Abstract
The vestibular system senses the movement and position of the head in space and uses this information to stabilize vision, control posture, perceive head orientation and self-motion in three-dimensional space, and modulate autonomic and limbic activity in response to locomotion and changes in posture. Most vestibular signals are not consciously perceived and are usually appreciated through effector pathways classically described as the vestibulo-ocular, vestibulo-spinal, vestibulo-collic and vestibulo-autonomic reflexes. The present study reviews some of the recent data concerning the connectivity and chemical anatomy of vestibular projections to autonomic sites that are important in the sympathetic control of blood pressure.
Keywords: Vestibular nuclei, vestibulo-sympathetic pathways, vestibulo-sympathetic reflex, vestibulo-autonomic control
1. Introduction
In all mammalian species, movements and changes in posture, particularly those involving alterations in head position with regard to gravity, trigger rapid cardiovascular adjustments in order to maintain physiological homeostasis. The existence of functional integration between blood pressure control mechanisms and the vestibular system, which senses head accelerations that occur during postural adjustments, was hypothesized in the 1920s on the basis of clinical observations [15] and is now supported by a substantial number of basic and clinical studies [8]. Current understanding of this integration is that arterial baroreceptors participate in a feedback regulatory mechanism that determines and stabilizes sympathetic tone while signals from the vestibular end organs drive a faster, feedforward mechanism that predictively counteracts the effects of postural adjustments on blood pressure (for review, see [129]). Despite the nearly century-old appreciation that the vestibular system participates in cardiovascular control, however, the chemical anatomy and connectivity of the underlying neural pathways remain largely unknown. This lack of fundamental information is a major obstacle to the development of evidence-based pharmacotherapeutics for the treatment of vestibulo-autonomic disorders, and to the development of more specific anti-hypertensive medications that do not elicit disabling vestibular side-effects such as dizziness and vertigo.
2. Vestibular influences on sympathetic control of blood pressure in humans
Research on humans has demonstrated that the vestibular system modulates blood pressure during body movements and postural adjustments. Caloric vestibular stimulation [22] and head pitch alter sympathetic nerve activity [89], most likely through the vestibulo-sympathetic “reflex” (VSR) pathway [90] (for review, see [129]). In addition, linear acceleration of human subjects, which specifically stimulates the otolith organs, causes transient changes in systolic blood pressure [21] that are attenuated in patients with bilateral vestibular deficits [128]. Similarly, otolith activation by off-vertical axis rotation produces an increase in muscle sympathetic nerve activity in-phase with the head-up tilt component and a decrease in such activity corresponding to the head-down tilt phase [60]. Overall it appears that the baroreflex and VSR are additive [88], although the VSR latency is estimated to be 3–10X faster than the baroreflex for effecting changes in blood pressure [45,60,119]. In fact, both shorter-and longer-latency components of the VSR have been detected in humans [60,87,119]. This suggests that the vestibular system contributes fast as well as slow temporal components to central sympathetic (as well as parasympathetic [86]) processing and can achieve blood pressure control during rapid linear head accelerations such as those that occur upon standing, as well as during slower head movements.
3. The role of primary vestibular afferents in the sympathetic control of blood pressure
The first study in experimental animals demonstrating blood pressure changes in response to postural adjustments was conducted in anesthetized and paralyzed cats, and found orthostatic intolerance during nose-up pitch following bilateral vestibular nerve transection [28]. Bilateral vestibular nerve lesions raise the probability of orthostatic intolerance in awake animals as well [1,32,58,78] (for review, see [133]), although cerebral blood flow is not compromised [122]. The generality of this finding was extended in studies showing that otolith-specific stimulation achieved through linear acceleration [2] or head-up tilt increased sympathetic nerve activity [131] and elevated blood pressure [123], while nose-down stimulation decreased this activity [78], as is observed in humans. Indeed, numerous studies in experimental animals have found that sympathetic nerve activity increases during electrical stimulation of primary vestibular afferent fibers [20] (for review, see [129]), although different vascular beds are differentially affected by this activation [135]. Thus, data from both humans and experimental animals indicate that vestibular nerve afferents contribute dynamically to the sympathetic control of blood pressure.
4. The role of the vestibular nuclear complex in the sympathetic control of blood pressure
Two regions of the vestibular nuclear complex (VNC) have been identified as major autonomic loci, primarily on the basis of tract-tracing studies in rabbits [5,6] and electrophysiological recordings in cats [130] (for review, see [7]). One area, comprised of the inferior vestibular nucleus and the caudal portion of the medial vestibular nucleus, receives primary vestibular afferent innervation from the otolith organs as well as the semicircular canals, and sends direct projections to caudal brainstem sites involved in the regulation of sympathetic outflow [71,130]. These sites include the dorsal motor vagal nucleus, nucleus ambiguus, the lateral medullary tegmental field, the lateral and ventrolateral subnuclei of the solitary nucleus (NTS), raphé magnus, the parabrachial nuclei, and the rostral and caudal ventrolateral medullary regions (RVLM and CVLM, respectively) [6,84]. The other autonomic region of the VNC is located more rostrally and projects to the parabrachial nuclei. This latter pathway is thought to be involved primarily in mediating more affective and emotional aspects of vestibulo-autonomic connectivity, such as the association between vertigo and panic [5,8, 62], although this projection may participate in blood pressure control as well [52]. The functional significance of the caudal vestibulo-autonomic pathway was demonstrated by experiments in which stereotaxic lidocaine injections into the inferior or caudal medial vestibular nucleus (to suppress local neural activity) resulted in the attenuation of sympathetic nerve responses to labyrinthine stimulation [63]. Similarly, early research in a variety of mammalian species showed that ablation of these regions of the VNC abolished the decrease in blood pressure (depressor response) that is normally observed during caloric stimulation [107,108] and increased the threshold level of activation necessary for eliciting sympathetic responses to electrical stimulation of the vestibular nerve [117]. Thus, the caudal portion of the VNC appears to be important for mediating the vestibular influence on cardiovascular activity in numerous mammalian species including humans.
5. The differing roles of RVLM and CVLM in vestibulo-autonomic connectivity
The RVLM region integrates diencephalic and brainstem signals that participate in sympathetic circuitry [11,26,48,82,93,94,98,99,116]. From the RVLM, a cytologically and chemoanatomically diverse population of output neurons send excitatory projections to preganglionic sympathetic neurons in the intermediolateral cell column of the spinal cord [17,24,48,65, 99,120]. Additional direct influences on sympathetic outflow from the spinal cord to the vasculature are derived from the caudal raphé nuclei, rostral ventro-medial medulla, the A5 noradrenergic cell group, locus coeruleus and the paraventricular hypothalamic nucleus [16,64,103]. The vasomotor RVLM cells receive direct monosynaptic GABAergic input from the CVLM region [10,19,51,57,82] that tonically inhibits these bulbospinal neurons [25,47,105]. As a result, some CVLM cells can be viewed as sympathoinhibitory interneurons in the vasomotor pathway controlling blood pressure [41,120].
Direct and polysynaptic pathways from the caudal vestibular nuclei to RVLM and CVLM, as well as to NTS, have been demonstrated anatomically (for review, see [7]) and electrophysiologically (for review, see [129]). However, the vestibular and baroreceptor terminal fields in NTS are distinct, with no significant convergence of these two inputs onto individual NTS neurons [6,110,130,131]. Further, despite the presence of second order vestibular terminals in both NTS and CVLM, neither of these cell groups responds appreciably to vestibular nerve stimulation. Moreover, NTS lesions have little impact on the sympathetic activity resulting from electrical stimulation of the vestibular nerve [110,131]. Thus, NTS and CVLM appear to be more directly involved in mediating the baroreflex than the VSR (see [63]).
In contrast, RVLM neurons receive significant convergent baroreceptor and vestibular input [129], and indeed chemical ablation of the RVLM in cats abolishes vestibular nerve stimulation-elicited effects on sympathetic nerve activity [132]. In addition, unlike CVLM and NTS, RVLM stands as an obligatory (and excitatory) relay between vestibular afferent activity and sympathetic preganglionic neurons in the spinal cord [132] (for review, see [127]). Since the RVLM is a requisite relay in the caudal vestibulo-sympathetic pathway, and since baroreflex activation strongly inhibits RVLM neurons, the RVLM is the most likely site of functional interplay between the baroreflex and the VSR (see [127]). In this light, the observation that baroreflex and VSR signals interact additively [88] can be interpreted as a reflection of the convergence of these two reflex arcs in the RVLM [131]. Figure 1 is a schematic diagram summarizing these connections. Functionally, an increase in blood pressure activates the baroreceptors, whose second order neurons are located in NTS [109]. NTS in turn sends excitatory projections to RVLM and to GABAergic cells in CVLM that project rostrally to inhibit RVLM neuronal activity [54,111]. This inhibitory influence on RVLM dampens VSR- as well as baroreflex-mediated excitation of the intermediolateral cell column [63]. It should be noted, however, that the response latency of RVLM neurons to vestibular stimulation appears to be too long for a di-synaptic labyrinthine input [134], suggesting that multi-synaptic projections are important in mediating at least some vestibulo-sympathetic responses. Moreover, since CVLM tonically inhibits RVLM even in the absence of baroreceptor (NTS) input, it is likely that there is a non-baroreceptor (and therefore non-NTS-mediated) excitatory input to CVLM that tonically activates the inhibitory (GABAergic) projection to RVLM [105]. Clearly, one putative source of such excitatory activity is the vestibular projection to CVLM [36].
Fig. 1.
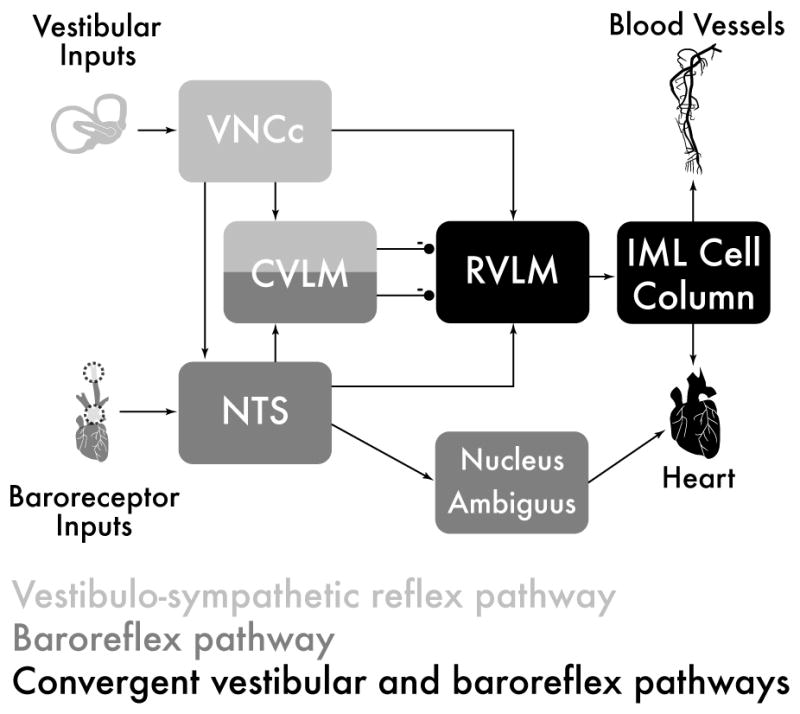
Schematic diagram of the major cell groups mediating vestibulo-autonomic and baroreflex pathways. Although there are vestibular projections to NTS and to CVLM, little converge of this pathway with baroreflex signals occurs prior to processing in the RVLM. Abbreviations: CVLM: caudal ventrolateral medullary region; IML: intermediolateral cell column; NTS: solitary nucleus; RVLM: rostral ventrolateral medulla; VNCc: caudal vestibular nuclear complex.
In this regard, it is interesting to consider the results of Galvanic vestibular stimulation (GVS) studies in human subjects. GVS entails low level DC galvanic current applied to the region of the mastoid processes. The cathodal stimulation selectively activates vestibular afferents, particularly those with irregular spontaneous firing rates [40,72], while afferents are inhibited at the anode (for review, see [33]). GVS has been used extensively in human studies [43] in which brief trains of sinusoidally modulated stimulation significantly increase muscle sympathetic nerve activity [9,119] and evoke frequency-dependent postural sway in standing subjects. There appears to be an optimal range of stimulus frequencies, at least in humans, the most effective being those lower than the cardiac rhythm [43]. While GVS does not appear to stimulate non-vestibular “graviceptors” (for review, see [23]), it does activate the entire vestibular labyrinth (for review, see [18]). Nevertheless, modulation of sympathetic activity through GVS appears to originate primarily from the otoliths and not the semicircular canals [90]. It has been suggested recently [43] that the frequency-dependent sinusoidal GVS modulation of muscle SNA reflects an independent vestibular input to RVLM, and that this input competes with baroreceptor afferents projecting to the RVLM via NTS and CVLM.
6. Chemoanatomy of vestibulo-autonomic connectivity
Glutamate is currently thought to be the principal neurotransmitter mediating the presympathetic vaso-motor pathway from the RVLM to the intermediolateral cell column [27,77] (for reviews, see [82,112]). Although the C1 group of catecholaminergic neurons [53] was originally posited as the chief purveyor of sympathoexcitation from the RVLM [94], immunotoxic lesions specifically targeting these cells produce only small reductions in mean arterial blood pressure [17, 68,106] and vesicular glutamate transporter type 2 has been localized in C1 as well as non-C1 barosensitive RVLM neurons [113,114]. Such findings have led to the current concept that the catecholaminergic neurons of the RVLM have a primarily modulatory function in blood pressure regulation, possibly contributing to sympathoexcitatory reflexes rather than to the maintenance of resting sympathetic vasomotor tone [48,112].
In addition to the catecholamines, a number of other neurotransmitters and modulators have been localized in RVLM cells, sometimes co-localizing in presumptive glutamatergic and/or catecholaminergic neurons [26,53,82,94,98,104,112]. These include calbindin, enkephalin, neuropeptide Y, substance P, cocaine- and amphetamine-regulated transcript, pituitary adenylate cyclase-activating polypeptide and nitric oxide (for review, see [112]). Similarly, numerous different receptor subtypes and systems have been investigated in the RVLM, including those of the excitatory and inhibitory amino acids, catecholamines, and peptides including angiotensin II receptors. Pharmacological investigations of these receptor systems have largely been directed toward identifying neuroactive compounds that alter blood pressure. Anti-hypertensive agents that contain an imidazoline ring, such as clonidine, moxonidine and rilmenidine, are ligands at alpha2 adrenergic receptors (a2ARs) and at imidazol(in)e binding sites (IRs), and lower blood pressure by inhibiting the tonically active sympatho-excitatory RVLM projection neurons [93]. Indeed, the RVLM is rich in both a2ARs and IRs, and microinjections of their ligands into the region produce hypotension [75], suggesting that the RVLM is an important site of action of anti-hypertensive agents [29,31,92]. IRs have been characterized pharmacologically and divided into three subtypes, I1- I2- and I3-Rs [29,50,76]. While the precise molecular identities of the I-Rs have yet to be established fully, a candidate I1-R protein termed Nischarin or IRAS (imidazoline receptor antisera-selected protein) has been cloned from human hippocampus [56, 81,115,125] and a second potential candidate I1-R be-longing to the sphingosine-1-phosphate receptor family has also been identified [73]. I1- and I3-Rs have been implicated in the regulation of blood pressure [85], but while imidazole and imidazoline receptors were postulated almost three decades ago [14,59], endogenous ligands for IRs such as agmatine (for review, see [49]) and the I-4 isomer of imidazoleacetic acid-ribotide (IAA-RP) [85] have been identified only recently.
We have reported that IAA-RP is a putative neuro-modulator in mammalian brain [85]. This ribotide displays high and low affinity binding to IRs and a2ARs, respectively, and microinjections of IAA-RP into the rat RVLM alter blood pressure. IAA-RP displays high affinity binding specifically to I1 and I3-Rs, producing physiologic effects that are blocked by their respective specific IR antagonists [85]. We have produced a polyclonal antiserum against IAA-RP, and have used it in immunocytochemical and immunofluorescence studies of the CNS [35], including the caudal VNC [69]. More recently, we have continued to explore the localization of IAA-RP-containing neurons in the ventrolateral medulla, in the context of vestibulo-autonomic projections.
7. IAA-RP-containing neurons in the RVLM
Using the polyclonal anti-IAA-RP antibody and immunofluorescence staining of sections through the caudal brainstem, we have observed a moderate density of IAA-RP-immunopositive neuronal cell bodies in the rat RVLM, as well as in other regions of the caudal medulla [35] (Fig. 2). Only a small subset of the neurons in each region are IAA-RP-immunostained, as previously observed in other CNS regions [35], and major white matter tracts of the brainstem including the pyramidal tract, spinal trigeminal tract and the inferior cerebellar peduncle are not immunolabeled.
Fig. 2.
IAA-RP immunofluorescence in the rat medulla. IAA-RP-immunopositive cell bodies are present in the rostral ventrolateral medullary region (RVLM). Subpopulations of neurons in other areas of the caudal brainstem are also IAA-RP-immunofluorescent, including the facial motor nucleus (7), the spinal trigeminal nucleus (Sp5), pars compacta of nucleus ambiguus (Amb). The inferior olivary complex (IOC) and solitary tract (sol) are identified as landmarks. Midline is to the right. Scale bar: 200 μm.
The distribution of IAA-RP-immunostained cells in the RVLM was assessed using a series of diaminobenzidine-horseradish peroxidase-stained sections through the RVLM region. Skip (1 every 6)-serial 50-μm-thick coronal Vibratome sections encompassing the entire rostro-caudal extent of the RVLM were immunostained for IAA-RP and visualized using diaminobenzidine. Each section was imaged at 2.5X, and the rostro-caudal (Bregma) position of each section was determined by comparison of the anatomical structures present in the ventral aspect of the section with anatomical, physiological and functional maps and atlases of the region [3, 12,13,17,26,39,41,42,79,99,100,120,121]. Each of the low magnification images was imported into Adobe Illustrator™, and was superimposed on the corresponding Bregma level drawing from the atlas [79], adjusting image position and size to match the atlas drawing. The entire extent of the RVLM in each section, as defined by the merged atlas/section images, was re-imaged as a 40X photomontage (6–20 fields/level). Each tissue section was then re-examined in the microscope at 40X; immunostained neurons with clearly visible nuclei were identified by direct examination of the sections and their locations were recorded on the printed 40X image montages. The location of each immunostained RVLM neuron was then transferred to the corresponding atlas drawing.
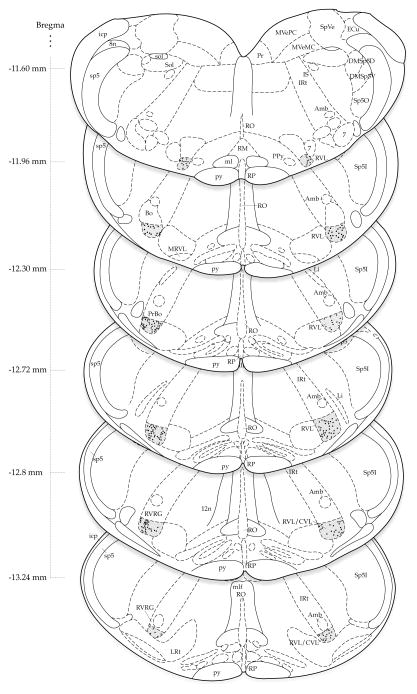
The stereotaxic atlas-based maps of immunopositive neurons demonstrate that IAA-RP-containing cells are present throughout the rostro-caudal extent of the RVLM (Fig. 3). Although the density (number per unit area) of immunolabeled neurons is consistent throughout much of the RVLM, lower densities are observed at the rostral and caudal poles of the region. No consistent medio-lateral or right-left asymmetries were observed.
Fig. 3.
The distribution of IAA-RP-immunolabeled cells in the rat RVLM of one representative subject. Labeled neurons in RVLM were mapped to transverse sections from the rat brain atlas of Paxinos and Watson [80]. Bregma coordinates for each of the levels are indicated to the left of each atlas section. The RVLM is shaded grey and individual stained neurons in this subject are mapped as dots on the atlas drawings. The density of immunolabeled neurons is consistent throughout much of the region, although lower densities are observed at the rostral and caudal poles. No consistent medio-lateral or right-left asymmetries are observed. Note that this map does not illustrate IAA-RP-immunostained cells in sites outside the RVLM. Abbreviations (from [80]): 7, facial nucleus; 8n, VIIIth nerve roots; 12n, XIIth nerve roots; Amb, nucleus ambiguus; Bo, Bötzinger complex; DMSp5D, dorsomedial spinal trigeminal nucleus, pars dorsalis; DMSp5V, dorsomedial spinal trigeminal nucleus, pars ventralis; ECu. external cuneate nucleus; icp; inferior cerebellar peduncle; IS, inferior salivatory nucleus; IRt, intermediate reticular nucleus; Li, linear nucleus; LPGi, lateral paragigantocellular nucleus; LRt, lateral reticular nucleus, ml, medial lemniscus; mlf, medial longitudinal fasciculus; MRVL, medial rostroventral lateral medulla; MVeMC, magnocellular medial vestibular nucleus; MVePC, parvocellular medial vestibular nucleus; PPy, parapyramidal nucleus; Pr, prepositus nucleus; PrBo, pre-Bötzinger complex; py, pyramidal tract; RM, raphé magnus; RO, raphé obscurus; RP, raphé pallidus; RVL, rostral ventrolateral medulla; RVL/CVL, rostral/caudal ventrolateral medulla; RVRG, rostral ventral respiratory group; sol, solitary tract; Sol, nucleus of the solitary tract; SpVe, spinal vestibular nucleus; sp5, spinal trigeminal tract; Sp5I, spinal trigeminal nucleus pars interpolaris; Sp5O, spinal trigeminal nucleus pars oralis.
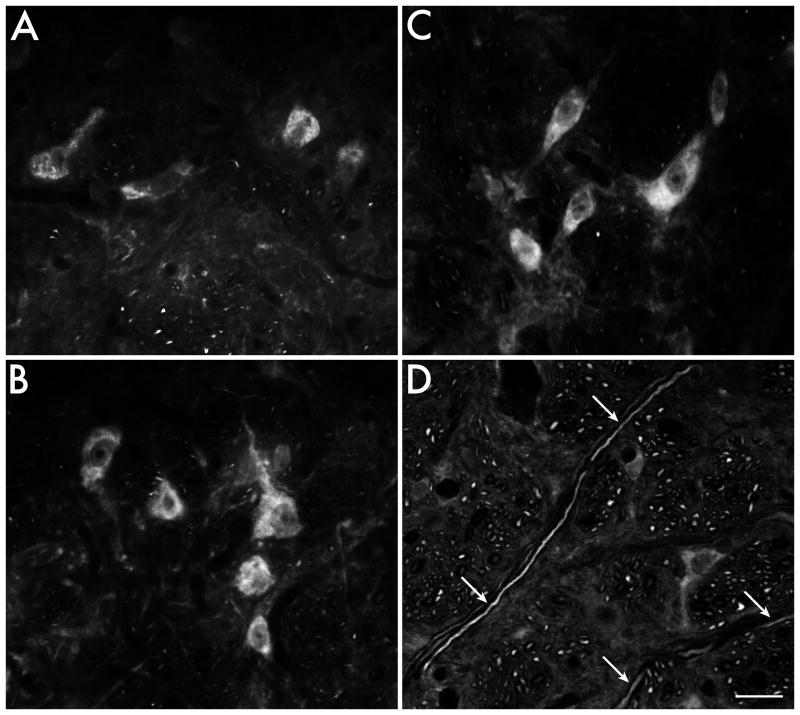
Based on both immunofluorescence and immunocytochemical observations, IAA-RP-immunolabeled cell bodies range from 10 to 35 μm in long-axis diameter and have 1–4 primary dendrites emerging from their somata, affording most neurons a fusiform or multipolar shape (Fig. 4A–C). Within the perikarya, IAA-RP immunolabeling usually appears in focal accumulations that give the cytoplasm a mottled appearance, as is observed in other regions of the nervous system [35], including the VNC [69]. IAA-RP is also present in axonal and dendritic processes in the RVLM. While the majority of these processes course rostro-caudally through the medulla, and are observed in cross-section in transverse brainstem sections, dorso-ventrally oriented fibers containing IAA-RP-immunostained processes are also apparent. Regardless of trajectory, only a few of the processes within a bundle are IAA-RP-immunopositive (Fig. 4D).
Fig. 4.
The morphology of IAA-RP-containing neurons and processes in the RVLM. A-C: Most IAA-RP-immunofluorescent somata in RVLM are 10–35 μm in diameter and are fusiform or multipolar in shape. The immunostain is not usually distributed homogeneously in the cytoplasm, but instead is concentrated in focal clumps in both the cell bodies and dendrites. In fusiform neurons, these clumps are often observed at the polar regions of the perikaryon. D: IAA-RP is present in processes in the RVLM. While most of these course rostro-caudally, dorso-ventrally oriented IAA-RP-immunostained processes are also apparent (arrows). Scale bar in D: 20 μm for all panels.
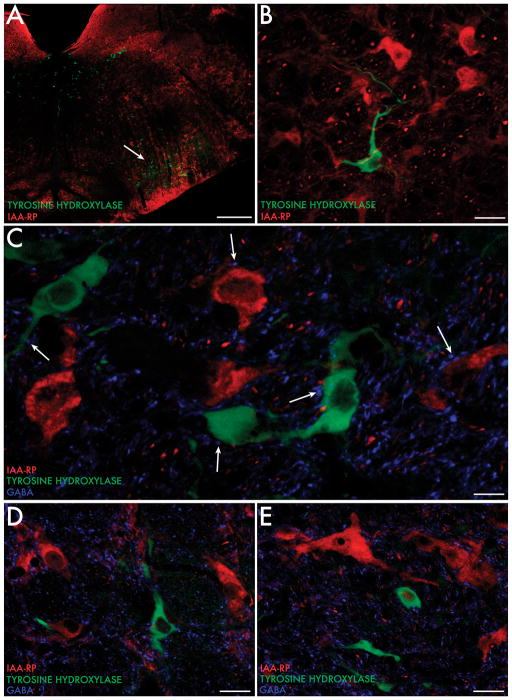
In order to visualize IAA-RP-containing neurons within the chemoanatomic framework of the RVLM, catecholaminergic neurons were identified using a commercial mouse monoclonal antibody against tyrosine hydroxylase (TH; Millipore, Billerica, MA), therefore potentially recognizing adrenergic, noradrenergic and dopaminergic phenotypes. As previously reported by others, the RVLM contains a discrete population of TH-immunopositive neurons [4,53,82,94,98, 104]. The perikarya of these neurons are approximately 20–25 μm in diameter and are morphologically diverse, including fusiform, polygonal, globular and stellate shaped somata, although all cytological types have extensive, radiating dendritic processes. Since IAA-RP-immunopositive neurons are distributed homogeneously throughout the RVLM, some of these cells are present in the region containing catecholaminergic (TH-immunopositive) perikarya (Fig. 5A). However, co-localization of TH- and IAA-RP-immunoreactivity in individual RVLM neurons is rarely observed (Fig. 5B).
Fig. 5.
IAA-RP, TH and GABA immunofluorescence in the RVLM. A, B: TH-immunoreactive neurons (feathered arrows) form a loosely organized cluster in the RVLM region. IAA-RP-immunofluorescent cells (simple arrows) are present in the same RVLM region as the catecholaminergic cells and fibers, but IAA-RP does not co-localize within the TH-positive neurons. C-E: GABA-immunofluorescent processes and puncta (wands) are present throughout the neuropil of the RVLM, and are contiguous with TH- (feathered arrows) and IAA-RP (simple arrows)-immunofluorescent cell bodies and proximal dendrites. Scale bars: 400 μm (A); 20 μm (B, D, E); 10 μm (C).
Using a monoclonal antibody against GABA [55], we observed a dense plexus of GABAergic fibers and terminals in the RVLM, which is likely to be at least partly attributable to the projections from CVLM [10, 19,25,47,51,82,105]. In addition to the GABA immunofluorescence in the general neuropil, GABAergic puncta are frequently observed surrounding TH-immunofluorescent and IAA-RP-immunolabeled cell bodies and proximal dendrites (Fig. 5C–E). However, this is not a unique morphological signature for either TH-positive or IAA-RP-positive cells since sheaths of axo-somatic boutons are also observed around phantom cell bodies that are not immunoreactive for IAA-RP or TH.
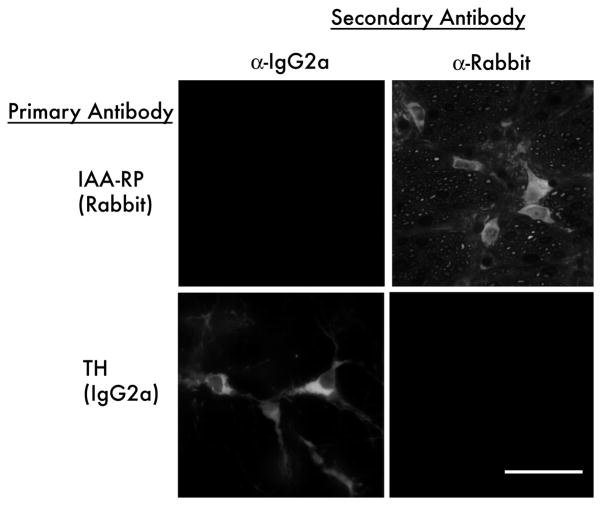
The multiple-label simultaneous fluorescence experiments all utilized combinations of primary antibodies distinguished by their host species and, for mixtures containing multiple mouse monoclonal antibodies, by their immunoglobin class and subclass. Secondary antibodies were combinations of species- and subclass-specific reagents selected such that each secondary recognized only one element of the primary antibody mixture. Control sections from the same animals were processed concurrently through all steps, with omission of the primary reagent(s) or pre-absorption with unconjugated or BSA-conjugated antigen; autofluorescence control sections were processed without primary and secondary antibodies. In addition, crosstalk among the secondary reagents was assessed in the experiments that used multiple primary and secondary antibodies by eliminating one primary at a time from stain combinations, or by staining with only one primary at a time, while using the entire corresponding combination of secondaries. In each case, the image channels corresponding to the missing primaries was blank, indicating that each secondary did not react with inappropriate primaries and that both tissue autofluorescence and nonspecific binding of secondaries were negligible (Fig. 6). In addition, for each primary within each stain combination, we commonly observed cell bodies and/or processes emitting fluorescence of only one color. This is an indication, seen within each specimen, that only one secondary antibody bound to the corresponding primary and that cross-reactivity from other secondaries was negligible.
Fig. 6.
Immunofluorescence controls for secondary antibody cross-reactivity. Brainstem sections were exposed to anti-imidazoleacetic acid-ribotide (IAA-RP; rabbit polyclonal; top row) or anti-tyrosine hydroxylase (TH, mouse monoclonal IgG2a; bottom row) followed a mixture of anti-mouse IgG2a-AlexaFluor 647 (left column) and species-specific anti-rabbit IgG-AlexaFluor 568 (right column) secondary antibodies. The same field is shown visualized for long red (AlexaFluor 647, left column) and red (AlexaFluor 568, right column). Rabbit (IAA-RP) labeled cells bind anti-rabbit secondary antibody (upper right) but not the anti-mouse IgG2a antibody (upper left). Tyrosine-hydroxlase labeled cells bind the anti-mouse IgG2a secondary antibody (lower left) but not the anti-rabbit IgG antibody (lower right). Scale bar: 20 μm.
To visualize vestibular projections to the RVLM, injections of the anterograde tracer Phaseolus vulgaris leucoagglutinin (PhaL) were placed in the caudal VNC. These experiments have demonstrated a direct projection from the caudal medial vestibular nucleus to the RVLM, conveyed by fine caliber, highly varicose axons (Fig. 7). In general, these axons appear to target non-catecholaminergic neurons. This leads to our current hypothesis, which remains to be tested, that the direct vestibulo-autonomic projection terminates on IAA-RP-containing cells in the RVLM.
Fig. 7.
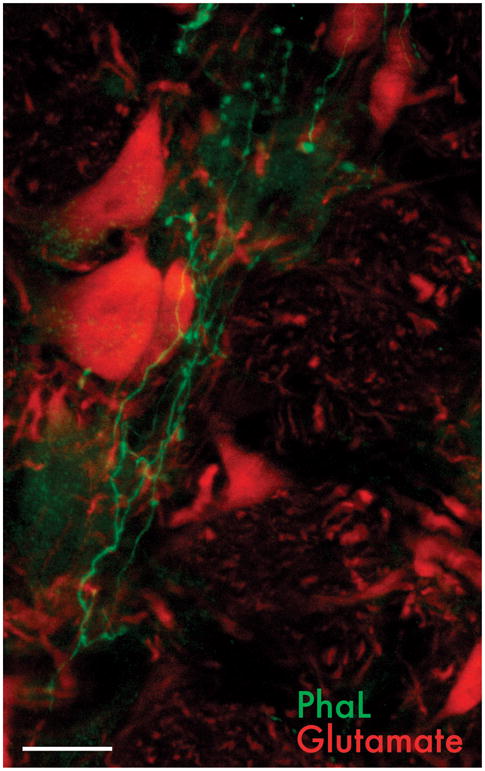
PhaL-immunofluorescent vestibular axons in the RVLM. The vestibular axons (white) are fine caliber and varicose, arborizing profusely in the RVLM. In this image, they ramify near the cell bodies of neurons immunolabeled for glutamate (gray), used as a generic cell marker. Scale bar: 20 μm.
8. The clinical significance of vestibulo-autonomic pathways
Vestibular disorders, particularly those of acute onset, include symptoms of abnormal autonomic function such as orthostatic hypotension (OH) and orthostatic intolerance [37]. In addition, the VSR is often impaired in the elderly [91], and this deficit becomes more prevalent and severe with orthostatic stress [74]. The decline in vestibulo-autonomic function with age is manifest in the high prevalences of OH, postural orthostatic tachycardia syndrome, dizziness and consequent falls in persons over age 65 [66,95]. Typically, individuals with OH have low blood pressure while standing, normal blood pressure while seated, and sometimes high blood pressure when lying down [95]. Estimates of the prevalence of OH vary widely, perhaps due to differences in the operational definition (e.g. symptomatic vs asymptomatic) utilized in different studies, as well as to differences in subject sampling, medication usage, degree of orthostatic stress and other study design factors. The lowest estimates are from the Honolulu Heart Program cohort of 3522 Japanese-Americans, which indicates progressively increasing prevalences from 5.1% in 71–74 yr olds to 10.9% in people 85 and older [70]. However, an epidemiological study (employing the consensus definition of OH: a fall in systolic/diastolic blood pressure of at least 20/10 mmHg within 3 min of standing or during head-up tilt [102]) of 1638 adults 20 years or older reported a prevalence of 15.8% [124]. Similarly, a longitudinal multi-center study of 5201 individuals aged 65 yr or older at their initial exam found the overall prevalence of asymptomatic OH to be 16.2% [101]. When the subject selection criteria were expanded to include individuals who became dizzy upon standing during the test, the prevalence estimate increased to 18.2%. After reviewing data from seven studies of generally healthy subjects older than 70, Low [66] estimated that the prevalence of OH is 10–30% in ambulatory subjects, but as much as 51.5% when the tests include increased orthostatic challenge. Despite the wide range in overall prevalence estimates, all studies consistently find that the prevalence of OH increases significantly with age [34,44,66,101]. These data suggest that OH is common even among otherwise healthy adults, and is even more prevalent in patients with hypertension, brainstem lesions, or neurological diseases such as Parkinson’s disease, multiple system atrophy and disorders that affect the release of norepinephrine [66,95].
OH has been associated with falling [46,83,118] (but see [38]), cognitive decline [30,126], cardiovascular morbidity, and mortality in the elderly [46,67,70]. In fact, after adjusting for other risk factors, OH in the Honolulu cohort was shown to be a significant independent predictor of four-year all-cause mortality [70]. This association has been replicated in another study population, in which all-cause mortality was 4.2% in subjects without OH, but 13.7% in OH patients [97].
OH patients usually seek medical attention for debilitating symptoms, and current treatment focuses on improving patients’ functional capacity and quality of life, but not on treating the disease itself [95]. Further research is needed to identify the neurotransmitters, modulators and receptors mediating this important facet of physiological regulation. Such research will be important for the development of more specific anti-hypertensive medications, and more rational pharmacologic treatments for vestibulo-autonomic disorders that directly address the disease process, thus potentially granting patients lifelong improvement. Receptor-specific pharmacotherapeutics for postural hypotension would be a substantial gain over current drug treatment options, which ameliorate some symptoms but which are often contra-indicated in the high proportion of elderly with resting hypertension [61,95,96]. This review is intended to highlight the current level of understanding of the chemical anatomy and connectivity of the vestibulo-sympathetic pathways since those projections are clearly important to the survival, and not just the wellbeing, of the population.
Acknowledgments
The authors are grateful to Dr. Ewa Kukielka, Mr. Tim Kang and Ms. Melissa Magrath for assistance with various aspects of the research. The research was supported by NIH grant 1R01 DC008846 from the National Institute on Deafness and Other Communication Disorders.
References
- 1.Abe C, Tanaka K, Awazu C, Morita H. Impairment of vestibular-mediated cardiovascular response and motor coordination in rats born and reared under hypergravity. Am J Physiol Regul Integr Comp Physiol. 2008;295:R173–R180. doi: 10.1152/ajpregu.00120.2008. [DOI] [PubMed] [Google Scholar]
- 2.Abe C, Tanaka K, Awazu C, Morita H. Galvanic vestibular stimulation counteracts hypergravity-induced plastic alteration of vestibulo-cardiovascular reflex in rats. J Appl Physiol. 2009;107:1089–1094. doi: 10.1152/japplphysiol.00400.2009. [DOI] [PubMed] [Google Scholar]
- 3.Aicher SA, Reis DJ, Ruggiero DA, Milner TA. Anatomical characterization of a novel reticulospinal vasodepressor area in the rat medulla oblongata. Neurosci. 1994;60:761–779. doi: 10.1016/0306-4522(94)90503-7. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong DM, Ross CA, Pickel VM, Joh TH, Reis DJ. Distribution of dopamine-. noradrenaline-, and adrenaline-containing cell bodies in the rat medulla oblongata: Demonstration by the immunocytochemical localization of catecholamine biosynthetic enzymes. J Comp Neurol. 1982;212:173–187. doi: 10.1002/cne.902120207. [DOI] [PubMed] [Google Scholar]
- 5.Balaban CD. Vestibular nucleus projections to the parabrachial nucleus in rabbits: implications for vestibular influences on the autonomic nervous system. Exp Brain Res. 1996;108:367–381. doi: 10.1007/BF00227260. [DOI] [PubMed] [Google Scholar]
- 6.Balaban CD, Beryozkin G. Vestibular nucleus projections to nucleus tractus solitarius and the dorsal motor nucleus of the vagus nerve: potential substrates for vestibular-autonomic interactions. Exp Brain Res. 1994;98:200–212. doi: 10.1007/BF00228409. [DOI] [PubMed] [Google Scholar]
- 7.Balaban CD, Porter JD. Neuroanatomical substrates for vestibulo-autonomic interactions. J Vest Res. 1998;8:7–16. [PubMed] [Google Scholar]
- 8.Balaban CD, Yates BJ. Vestibulo-autonomic Interactions: A teleological perspective. In: Highstein SH, Fay RR, Popper AN, editors. The Vestibular System. Vol. 19. Springer; Wein: 2004. [Google Scholar]
- 9.Bent LR, Bolton PS, Macefield VG. Modulation of muscle sympathetic bursts by sinusoidal galvanic vestibular stimulation in human subjects. Exp Brain Res. 2006;174:701–711. doi: 10.1007/s00221-006-0515-6. [DOI] [PubMed] [Google Scholar]
- 10.Blessing WW. Depressor neurons in rabbit caudal medulla act via GABA receptors in rostral medulla. Am J Physiol. 1988;254:H686–H692. doi: 10.1152/ajpheart.1988.254.4.H686. [DOI] [PubMed] [Google Scholar]
- 11.Blessing WW. Lower brain stem regulation of visceral, cardiovascular, and respiratory function. In: Paxinos G, Mai JK, editors. The Human Nervous System. 2. Elsevier Academic Press; Amsterdam: 2004. [Google Scholar]
- 12.Blessing WW, Reis DJ. Inhibitory cardiovascular function of neurons in the caudal ventrolateral medulla of the rabbit: relationship to the area containing A1 noradrenergic cells. Brain Res. 1982;253:161–171. doi: 10.1016/0006-8993(82)90683-7. [DOI] [PubMed] [Google Scholar]
- 13.Bourassa EA, Sved AF, Speth RC. Angiotensin modulation of rostral ventrolateral medulla (RVLM) in cardiovascular regulation. Mol Cel Endocrin. 2009;302:167–175. doi: 10.1016/j.mce.2008.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bousquet P, Feldman J, Schwartz J. Central cardiovascular effects of a-adrenergic drugs: Difference between catecholamines and imidazolines. J Pharmacol Exp Ther. 1984;230:232–236. [PubMed] [Google Scholar]
- 15.Bradbury S, Eggleston C. Postural hypotension. A report of three cases. Am Heart J. 1925;1:73–86. [Google Scholar]
- 16.Cano G, Card JP, Sved AF. Dual viral transneuronal tracing of central autonomic circuits involved in the innervation of the two kidneys in rat. J Comp Neurol. 2004;471:462–481. doi: 10.1002/cne.20040. [DOI] [PubMed] [Google Scholar]
- 17.Card JP, Sved JC, Craig B, Raizada M, Vazquez J, Sved AF. Efferent projections of rat rostroventrolateral medulla C1 catecholamine neurons: implications for the central control of cardiovascular regulation. J Comp Neurol. 2006;499:840–859. doi: 10.1002/cne.21140. [DOI] [PubMed] [Google Scholar]
- 18.Carter JR, Ray CA. Sympathetic responses to vestibular activation in humans. Am J Physiol Regul Integr Comp Physiol. 2008;294:R681–R688. doi: 10.1152/ajpregu.00896.2007. [DOI] [PubMed] [Google Scholar]
- 19.Chan RKW, Sawchenko PE. Organization and transmitter specificity of medullary neurons activated by sustained hypertension: implications for understanding baroreceptor reflex circuitry. J Neurosci. 1998;18:371–387. doi: 10.1523/JNEUROSCI.18-01-00371.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cobbold AF, Megirian D, Sherrey JH. Vestibular evoked activity in autonomic motor outflows. Arch Ital Biol. 1968;106:113–123. [PubMed] [Google Scholar]
- 21.Cui J, Iwase S, Mano T, Katayama N, Mori S. Sympathetic response to horizontally linear acceleration in humans. J Gravit Physiol. 1999;6:65–66. [PubMed] [Google Scholar]
- 22.Cui J, Mukai C, Iwase S, Sawasaki N, Kitazawa H, Mano T, Sugiyama Y, Wada Y. Response to vestibular stimulation of sympathetic outflow to muscle in humans. J Auton Nerv Syst. 1997;66:154–162. doi: 10.1016/s0165-1838(97)00077-5. [DOI] [PubMed] [Google Scholar]
- 23.Curthoys IS. A critical review of the neurophysiological evidence underlying clinical vestibular testing using sound, vibration and galvanic stimuli. Clin Neurophysiol. 2009 doi: 10.1016/j.clinph.2009.09.027. in press. [DOI] [PubMed] [Google Scholar]
- 24.Dampney RA, Goodchild AK, Robertson LG, Montgomery W. Role of ventrolateral medulla in vasomotor regulation: a correlative anatomical and physiological study. Brain Res. 1982;249:223–235. doi: 10.1016/0006-8993(82)90056-7. [DOI] [PubMed] [Google Scholar]
- 25.Dampney RA, Horiuchi J, Tagawa T, Fontes MA, Potts PD, Polson JW. Medullary and supramedullary mechanisms regulating sympathetic vasomotor tone. Acta Physiol Scand. 2003;177:209–218. doi: 10.1046/j.1365-201X.2003.01070.x. [DOI] [PubMed] [Google Scholar]
- 26.Dampney RAL. The subretrofacial vasomotor nucleus: Anatomical, chemical and pharmacological properties and role in cardiovascular regulation. Prog Neurobiol. 1994;42:197–227. doi: 10.1016/0301-0082(94)90064-7. [DOI] [PubMed] [Google Scholar]
- 27.Deuchars SA, Morrison SF, Gilbey MP. Medullary-evoked EPSPs in neonatal rat sympathetic preganglionic neurones in vitro. J Physiol. 1995;487:453–463. doi: 10.1113/jphysiol.1995.sp020892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doba N, Reis DJ. Role of the cerebellum and vestibular apparatus in regulation of orthostatic reflexes in the cat. Circ Res. 1974;34:9–18. doi: 10.1161/01.res.40.4.9. [DOI] [PubMed] [Google Scholar]
- 29.Eglen RM, Hudson AL, Kendall DA, Nutt DJ, Morgan NG, Wilson VG, Dillon MP. Seeing through a glass darkly: casting light on the imidazoline “I” sites. Trends Pharmacol Sci. 1998;19:381–390. doi: 10.1016/s0165-6147(98)01244-9. [DOI] [PubMed] [Google Scholar]
- 30.Elmstahl S, Rosen I. Postural hypotension and EEG variables predict cognitive decline: results from a 5-year follow-up of healthy elderly women. Dement Geriatr Cogn Disord. 1997;8:180–187. doi: 10.1159/000106629. [DOI] [PubMed] [Google Scholar]
- 31.Ernsberger P, Elliott HL, Weimann H-J, Raap A, Haxhiu MA, Hofferber E, Low-Kroger A, Reid JL, Mest HJ. Moxonidine: A second-generation central antihypertensive agent. Cardiovas Drug Rev. 1993;11:411–431. [Google Scholar]
- 32.Etard O, Reber A, Quarck G, Normand H, Mulder P, Denise P. Vestibular control of blood pressure during parabolic flights in awake rats. Neuro Report. 2004;15:2357–2360. doi: 10.1097/00001756-200410250-00011. [DOI] [PubMed] [Google Scholar]
- 33.Fitzpatrick RC, Day BL. Probing the human vestibular system with galvanic stimulation. J Appl Physiol. 2004;96:2301–2316. doi: 10.1152/japplphysiol.00008.2004. [DOI] [PubMed] [Google Scholar]
- 34.Forman DE, Lipsitz LA. Syncope in the elderly. Cardio Clin. 1997;15:295–311. doi: 10.1016/s0733-8651(05)70337-4. [DOI] [PubMed] [Google Scholar]
- 35.Friedrich VLJ, Martinelli GP, Prell GD, Holstein GR. Distribution and cellular localization of imidazoleacetic acid-ribotide, an endogenous ligand at imidazol(in)e and adrenergic receptors, in rat brain. J Chem Neuroanat. 2007;33:53–64. doi: 10.1016/j.jchemneu.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Friedrich VLJ, Martinelli GP, Prell GD, Holstein GR. Vestibular projections target catecholaminergic neurons in the caudal ventrolateral medullary region involved in blood pressure control. Soc Neurosci Annual Meeting Abstracts. 2009:Abst. #264.261. [Google Scholar]
- 37.Furman JM, Jacob RG, Redfern MS. Clinical evidence that the vestibular system participates in autonomic control. J Vest Res. 1998;8:27–34. [PubMed] [Google Scholar]
- 38.Ganz DA, Bao Y, Shekelle PG, Rubenstein LZ. Will my patient fall? JAMA. 2007;297:77–86. doi: 10.1001/jama.297.1.77. [DOI] [PubMed] [Google Scholar]
- 39.Golanov EV, Ruggiero DA, Reis DJ. A brainstem area mediating cerebrovascular and EEG responses to hypoxic excitation of rostral ventrolateral medulla in rat. J Physiol. 2000;529:413–429. doi: 10.1111/j.1469-7793.2000.00413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goldberg JM, Smith CE, Fernandez C. Relation between discharge regularity and responses to externally applied galvanic currents in vestibular nerve afferents of the squirrel monkey. J Neurophysiol. 1984;51:1236–1256. doi: 10.1152/jn.1984.51.6.1236. [DOI] [PubMed] [Google Scholar]
- 41.Goodchild AK, Moon EA. Maps of cardiovascular and respiratory regions of rat ventral medulla: Focus on the caudal medulla. J Chem Neuroanat. 2009;38:209–221. doi: 10.1016/j.jchemneu.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 42.Gordon FJ, McCann LA. Pressor responses evoked by microinjections of L-glutamate into the caudal ventrolateral medulla of the rat. Brain Res. 1988;457:251–258. doi: 10.1016/0006-8993(88)90693-2. [DOI] [PubMed] [Google Scholar]
- 43.Grewal T, James C, Macefield VG. Frequency-dependent modulation of muscle sympathetic nerve activity by sinusoidal galvanic vestibular stimulation in human subjects. Exp Brain Res. 2009;197:379–386. doi: 10.1007/s00221-009-1926-y. [DOI] [PubMed] [Google Scholar]
- 44.Groothius JT, Thijssen DHJ, Kooijman M, Paulus R, Hopman MTE. Attenuated peripheral vasoconstriction during an orthostatic challenge in older men. Age and Ageing. 2008;37:680–684. doi: 10.1093/ageing/afn195. [DOI] [PubMed] [Google Scholar]
- 45.Gulli G, CVL, Claydon VE, Hainsworth R. Prolonged latency in the baroreflex mediated vascular resistance response in subjects with postural related syncope. Clin Auton Res. 2005 doi: 10.1007/s10286-005-0273-8. [DOI] [PubMed] [Google Scholar]
- 46.Gupta V, Lipsitz LA. Orthostatic hypotension in the elderly: diagnosis and treatment. Am J Med. 2007;120:841–847. doi: 10.1016/j.amjmed.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 47.Guyenet PG. Role of ventral medulla oblongata in blood pressure regulation. In: Loewy AD, editor. Central Regulation of Autonomic Function. Oxford University Press; New York: 1990. pp. 145–167. [Google Scholar]
- 48.Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci. 2006;7:335–346. doi: 10.1038/nrn1902. [DOI] [PubMed] [Google Scholar]
- 49.Halaris A, Piletz J. Agmatine: metabolic pathway and spectrum of activity in brain. CNS Drugs. 2007;21:885–900. doi: 10.2165/00023210-200721110-00002. [DOI] [PubMed] [Google Scholar]
- 50.Head GA. Central imidazoline- and a2-receptors involved in the cardiovascular actions of centrally acting antihypertensive agents. Ann NY Acad Sci. 1999;881:279–286. doi: 10.1111/j.1749-6632.1999.tb09370.x. [DOI] [PubMed] [Google Scholar]
- 51.Heesch CM, Laiprasert JD, Kvochina L. RVLM glycine receptors mediate GABAa and GABAb independent sympathoinhibition from CVLM in rats. Brain Res. 2006;1125:46–59. doi: 10.1016/j.brainres.2006.09.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Henry RT, Connor JD, Balaban CD. Nodulus-uvula depressor response: central GABA-mediated inhibition of a-adrenergic outflow. Am J Physio. 1989;256:H1601–H1608. doi: 10.1152/ajpheart.1989.256.6.H1601. [DOI] [PubMed] [Google Scholar]
- 53.Hökfelt T, Füxe K, Goldstein M, Johansson O. Evidence for adrenaline neurons in the rat brain. Acta Physiol Scand. 1973;89:286–288. doi: 10.1111/j.1748-1716.1973.tb05522.x. [DOI] [PubMed] [Google Scholar]
- 54.Holstege G. Anatomical study of the final common pathway for vocalization in the cat. J Comp Neurol. 1989;284:242–252. doi: 10.1002/cne.902840208. [DOI] [PubMed] [Google Scholar]
- 55.Holstein GR, Martinelli GP, Henderson SC, Friedrich VLJ, Rabbitt RD, Highstein SM. Gamma-aminobutyric acid is present in a spatially discrete subpopulation of hair cells in the crista ampullaris of the toadfish, Opsanus tau. J Comp Neurol. 2004;471:1–10. doi: 10.1002/cne.11025. [DOI] [PubMed] [Google Scholar]
- 56.Ivanov TR, Jones JC, Dontenwill M, Bousquet P, Piletz JE. Characterization of a partial cDNA clone detected by imidazoline receptor-selective antisera. J Auton Nerv Syst. 1998;72:98–110. doi: 10.1016/s0165-1838(98)00094-0. [DOI] [PubMed] [Google Scholar]
- 57.Jeske I, Reis DJ, Milner TA. Neurons in the barosensory area of the caudal ventrolateral medulla project monosynaptically on to sympathoexcitatory bulbospinal neurons in the rostral ventrolateral medulla. Neurosci. 1995;65:343–353. doi: 10.1016/0306-4522(94)00470-p. [DOI] [PubMed] [Google Scholar]
- 58.Jian BJ, Cotter LA, Emanuel BA, Cass SP, Yates BJ. Effects of bilateral vestibular lesions on orthostatic tolerance in awake cats. J Appl Physiol. 1999;86:1552–1560. doi: 10.1152/jappl.1999.86.5.1552. [DOI] [PubMed] [Google Scholar]
- 59.Karppanen H. Interrelationships between clonidine and his-taminergic mechanisms. Trends Pharmacol Sci. 1981;2:35–37. [Google Scholar]
- 60.Kaufmann H, Biaggioni I, Voustianiouk A, Diedrich A, Costa F, Clarke R, Gizzi M, Raphan T, Cohen B. Vestibular control of sympathetic activity. An otolith-sympathetic reflex in humans. Exp Brain Res. 2002;143:463–469. doi: 10.1007/s00221-002-1002-3. [DOI] [PubMed] [Google Scholar]
- 61.Kawada T, Sugimachi M. Artificial neural interfaces for bionic cardiovascular treatments. J Artif Organs. 2009;12:17–22. doi: 10.1007/s10047-008-0438-z. [DOI] [PubMed] [Google Scholar]
- 62.Kerman IA, Akil H, Watson SJ. Rostral elements of sympatho-motor circuitry: A virally mediated transsynaptic tracing study. J Neurosci. 2006;26:3423–3433. doi: 10.1523/JNEUROSCI.5283-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kerman IA, Yates BJ. Regional and functional differences in the distribution of vestibulosympathetic reflexes. Am J Physiol. 1998;275:R824–R835. doi: 10.1152/ajpregu.1998.275.3.R824. [DOI] [PubMed] [Google Scholar]
- 64.Lee TK, Lois JH, Troupe JH, Wilson TD, Yates BJ. Transneuronal tracing of neural pathways that regulate hindlimb muscle blood flow. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1532–R1541. doi: 10.1152/ajpregu.00633.2006. [DOI] [PubMed] [Google Scholar]
- 65.Lipski J, Kanjhan R, Kruszewska B, Smith M. Barosensitive neurons in the rostral ventrolateral medulla of the rat in vivo: morphological properties and relationship to C1 adrenergic neurons. Neurosci. 1995;69:601–618. doi: 10.1016/0306-4522(95)92652-z. [DOI] [PubMed] [Google Scholar]
- 66.Low PA. Prevalence of orthostatic hypotension. Clin Auton Res. 2008;18:8–13. doi: 10.1007/s10286-007-1001-3. [DOI] [PubMed] [Google Scholar]
- 67.Luukinen H, Koski K, Laippala P, Kivela SL. Prognosis of diastolic and systolic orthostatic hypotension in older persons. Arch Intern Med. 1999;159:273–280. doi: 10.1001/archinte.159.3.273. [DOI] [PubMed] [Google Scholar]
- 68.Madden CJ, Sved AF. Rostral ventrolateral medulla C1 neurons and cardiovascular regulation. Cell Mol Neurobio. 2003;23:739–749. doi: 10.1023/A:1025000919468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Martinelli GP, Friedrich VLJ, Prell GD, Holstein GR. Vestibular neurons in the rat contain imidazoleacetic acid-ribotide, a putative neurotransmitter involved in blood pressure regulation. J Comp Neurol. 2007;501:568–581. doi: 10.1002/cne.21271. [DOI] [PubMed] [Google Scholar]
- 70.Masaki KH, Schatz IJ, Burchfiel CM, Sharp DS, Chiu D, Foley D, Curb JD. Orthostatic hypotension predicts mortality in elderly men: the Honolulu Heart Program. Circulation. 1998;98:2290–2295. doi: 10.1161/01.cir.98.21.2290. [DOI] [PubMed] [Google Scholar]
- 71.Miller DM, Cotter LA, Gandhi NJ, Schor RH, Cass SP, Huff NO, Raj SG, Shulman JA, Yates BJ. Responses of caudal vestibular nucleus neurons of conscious cats to rotations in vertical planes, before and after a bilateral vestibular neurectomy. Exp Brain Res. 2008;188:175–186. doi: 10.1007/s00221-008-1359-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Minor LB, Goldberg JM. Vestibular-nerve inputs to the vestibulo-ocular reflex: a functional-ablation study in the squirrel monkey. J Neurosci. 1991;11:1636–1648. doi: 10.1523/JNEUROSCI.11-06-01636.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Molderings GJ, Bönish H, Brüss M, Wolf C, von Kügelgen I, Göthert M. S1P-receptors in PC12 and transfected HEK293 cells: molecular targets of hypotensive imidaoline I(1) receptor ligands. Neurochem Int. 2007;51:476–485. doi: 10.1016/j.neuint.2007.04.022. [DOI] [PubMed] [Google Scholar]
- 74.Monahan KD, Ray CA. Vestibulosympathetic reflex during orthostatic challenge in aging humans. Am J Physio. 2002;283:R1027–R1032. doi: 10.1152/ajpregu.00298.2002. [DOI] [PubMed] [Google Scholar]
- 75.Moreira TS, Takakura AC, Menani JV, Sato MA, Colombari E. Central blockade of nitric oxide synthesis reduces moxonidine-induced hypotension. Brit J Pharmacol. 2004;142:765–771. doi: 10.1038/sj.bjp.0705853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Morgan NG. Imidazoline receptors: new targets for anti-hyperglycemic drugs. Exp Opin Invest Drugs. 1999;8:575–584. doi: 10.1517/13543784.8.5.575. [DOI] [PubMed] [Google Scholar]
- 77.Morrison SF. Glutamate transmission in the rostral ventrolateral medullary sympathetic premotor pathway. Cell and Mol Neurobiol. 2003;23:761–772. doi: 10.1023/A:1025005020376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nakamura Y, Matsuo S, Hosogai M, Kawai Y. Vestibular control of arterial blood pressure during head-down postural change in anesthetized rabbits. Exp Brain Res. 2009;194:563–570. doi: 10.1007/s00221-009-1732-6. [DOI] [PubMed] [Google Scholar]
- 79.Paxinos G, Carrive P, Wang H, Wang P-Y. Chemoarchitectonic Atlas of The Rat Brainstem. Academic Press; San Diego: 1999. [Google Scholar]
- 80.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; San Diego: 1998. [Google Scholar]
- 81.Piletz JE, Ivanov TR, Sharp JD, Ernsberger P, Chang CH, Pickard RT, Gold G, Roth B, Zhu H, Jones JC, Baldwin J, Reis DJ. Imidazoline receptor antisera-selected (IRAS) cDNA: cloning and characterization. DNA Cell Biol. 2000;19:319–329. doi: 10.1089/10445490050043290. [DOI] [PubMed] [Google Scholar]
- 82.Pilowsky PM, Goodchild AK. Baroreceptor reflex pathways and neurotransmitters: 10 years on. J Hypertens. 2002;20:1675–1688. doi: 10.1097/00004872-200209000-00002. [DOI] [PubMed] [Google Scholar]
- 83.Poon IO, Braun U. High prevalence of orthostatic hypotension and its correlation with potentially causative medications among elderly veterans. J Clin Pharm Ther. 2005;301:173–178. doi: 10.1111/j.1365-2710.2005.00629.x. [DOI] [PubMed] [Google Scholar]
- 84.Porter JD, Balaban CD. Connections between the vestibular nuclei and regions that mediate autonomic function in the rat. J Vest Res. 1997;7:63–76. [PubMed] [Google Scholar]
- 85.Prell GD, Martinelli GP, Holstein GR, Matulic-Adamic J, Watanabe KA, Chan SL, Morgan NG, Haxhiu MA, Ernsberger P. Imidazoleacetic acid-ribotide: an endogenous ligand that stimulates imidazol(in)e receptors. Proc Natl Acad Sci U S A. 2004;101:13677–13682. doi: 10.1073/pnas.0404846101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Radtke A, Popov K, Bronstein AM, Gresty MA. Evidence for a vestibulo-cardiac reflex in man. The Lancet. 2000;356:736–737. doi: 10.1016/S0140-6736(00)02635-0. [DOI] [PubMed] [Google Scholar]
- 87.Radtke A, Popov K, Bronstein AM, Gresty MA. Vestibulo-autonomic control in man: Short- and long-latency vestibular effects on cardiovascular function. J Vest Res. 2003;13:25–37. [PubMed] [Google Scholar]
- 88.Ray CA. Interaction of the vestibular system and barore-flexes on sympathetic nerve activity in humans. Am J Physiol Heart Circ Physiol. 2000;279 :H2399–H2404. doi: 10.1152/ajpheart.2000.279.5.H2399. [DOI] [PubMed] [Google Scholar]
- 89.Ray CA, Carter JR. Review: Vestibular activation of sympathetic nerve activity. Acta Physiol Scand. 2003;177:313–319. doi: 10.1046/j.1365-201X.2003.01084.x. [DOI] [PubMed] [Google Scholar]
- 90.Ray CA, Hume KM, Steele SL. Sympathetic nerve activity during natural stimulation of horizontal semicircular canals in humans. Am J Physio. 1998;275:R1274–R1278. doi: 10.1152/ajpregu.1998.275.4.R1274. [DOI] [PubMed] [Google Scholar]
- 91.Ray CA, Monahan KD. Aging attenuates the vestibulosympathetic reflex in humans. Circulation. 2002;105:956–961. doi: 10.1161/hc0802.104289. [DOI] [PubMed] [Google Scholar]
- 92.Regunathan S, Reis DJ. Imidazoline receptors and their endogenous ligands. Ann Rev Pharmacol Toxicol. 1996;36:511–544. doi: 10.1146/annurev.pa.36.040196.002455. [DOI] [PubMed] [Google Scholar]
- 93.Reis DJ. Neurons and receptors in the rostroventrolateral medulla mediating the antihypertensive actions of drugs acting at imidazoline receptors. J Cardiovasc Pharmacol. 1996;27(Suppl 3):S11–S18. doi: 10.1097/00005344-199627003-00003. [DOI] [PubMed] [Google Scholar]
- 94.Reis DJ, Morrison SF, Ruggiero D. The C1 area of the brainstem in tonic and reflex control of blood pressure. Hypertens. 1988;11:I8–I13. doi: 10.1161/01.hyp.11.2_pt_2.i8. [DOI] [PubMed] [Google Scholar]
- 95.Robertson D. The pathophysiology and diagnosis of orthostatic hypotension. Clin Auton Res. 2008;18:2–7. doi: 10.1007/s10286-007-1004-0. [DOI] [PubMed] [Google Scholar]
- 96.Robertson D, Davis TL. Recent advances in the treatment of orthostatic hypotension. Neurology. 1995;45:S26–S32. [PubMed] [Google Scholar]
- 97.Rose KM, Eigenbrodt ML, Biga RL, Couper DL, Light KC, Sharrett AR, Heiss G. Orthostatic hypotension predicts mortality in middle-aged adults: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2006;114:630–636. doi: 10.1161/CIRCULATIONAHA.105.598722. [DOI] [PubMed] [Google Scholar]
- 98.Ross CA, Ruggiero DA, Joh TH, Park DH, Reis DJ. Rostral ventrolateral medulla: selective projections to the thoracic autonomic cell column from the region containing C1 adrenaline neurons. J Comp Neurol. 1984;228:168–185. doi: 10.1002/cne.902280204. [DOI] [PubMed] [Google Scholar]
- 99.Ross CA, Ruggiero DA, Park DH, Joh TH, Sved AF, Fernandez-Pardal J, Saavedra JM, Reis DJ. Tonic vasomotor control by the rostral ventrolateral medulla: Effect of electrical or chemical stimulation of the area containing C1 adrenaline neurons on arterial pressure, heart rate, and plasma catecholamines and vasopressin. J Neurosci. 1984;4:474–494. doi: 10.1523/JNEUROSCI.04-02-00474.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ruggiero DA, Cravo SL, Golanov E, Gomez R, Anwar M, Reis DJ. Adrenergic and non-adrenergic spinal projections of a cardiovascular-active pressor area of medulla oblongata: quantitative topographic analysis. Brain Res. 1994;663:107–120. doi: 10.1016/0006-8993(94)90468-5. [DOI] [PubMed] [Google Scholar]
- 101.Rutan GH, Hermanson B, Bild DE, Kittner SJ, LaBaw F, Tell GS. Orthostatic hypotension in older adults. The Cardiovascular Helath Study CHS Collaborative Research Group. Hypertension. 1992;19:508–519. doi: 10.1161/01.hyp.19.6.508. [DOI] [PubMed] [Google Scholar]
- 102.Schatz IJ, Bannister R, Freeman RL, Goetz CG, Jankovic J, Kaufmann HC, Koller WC, Low PA, Mathias CJ, Polinsky RJ, Quinn NP, Robertson D, Streeten DHP. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure, and multiple system atrophy. Neurology. 1996;46:1470. doi: 10.1212/wnl.46.5.1470. [DOI] [PubMed] [Google Scholar]
- 103.Schramm LP, Strack A, Platt MKB, Loewy AD. Peripheral and central pathways regulating the kidney: a study using pseudorabies virus. Brain Res. 1993;616:251–262. doi: 10.1016/0006-8993(93)90216-a. [DOI] [PubMed] [Google Scholar]
- 104.Schreihofer AM, Guyenet PG. Identification of C1 presympathetic neurons in rat rostral ventrolateral medulla by juxtacellular labeling in vivo. J comp Neurol. 1997;387:524–536. doi: 10.1002/(sici)1096-9861(19971103)387:4<524::aid-cne4>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 105.Schreihofer AM, Ito S, Sved AF. Brain stem control of arterial pressure in chronic arterial baroreceptor-denervated rats. Am J Physio. 2005;289:R1746–R1755. doi: 10.1152/ajpregu.00307.2005. [DOI] [PubMed] [Google Scholar]
- 106.Schreihofer AM, Stornetta RL, Guyenet PG. Regulation of sympathetic tone and arterial pressure by rostral ventrolateral medulla after depletion of C1 cells in rat. J Physiol. 2000;529:221–236. doi: 10.1111/j.1469-7793.2000.00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Spiegel EA, Démétriades TD. Beiträge zum studium des vegetativen nervensystems. III. Metteilung. Der ein-flub des vestibularapparates auf das GefäBsystem. Pflüger’s Achiv. 1922;196:185–199. [Google Scholar]
- 108.Spiegel EA, Démétriades TD. Beiträge zum studium des vegetativen nervensystems. VII. Metteilung. Der zen-trale mechanismus der vestibulären blutdrucksenkung und ihre bedeutung für die entstehung des labyrinthschwindels. Pflüger’s Achiv. 1924;205:328–337. [Google Scholar]
- 109.Spyer KM. Neural organisation and control of the baroreceptor reflex. Rev Physiol Biochem Pharmacol. 1981;88:24–124. [PubMed] [Google Scholar]
- 110.Steinbacher BC, Yates BJ. Processing of vestibular and other inputs by the caudal ventrolateral medullary reticular formation. Am J Physiol. 1996;271:R1070–R1077. doi: 10.1152/ajpregu.1996.271.4.R1070. [DOI] [PubMed] [Google Scholar]
- 111.Stocker SD, Steinbacher BC, Balaban CD, Yates BJ. Connections of the caudal ventrolateral medullary reticular formation in the cat brainstem. Exp Brain Res. 1997;116:270–282. doi: 10.1007/pl00005755. [DOI] [PubMed] [Google Scholar]
- 112.Stornetta RL. Neurochemistry of bulbospinal presympathetic neurons of the medulla oblongata. J Chem Neuroanat. 2009;38:222–230. doi: 10.1016/j.jchemneu.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Stornetta RL, Sevigny CP, Guyenet PG. Vesicular glutamate transporter DNPI/VGLUT2 mRNA is present in C1 and several other groups of brainstem catecholaminergic neurons. J Comp Neurol. 2002;444:191–206. doi: 10.1002/cne.10141. [DOI] [PubMed] [Google Scholar]
- 114.Stornetta RL, Sevigny CP, Schreihofer AM, Rosin DL, Guyenet PG. Vesicular glutamate transporter DNPI/VGLUT2 is expressed by both C1 adrenergic and non-adrenergic presympathetic vasomotor neurons of the rat medulla. J Comp Neurol. 2002;444:207–220. doi: 10.1002/cne.10142. [DOI] [PubMed] [Google Scholar]
- 115.Sun Z, Chang CH, Ernsberger P. Identification of IRAS/Nischarin as an I1-imidazoline receptor in PC12 pheochromocytoma cells. J Neurochem. 2007;101:99–108. doi: 10.1111/j.1471-4159.2006.04413.x. [DOI] [PubMed] [Google Scholar]
- 116.Sved AF, Ito S, Sved JC. Brainstem mechanisms of hypertension: Role of the rostral ventrolateral medulla. Curr Hypertens Rep. 2003;5:262–268. doi: 10.1007/s11906-003-0030-0. [DOI] [PubMed] [Google Scholar]
- 117.Uchino Y, Kudo N, Tsuda K, Iwamura Y. Vestibular inhibition of sympathetic nerve activities. Brain Res. 1970;22:195–206. doi: 10.1016/0006-8993(70)90004-1. [DOI] [PubMed] [Google Scholar]
- 118.van Hensbroek PB, van Dijk N, van Breda GF, Scheffer AC, van der Cammen TJ, Lips P, Goslings JC, de Rooij SE. The CAREFALL triage instrument identifying risk factos for recurrent falls in elderly patients. Am J Emerg MEd. 2009;27:23–36. doi: 10.1016/j.ajem.2008.01.029. [DOI] [PubMed] [Google Scholar]
- 119.Voustianiouk A, Kaufmann H, Diedrich A, Raphan T, Biaggioni I, MacDougall H, Ogorodnikov D, Cohen B. Electrical activtion of the human vestibulo-sympathetic reflex. Exp Brain Res. 2005 doi: 10.1007/s00221-005-0266-9. [DOI] [PubMed] [Google Scholar]
- 120.Willette RN, Barcas PP, Krieger AJ, Sapru HN. Vasopressor and depressor areas in the rat medulla. Identification by microinjection of L-glutamate. Neuropharm. 1983;22:1071–1079. doi: 10.1016/0028-3908(83)90027-8. [DOI] [PubMed] [Google Scholar]
- 121.Willette RN, Punnen-Grandy S, Krieger AJ, Sapru HN. Differential regulation of regional vascular resistance by the rostral and caudal ventrolateral medulla in the rat. J Auton Nerv Syst. 1987;18:143–151. doi: 10.1016/0165-1838(87)90101-9. [DOI] [PubMed] [Google Scholar]
- 122.Wilson TD, Cotter LA, Draper JA, Misra SP, Rice CD, CSP, Yates BJ. Effects of postural changes and removal of vestibular inputs on blood flow to the head of conscious felines. J Appl Physiol. 2006;100:1475–1482. doi: 10.1152/japplphysiol.01585.2005. [DOI] [PubMed] [Google Scholar]
- 123.Woodring SF, Rossiter CD, Yates BJ. Pressor response elicited by nose-up vestibular stimulation in cats. Exp Brain Res. 1997;113:165–168. doi: 10.1007/BF02454153. [DOI] [PubMed] [Google Scholar]
- 124.Wu JS, Yang YC, JLu FH, Wu CH, Chang CJ. Population-based study on the prevalence and correlates of orthostatic hypotension/hypertension and orthostatic dizziness. Hypertension Res. 2008;31:897–904. doi: 10.1291/hypres.31.897. [DOI] [PubMed] [Google Scholar]
- 125.Wu N, Su R-B, Li J. Agmatine and imidazoline receptors: Their role in opioid analgesia, tolerance and dependence. Cell Mol Neurobiol. 2008;28:629–641. doi: 10.1007/s10571-007-9164-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yap PLK, Niti M, Yap KB, Ng TP. Orthostatic hypotension, hypotenion and cognitive status: Early comorbid markers of primary demintia? Dement Geriatr Cogn Disord. 2008;26:239–246. doi: 10.1159/000160955. [DOI] [PubMed] [Google Scholar]
- 127.Yates BJ. Vestibular influences on the autonomic nervous system. Ann NY Acad Sci. 1996;781:458–473. doi: 10.1111/j.1749-6632.1996.tb15720.x. [DOI] [PubMed] [Google Scholar]
- 128.Yates BJ, Aoki M, Burchill P, Bronstein AM, Gresty MA. Cardiovascular responses elicited by linear acceleration in humans. Exp Brain Res. 1999;125:476–484. doi: 10.1007/s002210050705. [DOI] [PubMed] [Google Scholar]
- 129.Yates BJ, Bronstein AM. The effects of vestibular system lesions on autonomic regulation: Observations, mechanisms, and clinical implications. J Vest Res. 2005;15:119–129. [PubMed] [Google Scholar]
- 130.Yates BJ, Grélot L, Kerman IA, Balaban CD, Jakus J, Miller AD. Organization of vestibular inputs to nucleus tractus solitarius and adjacent structures in cat brain stem. Am J Physiol. 1994;267:R974–R983. doi: 10.1152/ajpregu.1994.267.4.R974. [DOI] [PubMed] [Google Scholar]
- 131.Yates BJ, Miller AD. Properties of sympathetic reflexes elicited by natural vestibular stimulation: implications for cardiovascular control. J Neurophysiol. 1994;71:2087–2092. doi: 10.1152/jn.1994.71.6.2087. [DOI] [PubMed] [Google Scholar]
- 132.Yates BJ, Siniaia MS, Miller AD. Descending pathways necessary for vestibular influences on sympathetic and inspiratory outflow. Am J Physiol. 1995;268:R1381–R1385. doi: 10.1152/ajpregu.1995.268.6.R1381. [DOI] [PubMed] [Google Scholar]
- 133.Yates BJ, Stocker SD. Integration of somatic and visceral inputs by the brainstem. Functional considerations. Exp Brain Res. 1998;119:269–275. doi: 10.1007/s002210050342. [DOI] [PubMed] [Google Scholar]
- 134.Yates BJ, Yamagata Y, Bolton PS. The ventrolateral medulla of the cat mediates vestibulosympathetic reflexes. Brain Res. 1991;552:265–272. doi: 10.1016/0006-8993(91)90091-9. [DOI] [PubMed] [Google Scholar]
- 135.Yavorcik KJ, Reighard DA, Misra SP, Cotter LA, Cass SP, Wilson TD, Yates BJ. Effects of postural changes and removal of vestibular inputs on blood flo to and from the hindlimb of conscious felines. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1777–R1784. doi: 10.1152/ajpregu.00551.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]