Abstract
There has been considerable interest in the use of creatine (Cr) supplementation to treat neurological disorders. However, in contrast to muscle physiology, there are relatively few studies of creatine supplementation in the brain. In this report, we use high-field MR 31P and 1H spectroscopic imaging of human brain with a 7-day protocol of oral Cr supplementation to examine its effects on cerebral energetics (phosphocreatine, PCr; ATP) and mitochondrial metabolism (N-acetyl aspartate, NAA; and Cr). We find an increased ratio of PCr/ATP (day 0, 0.80 ± 0.10; day 7, 0.85 ± 09), with this change largely due to decreased ATP, from 2.7 ± 0.3 mM to 2.5 ± 0.3 mM. The ratio of NAA/Cr also decreased (day 0, 1.32 ± 0.17; day 7 1.18 ± 0.13), primarily from increased Cr (9.6 ± 1.9 to 10.1 ± 2.0 mM). The Cr-induced changes significantly correlated with the basal state, with the fractional increase in PCr/ATP negatively correlating with the basal PCr/ATP value (R = −0.74, P < 0.001). As NAA is a measure of mitochondrial function, there was also a significant negative correlation between basal NAA concentrations with the fractional change in PCr and ATP. Thus healthy human brain energetics is malleable and shifts with 7 days of Cr supplementation, with the regions of initially low PCr showing the largest increments in PCr. Overall, Cr supplementation appears to improve high-energy phosphate turnover in healthy brain and can result in either a decrease or an increase in high-energy phosphate concentrations.
Keywords: high-energy phosphates, brain, N-acetyl aspartate, metabolism
SEVERAL GROUPS HAVE SHOWN that oral creatine (Cr) supplementation can affect energy dynamics and performance in muscle (4, 17, 22) and brain (7, 12, 16, 24). In both of these tissues where energy demand and ATP consumption may be temporally dynamic, relatively higher concentrations of creatine and phosphocreatine are found, presumably for its physiological role as a buffer for ATP. Also found in these tissues are mitochondrial and cytosolic creatine kinase isoforms that serve to transfer high-energy phosphate equivalents between the sites of production and consumption. Thus, as suggested in muscle (3, 18), Cr supplementation may enhance both the spatial and chemical buffering of high-energy phosphates, and this effect may contribute to the functional consequences of supplementation. For example, Watanabe et al. (24) used a double-blind placebo-controlled study of short-period creatine supplementation (5 days) to affect cognitive performance, finding that the cognitive decline associated with mental fatigue was reduced with a brief period of creatine supplementation (8 g/day for 5 days). Although these considerations have contributed to the therapeutic use of creatine in neurological diseases in which metabolic and mitochondrial dysfunction is pertinent [e.g., Huntington's disease, amyotrophic lateral sclerosis (8, 11)], what governs the extent of Cr-induced change in the brain has not been evaluated. This may be important, as it may provide an estimate of who may benefit from creatine, as well as provide insight as to the malleability of brain energetics.
In this study, we examine the metabolic and energetic effects of creatine supplementation in human brain using in vivo quantitative 1H and 31P spectroscopic imaging. We determine hippocampal measurements of total creatine, N-acetyl aspartate (NAA) and their spectroscopic ratio NAA/Cr. NAA has been shown by Bates et al. (2) and Patel and Clark (15) to be sensitive to oxygen consumption and neuronal ATP synthesis, with its synthesis well correlated with mitochondrial oxidation and has thus been interpreted as a measure of neuronal mitochondrial function. NAA has thus been substantively studied with numerous neurological disorders finding significantly depressed values for NAA/Cr (1, 10, 19). In the present 31P studies, we determine the in vivo concentrations of the high-energy phosphates phosphocreatine (PCr), ATP, and their raw spectroscopic ratio. With these measurements, we studied n = 12 healthy volunteers to evaluate the basal state and changes in these compounds before and after a 7-day period of 20 g/day creatine supplementation.
METHODS
Healthy volunteers (n = 12; 5 women; mean age 32 ± 9 years) and not previously taking creatine within the past year were studied. Studies were performed at baseline and after 7 days of creatine supplementation at 20 g/day. All volunteers were asked to take creatine monohydrate (Avicena Group, Palo Alto CA) at a dosing of 10 g twice daily as mixed in a juice drink. Volunteers were asked to maintain a consistent daily schedule of work/activity so as to optimize data consistency. All volunteers were MR screened before participation so as to be familiar with the magnet environment. All studies were performed with the approval of the local Institutional Review Board for compliance with ethical research in humans.
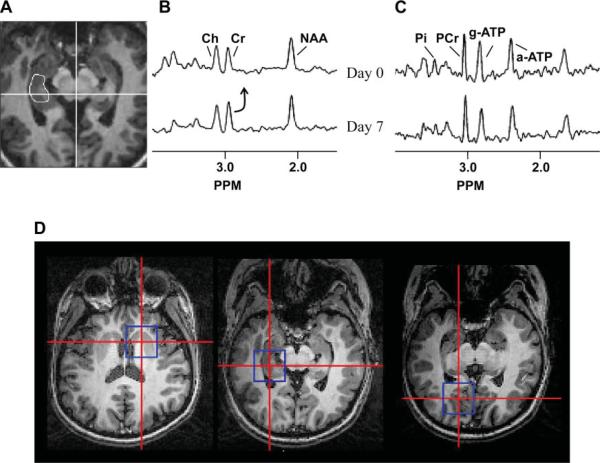
All imaging data were acquired using a high-field (4 T) human whole body MR system (Varian Inova, Vernon Hills, IL) with volume transverse electromagnetic detectors (1H and double tuned, 31P-1H) (20). For each volunteer, the two MR studies (1H, 31P) were performed sequentially within a 3-h period. In both studies, the imaging plane was angulated along the planum temporale. The 1H spectroscopic imaging data were acquired using a three-dimensional localized adiabatic refocusing sequence (10), TE/TR 72/2000 with two dimensions of 24 × 24 spatial encoding over a field of view (FOV) of 19.2 × 19.2 cm (nominal voxel size of 0.64 cc, 20-min acquisition). Quantification of the 1H spectra was performed relative to cerebrospinal fluid in the superior cistern measured in a high-resolution proton density image. The proton density image then was referenced to a nonsuppressed water spectroscopic image [a modification of the methods described by Pan et al. (14)]. For both the 1H and 31P data, quantification was performed accounting for tissue volume, coil loading, and relaxation. Tissue volumes were determined from semiautomated image segmentation of quantitative T1-weighted images. The typical duration of the 1H study was 65 min. Analysis was performed as previously described in each hippocampus with reproducibility of these measurements between 3 and 9% (6). Fig. 1A shows a typical example of the hippocampal locations and spectra.
Fig. 1.
Scout showing the location of the right 1H hippocampal measurement (A), 1H spectra (B) and 31P spectra (C) are shown from a single volunteer. The baseline data set (top spectra) are compared with the day 7 data set (bottom spectra) for both 1H and 31P acquisitions. From the same volunteer, scouts (D) are shown with the locations of the 31P data, striatum (left), medial temporal lobe (middle) and parasagittal occipital lobe (right). PCr, phosphocreatine; Cr, creatine; NAA, N-acetyl asparate; and Ch, choline.
31P spectroscopic images were collected using a one-pulse acquisition and three dimensions of phase encoding with spherical sampling 13 × 13 × 13, FOV = 24 × 24 × 24 cm). To provide anatomical reference positions, high-contrast whole brain images were acquired. Data were analyzed using in-house written software spectroscopic imaging display with a single-voxel reconstruction previously described (5, 10), in which voxels are anatomically positioned using the infrared gradient echo scout image resolution. Reproducibility of the 31P data acquisition is 10%. Spatial processing was performed with a Hanning spatial filter, which resulted in a negligible contribution of skeletal muscle tissue volume to the hippocampal voxels. The typical duration of the 31P study was 75 min. Regional analysis was performed on three locations (Fig. 1B), mesial temporal lobe (left and right hippocampus and surrounding temporal lobe), neocortical tissue (a mean of measurements made from the left and right parasagittal occipital lobe), and the striatum (subcortical tissue, left and right caudate and putamen). The 1H and 31P magnetic resonance spectroscopy data were acquired as separate studies; their analyses for ratios and concentrations were also independent. The calculation of ADP concentrations, however, does inter-relate the 1H and 31P data (23), as it is based on the creatine kinase equilibrium, which uses the total creatine values from 1H data and the high-energy phosphate values from 31P data. ADP was, therefore, only determined from medial temporal lobe data.
All volunteers were studied in the morning after an overnight fast, with the 31P and 1H studies performed before and following breakfast, respectively. Volunteers were permitted to sleep during MR data acquisition.
Group data were compared using a paired Student's two-tailed t-test at P < 0.05. Pearson correlations were performed between parameters acquired at day 0 (D0) with their subsequent fractional [day 7 (D7)−D0]/D0 changes with significance taken at P < 0.05. As ad hoc measurements based on hypothesis testing, cross-correlations were also tested with significance taken at P < 0.05.
RESULTS
Group results between D0 and D7
As a group, volunteers gained a small amount of weight (1.1 ± 0.5 lb) over the 7-day period. Complaints were all mild in severity, consisting of diarrhea (n = 2), muscle cramps (n = 3), and headache (n = 2). No major changes in laboratory values of renal or liver function were noted. None of the volunteers needed to discontinue the study due to difficulties with creatine.
Figure 1 shows typical 1H and 31P data acquired from a single volunteer. The approximate tissue sizes of the spectroscopic measurements are shown in the scouts, with the smallest voxel size achieved with the 1H measurements.
Tables 1 and 2 show the 1H and 31P metabolite ratios and concentrations of the measured compounds, respectively. On D0, the mean NAA/Cr was 1.32 ± 0.17, which declined to 1.18 ± 0.13 by D7 (P < 0.001). Although an increase in Cr (D0, 9.6 ± 1.9; D7, 10.1 ± 2.0, P < 0.05) was the main cause for the lowered ratio, it is notable that NAA/Ch (NAA:choline ratio) also declined by D7. With quantification, there was a trend for a decline in NAA (P = 0.11), but this was not significant, while choline did not change.
Table 1.
Hippocampal 1H measurements
| NAA/Cr |
NAA/Ch |
NAA |
Cr |
Ch |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| D0 | D7 | D0 | D7 | D0 | D7 | D0 | D7 | D0 | D7 | |
| Mean | 1.32 | 1.18 | 1.43 | 1.32 | 9.3 | 8.9 | 9.6 | 10.1 | 2.3 | 2.4 |
| SD | 0.17 | 0.13 | 0.26 | 0.18 | 1.3 | 1.8 | 1.9 | 2.0 | 0.5 | 0.5 |
Values are means ± SD. Bolded values indicate significantly difference between day 0 (D0) and day 7 (D7), two tailed paired t-test; P < 0.05. NAA, N-acetylaspartate; Cr, creatine; Ch, chorine.
Table 2.
31P measurements
| PCr/ATP |
PCr, mM |
ATP, mM |
Calculated ADP, μM |
|||||
|---|---|---|---|---|---|---|---|---|
| D0 | D7 | D0 | D7 | D0 | D7 | D0 | D7 | |
| Occipital lobe | 0.81±0.11 | 0.82±0.10 | 3.2±0.4 | 3.1±0.3 | 2.6±0.3 | 2.5±0.3 | ||
| Medial temporal lobe (hippocampus) | 0.80±0.10 | 0.85±0.09 | 3.3±0.4 | 3.3±0.3 | 2.7±0.3 | 2.5±0.3 | 27±8 | 28±10 |
| Striatum | 0.69±0.10 | 0.74±0.09 | 2.5±0.5 | 2.5±0.3 | 2.4±0.3 | 2.2±0.3 | ||
Values are means ± SD. Bolded values indicate significantly difference between D0 and D7, two-tailed t-test P < 0.05.
As a group, significant increases in PCr/ATP were found in the medial temporal lobe and striatum (in the temporal lobe, D0, 0.80 ± 0.10; D7, 0.85 ± 0.09; P < 0.01). Although measurement error is approximately the same in the occipital lobe relative to the subcortical locations, the occipital change in the ratio was less. In these two regions, a group decline in ATP (temporal lobe, D0, 2.7 ± 0.03; and D7, 2.5 ± 0.3, P < 0.02) appeared to be driving the increased ratio with no major increases seen in PCr.
Correlations between basal state (D0) with extent of change 31P data
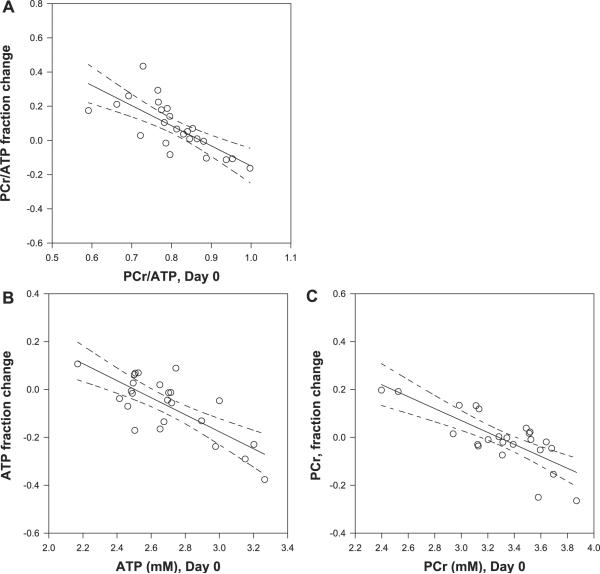
There was variability in the energetic changes and uptake of creatine. In considering what may be some of the causes of this variability, we considered whether interindividual variability in basal state may be contributing. Figure 2A shows that the ratio of medial temporal lobe PCr/ATP at baseline is significantly correlated with the fractional decline in PCr/ATP over the 7-day period, R = −0.74, P < 0.001. (If the null hypothesis were true with no change between D0 and D7, the correlation coefficient should be zero). In particular, those with the lowest basal PCr/ATP generated the greatest increase in PCr/ATP over the 7 days, while regions with a higher basal PCr/ATP value showed negligible change. Similar results were found for the striatum and occipital lobe (R = −0.74, P < 0.001, R = −0.57, P < 0.05 respectively, data not shown).
Fig. 2.
Basal PCr/ATP correlates well with creatine-induced changes in PCr/ATP (R = − 0.74, P < 0.001; A), with the individual components ATP (B) and PCr (C) show similar correlations, ATP, R = −0.71, P < 0.001; PCr R = −0.72, P < 0.001. Shown are the linear regressions with 95% confidence intervals on the predicted changes in PCr/ATP or NAA/Cr. All of these data were acquired from the medial temporal lobe.
The changes in PCr/ATP may be resulting from changes in PCr and/or ATP. Figure 3 shows that both the basal PCr and ATP correlated significantly and negatively with their fractional changes, with R = −0.79 P < 0.001; R = −0.76 P < 0.001, respectively. A linear regression of these data with the independent variable being D0 PCr and D0 ATP demonstrated x-intercepts (“crossover” points) at 3.29 mM and 2.51 mM, that is, above these concentrations, PCr and ATP decline with creatine supplementation; below these concentrations, they increase. As above, similar negative correlations were found in the striatum and occipital lobe.
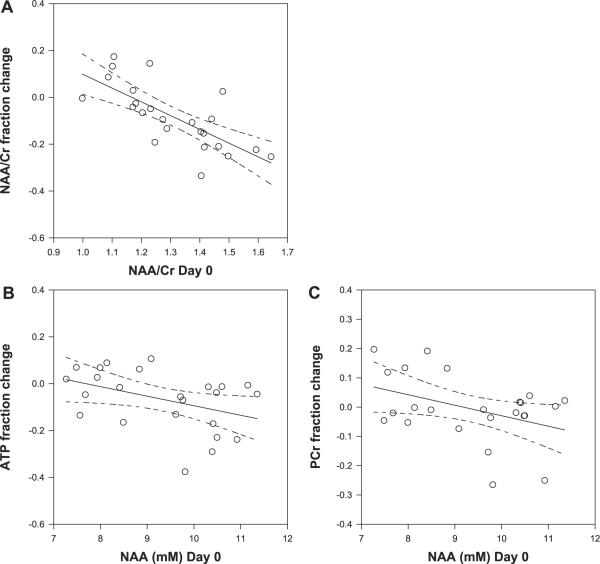
Fig. 3.
Basal NAA/Cr correlates with fractional change in NAA/Cr (A), R = −0.73 P < 0.001. Basal NAA correlates with fractional changes in ATP R = −0.42 P < 0.05 (B) and with PCr R = −0.41 P < 0.05 (C). Shown are the linear regression lines with the 95% confidence intervals with the predicted changes.
Correlations between basal state (D0) with extent of change: 1H and 31P data
Similarly, the basal hippocampal NAA/Cr also correlated significantly with the fractional change in hippocampal NAA/Cr (R = −-0.73 P < 0.001 Fig. 3A). Specifically, regions with lower basal NAA/Cr demonstrated increases in NAA/Cr, while regions with higher basal NAA/Cr showed declines. Although it is possible that this ratio shift is due to creatine changes directly, neither NAA nor Cr demonstrated correlations with their respective changes. However, with NAA as a measure of neuronal mitochondrial function, we considered that baseline D0 NAA would influence the extent of Cr-induced high-energy phosphate changes. Thus an ad hoc comparison of hippocampal D0 NAA with fractional changes in medial temporal lobe PCr and ATP were both found to correlate negatively, R = −0.42 P < 0.05 and R = −0.41 P < 0.05, respectively. No such correlations were seen with D0 Cr.
DISCUSSION
Oral creatine supplementation affects high-energy phosphates and NAA, Cr values
Consistent with literature data (12), we find that a 7-day protocol of oral creatine supplementation results in an increase in PCr/ATP. The spectroscopic imaging results demonstrate that the increase is most clearly observed in deeper gray matter structures, including the medial temporal lobe (hippocampus) and subcortical nuclei, while occipital lobe tissue showed similar but nonsignificant changes (Table 2). As a group, quantification of these changes showed that the major change was a decline in ATP, while there was relatively little group change in PCr. At the same time, in the hippocampus, a significant decline in NAA/Cr was seen, also consistent with literature (7, 21). As expected, the major cause for the change in NAA/Cr is an increase in creatine; however, there was a tendency for NAA to decline as well (Table 1).
Extent of high-energy phosphate changes depend on the basal state
The cerebral effect of creatine supplementation varied between individuals and also varied depending on the local energetic state of the brain. We found that those regions with low PCr and ATP values prior to supplementation increased their concentrations, while regions with high PCr, ATP values decreased their concentrations. Because of a difference in the y-intercept (crossover points) in PCr and ATP compared with their mean values (e.g., the measured mean ATP level of 2.68 mM is larger than the ATP crossover value of 2.51 mM, Fig. 2, B and C), as a group, ATP concentrations dropped while PCr concentrations stayed roughly the same. Consequently, the ratio of PCr/ATP increased with supplementation, but also as a function of baseline PCr/ATP (R = −0.74 P < 0.001), the largest increases occurring with lower D0 PCr/ATP.
NAA and high-energy phosphates
As a measure of neuronal mitochondrial function, we anticipated that similar findings would occur with NAA. Our group previously used quantitative MR spectroscopic imaging to find that interindividual differences in NAA concentrations correlated positively with calculated ADP (13), a finding that we interpreted in terms of the relation between mitochondrial function and a key regulator of oxidative function. The present D0 data reiterate this finding (data not shown); furthermore, as an ad hoc examination, we found that the Cr-induced fractional changes in ADP concentrations positively correlated with the fractional change in NAA concentrations, R = +0.49, P < 0.02 (Fig. 4). One interpretation of this is that in the healthy brain, ADP regulates NAA, i.e., that cellular energetic state regulates mitochondrial function as suggested earlier.
Fig. 4.
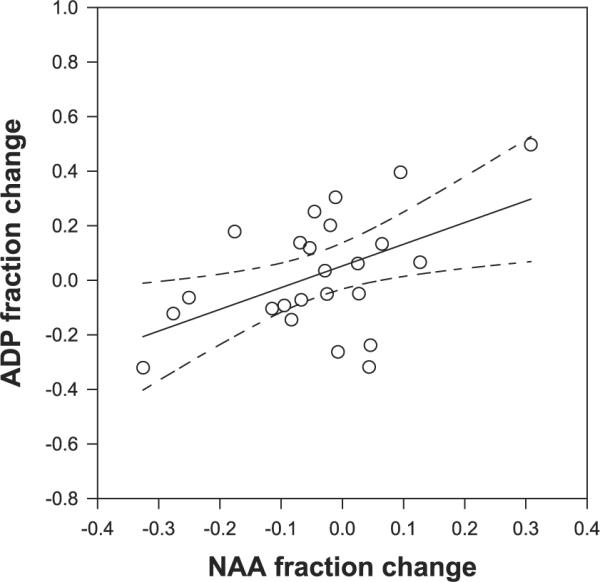
The changes in NAA and ADP induced by the 7-ay protocol correlate positively at R = +0.49, P < 0.02.
Conversely, given the negative correlations between D0 NAA with fractional changes in PCr and ATP (Fig. 3, B and C), NAA may influence the extent of Cr-mediated effect on high-energy phosphates, with a greater D0 NAA resulting in larger high-energy phosphate declines. However, given the relationship of NAA with ADP, it is likely that the multiple changes in NAA, PCr, and ATP are concerted events occurring during the 7-day protocol.
Creatine supplementation in brain and muscle
Integrating these data, several findings are notable. First, ATP, PCr, and NAA concentrations and their ratios in the healthy brain are not static. Second, oral creatine supplementation modifies the available pool of high-energy phosphates in a relatively systematic manner that is not solely based on the creatine kinase equilibrium. Given this equilibrium,
it is possible that increasing Cr could have several different outcomes. However, in tissues with low initial PCr/ATP, increasing available Cr does not increase PCr or ADP (or NAA, which appears to be linked to ADP), but rather decreases ATP, with variably decreased ADP (and NAA). In tissues with high initial PCr/ATP, increasing available Cr results in small increases in ATP, with variably increased ADP (and NAA). In both of these situations, Cr may be described as improving high-energy phosphate turnover, either from enhancing efficiency of coupling between production and consumption [mitochondria and cytosol, as suggested originally in muscle by (3, 18)], or from increasing production and consumption directly. These energetic effects may contribute to the apparent improvement of cognitive performance in the functional studies of Watanabe et al. (24) and Rae et al. (16). The situation of enhanced efficiency characterizes the predominant situation in the present data, as the majority of the data demonstrated a decline in ATP. It should be noted in general, however, that the interpretation of these data assumes the premise that the average amount of cerebral work performed by our volunteers was not different between D0 and D7 (none of our volunteers experienced major environmental change or stressors during this period).
Importantly, while these data demonstrate a systematic effect on creatine use in the brain, what is guiding these effects is unclear. One possibility is that the adjustments in high-energy phosphates adhere to a preexisting level of free energy of ATP hydrolysis. This will need to be examined more carefully with studies that include quantification of inorganic phosphate (Pi) and direct calculation of the free energy. Notably, although the present data acquisition was designed to robustly examine PCr and ATP, the accuracy of the Pi resonance was relatively less. The concentration of D0 Pi was 0.85 ± 0.28 mM, and ad hoc consideration of the Pi resonance, however, did not reveal any specific relationships. Better methods (such as using a spin echo acquisition and/or higher magnetic field) exist to specifically target Pi and may be helpful for future studies.
Our present data are similar to muscle studies by Rawson et al. (17), who found that tissues with preexisting low levels of PCr showed greater creatine-mediated PCr increases (i.e., a negative correlation). Notably, however, in that cohort, no clear decline in muscle PCr was seen (range of increase was 2–9 mM). This contrasts to the present study in which we have seen both increases and decreases in PCr, ATP, and NAA. The etiology of the difference between muscle and brain may be complex, possibly a consequence of restricted brain osmolarity, which is much less of an issue for muscle.
For neurological disorders that are manifest with depressed high-energy phosphates, creatine supplementation may be constructive. Nonetheless, it should be noted that depending on the initial tissue state, it may have the appearance of dropping high-energy phosphate levels rather than increasing them, this because (for presumably the same degree of cerebral work) Cr supplementation allows more efficient coupling between mitochondrial production and energy consumption and thus allows the same functionality at lower high-energy phosphate concentrations. However, it is in this context, under conditions in which immediate energetic supply may be limited and mitochondrial damage may be self-propagating (for example, such as that seen in seizures), that Cr may change the probabilities of neuronal injury. (Table 2)
ACKNOWLEDGMENTS
The authors thank Dr. H. Hetherington for helpful discussions and L. Popivker for administrative assistance.
GRANTS This work is funded by: NIH NCCAM R21 AT0002984 (to J. W. Pan and K. Takahashi).
REFERENCES
- 1.Abe K, Takanashi M, Watanabe Y, Tanaka H, Fujita N, Hirabuki N, Yanagihara T. Decrease in N-acetylaspartate/creatine ratio in the motor area and the frontal lobe in amyotrophic lateral sclerosis. Neuroradiology. 2001;43:537–541. doi: 10.1007/s002340000521. [DOI] [PubMed] [Google Scholar]
- 2.Bates TE, Strangward M, Keelan J, Davey GP, Munro PM, Clark JB. Inhibition of N-acetylaspartate production: implications for HMRS studies in vivo. Neuroreport. 1996;7:1397–1400. [PubMed] [Google Scholar]
- 3.Bessman SP, Geiger PJ. Transport of energy in muscle: the phosphorylcreatine shuttle. Science. 1981;211:448–452. doi: 10.1126/science.6450446. [DOI] [PubMed] [Google Scholar]
- 4.Casey A, Constantin-Teodosiu D, Howell S, Hultman E, Greenhaff PL. Creatine ingestion favorably affects performance, and muscle metabolism during maximal exercise in humans. Am J Physiol Endocrinol Metab. 1996;271:E31–E37. doi: 10.1152/ajpendo.1996.271.1.E31. [DOI] [PubMed] [Google Scholar]
- 5.Chu WJ, Mason GF, Hetherington HP. Phosphorus metabolic differences in gray, and white matter: 31P MR spectroscopic imaging studies of human brain at 4.1 T. Proceedings of the Society of Magnetic Resonance 5th Annual Meeting; Berkeley, CA: International Society for Magnetic Resonance in Medicine; 1997. p. 1407. [Google Scholar]
- 6.Chu WJ, Pan C, Pan JW, Hetherington HP. Reproducibility of 1H MR spectroscopic imaging of the human hippocampus. Proceedings of the International Society of Magnetic Resonance Medicine, 12th Annual Meeting; Kyoto Japan. 2004. p. 105. [Google Scholar]
- 7.Dechent P, Pouwels PJ, Wilken B, Hanefeld F, Frahm J. Increase of total creatine in human brain after oral supplementation of creatinemonohydrate. Am J Physiol Regul Integr Comp Physiol. 1999;277:R698–R704. doi: 10.1152/ajpregu.1999.277.3.R698. [DOI] [PubMed] [Google Scholar]
- 8.Dedeoglu A, Kubilus JK, Yang L, Ferrante KL, Hersch SM, Beal MF, Ferrante RJ. Creatine therapy provides neuroprotection after onset of clinical symptoms in Huntington's disease transgenic mice. J Neurochem. 2003;85:1359–1367. doi: 10.1046/j.1471-4159.2003.01706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hetherington H, Kuzniecky R, Pan J, Mason G, Morawetz R, Harris C, Faught E, Vaughan T, Pohost G. Proton nuclear magnetic resonance spectroscopic imaging of human temporal lobe epilepsy at 4.1 T. Ann Neurol. 1995;38:396–404. doi: 10.1002/ana.410380309. [DOI] [PubMed] [Google Scholar]
- 10.Hetherington HP, Kim JH, Pan JW, Spencer DD. 1H and 31P spectroscopic imaging of epilepsy: spectroscopic and histological correlations. Epilepsia. 2004;45(S4):17–23. doi: 10.1111/j.0013-9580.2004.04004.x. [DOI] [PubMed] [Google Scholar]
- 11.Klivenyi P, Ferrante RJ, Matthews RT, Bogdanov MB, Klein AM, Andreassen OA, Mueller G, Wermer M, Kaddurah-Daouk R, Beal MF. Neuroprotective effects of creatine in a transgenic animal model of amyotrophic lateral sclerosis. Nat Med. 1999;5:347–350. doi: 10.1038/6568. [DOI] [PubMed] [Google Scholar]
- 12.Lyoo IK, Kong SW, Sung SM, Hirashima F, Parow A, Hennen J, Cohen BM, Renshaw PF. Multinuclear magnetic resonance spectroscopy of high-energy phosphate metabolites in human brain following oral supplementation of creatine-monohydrate. Psychiatry Res. 2003;123:87–100. doi: 10.1016/s0925-4927(03)00046-5. [DOI] [PubMed] [Google Scholar]
- 13.Pan JW, Takahashi K. Inter-dependence of NAA and high energy phosphates in human brain. Ann Neurol. 2005;57:92–97. doi: 10.1002/ana.20317. [DOI] [PubMed] [Google Scholar]
- 14.Pan JW, Twieg DB, Hetherington HP. Quantitative spectroscopic imaging of the human brain. Magn Reson Med. 1998;40:363–369. doi: 10.1002/mrm.1910400305. [DOI] [PubMed] [Google Scholar]
- 15.Patel TB, Clark JB. Synthesis of N-acetyl-l-aspartate by rat brain mitochondria and its involvement in mitochondrial/cytosolic carbon transport. Biochem J. 1979;184:539–546. doi: 10.1042/bj1840539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rae C, Digney AL, McEwan SR, Bates TC. Oral creatine monohydrate supplementation improves brain performance: a double-blind, placebo-controlled, cross-over trial. Proc R Soc Lond B Biol Sci. 2003;270:2147–2150. doi: 10.1098/rspb.2003.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rawson ES, Clarkson PM, Price TB, Miles MP. Differential response of muscle phosphocreatine to creatine supplementation in young, and old subjects. Acta Physiol Scand. 2002;174:57–65. doi: 10.1046/j.1365-201x.2002.00924.x. [DOI] [PubMed] [Google Scholar]
- 18.Saks VA, Rosenshtraukh LV, Smirnov VN, Chazov EI. Role of creatine phosphokinase in cellular function, and metabolism. Can J Physiol Pharmacol. 1978;56:691–706. doi: 10.1139/y78-113. [DOI] [PubMed] [Google Scholar]
- 19.Suhy J, Rooney WD, Goodkin DE, Capizzano AA, Soher BJ, Maudsley AA, Waubant E, Andersson PB, Weiner MW. 1H MRSI comparison of white matter, and lesions in primary progressive and relapsing-remitting MS. Mult Scler. 2000;6:148–155. doi: 10.1177/135245850000600303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vaughan T, Hetherington HP, Harrison JG, Out JO, Pan JW, Noa PJ, den Hollander JA, Pohost GM. High-frequency coils for clinical NMR imaging, and spectroscopy. Physcia Medica. 1993;9:147–153. [Google Scholar]
- 21.Vielhaber S, Kaufmann J, Kanowski M, Sailer M, Feistner H, Tempelmann C, Elger CE, Heinze HJ, Kunz WS. Effect of creatine supplementation on metabolite levels in ALS motor cortices. Exp Neurol. 2001;172:377–382. doi: 10.1006/exnr.2001.7797. [DOI] [PubMed] [Google Scholar]
- 22.Volek JS, Rawson ES. Scientific basis, and practical aspects of creatine supplementation for athletes. Nutrition. 2004;20:609–614. doi: 10.1016/j.nut.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 23.Wackerhage H, Hoffmann U, Essfeld D, Leyk D, Mueller K, Zange J. Recovery of free ADP, Pi, and free energy of ATP hydrolysis in human skeletal muscle. J Appl Physiol. 1998;85:2140–2145. doi: 10.1152/jappl.1998.85.6.2140. [DOI] [PubMed] [Google Scholar]
- 24.Watanabe A, Kato N, Kato T. Effects of creatine on mental fatigue, and cerebral hemoglobin oxygenation. Neurosci Res. 2002;42:279–285. doi: 10.1016/s0168-0102(02)00007-x. [DOI] [PubMed] [Google Scholar]