Abstract
Objective
The purpose of this study was to evaluate clinical outcomes associated with the initiation of treatment for urgency-predominant incontinence in women diagnosed by a simple 3-item questionnaire.
Study Design
We conducted a multicenter, double-blinded, 12-week randomized trial of pharmacologic therapy for urgency-predominant incontinence in ambulatory women diagnosed by the simple 3-item questionnaire. Participants (N = 645) were assigned randomly to fesoterodine therapy (4-8 mg daily) or placebo. Urinary incontinence was assessed with the use of voiding diaries; postvoid residual volume was measured after treatment.
Results
After 12 weeks, women who had been assigned randomly to fesoterodine therapy reported 0.9 fewer urgency and 1.0 fewer total incontinence episodes/day, compared with placebo (P ≤ .001). Four serious adverse events occurred in each group, none of which was related to treatment. No participant had postvoid residual volume of ≥250 mL after treatment.
Conclusion
Among ambulatory women with urgency-predominant incontinence diagnosed with a simple 3-item questionnaire, pharmacologic therapy resulted in a moderate decrease in incontinence frequency without increasing significant urinary retention or serious adverse events, which provides support for a streamlined algorithm for diagnosis and treatment of female urgency-predominant incontinence.
Keywords: antimuscarinic therapy, diagnostic algorithm, fesoterodine, urgency incontinence
Urinary incontinence affects up to one-third of adult women and is associated with depression, social isolation, physical inactivity, and institutionalization.1-4 Despite recommendations that nonspecialist clinicians assume a greater role in diagnosing and treating incontinence,5,6 rates of diagnosis and treatment outside of urology or urogynecology remain low.7-11
One obstacle to the diagnosis and treatment of female incontinence is that professional organizations traditionally have recommended an extended evaluation to distinguish between the 2 most common types of incontinence in women: urgency and stress.12,13 In addition to a clinical history and urinalysis test, this evaluation includes a voiding diary, neurologic examination, pelvic examination, measurement of postvoid residual (PVR) volume, and cough stress test.12,13 Because there are approved medications to treat urgency but not stress incontinence,12,13 classification of incontinence has implications for treatment. However, the traditional extended evaluation to classify incontinence in women is not performed easily in primary care or general gynecology settings, which creates a barrier to treatment.16
To address this problem, a simple 3-item, self-administered questionnaire (the 3 Incontinence Questions [3IQ]) was developed to identify and classify female incontinence (Appendix, Supplementary Figure). In a sample of 301 generally healthy women with ongoing incontinence symptoms, the 3IQ demonstrated good sensitivity and specificity in distinguishing between urgency and stress incontinence, compared with an extended evaluation.16 To examine the clinical consequences of using the 3IQ to guide treatment, we sought to examine the efficacy and safety of initiating pharmacologic therapy for urgency incontinence in women using a streamlined algorithm that was based on the 3IQ.
Materials and Methods
Study population
Participants were ambulatory women who were ≥18 years old who were recruited from the general community surrounding 13 clinical sites in the United States (Supplementary Table 1). Women who reported clinically frequent incontinence during preliminary telephone screening (ie, ≥7 incontinence episodes per week in the past 3 months) were invited to come to an in-person visit to complete the 3IQ on paper to self-diagnose incontinence. Those who self-diagnosed as having urgency-predominant incontinence on the 3IQ (ie, those who indicated that they had incontinence that occurred most often when they “had the urge or the feeling that [they] needed to empty your bladder but could not get to the toilet fast enough”) were eligible to continue. Therefore, the study population consisted of women who indicated that they had either isolated urgency incontinence or mixed incontinence that was associated predominantly with urgency. Women completed the 3IQ on their own and did not receive assistance from research staff in diagnosing or classifying their incontinence. Consistent with the proposed use of the 3IQ in clinical practice,16 women subsequently underwent dipstick urinalysis testing to rule out urinary-tract infection or hematuria before enrollment; those who tested positive could return after completing treatment. Self-report bladder diaries were used to document baseline frequency of incontinence; those women whose diaries confirmed that they had at least 3 incontinence episodes in 3 days were eligible to continue.
Other eligibility criteria were selected to define a community-dwelling sample of women who would be considered appropriate for evaluation and treatment in primary care. Specifically, women were excluded if they self-reported complex medical histories that automatically would require a specialist evaluation for incontinence, such as antiincontinence surgery in the past 5 years, other pelvic surgery in the past 6 months, >3 urinary tract infections in the past year, lower urinary tract or rectal fistula, interstitial cystitis, symptomatic pelvic organ prolapse, urogenitalcancer or radiation, congenital abnormality that leads to incontinence, or major neurologic disorder.
Because of the pharmacologic intervention that was used in this study, participants could not have specific contraindications to fesoterodine therapy (such as urinary or gastric retention, uncontrolled narrow-angle glaucoma, myasthenia gravis, severe ulcerative colitis, clinically significant hepatic or renal disease, toxic megacolon, potent CYP3A4 inhibitor treatment in the last 2 weeks, or pregnancy or nursing).
Randomization, masking, and treatments
Eligible women were allocated randomly in a 1:1 ratio to receive 12 weeks of pharmacologic treatment with flexible-dose fesoterodine therapy (Toviaz; Pfizer, Inc, New York, NY) 4-8 mg (fesoterodine group) or an identical placebo pill (placebo group) daily. Randomization was performed by computer in permuted blocks of 2-4 without stratification for clinical site. Active and placebo tablets were prepared by the University of California San Francisco pharmacy, where they were labeled by a pharmacist with randomization numbers and then distributed to clinical sites. Participants, clinical personnel, and statistical staff were masked to treatment assignment, and no unmasking occurred during the trial. All participants were asked to forgo other pharmacologic incontinence treatments and pelvic floor or bladder physical therapy for the 12-week trial period to avoid contamination of treatment effects.
According to previously established protocols for participant-directed dosing,17 participants were started initially on either fesoterodine 4 mg or an identical placebo pill daily. At their 2-week telephone call and their 4-week follow-up visit, women were offered the option of increasing their dose to fesoterodine 8 mg or an identical placebo daily. At their 8-week telephone call, they were invited to readjust their dose to a maximum of 8 or minimum of 4 mg daily.
Clinical efficacy outcomes
All clinical efficacy outcomes were assessed at baseline, 4 weeks, and 12 weeks. The primary efficacy outcome was a 12-week change in the average number of self-reported urgency incontinence episodes per day that were documented by a validated 3-day voiding diary in which women recorded all incontinence and voiding episodes and indicated which episodes were associated with a sensation of urgency (rated as none, mild, moderate, or severe).18,19 Secondary efficacy outcomes included a 12-week change in total incontinence frequency, diurnal and nocturnal voiding frequency, and frequency of voiding episodes that were associated with at least a moderate or severe sensation of urgency, also recorded in the voiding diary.
Additional secondary efficacy outcomes also included 12-week improvement in scores on validated questionnaires that assess the self-reported impact of women's bladder symptoms: (1) the Overactive Bladder Questionnaire,20 a 33-iteminstrument that assesses symptom impact with a 100-point scale; (2) the Patient Perception of Bladder Condition,20,21 a single-item that assesses bladder problems with a 6-point Likert scale; (3) and the Patient Perception of Urgency Scale,22 a single-item measure that assesses perception of urinary urgency with a 3-point Likert scale.
Safety monitoring
Adverse events were assessed at all follow-up assessments at 2, 4, 8, and 12 weeks by asking participants to report any negative changes in their health. Adverse events that involved self-reported dry mouth, constipation, drowsiness, tachycardia, or urinary hesitancy or retention were classified as “potentially associated with antimuscarinic therapy.” Adverse events were considered to be “severe” if they prevented women from participating in any daily activities. Serious adverse events were defined as those that resulted in death, disability, or hospitalization. For all serious adverse events, site investigators used a standardized attribution scale to rate the likelihood of relationship to treatment.
Measurement of PVR volume was performed by bladder ultrasound scanning or catheterization at 12 weeks or early termination to provide objective assessment of posttreatment urinary retention. The protocol required women with a posttreatment PVR volume of ≥250 mL (confirmed by repeat assessment) to undergo an extended evaluation by a urology or urogynecology specialist at their site. Additionally, site investigators could refer participants for extended evaluations at any time in the event of a safety concern. The study was approved by the institutional review board at each site, and all participants provided written informed consent before enrollment. This trial was registered with clinicaltrials.gov (NCT00862745).
Statistical analysis
A sample size of 636 participants was estimated to provide 90% power to detect a net reduction in the primary outcome of urgency incontinence frequency with a 2 sample t test and the assumption of a 15% drop-out rate. The effect size was based on pooled data from 2 previous trials that reported an average effect size of 0.92 episodes per day and a standard deviation of 3.2 episodes per day.23,24
Baseline characteristics of participants in each treatment group were compared by the use of analysis of covariance models that were adjusted for clinical site. Changes in incontinence and voiding outcomes over 12 weeks were also examined using analysis of covariance, with adjustment for baseline values and site. Improvement in bladder-specific impact questionnaires was assessed by analysis of covariance to examine continuous change in questionnaires scores and by logistic regression to examine the odds of clinically meaningful improvement in scores (defined as an increase at least 10 points on the Overactive Bladder Questionnaire and at least 1 point on the Patient Perception of Bladder Condition and Patient Perception of Urgency Scale). For the primary analyses, only participants who took at least 1 dose of medication and provided follow-up data at ≥ 1 visits were included. Among those women who terminated the study early, the last postbaseline value of each outcome was carried forward to replace missing data at 12 weeks. Analyses were conducted without regard to adherence or final medication dosage.
Two additional sensitivity analyses were performed to address potential bias that was due to missing data through attrition or nonresponse. First, missing imputation analyses were performed on all participants with intent to treat.25 Twenty multiply-imputed datasets were created wit Twenty multiply-imputed datasets were created with the use of the Markov chain Monte Carlo method. Imputation models included demographics, treatment assignment, and interim (4-week) outcomes. Summary effect estimates and standard errors were computed by standard methods for imputed data. Second, complete case analyses were performed in which only participants with complete baseline and 12-week data were included.
Safety analyses compared the rates of (1) ≥1 adverse events, (2) ≥1 adverse events that were “potentially associated with antimuscarinic therapy,” (3) serious adverse events, and (4) posttreatment PVR volume of >250 mL between treatment groups, with the use of Fisher exact tests. All analyses were performed with SAS statistical software (version 9.1; SAS Institute Inc, Cary, NC).
Results
Participants and adherence
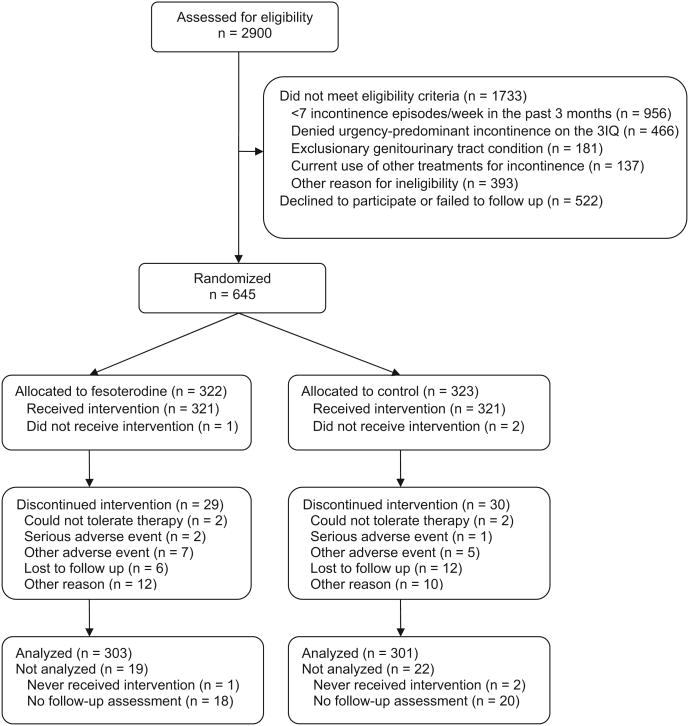
Between February 2009 and January 2010, 322 women were assigned randomly to receive fesoterodine therapy and 323 women were assigned randomly to receive placebo (Figure). All but 1 woman who was assigned to fesoterodine therapy and 2 women who were assigned to placebo took at least 1 dose of medication. Baseline characteristics of participants did not differ significantly by treatment assignment (Table 1). The mean (±SD) age of participants was 56 ± 14 years old; the mean baseline frequency of urgency incontinence was 3.9 ± 3.0 episodes per day.
Figure. Recruitment, randomization, and retention in the BRIDGES trial.
BRIDGES, BRinging simple urge Incontinence DiaGnosis and treatment to providerS; 3IQ, 3 incontinence questions.
Huang. Simplified diagnosis and treatment for urgency incontinence in women. Am J Obstet Gynecol 2012.
Table 1. Baseline characteristics of randomized participants by treatment assignment.
| Variable | Fesoterodine (n = 322) | Placebo (n = 323) |
|---|---|---|
| Demographic | ||
| Age, ya | 56.2 ± 14.7 | 55.9 ±14.2 |
| Race/ethnicity, nb | ||
| White | 215(66.8) | 212(65.6) |
| Black | 73 (22.7) | 71 (22.0) |
| Latina/Hispanic | 18(5.6) | 28 (8.7) |
| Asian/Pacific Islander | 9 (3.0) | 6(1.9) |
| Multiethnic/other | 7 (2.3) | 6(1.9) |
| Married, n (%) | 141 (43.8) | 133(41.2) |
| Clinical | ||
| Excellent or very good overall health, n (%)c | 255 (79.2) | 252 (78.0) |
| Previous childbirth: parity ≥1, n (%) | 256 (79.5) | 256 (79.3) |
| No current menstrual periods, n (%) | 229 (71.3) | 229(71.1) |
| History of hysterectomy, n (%) | 99 (30.7) | 95 (29.4) |
| Self-reported urinary tract infection in the past year, n (%) | 50(15.5) | 50(15.5) |
| Current cigarette smoking, n (%) | 48(14.9) | 44(13.7) |
| Current weekly alcohol consumption, n (%) | 96 (29.9) | 99 (30.7) |
| Current systemic hormone therapy, n (%) | 35 (7.8) | 27 (8.4) |
| Current stable diuretic therapy, n (%) | 46(14.3) | 52(16.1) |
| Incontinence/micturition | ||
| Urgency incontinence episodes per daya | 3.8 ± 2.9 | 4.0 ± 3.0 |
| Total incontinence episodes per daya | 4.5 ± 3.4 | 4.8 ± 3.4 |
| Diurnal voiding episodes per daya | 8.6 ± 2.7 | 8.8 ± 3.1 |
| Nocturnal voiding episodes per nighta | 1.3 ±1.3 | 1.2 ±1.2 |
| Moderate urgency-associated voids per daya,d | 7.5 ± 4.1 | 7.8 ± 4.5 |
| Severe urgency-associated voids per daya,e | 3.5 ± 3.3 | 3.7 ± 3.6 |
| Bladder-specific questionnaires | ||
| Overactive Bladder Questionnaire scorea,f | 36.4 ± 20.8 | 36.8 ±19.2 |
| Patient perception of Bladder Condition scoreg,h | 3.0 (3.0–4.0) | 3.0 (2.0–4.0) |
| Patient perception of Urgency Scale scoreg,i | 1.0 (1.0–2.0) | 1.0 (1.0–2.0) |
P > .05 for comparison of intervention and placebo groups for all variables listed.
Data are given as mean ± SD;
Participants self-reported their primary racial/ethnic group as white/caucasian, black/African American, Latina/Hispanic, Asian, Pacific Islander, Native American/American Indian, or multiethnic;
Assessed by asking women to rate their overall health as excellent, very good, good, fair, or poor;
Defined as voiding episodes that were associated with at least a “moderate” sensation of urgency on voiding diary;
Defined as voiding episodes that were associated with a “severe” sensation of urgency on voiding diary;
Scores range from 0-100; higher scores indicate more severe or bothersome overactive bladder symptoms20;
Data are given as median (interquartile range);
Scores range from 1–3, with higher scores indicating greater urgency.22
Huang. Simplified diagnosis and treatment for urgency incontinence in women. Am J Obstet Gynecol 2012.
Adherence to medication (which was assessed through pill counts) was similar in both treatment groups; 86.3% of women in the fesoterodine group and 87.0% in the placebo group completed 80% of the administrations (P= .82). Of those in the fesoterodine group, final medication dosage was confirmed for 281 women who returned their unused pills. Ninety women (32.0%) remained at 4 mg dose for the entire study; 152 women (54.1%) increased to 8 mg and remained at this dose throughout the study, and 39 women (13.9%) increased to 8 mg but returned to 4 mg before the end of the study. Twenty-nine women in the fesoterodine group and 30 women in the placebo group discontinued the study medication during the 12-week trial (Figure).
Clinical efficacy outcomes
Follow-up data on urinary incontinence were obtained for 303 women in the fesoterodine group (94.4%) and 301 women in the placebo group (93.2%). Women with missing follow-up data tended to be younger (mean age, 50 ± 18 vs 56 ± 14 years old; P = .04), nonwhite (58.5% vs 32.1%; P = .02), and unmarried (77.0% vs 56.1%; P = .005) but did not differ from women who contributed follow-up data with respect to other characteristics, which included baseline incontinence frequency or bladder-specific questionnaire scores.
Over the 12-week study period, urgency incontinence frequency decreased by 0.9 more episodes per day among women in the fesoterodine group vs the placebo group (P < .001; Table 2). Compared with placebo, women who were assigned to fesoterodine therapy also reported greater decreases in total incontinence frequency, diurnal and nocturnal voiding frequency, and frequency of voids that were associated with moderate or severe urgency, and also reported greater improvement in scores on bladder-specific impact questionnaires (Table 3). Treatment effects on incontinence frequency were not changed significantly in analyses that used multiple imputation to account for missing data in participants with intent to treat (Supplementary Table 2) or in complete case analyses in which women without complete data were excluded (Supplementary Table 3).
Table 2. Change in urinary incontinence and other voiding outcomes per day over 12 weeks by treatment assignment.
| Variable | Fesoterodine (n = 303) | Placebo (n = 301) | Least square mean difference (95% CI)a | P valuea |
|---|---|---|---|---|
| Urgency incontinence episodes per day | ||||
| Mean ± SD | −2.5 ± 2.5 | −1.8 ±2.7 | −0.9 (−1.2 to −0.5) | < .001 |
| Median (IQR) | −2.0 (−3.7 to −1.0) | −1.3 (−2.7 to −0.3) | ||
| Total incontinence episodes per day | ||||
| Mean ± SD | −2.9 ± 2.7 | −2.1 ± 2.9 | −1.0 (−1.3 to −0.6) | < .001 |
| Median (IQR) | −2.3 (−3.7 to −1.0) | −1.7 (−3.3 to −0.7) | ||
| Diurnal voiding episodes per day | ||||
| Mean ± SD | −0.8 ± 2.3 | −0.5 ± 2.3 | −0.4 (−0.7 to −0.1) | .03 |
| Median (IQR) | −1.0 (−2.0 to −0.7) | −0.3 (−1.3 to −0.7) | ||
| Nocturnal voiding episodes per day | ||||
| Mean ± SD | −0.5 ±1.1 | −0.2 ±1.2 | −0.2 (−0.4 to −0.1) | .006 |
| Median (IQR) | −0.3 (−1.0 to 0.0) | −0.3 (−0.7 to −0.3) | ||
| Voids associated with at least moderate urgencyb | ||||
| Mean ± SD | −2.1 ± 3.8 | −1.4 ±3.9 | −0.9 (−1.4 to −0.3) | .001 |
| Median (IQR) | −1.7 (−4.0 to 0.0) | −0.7 (−3.0 to 0.7) | ||
| Voids associated with severe urgencyc | ||||
| Mean ± SD | −1.7 ±2.9 | −1.4 ±3.0 | −0.6 (−0.9 to −0.2) | .005 |
| Median (IQR) | −1.3 (−3.0 to 0.0) | −1.0 (−2.3 to 0.0) |
CI, confidence interval; IQR, interquartile range.
Derived from analysis of covariance models, adjusted for baseline level of symptoms as well as clinical site;
Defined as voiding episodes that were associated with either a “moderate” or “severe” sensation of urgency on voiding diary;
Defined as voiding episodes that were associated with a “severe” sensation of urgency on voiding diary.
Huang. Simplified diagnosis and treatment for urgency incontinence in women. Am J Obstet Gynecol 2012.
Table 3. Improvement in Bladder-Specific Impact Questionnaire scores over 12 weeks by treatment assignment.
| Questionnaire | Average change in questionnaire scores | Participants with “meaningful” improvement in scoresa | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Fesoterodine (n = 303)b | Placebo (n = 301)b | Least square mean difference (95% CI)c | Fesoterodine (n = 303)b | Placebo (n = 301)a,d | Odds ratio (95% CI)e | |
| Overactive Bladder Questionnairef | −17.1 ± 17.6 | −12.0 ± 16.6 | −5.58 (−8.01 to −3.16) | 181 (63.1) | 140 (49.3) | 1.81 (1.19−2.77) |
|
| ||||||
| Patient perception of bladder conditiong | −1.2 ± 1.2 | −0.6 ± 1.2 | −0.50 (−0.69 to −0.32) | 188 (65.5) | 142 (49.8) | 2.04 (1.42−2.93) |
|
| ||||||
| Patient perception of urgency scaleh | −0.5 ± 0.8 | −0.3 ± 0.6 | −0.21 (−0.12 to −0.30) | 132 (46.0) | 90 (31.6) | 2.61 (1.73−3.96) |
CI, confidence interval.
Defined as at least 10-point increase in Overactive Bladder Questionnaire score, at least 1-point increase in Patient Perception of Bladder Condition score, and at least 1-point increase in Patient Perception of Urgency Scale score;
Data are given as mean ± SD;
Derived from analysis of covariance models, adjusted for baseline questionnaire scores and clinical site;
Data are given as number (percentage);
Obtained through logistic regression models, adjusted for baseline proportion of the outcome and clinical site;
A 31-item instrument in which scores range from 0-100; higher scores indicate more severe or bothersome overactive bladder symptoms20;
A single-item measure scored on a 6-point Likert scale; higher scores indicate more severe bladder-related problems20,21;
A single-item measure scored on a 3-point Likert scale; higher scores indicate greater urgency.22
Huang. Simplified diagnosis and treatment for urgency incontinence in women. Am J Obstet Gynecol 2012.
After 12 weeks of treatment, 106 women in the fesoterodine group (35.0%) reported complete resolution of urgency-type incontinence, compared with 52 women (17.3%) in the placebo group (P < .001). With regard to any urinary incontinence, 79 women in the fesoterodine group (26.1%) reported being free of all urinary incontinence after 12 weeks, compared with 34 women (11.3%) in the placebo group (P < .001).
Safety outcomes
Four serious adverse events were reported in the fesoterodine group, and 4 were reported in the placebo group (Table 4). All serious adverse events in the fesoterodine group were judged by blinded site investigators as unlikely or unrelated to treatment. No participants were found to have a posttreatment PVR volume of >250 mL, and no participants met criteria for referral for an extended evaluation. An independent, unmasked data safety monitor also reviewed adverse events at 6-month intervals to evaluate safety throughout the trial.
Table 4. Adverse events and posttreatment postvoid residual volume by treatment assignment.
| Variable | Fesoterodine (n = 303) | Placebo (n = 301) | P valuea |
|---|---|---|---|
| Adverse events, n (%)b | |||
| Reported at least 1 adverse event | 187(61.7) | 149(49.5) | .003 |
| Reported at least 1 potentially anticholinergic adverse eventc | 111 (36.6) | 41 (13.6) | < .001 |
| Reported at least 1 “severe” potentially anticholinergic adverse eventd | 18 (16.2) | 4 (9.8) | .23 |
| Reported 1 serious adverse evente | 4(1.3) | 4(1.3) | 1.00 |
| Serious adverse event “possibly” related to treatmentf | 0 | 1 (0.3) | .50 |
| Postvoid residual volumeg | |||
| Volumeh | 39.1 (48.0) | 31.2(39.0) | .04 |
| Volume ≥250 mL, n | 0 | 0 | — |
Estimated by Fisher exact tests for adverse events and postvoid residual volume ≥250 mL; obtained from analysis of covariance for mean postvoid residual volume, with adjustment for clinical site and cube root transformation to accommodate the nonnormal distribution of postvoid residual volumes;
Assessed in the 303 women in the fesoterodine group and 301 women in the placebo group who took at least 1 dose of study drug and completed at least 1 follow-up visit;
Defined as constipation, dry mouth, tachycardia, drowsiness, or urinary hesitancy or retention;
Considered to be “severe” if they prevented participants from participating in one or more daily activities;
Defined as adverse events that resulted in death, disability, or hospitalization;
Defined as serious adverse events that were rated by site investigators as having a possible, probable, or definite relationship to study medication;
Measured at 12 weeks or early termination among women receiving at least 1 dose of study medication; data on posttreatment postvoid residual were missing for 39 treated women in the fesoterodine group and 42 in the placebo group; 36 women were lost to follow-up evaluation; 8 women discontinued the study early and refused an early termination visit, and 37 women attended a 12-week or early termination visit but declined to undergo postvoid residual measurement or left before postvoid residual volume could be measured;
Data are given as mean ± SD.
Huang. Simplified diagnosis and treatment for urgency incontinence in women. Am J Obstet Gynecol 2012.
Comment
This study provides new insight into the efficacy and safety of initiating pharmacologic treatment for urgency urinary incontinence in ambulatory women on the basis of a simplified diagnostic algorithm rather than a traditional extended evaluation. Among women with uncomplicated urgency incontinence that was diagnosed by a simple 3-item measure, flexible-dose pharmacologic therapy resulted in moderate improvement in frequency of urgency incontinence, compared with placebo. In addition, pharmacologic therapy was associated with similar improvements in frequency of total incontinence, diurnal and nocturnal voiding, and urgency-associated voiding and with bladder-specific impact questionnaires. Serious adverse events were uncommon and were unrelated to treatment; no women were found to have a posttreatment PVR volume of ≥250 mL or required referral for an extensive evaluation because of a safety concern.
Despite recommendations that non-specialist clinicians assume a greater role in managing incontinence,5 the rates of diagnosis and treatment for incontinence outside of urogynecology and urology practice remain low.7-11 One explanation is that the traditional extended, time-consuming evaluation for incontinence is impractical to perform in primary care or general gynecology settings, where clinicians frequently are required to address multiple health complaints during relatively brief visits. Recently, some experts have suggested that noninvasive treatments for incontinence may be initiated in uncomplicated conditions before complex evaluation, which would include assessment of PVR volume.26-29 However, data to support a streamlined approach to diagnosing and treating incontinence in women have been lacking. Our findings suggest that, among ambulatory women who would be appropriate for primary care or general gynecology treatment, clinicians may be able to use the 3IQ and a urine dipstick to initiate pharmacologic therapy for urgency incontinence with reasonable expectation of good clinical outcomes.
Although women who were assigned randomly to fesoterodine therapy reported an average reduction in urgency incontinence frequency of 2.5 episodes per day, substantial reductions in incontinence were also observed in the placebo group, which resulted in only a moderate net improvement in the primary outcome. Comparisons with the 4 previously published phase III trials of fesoterodine therapy are complicated by differences in study population (all adults vs women only), medication dosage (fixed vs flexible dosing), and baseline symptoms (ie, inclusion of women with multiple bladder symptoms vs urgency incontinence only; Supplementary Table 4). Nevertheless, in these earlier trials in which women were selected through a more extended evaluation that included assessment of PVR volume, comparable effect sizes for urgency incontinence were reported, with net improvements in urgency incontinence frequency that ranged from 0.3–1.2 episodes per day.17,23,24,30 As a result, among ambulatory women without a history of urologic or neurologic complications, our study suggests that the use of a simplified diagnostic algorithm results in similar efficacy of pharmacologic treatment compared to an extended evaluation.
Several limitations of this study should be noted. First, because the 3IQ was developed originally for use in nonspecialist settings, women were excluded if they reported major comorbidities that would automatically require urogynecology or urology evaluation (such as recent urologic surgery, major neurologic disease, kidney failure, or pelvic cancer). As a result, this study evaluated outcomes associated with the use of the 3IQ in generally well-functioning women who would be most appropriate for evaluation in primary care or general gynecology, and results should not be extrapolated to women with more complicated histories or to men with urgency incontinence. Second, our study did not include women with urinary urgency in the absence of incontinence or women with equally mixed incontinence symptoms. Therefore, results cannot be extrapolated to patients with “dry” overactive bladder symptoms or to women with equally mixed stress and urgency leakage. Additionally, participants did not undergo a confirmatory extended evaluation to examine the accuracy of their initial diagnosis by the 3IQ. Consequently, we cannot assess whether women who showed less response to treatment would have been classified differently by an initial extended evaluation. As noted earlier, however, the predictive value of the 3IQ in comparison with extended evaluation was validated in earlier research.16 Furthermore, the magnitude of the treatment effect that was observed in this study was not substantially different from previous trials of fesoterodine that involved more extensive evaluation.
Overall, this study provides evidence to support a streamlined algorithm for diagnosis and treatment of urgency urinary incontinence in ambulatory women with no major comorbidities. In this population, clinicians may be able to evaluate incontinence using a urine screen to exclude infection or hematuria followed by the use of the 3IQ to classify incontinence type. If the urinalysis is normal, study results indicate that the initiation of treatment for urgency incontinence on the basis of the 3IQ can result in improved 12-week incontinence outcomes. Integration of a streamlined algorithm into primary care and general gynecology settings has the potential to improve access to treatment for this common condition in women.
Supplementary Material
Supplementary Figure: The 3 incontinence questions (3IQ).
Supplementary Table 1: Participating clinical sites in the BRIDGES trial, in alphabetical order
BRIDGES, BRinging simple urge Incontinence DiaGnosis and treatment to providerS.
Supplementary Table 2: Change in voiding outcomes, with multiple imputation
CI, confidence interval. a Data are given as mean ± SE; b Derived from analysis of covariance models, adjusted for baseline level of symptoms as well as site, using multiple imputation; c Defined as voiding episodes associated with either a “moderate” or “severe” sensation of urgency on voiding diary; d Defined as voiding episodes associated with a “severe” sensation of urgency on voiding diary.
Supplementary Table 3: Change in voiding outcomes, excluding participants without complete data
CI, confidence interval. a Data are given as mean ± SD; b Derived from analysis of covariance models, adjusted for baseline level of symptoms as well as clinical site; c Defined as voiding episodes associated with either a “moderate” or “severe” sensation of urgency on voiding diary; d Defined as voiding episodes associated with a “severe” sensation of urgency on voiding diary.
Supplementary Table 4: Published phase III randomized controlled trials of fesoterodine therapy for urgency incontinencea
aIdentified through an English-language search of PubMed, EMBASE, and the Cochrane Library.
Acknowledgments
Pfizer, Inc, provided funding for the study and the study medication but did not provide other input into the design of the study; collection, analysis, or interpretation of data; writing of the report; or the decision to submit the paper for publication. A.J.H. was additionally supported by grants RR024130 and 1K23AG038335-01A1 from the US National Institutes of Health; however, the views expressed in this article do not necessarily represent those of the National Institutes of Health. A.J.H. and J.S.B. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. No manuscript preparation assistance was provided by the study funders.
A.J.H. has received a University of California San Francisco research grant from Pfizer, Inc, to conduct research related to urinary incontinence. L.A.A. has received a research grant from Pfizer, Inc. H.E.R. has received a research grant and participated in a speaker's bureau for Pfizer, Inc, received a research grant and participated in an advisory board for Astellas, and served as a consultant for Uromedica and GlaxoSmithKline. C.S.B. has served as a consultant for Astellas and for GlaxoSmithKline. S.R.K. has served as a consultant for Pfizer, Inc, and Allergan and has been a course director and teaching faculty member for Laborie. J.S.B. has received research grants related to incontinence through University of California San Francisco from Pfizer, Inc, and Mytrus, Inc.
Footnotes
No other authors report any potential conflict of interest.
Presented in abstract form at the 34th annual meeting of the Society of General Internal Medicine, Phoenix, AZ, May 4-7, 2011.
Contributor Information
Dr Alison J. Huang, Department of Medicine, University of California San Francisco School of Medicine, San Francisco, CA.
Dr Rachel Hess, Department of Medicine, University of Pittsburgh School of Medicine, Pittsburgh, PA.
Dr Lily A. Arya, Department of Obstetrics and Gynecology, University of Pennsylvania School of Medicine, Philadelphia, PA.
Dr Holly E. Richter, Department of Obstetrics and Gynecology, University of Alabama at Birmingham School of Medicine, Birmingham, AL.
Dr Leslee L. Subak, Departments of Obstetrics, Gynecology, and Reproductive Sciences, University of California San Francisco School of Medicine, San Francisco, CA.
Dr Catherine S. Bradley, Department of Obstetrics and Gynecology, Carver College of Medicine, University of Iowa, Iowa City, IA.
Dr Rebecca G. Rogers, Department of Obstetrics and Gynecology, University of New Mexico Health Sciences Center, Albuquerque, NM.
Dr Deborah L. Myers, Department of Obstetrics and Gynecology, Alpert Medical School, Brown University, Providence, RI.
Dr Karen C. Johnson, Department of Preventive Medicine, University of Tennessee Health Sciences Center, Memphis, TN.
Dr W. Thomas Gregory, Department of Obstetrics and Gynecology, Oregon Health Sciences University, Portland, OR.
Dr Stephen R. Kraus, Department of Urology, University of Texas Health Science Center at San Antonio, San Antonio, TX.
Dr Michael Schembri, Departments of Obstetrics, Gynecology, and Reproductive Sciences, University of California San Francisco School of Medicine, San Francisco, CA.
Dr Jeanette S. Brown, Departments of and Obstetrics, Gynecology, and Reproductive Sciences, University of California San Francisco School of Medicine, San Francisco, CA.
References
- 1.Melville JL, Katon W, Delaney K, Newton K. Urinary incontinence in US women: a population-based study. Arch Intern Med. 2005;165:537–42. doi: 10.1001/archinte.165.5.537. [DOI] [PubMed] [Google Scholar]
- 2.Grodstein F, Fretts R, Lifford K, Resnick N, Curhan G. Association of age, race, and obstetric history with urinary symptoms among women in the Nurses' Health Study. Am J Obstet Gynecol. 2003;189:428–34. doi: 10.1067/s0002-9378(03)00361-2. [DOI] [PubMed] [Google Scholar]
- 3.Fultz NH, Fisher GG, Jenkins KR. Does urinary incontinence affect middle-aged and older women's time use and activity patterns? Obstet Gynecol. 2004;104:1327–34. doi: 10.1097/01.AOG.0000143829.21758.3c. [DOI] [PubMed] [Google Scholar]
- 4.Nygaard I, Turvey C, Burns TL, Crischilles E, Wallace R. Urinary incontinence and depression in middle-aged United States women. Obstet Gynecol. 2003;101:149–56. doi: 10.1016/s0029-7844(02)02519-x. [DOI] [PubMed] [Google Scholar]
- 5.Newman DK. Aging and the problem of urinary incontinence: AHCPR's 1996 clinical practice guidance. Urol Nurs. 1996;16:121–2. [PubMed] [Google Scholar]
- 6.Holroyd-Leduc JM, Straus SE. Management of urinary incontinence in women: clinical applications. JAMA. 2004;291:996–9. doi: 10.1001/jama.291.8.996. [DOI] [PubMed] [Google Scholar]
- 7.Jones TV, Bunner SH. Approaches to urinary incontinence in a rural population: a comparison of physician assistants, nurse practitioners, and family physicians. J Am Board Fam Pract. 1998;11:207–15. doi: 10.3122/15572625-11-3-207. [DOI] [PubMed] [Google Scholar]
- 8.Shaw C, Tansey R, Jackson C, Hyde C, Allan R. Barriers to help seeking in people with urinary symptoms. Fam Pract. 2001;18:48–52. doi: 10.1093/fampra/18.1.48. [DOI] [PubMed] [Google Scholar]
- 9.Du Moulin M. Prevalence of undiagnosed urinary incontinence in women is 53% in the preceding year and 39% in the preceding week in a US managed-care population. Evid Based Nurs. 2010;13:79–80. doi: 10.1136/ebn1056. [DOI] [PubMed] [Google Scholar]
- 10.Shaw C, Atwell C, Wood F, Brittain K, Williams K. A qualitative study of the assessment and treatment of incontinence in primary care. Fam Pract. 2007;24:461–7. doi: 10.1093/fampra/cmm041. [DOI] [PubMed] [Google Scholar]
- 11.Wenger NS, Roth CP, Hall WJ, et al. Practice redesign to improve care for falls and urinary incontinence: primary care intervention for older patients. Arch Intern Med. 2009;170:1765–72. doi: 10.1001/archinternmed.2010.387. [DOI] [PubMed] [Google Scholar]
- 12.Managing acute and chronic urinary incontinence: AHCPR Urinary Incontinence in Adults Guideline Update Panel. Am Fam Physician. 1996;54:1661–72. [PubMed] [Google Scholar]
- 13.Assessment and treatment of urinary incontinence: Scientific Committee of the First International Consultation on Incontinence. Lancet. 2000;355:2153–8. [PubMed] [Google Scholar]
- 14.Roe B, Williams K, Palmer M. Bladder training for urinary incontinence in adults. Cochrane Database Syst Rev. 2000:CD001308. doi: 10.1002/14651858.CD001308. [DOI] [PubMed] [Google Scholar]
- 15.Haeusler G, Leitich H, van Trotsenburg M, Kaider A, Tempfer CB. Drug therapy of urinary urge incontinence: a systematic review. Obstet Gynecol. 2002;100:1003–16. doi: 10.1016/s0029-7844(02)02238-x. [DOI] [PubMed] [Google Scholar]
- 16.Brown JS, Bradley CS, Subak LL, et al. The sensitivity and specificity of a simple test to distinguish between urge and stress urinary incontinence. Ann Intern Med. 2006;144:715–23. doi: 10.7326/0003-4819-144-10-200605160-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dmochowski RR, Peters KM, Morrow JD, et al. Randomized, double-blind, placebo-controlled trial of flexible-dose fesoterodine in subjects with overactive bladder. Urology. 2009;75:62–8. doi: 10.1016/j.urology.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 18.Anderson RU, Mobley D, Blank B, Saltzstein D, Susset J, Brown JS. Once daily controlled versus immediate release oxybutynin chloride for urge urinary incontinence: OROS Oxybutynin Study Group. J Urol. 1999;161:1809–12. [PubMed] [Google Scholar]
- 19.Brown JS, McNaughton KS, Wyman JF, et al. Measurement characteristics of a voiding diary for use by men and women with overactive bladder. Urology. 2003;61:802–9. doi: 10.1016/s0090-4295(02)02505-0. [DOI] [PubMed] [Google Scholar]
- 20.Coyne KS, Matza LS, Kopp Z, Abrams P. The validation of the patient perception of bladder condition (PPBC): a single-item global measure for patients with overactive bladder. Eur Urol. 2006;49:1079–86. doi: 10.1016/j.eururo.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 21.Matza LS, Thompson CL, Krasnow J, Brewster-Jordan J, Zyczynski T, Coyne KS. Test-retest reliability of four questionnaires for patients with overactive bladder: the overactive bladder questionnaire (OAB-q), patient perception of bladder condition (PPBC), urgency questionnaire (UQ), and the primary OAB symptom questionnaire (POSQ) Neurourol Urodyn. 2005;24:215–25. doi: 10.1002/nau.20110. [DOI] [PubMed] [Google Scholar]
- 22.Cardozo L, Coyne KS, Versi E. Validation of the urgency perception scale. BJU Int. 2005;95:591–6. doi: 10.1111/j.1464-410X.2005.05345.x. [DOI] [PubMed] [Google Scholar]
- 23.Chapple C, Van Kerrebroeck P, Tubaro A, et al. Clinical efficacy, safety, and tolerability of once-daily fesoterodine in subjects with overactive bladder. Eur Urol. 2007;52:1204–12. doi: 10.1016/j.eururo.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 24.Nitti VW, Dmochowski R, Sand PK, et al. Efficacy, safety and tolerability of fesoterodine for overactive bladder syndrome. J Urol. 2007;178:2488–94. doi: 10.1016/j.juro.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 25.Little RJA, Rubin DB. Statistical analysis with missing data. Vol. 1987. New York: Wiley-Inter-science; pp. 209–14. [Google Scholar]
- 26.Gibbs CF, Johnson TM, 2nd, Ouslander JG. Office management of geriatric urinary incontinence. Am J Med. 2007;120:211–20. doi: 10.1016/j.amjmed.2006.03.044. [DOI] [PubMed] [Google Scholar]
- 27.Abrams P, Cardozo L, Wein A, editors. Fourth International Consultation on Incontinence. Paris: Health Publication Ltd; 2008. The utility of post-voiding residual urine volume determination in the initial assessment of incontinent patients; pp. 338–9. [Google Scholar]
- 28.Ouslander JG. Management of overactive bladder. N Engl J Med. 2004;350:786–99. doi: 10.1056/NEJMra032662. [DOI] [PubMed] [Google Scholar]
- 29.Nygaard I. Clinical practice. Idiopathic urgency urinary incontinence. N Engl J Med. 2010;363:1156–62. doi: 10.1056/NEJMcp1003849. [DOI] [PubMed] [Google Scholar]
- 30.Herschorn S, Swift S, Guan Z, et al. Comparison of fesoterodine and tolterodine extended release for the treatment of overactive bladder: a head-to-head placebo-controlled trial. BJU Int. 2010;105:58–66. doi: 10.1111/j.1464-410X.2009.09086.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure: The 3 incontinence questions (3IQ).
Supplementary Table 1: Participating clinical sites in the BRIDGES trial, in alphabetical order
BRIDGES, BRinging simple urge Incontinence DiaGnosis and treatment to providerS.
Supplementary Table 2: Change in voiding outcomes, with multiple imputation
CI, confidence interval. a Data are given as mean ± SE; b Derived from analysis of covariance models, adjusted for baseline level of symptoms as well as site, using multiple imputation; c Defined as voiding episodes associated with either a “moderate” or “severe” sensation of urgency on voiding diary; d Defined as voiding episodes associated with a “severe” sensation of urgency on voiding diary.
Supplementary Table 3: Change in voiding outcomes, excluding participants without complete data
CI, confidence interval. a Data are given as mean ± SD; b Derived from analysis of covariance models, adjusted for baseline level of symptoms as well as clinical site; c Defined as voiding episodes associated with either a “moderate” or “severe” sensation of urgency on voiding diary; d Defined as voiding episodes associated with a “severe” sensation of urgency on voiding diary.
Supplementary Table 4: Published phase III randomized controlled trials of fesoterodine therapy for urgency incontinencea
aIdentified through an English-language search of PubMed, EMBASE, and the Cochrane Library.