Abstract
Essential tremor (ET) is one of the most common tremor disorders in the world. Despite this, only two medications have received Level A recommendations from the American Academy of Neurology to treat it (primidone and propranolol). Even though these medications provide relief to a large group of ET patients, up to 50% of patients are non-responders. Additional medications to treat ET are needed. This review discusses some of the methodological issues that should be addressed for quality clinical drug development in ET.
Keywords: Essential tremor, methodology, tremor, etiology
Introduction
Essential tremor (ET) is the most common tremor disorder and is characterized by postural and kinetic components.1 It reportedly occurs in 0.9% of the general population and affects approximately 5% of adults aged 65 years and older.2 Although ET was considered a “benign” condition in the past, it is now recognized as a progressive neurological disease, with tremor severity varying widely across patients.3,4 Tremor amplitude worsens with time, often leading to difficulties when carrying out activities of daily living, including writing, drinking, eating, and speaking.5,6
The exact pathophysiology of ET is unknown,4 and developing effective drugs specifically for ET has been difficult. Drug discovery for ET has largely depended on clinical observations with medications that were originally developed to treat other disorders. Only two drugs have been given Level A “effective” recommendations by the American Academy of Neurology Practice Parameter, which was published in 2005 and updated in 2011: the β-adrenergic blocker propranolol and the anticonvulsant primidone.7 Because approximately 30–50% of ET patients will not respond adequately to either primidone or propranolol,8 new therapies for ET are warranted.
This review will describe the methodological issues found in clinical trials of ET.
Diagnostic criteria
An accurate diagnosis of ET is essential in clinical trials. The erroneous diagnosis of ET can confound the description of active treatment response. Estimates suggest that 30–50% of ET cases are misdiagnosed.9–12 In particular, dystonic tremor, Parkinson's disease tremor, and enhanced physiologic tremor are commonly mistaken as ET. This may be partly explained by similarities in frequency (4–11 Hz) between these tremor etiologies.13 There are currently no definitive diagnostic tests or biomarkers for the diagnosis of ET, but dopamine transporter imaging is helpful in distinguishing ET from Parkinson's disease.14 Clinical diagnostic criteria for ET are still the primary method of diagnosis.
Tremor researchers have developed several sets of diagnostic criteria to distinguish ET from other forms of tremor. All define ET as a pure tremor disorder, affecting the upper limbs in nearly all cases, without signs of dystonia and parkinsonism. The Tremor Research Investigation Group (TRIG) criteria for definite ET exclude isolated head tremor and other focal and task-specific tremors, and they require at least a 5-year history of tremor, so as to reduce the chance of other tremor disorders.1,4,15,16 The 1996 NIH Collaborative Genetic Criteria (NIHCGC) include a 0–4 rating of tremor amplitude to reduce the chance of including enhanced physiologic tremor. Unlike the TRIG criteria, the NIHCGC allow the inclusion of coexistent dystonia in both “definite” and “probable” ET, and Parkinson's disease in “probable” ET, as long as a confirmed history of pre-existing ET is available.17,18 The ET criteria of an ad hoc committee of the Movement Disorder Society include isolated head tremor if there is abnormal posturing and do not specify a minimum time for pure tremor to be present.1 The Washington Heights-Inwood Genetic Study of Essential Tremor (WHIGET) Scale uses 0–3 or 0–4 ratings of upper extremity tremor during horizontal extension, pouring, drinking, using a spoon, finger-to-nose testing, and spirography to distinguish ET from enhanced physiologic tremor.19
Since 1997, nearly all treatment and genetic studies of ET have used one or more of these sets of diagnostic criteria. All of these diagnostic schemes are subject to investigator bias and inexperience, and misdiagnosis is still common.12 Even the definition of ET continues to be debated, and it is clear that ET is not a specific disease.3
Limitations of published clinical trials
Published clinical trials of ET differ widely with respect to patient selection, cohort size, type(s) of controls, use of concomitant antitremor medications, methods and locations of tremor assessment, and duration of treatment. For example, clinical trials of gabapentin have produced inconsistent results, depending on whether gabapentin was used as a monotherapy or as an adjunct therapy.20–23 The mean (range) number of patients in published ET drug trials is 18.9 (1–208), and few studies have involved more than one study site.4 Only topiramate, botulinum toxin, and carisbamate have been studied in large double-blind placebo-controlled trials. The mean (range) duration of treatment in published ET drug trials is only 5.4 weeks (0–120). Prior to 1993, rating scales differed widely among studies, and none had been validated.6 Accelerometry of postural hand tremor was common in studies prior to 1993, but postural tremor is a very limited measure of ET,24 and the results are difficult to compare with rating scales.4 Finally, many studies did not have suitable controls, and the ET literature is full of anecdotal reports of efficacy that were not confirmed in controlled studies.7,25
The intrarater reliability of the Fahn–Tolosa–Marín Tremor Rating Scale is such that a minimum of 15 patients should be adequate in a double-blind placebo-controlled crossover study design.4 Deuschl et al.4 recommended that this study design should be used in pilot studies of promising agents. Positive results should then be confirmed in placebo-controlled, double-blind multicenter parallel study design, with a minimum of 60 patients.
Randomization and allocation concealment
Patient bias can be especially difficult to prevent in randomized controlled studies of treatments, which have side effects that are common and unique. The blinded assessments in botulinum toxin A studies of ET are hampered by the common occurrence of muscle weakness.26,27 Weight loss is common with topiramate, so the large multicenter trial used raters who were blinded to all other clinical assessments.28
Deep brain stimulation (DBS) of the ventral intermediate nucleus of the thalamus has a high magnitude of effect in treating ET, estimated at 60–90% by various clinical rating scales.7 Sham surgeries have not been done. Assessments by blinded raters with the stimulator on and off have been employed, but this does not eliminate patient bias. The lack of a complete blind makes the true incidence of side effects difficult to determine.4 Stereotactic targets other than the ventrolateral thalamus are being explored for ET,4 and future randomized controlled trials comparing two targets might provide a more reliable assessment of efficacy and side effects.
Correlating tremor measurement to treatment outcomes
Motion transducers and rating scales have been employed in ET trials. Accelerometers, gyroscopic transducers, digitizing tablets, and other motion transducers produce precise measures of tremor amplitude and frequency. The Fahn–Tolosa–Marin Tremor Rating Scale (FTM-TRS), the Essential Tremor Rating Assessment Scale (TETRAS), the WHIGET Scale, and the tremor scale of Bain et al. have all been validated in patients with ET.
Accelerometers and gyroscopic transducers provide precise but incomplete measures of body motion in three-dimensional space, and accelerometers are affected by gravitational artifact.28 Postural tremor is assessed more completely, but changes in postural tremor may not be the most valid measure of a treatment effect. For example, a double-blind placebo-controlled randomized trial of zonisamide found no significant improvement in the FTM-TRS, but a statistically significant 40% tremor reduction in postural tremor was measured with accelerometry.29 Similarly, a double-blind, placebo-controlled, randomized trial of pregabalin in 22 ET patients yielded significant improvements in postural tremor measured with accelerometry but no significant improvement in FTM-TRS scores.30 Insensitivity of the FTM-TRS to modest changes in tremor severity is one explanation for these results. It is also possible that postural tremor is more sensitive to these medications than kinetic tremor, which is usually more disabling.
Clinical ratings of tremor are proportional to the logarithm of tremor amplitude, measured with a motion transducer, as predicted by the Weber–Fechner law of psychophysics.31,32 The percentage change in tremor amplitude T is related to the change in tremor rating (TRS) according to the following equation, where subscripts 1 and 2 denote the initial and final measurements:
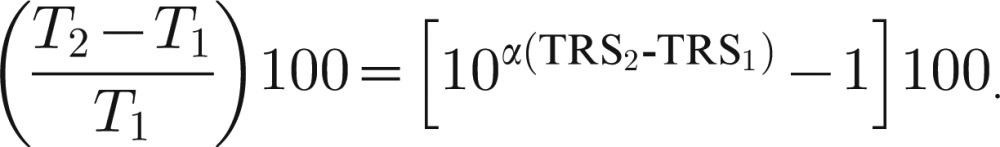 |
The value of α for upper extremity tremor is approximately 0.3–0.5 for a 0–4 (5-point) tremor rating.31 Thus, for a 1-point reduction in a 5-point rating, the percentage reduction in tremor amplitude is 68%, 60%, and 50% for α = 0.5, 0.4, and 0.3 respectively.
Deuschl et al.4 discussed the lack of a clearly defined minimum clinically significant effect as a methodologic issue in ET trials. Few studies have included a global clinical impression of treatment effect that can be used to compute the minimum clinically significant change in rating scale and transducer measures of tremor.4,33,34
Chronic management of ET
There are no long-term randomized controlled studies of any medication for ET, and the average duration of treatment in published studies of propranolol and primidone is less than 1 month.4 Future studies should address the long-term benefit of drugs for ET. Studies assessing the long-term efficacy of propranolol and primidone up to a year after initial treatment have yielded positive results on the control of tremor.8,35–37 However, it is now clear that these medications may lose efficacy over time, requiring higher doses to achieve their tremorolytic effect, and may cause side effects with both acute and chronic use.8,35,36,38 Up to 50% of ET patients will not respond to primidone or propranolol.8
Assessing the impact of therapy on quality of life
It is not uncommon for patients with ET to be forced into an early retirement, a result of the increasing impact of disease progression on their ability to lead a self-sufficient life.39 Assessments of the patient's quality of life become paramount in the clinical evaluation of therapeutic efficacy. Ferrara et al.40 recently included quality of life as an outcome measure in a trial of pregabalin for ET. Quality of life should be assessed for all ET treatments, given their side effects, cost, and modest benefit.
Although the SF-36 scale for assessing quality of life is valid and widely used,41 the Questionnaire for Essential Tremor (QUEST) is a newer, more specific assessment of quality of life changes associated with ET. Developed in 2005 by Tröster et al.,42 QUEST was designed as a clinical tool for correlating changes in 30 aspects of tremor severity, social and personal disability, and perception of health. An independent validation study of the QUEST performed by Martinez-Martin et al.43 concluded that most of the psychometric parameters were found to be satisfactory in their ability to assess the impact of ET on the patients' quality of life. The use of QUEST in clinical trials will give clinicians important information for estimating the clinical relevance of a response to therapy.
Conclusion
Thus far, clinical trials in ET have provided information to patients and clinicians alike regarding management of this debilitating disorder. While propranolol and primidone provide symptomatic relief to a substantial number of ET patients, up to half of those afflicted do not receive clinically significant benefit from these drugs; this is a clear sign that ET-specific medications are needed. As the ongoing efforts toward understanding the pathophysiology of ET continue, investigators will hopefully open a new chapter in the pharmaceutical development of more effective ET treatments.
Footnotes
Funding: None.
Financial Disclosures: None.
Conflict of Interest: The authors report no conflict of interest.
References
- 1.Deuschl G, Bain P, Brin M. Consensus statement of the Movement Disorder Society on tremor. Ad Hoc Scientific Committee. Mov Disord. 1998;13((Suppl 3)):2–23. doi: 10.1002/mds.870131303. [DOI] [PubMed] [Google Scholar]
- 2.Louis ED, Ferreira JJ. How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Mov Disord. 2010;25:534–541. doi: 10.1002/mds.22838. [DOI] [PubMed] [Google Scholar]
- 3.Deuschl G, Elble R. Essential tremor-neurodegenerative or nondegenerative disease towards a working definition of ET. Mov Disord. 2009;24:2033–2041. doi: 10.1002/mds.22755. [DOI] [PubMed] [Google Scholar]
- 4.Deuschl G, Raethjen J, Hellriegel H, Elble R. Treatment of patients with essential tremor. Lancet Neurol. 2011;10:148–161. doi: 10.1016/S1474-4422(10)70322-7. [DOI] [PubMed] [Google Scholar]
- 5.Koller WC, Biary N, Cone S. Disability in essential tremor: effect of treatment. Neurology. 1986;36:1001–1004. doi: 10.1212/WNL.36.7.1001. [DOI] [PubMed] [Google Scholar]
- 6.Bain PG, Findley LJ, Atchison P, et al. Assessing tremor severity. J Neurol Neurosurg Psychiatry. 1993;56:868–87. doi: 10.1136/jnnp.56.8.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zesiewicz TA, Elble R, Louis ED, et al. Practice parameter: therapies for essential tremor: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2005;64:2008–2020. doi: 10.1212/01.WNL.0000163769.28552.CD. [DOI] [PubMed] [Google Scholar]
- 8.Koller WC, Vetere-Overfield B. Acute and chronic effects of propranolol and primidone in essential tremor. Neurology. 1989;39:1587–1588. doi: 10.1212/WNL.39.12.1587. [DOI] [PubMed] [Google Scholar]
- 9.Schrag A, Münchau A, Bhatia KP, et al. Essential tremor: an overdiagnosed condition. J Neurol. 2000;247:955–959. doi: 10.1007/s004150070053. [DOI] [PubMed] [Google Scholar]
- 10.Jain S, Lo SE, Louis ED. Common misdiagnosis of a common neurological disorder: how are we misdiagnosing essential tremor. Arch Neurol. 2006;63:1100–1104. doi: 10.1001/archneur.63.8.1100. [DOI] [PubMed] [Google Scholar]
- 11.Benito-Leon J, Louis ED. Essential tremor: emerging views of a common disorder. Nat Clin Pract Neurol. 2006;2:666–78. doi: 10.1038/ncpneuro0347. [DOI] [PubMed] [Google Scholar]
- 12.Quinn NP, Schneider SA, Schwingenschuh P, Bhatia KP. Tremor-some controversial aspects. Mov Disord. 2011;26:18–23. doi: 10.1002/mds.23289. [DOI] [PubMed] [Google Scholar]
- 13.Elble RJ, Deuschl G. Milestones in tremor research. Mov Disord. 2011;26:1096–1105. doi: 10.1002/mds.23579. [DOI] [PubMed] [Google Scholar]
- 14.Isaias IU, Marotta G, Hirano S, et al. Imaging essential tremor. Mov Disord. 2010;25:679–686. doi: 10.1002/mds.22870. [DOI] [PubMed] [Google Scholar]
- 15.Bermejo-Pareja F. Essential tremor—a neurodegenerative disorder associated with cognitive defects. Nat Rev Neurol. 2011;7:1–10. doi: 10.1038/nrneurol.2011.44. [DOI] [PubMed] [Google Scholar]
- 16.Findley LJ, Koller WC. Definitions and behavioral classifications. In: Findley LJ, Koller WC, editors. Handbook of Tremor Disorders. New York: Dekker; 1994. pp. 1–5. [Google Scholar]
- 17.Chandran P, Pal PK, Reddy JYC, Thennarasu K, Yadav R, Shivashankar N. Non-motor features in essential tremor. Acta Neurol Scand. 2012;125:332–337. doi: 10.1111/j.1600-0404.2011.01573.x. [DOI] [PubMed] [Google Scholar]
- 18.Chouinard S, Louis ED, Fahn S. Agreement among movement disorder specialists on the clinical diagnosis of essential tremor. Mov Disord. 1997;12:973–976. doi: 10.1002/mds.870120621. [DOI] [PubMed] [Google Scholar]
- 19.Louis ED, Ottman R, Ford B, et al. The Washington Heights-Inwood Genetic Study of Essential Tremor: methodological issues in essential tremor research. Neuroepidemiology. 1997;16:124–133. doi: 10.1159/000109681. [DOI] [PubMed] [Google Scholar]
- 20.Pahwa R, Lyons K, Hubble JP, et al. Double-blind controlled trial of gabapentin in essential tremor. Mov Disord. 1998;13:465–467. doi: 10.1002/mds.870130315. [DOI] [PubMed] [Google Scholar]
- 21.Giornell A, Kulisevsky J, Barbanok M, et al. A randomized placebo-controlled comparative trial of gabapentin and propranolol in essential tremor. Arch Neurol. 1999;56:475–480. doi: 10.1001/archneur.56.4.475. [DOI] [PubMed] [Google Scholar]
- 22.Ondo W, Hunter C, Vuong KD, et al. Gabapentin for essential tremor: a multiple-dose, double-blind, placebo-controlled trial. Mov Disord. 2000;15:678–682. doi: 10.1002/1531-8257(200007)15:4<678::AID-MDS1012>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 23.Lopez del Val, Santos S. Gabapentin in the treatment of tremor. Rev Neurol. 2003;36:322–326. [PubMed] [Google Scholar]
- 24.Bain PG, Mally J, Gresty M, Findley LJ. Assessing the impact of essential tremor on upper limb function. J Neurol. 1993;241:54–61. doi: 10.1007/BF00870673. [DOI] [PubMed] [Google Scholar]
- 25.Zesiewicz TA, Elble RJ, Louis ED, et al. Evidence-based guideline update: treatment of essential tremor: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2011;77:1752–1755. doi: 10.1212/WNL.0b013e318236f0fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brin MF, Lyons KE, Doucette J, et al. A randomized, double masked, controlled trial of botulinum toxin type A in essential hand tremor. Neurology. 2001;56:1523–1528. doi: 10.1212/WNL.56.11.1523. [DOI] [PubMed] [Google Scholar]
- 27.Jankovic J, Schwartz K, Clemence W, et al. A randomized, double-blind, placebo-controlled study to evaluate botulinum toxin type A in essential hand tremor. Mov Disord. 1996;11:250–256. doi: 10.1002/mds.870110306. [DOI] [PubMed] [Google Scholar]
- 28.Ondo WG, Jankovic J, Connor GS, et al. Topiramate in essential tremor: a double-blind, placebo-controlled trial. Neurology. 2006;66:672–677. doi: 10.1212/01.wnl.0000200779.03748.0f. [DOI] [PubMed] [Google Scholar]
- 29.Elble RJ. Gravitational artifact in accelerometric measurements of tremor. Clin Neurophysiol. 2005;116:1638–1643. doi: 10.1016/j.clinph.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 30.Zesiewicz TA, Ward CL, Hauser RA, et al. A double-blind placebo-controlled trial of zonisamide (Zonegram) in the treatment of essential tremor. Mov Disord. 2007;22:279–282. doi: 10.1002/mds.21282. [DOI] [PubMed] [Google Scholar]
- 31.Zesiewicz TA, Ward CL, Hauser RA, et al. A pilot, double-blind, placebo-controlled trial of pregabalin (Lyrica) in the treatment of essential tremor. Mov Disord. 2007;22:1660–1663. doi: 10.1002/mds.21629. [DOI] [PubMed] [Google Scholar]
- 32.Haubenberger D, Kalowitz D, Nahab FB, et al. Validation of digital spiral analysis as outcome parameter for clinical trials in essential tremor. Mov Disord. 2011;26:2073–2080. doi: 10.1002/mds.23808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elble RJ, Pullman SL, Matsumoto JY, et al. Tremor amplitude is logarithmically related to 4-point and 5-point tremor rating scales. Brain. 2006;129:2660–2666. doi: 10.1093/brain/awl190. [DOI] [PubMed] [Google Scholar]
- 34.Zhang K, Bhatia S, Oh MY, et al. Long-term results of thalamic deep brain stimulation for essential tremor. J Neurosurg. 2010;112:1271–1276. doi: 10.3171/2009.10.JNS09371. [DOI] [PubMed] [Google Scholar]
- 35.Sasso E, Perucca E, Fava R, Calzetti S. Primidone in the long-term treatment of essential tremor: a prospective study with computerized quantitative analysis. Clin Neuropharmacol. 1990;13:67–76. doi: 10.1097/00002826-199002000-00007. [DOI] [PubMed] [Google Scholar]
- 36.Calzetti S, Sasso E, Baratti M, Fava R. Clinical and computer-based assessment of long-term therapeutic efficacy of propranolol in essential tremor. Acta Neurol Scan. 1990;81:392–6. doi: 10.1111/j.1600-0404.1990.tb00982.x. [DOI] [PubMed] [Google Scholar]
- 37.Serrano-Duenas M. Use of primidone in low doses (250 mg/day) versus high doses (750 mg/day) in the management of essential tremor. Double-blind comparative study with one-year follow-up. Parkinsonism Relat Disord. 2003;10:29–33. doi: 10.1016/S1353-8020(03)00070-1. [DOI] [PubMed] [Google Scholar]
- 38.Gorman WP, Cooper R, Pocock P, Campbell MJ. A comparison of primidone, propranolol, and placebo in essential tremor, using quantitative analysis. J Neurol Neurosurg Psychiatry. 1986;49:64–68. doi: 10.1136/jnnp.49.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deuschl G, Koller WC. Introduction. Essential tremor. Neurology. 2000;54((Suppl 4)):S1. [PubMed] [Google Scholar]
- 40.Ferrara JM, Kenney C, Davidson AL, et al. Efficacy and tolerability of pregabalin in essential tremor: a randomized, double-blind, placebo-controlled, crossover trial. J Neurol Sci. 2009;285:195–197. doi: 10.1016/j.jns.2009.06.044. [DOI] [PubMed] [Google Scholar]
- 41.Lorenz D, Schweiger D, Moises H, Deuschl G. Quality of life and personality in essential tremor patients. Mov Disord. 2006;21:1114–1118. doi: 10.1002/mds.20884. [DOI] [PubMed] [Google Scholar]
- 42.Tröster Al, Pahwa R, Fields JA, Tanner CM, Lyons KE. Quality of life in essential tremor questionnaire (QUEST): development and initial validation. Parkinsonism Relat Disord. 2005;11:367–373. doi: 10.1016/j.parkreldis.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 43.Martinez-Martin P, Jimenez-Jimenez FJ, Carroza GE, et al. Most of the quality of life in essential tremor questionnaire (QUEST) psychometric properties resulted in satisfactory values. J Clin Epidemiol. 2010;63:767–773. doi: 10.1016/j.jclinepi.2009.09.001. [DOI] [PubMed] [Google Scholar]


