlDehni F, Ousingsawat J, Kongsuphol P, Rock JR, et al. TMEM16 proteins produce volume regulated chloride currents that are reduced in mice lacking TMEM16A. J Biol Chem. 2009;284:28571–28578. doi: 10.1074/jbc.M109.010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aruoma OI. Antioxidant actions of plant foods: use of oxidative DNA damage as a tool for studying antioxidant efficacy. Free Radic Res. 1999;30:419–427. doi: 10.1080/10715769900300461. [DOI] [PubMed] [Google Scholar]
- Bachhuber T, König J, Voelcker T, Mürle B, Schreiber R, Kunzelmann K. Chloride interference with the epithelial Na+ channel ENaC. J Biol Chem. 2005;280:31587–31594. doi: 10.1074/jbc.M504347200. [DOI] [PubMed] [Google Scholar]
- Belkaid A, Currie JC, Desgagnes J, Annabi B. The chemopreventive properties of chlorogenic acid reveal a potential new role for the microsomal glucose-6-phosphate translocase in brain tumor progression. Cancer Cell Int. 2006;6:7–19. doi: 10.1186/1475-2867-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caputo A, Caci E, Ferrera L, Pedemonte N, Barsanti C, Sondo E, et al. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science. 2008;322:590–594. doi: 10.1126/science.1163518. [DOI] [PubMed] [Google Scholar]
- Carew MA, Yang X, Schultz C, Shears SB. myo-inositol 3,4,5,6-tetrakisphosphate inhibits an apical calcium-activated chloride conductance in polarized monolayers of a cystic fibrosis cell line. J Biol Chem. 2000;275:26906–26913. doi: 10.1074/jbc.M002316200. [DOI] [PubMed] [Google Scholar]
- Chamberlain PP, Qian X, Stiles AR, Cho J, Jones DH, Lesley SA, et al. Integration of inositol phosphate signaling pathways via human ITPK1. J Biol Chem. 2007;282:28117–28125. doi: 10.1074/jbc.M703121200. Available at: http://www.ncbi.nlm.nih.gov/pubmed/17616525 (accessed 11/7/2012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan HC, Kaetzel MA, Gotter AL, Dedman JR, Nelson DJ. Annexin IV inhibits calmodulin-dependent protein kinase II-activated chloride conductance. A novel mechanism for ion channel regulation. J Biol Chem. 1994;269:32464–32468. [PubMed] [Google Scholar]
- Chang YT, Choi G, Bae YS, Burdett M, Moon HS, Lee JW, et al. Purine-based inhibitors of inositol-1,4,5-trisphosphate-3-kinase. Chembiochem. 2002;3:897–901. doi: 10.1002/1439-7633(20020902)3:9<897::AID-CBIC897>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- DeLisle S, Radenberg T, Wintermantel MR, Tietz C, Parys JB, Pittet D, et al. Second messenger specificity of the inositol trisphosphate receptor: reappraisal based on novel inositol phosphates. Am J Physiol. 1994;266:C429–C436. doi: 10.1152/ajpcell.1994.266.2.C429. [DOI] [PubMed] [Google Scholar]
- Fallah G, Roemer T, Detro-Dassen S, Braam U, Markwardt F, Schmalzing G. TMEM16A(a)/anoctamin-1 shares a homodimeric architecture with CLC chloride channels. Mol Cell Proteomics. 2010;79:649–661. doi: 10.1074/mcp.M110.004697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente R, Namkung W, Mills A, Verkman AS. Small molecule screen identifies inhibitors of a human intestinal calcium activated chloride channel. Mol Pharmacol. 2007;73:758–768. doi: 10.1124/mol.107.043208. [DOI] [PubMed] [Google Scholar]
- Fulcher ML, Gabriel S, Burns KA, Yankaskas JR, Randell SH. Well-differentiated human airway epithelial cell cultures. Methods Mol Med. 2005;107:183–206. doi: 10.1385/1-59259-861-7:183. [DOI] [PubMed] [Google Scholar]
- Gerlach AC, Syme CA, Giltinan L, Adelman JP, Devors DC. ATP-dependent activation of the intermediate conductance, Ca2+-activated K+ channel, hIK1, is conferred by a C-terminal domain. J Biol Chem. 2001;276:10963–10970. doi: 10.1074/jbc.M007716200. [DOI] [PubMed] [Google Scholar]
- Hartzell HC, Putzier I, Arreola J. Calcium-activated chloride channels. Annu Rev Physiol. 2005;67:719–758. doi: 10.1146/annurev.physiol.67.032003.154341. [DOI] [PubMed] [Google Scholar]
- Ho MW, Shears SB, Bruzik KS, Duszyk M, French AS. Ins(3,4,5,6)P4 specifically inhibits a receptor-mediated Ca2+-dependent Cl- current in CFPAC-1 cells. Am J Physiol. 1997;272:C1160–C1168. doi: 10.1152/ajpcell.1997.272.4.C1160. [DOI] [PubMed] [Google Scholar]
- Ho MW, Kaetzel MA, Armstrong DL, Shears SB. Regulation of a human chloride channel. A paradigm for integrating input from calcium, type ii calmodulin-dependent protein kinase, and inositol 3,4,5,6-tetrakisphosphate. J Biol Chem. 2001;276:18673–18680. doi: 10.1074/jbc.M101128200. [DOI] [PubMed] [Google Scholar]
- Huang CL, Feng S, Hilgemann DW. Direct activation of inward rectifier potassium channels by PIP2 and its stabilization by Gbetagamma. Nature. 1998;391:803–806. doi: 10.1038/35882. [DOI] [PubMed] [Google Scholar]
- Kachintorn U, Vajanaphanich M, Traynor-Kaplan AE, Dharmsathaphorn K, Barrett KE. Activation by calcium alone of chloride secretion in T84 epithelial cells. Br J Pharmacol. 1993;109:510–517. doi: 10.1111/j.1476-5381.1993.tb13599.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaetzel MA, Chan HC, Dubinsky WP, Dedman JR, Nelson DJ. A role for annexin IV in epithelial cell function. Inhibition of calcium-activated chloride conductance. J Biol Chem. 1994;269:5297–5302. [PubMed] [Google Scholar]
- Kunzelmann K, Bachhuber T, Regeer RR, Markovich D, Sun J, Schreiber R. Purinergic inhibition of the epithelial Na+ channel ENaC via hydrolysis of PIP2. FASEB J. 2005;18:142–163. doi: 10.1096/fj.04-2314fje. [DOI] [PubMed] [Google Scholar]
- Kunzelmann K, Kongsuphol P, AlDehni F, Tian Y, Ousingsawat J, Warth R, et al. Bestrophin and TMEM16 – Ca2+ activated Cl- channels with different functions. Cell Calcium. 2009;46:233–241. doi: 10.1016/j.ceca.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Kunzelmann K, Tian Y, Martins JR, Faria D, Kongsuphol P, Ousingsawat J, et al. Anoctamins. Pflugers Arch. 2011;462:195–208. doi: 10.1007/s00424-011-0975-9. [DOI] [PubMed] [Google Scholar]
- Martins JR, Faria D, Kongsuphol P, Reisch B, Schreiber R, Kunzelmann K. Anoctamin 6 is an essential component of the outwardly rectifying chloride channel. Proc Natl Acad Sci USA. 2011;108:18168–18172. doi: 10.1073/pnas.1108094108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menniti FS, Oliver KG, Nogimori K, Obie JF, Shears SB, Putney JW., Jr Origins of myo-inositol tetrakisphosphates in agonist-stimulated rat pancreatoma cells. Stimulation by bombesin of myo-inositol 1,3,4,5,6-pentakisphosphate breakdown to myo-inositol 3,4,5,6-tetrakisphosphate. J Biol Chem. 1990;265:11167–11176. [PubMed] [Google Scholar]
- Moody M, Pennington C, Schultz C, Caldwell R, Dinkel C, Rossi MW, et al. Inositol polyphosphate derivative inhibits Na+ transport and improves fluid dynamics in cystic fibrosis airway epithelia. Am J Physiol Cell Physiol. 2005;289:C512–C520. doi: 10.1152/ajpcell.00591.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris AP, Kirk KL, Frizzell RA. Simultaneous analysis of cell Ca2+ and Ca2(+)-stimulated chloride conductance in colonic epithelial cells (HT-29) Cell Regul. 1990;1:951–963. doi: 10.1091/mbc.1.12.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namkung W, Yao Z, Finkbeiner WE, Verkman AS. Small-molecule activators of TMEM16A, a calcium-activated chloride channel, stimulate epithelial chloride secretion and intestinal contraction. FASEB J. 2011;25:4048–4062. doi: 10.1096/fj.11-191627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ousingsawat J, Martins JR, Schreiber R, Rock JR, Harfe BD, Kunzelmann K. Loss of TMEM16A causes a defect in epithelial Ca2+ dependent chloride transport. J Biol Chem. 2009;284:28698–28703. doi: 10.1074/jbc.M109.012120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock JR, O'Neal WK, Gabriel SE, Randell SH, Harfe BD, Boucher RC, et al. Transmembrane protein 16A (TMEM16A) is a Ca2+ regulated Cl- secretory channel in mouse airways. J Biol Chem. 2009;284:14875–14880. doi: 10.1074/jbc.C109.000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolf MT, Li WH, Wolfson N, Traynor-Kaplan AE, Schultz C. 2-Deoxy derivative is a partial agonist of the intracellular messenger inositol 3,4,5,6-tetrakisphosphate in the epithelial cell line T84. J Med Chem. 1998;41:3635–3644. doi: 10.1021/jm970781n. [DOI] [PubMed] [Google Scholar]
- Rudolf MT, Dinkel C, Traynor-Kaplan AE, Schultz C. Antagonists of myo-inositol 3,4,5,6-tetrakisphosphate allow repeated epithelial chloride secretion. Bioorg Med Chem. 2003;11:3315–3329. doi: 10.1016/s0968-0896(03)00188-3. [DOI] [PubMed] [Google Scholar]
- Saiardi A, Cockcroft S. Human ITPK1: a reversible inositol phosphate kinase/phosphatase that links receptor-dependent phospholipase C to Ca2+-activated chloride channels. Sci Signal. 2008;1:pe5. doi: 10.1126/stke.14pe5. [DOI] [PubMed] [Google Scholar]
- Schreiber R, Uliyakina I, Kongsuphol P, Warth R, Mirza M, Martins JR, et al. Expression and function of epithelial anoctamins. J Biol Chem. 2010;285:7838–7845. doi: 10.1074/jbc.M109.065367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder BC, Cheng T, Jan YN, Jan LY. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell. 2008;134:1019–1029. doi: 10.1016/j.cell.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shears SB. Can intervention in inositol phosphate signalling pathways improve therapy for cystic fibrosis? Expert Opin Ther Targets. 2005;9:1307–1317. doi: 10.1517/14728222.9.6.1307. [DOI] [PubMed] [Google Scholar]
- Sheridan JT, Worthington EN, Yu K, Gabriel SE, Hartzell HC, Tarran R. Characterization of the oligomeric structure of the Ca2+-activated Cl- channel Ano1/TMEM16A. J Biol Chem. 2010;286:1381–1388. doi: 10.1074/jbc.M110.174847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S, Choudhury P, Li Z, Liu G, Nadkarni V, Ko K, et al. Phosphatidylinositol 3-phosphate indirectly activates KCa3.1 via 14 amino acids in the carboxy terminus of KCa3.1. Mol Biol Cell. 2006;17:146–154. doi: 10.1091/mbc.E05-08-0763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y, Kongsuphol P, Hug MJ, Ousingsawat J, Witzgall R, Schreiber R, et al. Calmodulin-dependent activation of the epithelial calcium-dependent chloride channel TMEM16A. FASEB J. 2011;25:1058–1068. doi: 10.1096/fj.10-166884. [DOI] [PubMed] [Google Scholar]
- Tian Y, Schreiber R, Kunzelmann K. Anoctamins are a family of Ca2+ activated Cl- channels with permeability for cations. J Cell Sci. 2012 doi: 10.1242/jcs.109553. in press. [DOI] [PubMed] [Google Scholar]
- Traynor-Kaplan AE, Moody M, Nur M, Gabriel SE, Majerus PW, Drumm ML, et al. INO-4995 therapeutic efficacy is enhanced with repeat dosing in CF KO mice and human epithelia. Am J Respir Cell Mol Biol. 2010;42:105–112. doi: 10.1165/rcmb.2008-0380OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vajanaphanich M, Schultz C, Rudolf MT, Wasserman M, Enyedi P, Craxton A, et al. Long-term uncoupling of chloride secretion from intracellular calcium levels by Ins(3,4,5,6)P4. Nature. 1994;371:711–714. doi: 10.1038/371711a0. [DOI] [PubMed] [Google Scholar]
- Worrell RT, Frizzell RA. CaMKII mediates stimulation of chloride conductance by calcium in T84. Am J Physiol. 1991;260:C877–C882. doi: 10.1152/ajpcell.1991.260.4.C877. [DOI] [PubMed] [Google Scholar]
- Xie W, Kaetzel MA, Bruzik KS, Dedman JR, Shears SB, Nelson DJ. Inositol 3,4,5,6-tetrakisphosphate inhibits the calmodulin-dependent protein kinase II-activated chloride conductance in T84 colonic epithelial cells. J Biol Chem. 1996;271:14092–14097. doi: 10.1074/jbc.271.24.14092. [DOI] [PubMed] [Google Scholar]
- Xie W, Solomons KR, Freeman S, Kaetzel MA, Bruzik KS, Nelson DJ, et al. Regulation of Ca2+-dependent Cl- conductance in a human colonic epithelial cell line (T84): cross-talk between Ins(3,4,5,6)P4 and protein phosphatases. J Physiol. 1998;510:661–673. doi: 10.1111/j.1469-7793.1998.661bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Reece J, Gabriel SE, Shears SB. Apical localization of ITPK1 enhances its ability to be a modifier gene product in a murine tracheal cell model of cystic fibrosis. J Cell Sci. 2006;119:1320–1328. doi: 10.1242/jcs.02836. [DOI] [PubMed] [Google Scholar]
- Yang YD, Cho H, Koo JY, Tak MH, Cho Y, Shim WS, et al. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature. 2008;455:1210–1215. doi: 10.1038/nature07313. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


