Summary
In vitro bladder contractions in response to cumulative carbachol doses were measured in the presence of selective muscarinic antagonists from rats which had their major pelvic ganglion bilaterally removed (denervation, DEN) or from rats in which the spinal cord was injured (SCI) via compression. DEN induced both hypertrophy (505±51 mg bladder weight) and a supersensitivity of the bladders to carbachol (EC50=0.7±0.1 uM). Some of the SCI rats regained the ability to void spontaneously (SPV). The bladders of these animals weighed 184±17 mg, significantly less than the bladders of non voiding rats (NV, 644±92 mg). The potency of carbachol was greater in bladder strips from NV SCI animals (EC50=0.54±0.1 uM) than either bladder strips from SPV SCI (EC50=0.93±0.3 μM), DEN or control (EC50=1.2±0.1 μM) animals. Antagonist affinities in control bladders for antagonism of carbachol induced contractions were consistent with M3 mediated contractions. Antagonist affinities in DEN bladders for 4-diphenlacetoxy-N-methylpiperidine methiodide (CDAMP, 8.5) and para fluoro hexahydrosilodifenidol (p-F-HHSiD, 6.6); were consistent with M2 mediated contractions, although the methoctramine aflinity (6.5) was consistent with MS mediated contractions. p-F-HHSiD inhibited carbachol induced contraction with an aflinity consistent with M2 receptors in bladders from NV SCI (pKb=6.4) animals and M3 receptors in bladders from SPV SCI animals (pKb=7.9). Subtype selective immunoprecipitation of muscarinic receptors revealed an increase in total and an increase in M2 receptor density with no change in M3 receptor density in bladders from DEN and NV SCI animals compared to normal or sham operated controls. M3 receptor density was lower in bladders from SPV SCI animals while the M2 receptor density was not different from control. This increase in M2 receptor density is consistent with the change in affinity of the antagonists for inhibition of carbachol induced contractions and may indicate that M2, receptors or a combination of M2 and M3 receptors directly mediate smooth muscle contraction in bladders from DEN and NV SCI rats.
Keywords: muscarinic receptors, bladder, spinal cord injury, incontinence, denervation
Acetylcholine, through its action at muscarinic receptors on smooth muscle cells, is the primary neurotransmitter controlling bladder voiding (1,2). Muscarinic receptor density and the contracile response of bladder smooth muscle to muscarinic stimulation are greatest in the dome and lowest in the base, allowing efficient bladder emptying (3). Pharmacological, biochemical and molecular data provide ample evidence that muscarinic receptors are heterogenous in nature (4, 5, 6). Pharmacologic data, based on the actions of subtype selective antimuscarinic agents, can distinguish at least three distinct subtypes of muscarinic acetylcholine receptors (M1, M2, and M3, 7). Molecular techniques have identified five muscarinic receptor subtypes (6) arising from five separate genes. Immunological and molecular studies revealed that most tissues including the urinary bladder express a mixture of subtypes (8,9).
Binding and subtype selective immunoprecipitation studies demonstrate that the majority of muscarinic receptors in the urinary bladder are of the M2, subtype (10, 11). On the other hand, pharmacological studies using subtype selective antagonists indicate that the M3 receptor subtype mediates smooth muscle contraction (10). The M2 receptor may be involved in inhibition of β-adrenergic receptor induced relaxation. A contribution of the M2 receptor to contraction in normal bladder tissue can only be demonstrated indirectly, when the majority of M3 receptors are inactivated in an environment of increased β-adrenergic receptor activation and when the tissue is prestimulated with a contractile agent such as potassium chloride (12, 13). The presence of prejunctional M1 facilitory and M2 inhibitory receptors on parasympathetic nerves innervating the rat bladder has been demonstrated both by acetylcholine release (14, 15) and muscle contraction studies (16).
Unlike other mammalian species, the normal rat bladder does not contain intramural ganglia (17). Bilateral ablation of the rat major pelvic ganglion (denervation, DEN) results in degeneration of bladder axons (18) and a rat unable to void. When the spinal cord of rats is damaged at T9 (SCI-decentralized), the rats frequently lose the ability to void spontaneously. The nonvoiding rat bladders hypertrophy. Consequently, both DEN and SCI results in hypertrophy of the urinary bladder. SCI rats have intact peripheral innervation of the bladder, although many can not void spontaneously apparently due to damage of the spinal cord which blocks the message to void from the micturition center. Thus, DEN and SCI are two different models of neurologic damage which can result in a nonvoidiig animal. We compared the fbnction and density of muscarinic receptor subtypes from the urinary bladders from SCI and DEN rats.
We measured the density of total, M2 and M3 receptors by subtype selective immunoprecipitation and calculated affinity values for a panel of muscarinic antagonists for inhibition of carbachol induced contractions three weeks after bladder denervation. Similar studies were performed on rats 10 days after SCI in order to compare the effects of DEN with decentralization on the density and function of muscarinic receptor subtypes in the rat urinary bladder.
Methods
Materials
Frozen normal rat bladders were purchased from Pel Freeze Biologic (Rogers, AR). The following drugs or chemicals were obtained from the sources indicated: carbachol, sodium cholate, protease inhibitors, atropine (Sigma Chemical Company, St. Louis, MO), methoctramine, 4-DAMP, 4-DAMP mustard, and p-F-HHSiD (Research Biochemicals International, Natick, MA), [3H]QNB (43 Ci/mM Dupont-New England Nuclear Research Products, Wilmington, DE), pansorbin (Calbiochem Inc., La Jolla, CA), digitonin (Gallard-Schlesinger Industries Inc., Carle Place, NY).
Surgery
Rats (200-250 g female Sprague-Dawley rats from Ace Animals Inc., Boyertown, PA) were anesthetized with 2% isoflurane in oxygen and a midline incision was made in the lower abdomen. For bilateral denervation, both the left and right major pelvic ganglion were cauterized with a Valleylab Inc.(Boulder, CO) handstitching pencil attached to a Model SSE 2 solid state electrosurgery device (Valleylab Inc., Boulder, CO). For sham operated animals, the plexus was exposed but left intact. For spinal cord injury a T8-T10 laminectomy was performed and the cord was compressed with a 35 g weight for 10 min at T9. After surgery, urine was expressed by the use of manual pressure on the lower abdomen of the animals twice daily.
Muscle Strips
Urinary bladders were removed from rats euthanized by decapitation. The urinary bladder body (tissue above the ureteral orifices) was dissected free of the serosa and surrounding fat. The bladder was divided in the mid-sagittal plane, then cut into longitudinal smooth muscle strips (approximately 4 mm × 10 mm). The muscle strips were then suspended with 1 g of isometric tension in tissue baths containing 15 ml of modified Tyrodes solution (125 mM NaCl, 2.7 mM KCl, 0.4 mM NaH2PO4, 1.8 mM CaCl2, 0.5 mM MgCl2, 23.8 mM NaHCO3, and 5.6 mM glucose) and equilibrated with 95/5% O2/CO2 at 37 °C. The strips were tested for their ability to contract in response to electric field stimulation of 8 volts, 30 Hz, 1 ms duration. The electric field stimulation was generated by a solid-state square wave stimulator (Model S88, Grass Instruments, Quincy MA) interfaced through a stimulus power booster (Stimu-Splitter II, Med-Lab Instruments, Loveland, CO) in order to maintain the amplitude, duration, and shape of the stimulus signal which is transmitted to 12 tissue baths in parallel simultaneously. The 2.5 cm long serpentine shaped platinum electrodes are situated parallel to the long axis of the muscle strips approximately 1.25 cm apart in 15 ml organ baths (Radnoti Glass Technology, Monrovia, CA).
Carbachol Concentration-Effect
Following equilibration to the bath solution for 30 minutes, bladder strips were incubated for 30 minutes in the presence or absence of antagonist. Concentration-effect curves were derived from the peak tension developed following cumulative addition of carbachol (10 nM to 300 μM final bath concentration). An EC50 value for each strip was determined from an nonlinear least squares sigmoidal curve fit of the data (Origin MicroCal Software, Inc., Northampton, Mass). The EC50 values determined in the presence of antagonist were used to generate Schild plots in order to calculate pA2 values for each antagonist. If the slope of the Schild plot was not significantly different from unity, then the slope of the Schild plot was constrained to unity in order to calculate the pKb value. When the slope of the Schild plot was significantly less than unity, pA2 values with slope are reported.
Immunoprecipitations
A two step solubilization procedure for immunoprecipitation (19) and antibodies (10) were used as previously described. Muscarinic receptor density is reported as fmoles/mg protein in the solubilized receptor preparations.
Statistical and data analysis
Results are reported as means ± S.E.M. The contractility data curves were generated by a curve fitting program (Origin, MicroCal Software, Inc., Northampton, Mass) based on a sigmoidal fit of the data for the concentration-effect curves and a linear fit for the Schild plots. Statistical analysis of multiple group comparison was performed by analysis of variance (ANOVA) with a post hoc Scheffé test (GB-STAT, Dynamic Microsystems, Silver Spring, MD) or Student’s t test where appropriate. Statistically significant differences in the afbnity values and departure from unity in the slopes derived from the Schild plots were determined using 95% confidence intervals.
Results
General Findings
DEN induced hypertrophy of the rat urinary bladder. DEN bladders weighed on average 505±51 mg (n=9) which was significantly more (p<0.01) than sham operated DEN bladders (98±5 mg, n=10). The bladders of SPV SCI animals weighed 184±17 mg (n=14), significantly less than the bladders of NV SCI rats (644±92 mg, n=10). Bladders from both SPV and NV SCI animals weighed significantly more than bladders from sham spinal cord injured animals (105±5 mg, n=5). While the bladders from SCI animals contracted normally to electric field stimulation, DEN bladders did not contract (data not shown).
Agonist Affinity
There was no difference between the EC50 values of carbachol for inducing contractions in control and sham operated bladder strips (data not shown). As a consequence these values were pooled for comparison to DEN bladders. The EC50 of carbachol for inducing contractions in DEN bladders (0.71±0.09 μM) was significantly lower (p<0.05) than pooled sham operated and control bladders (1.26±0.21 μM). The EC50 of carbachol for inducing contractions in SPV SCI bladders (EC50=0.93±0.3 μM) was not different from normal control or sham operated control SCI bladders (1.8±0.3 μM), however the EC50 of carbachol for inducing contractions in NV SCI bladders (EC50=0.54±0.1 uM) was significantly lower than either control.
Antagonist Affinities
Schild analysis of the shift in the carbachol dose response curves for a series of muscarinic receptor antagonists revealed a dose dependent competitive inhibition of bladder muscle contraction. As previously shown using PZP, methoctramine, 4-DAMP, and p-F-HHSiD, muscarinic receptor antagonists inhibited carbachol stimulated muscle contractions in control bladders at a concentration consistent with M3 receptors directly mediating muscle contraction (10, 16). However, as previously shown (20), in DEN bladders the affinities of 4-DAMP (pKb=8.5±0.2) and p-F-HHSiD (pKb=6.5±0.4) for inhibiting carbachol induced contractions are consistent with M2 receptors directly mediating muscle contraction (7, 12). The affinity of methoctramine for inhibiting carbachol induced contractions in the DEN rat bladder (pA2=6.5±0.5) is consistent with M3 receptors directly mediating muscle contraction. This affinity is not different from the affinity of methoctramine in control bladders. However, the slope of the Schild plot for DEN bladders was 0.60, significantly less than unity, as opposed to a slope not different from unity in control bladders. In sham operated controls, the affinities of methoctramine (pKb=6.2±0.4) and p-F-HHSiD (pKb=7.7±0.6) for inhibiting carbachol induced contractions are consistent with M3 receptors directly mediating muscle contraction as is the case in normal bladders. The affinity of p-F-HHSiD for inhibiting carbachol induced contractions in bladders from NV SCI rats is consistent with M2 receptors directly mediating muscle contraction (pKb=6.4±0.2). However, in bladders from SPV SCI and sham operated SCI animals, the affinity of p-F-HHSD (pA2=7.9±0.2, slope=0.65, pKb=7.5±0.2, respectively) is consistent with M3 receptors mediating this response or possibly M2 and M3 receptors in SPV SCI animals (Figures 1, 2).
Fig. 1.
Carbachol Dose-Response Displacement curves and Schild plot (inserts) for p-F-HHSiD, Effect on Bladder Strips From Chronic Spinal Cord Injured Rats in-vitro. Each curve represents the average responses of muscle strip preparations expressed as the percent of each individual strip’s maximal carbachol response. These maximal responses (average g ± S.E.M.) for SPV SCI bladders were 2.5±0.3 for control (n=15); 3.1±0.3 (n=14) and 2.2±0.2 (n=14) for 0.3 and 3 μM p-F-HHSiD respectively. The maximal responses for NV SCI bladder strips were 2.4± 0.3 for control (n=17); 2.7±0.3 (n=6), 2.1± 0.2 (n=11), 2.6±0.5 (n=13) and 2.5± 0.6 (n=8) for 0.1, 0.3, 3, and 10 μM p-F-HHSiD respectively. There was no significant difference in maximum between groups.
Fig. 2.
Antagonist Affinity for Inhibition of in-vitro Bladder Strip Contraction from Spinal Cord Injured and Denervated Rats. Affinities were determined as described in methods.
Immunoprecipitation
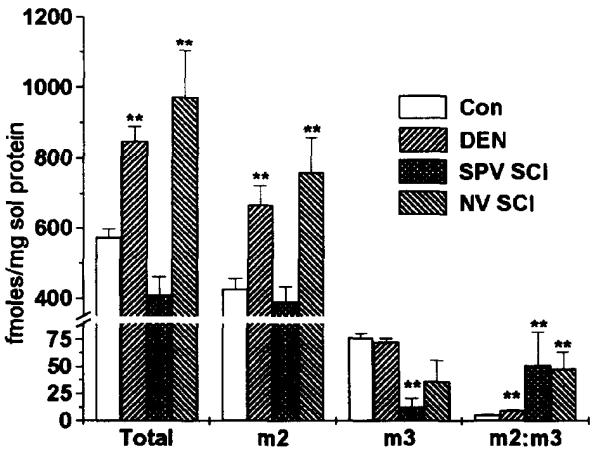
The total muscarinic receptor density (fmoles/mg solubilized protein) in DEN bladders was significantly (p<0.01) higher than in either sham operated or in unoperated control bladders. Also as can be seen in figure 3, the density of M2 receptors was also signficantly higher in denervated bladders than in either sham operated (p<0.05) or unoperated controls (p<0.01). There was no difference in the density of M3 receptors. The sum of the M2 and M3 receptors precipitated accounted for 87%, 92%, and 87% of the total receptors solubilized for unoperated control, sham operated control, and denervated bladders, respectively.
Fig. 3.
Precipitation of M2 and M3 Muscarinic Receptor Subtypes from the Bladder of Control, DEN, SPV SCI, and NV SCI Rats. Receptors were labeled with [3H] QNB and solubilized as described in Luthin et. al. (19). Data shown are average fmoles of receptor/mg solubilized protein ± S.E.M. from individual DEN (n=4) and NV SCI (n=6) bladders, pooled normal (n=4) and pooled SPV SCI bladders (n=2) or the ratio of m2:m3 receptors. Protein concentration in the solubilized receptor preparation was approximately 8% of the protein concentration in the crude homogenate. As compared to filtration binding, approximately 50% of the muscarinic receptors were solubilized (data not shown). ** denotes significant difference (p<0.01) from control.
Total muscarinic receptor density in bladders from NV SCI animals was significantly higher (p<0.01) than in control bladders. The increase in M2 receptor density accounted for all this increase, with no change in M3 receptor density. Bladders from SPV SCI animals showed no change in total receptor, or M2 receptor density although there was a decrease in M3 receptor density. The sum of the M2 and M3 receptors precipitated accounted for 83%, 89%, and 87% of the total receptors solubilized in bladders from NV SCI, SPV SCI, and control animals, respectively.
Discussion
Bilateral ablation of the major pelvic ganglion produced rats unable to void. The bladders of these animals were severely hypertrophied weighing 515% of control bladders. Compression injury to the spinal cord of rats at T9 yielded two groups of animals with respect to their ability to void. Some of the animals regained the ability to void spontaneously. The bladders from the SPV SCI animals were mildly hypertrophied (187% of control). The rats that regained the ability to void spontaneously on days 1 and 2 post-injury tended to have smaller bladders than the rats whose spontaneous voiding recovered later (data not shown). The bladders from NV SCI rat were severely hypertrophied weighing 657% of control bladders. Consistent with other reports, in bladders from both NV SCI and DEN rats, we observed a shift to the leg in the carbachol concentration effect curve termed “increased responsiveness” or “denervation induced supersensitivity” (21, 22). The EC50 of carbachol for inducing contractions in these bladders was significantly lower than in control bladders. The bladders from SPV SCI rats did not show a supersensitivity to carbachol.
Despite the predominance of M2 subtypes in rat bladder, pharmacologic evidence based on the affinity of a panel of subtype selective muscarinic antagonists is most consistent with M3 muscarinic receptors directly mediating smooth muscle contraction (10, 20). Baaed on the pharmacological data obtained with DEN and NV SCI rat bladders compared with normal and sham operated control rat bladders, it appears that in these bladders, M2 receptors provide a contractile fbnction which is mediated by M3 receptors in normal and spontaneously voiding SCI bladders (Figure 2). The reason that both 4-DAMP and p-F-HHSiD produced affinities consistent with M2 mediated contraction while methoctramine yielded an affinity consistent with M3 mediated contraction in DEN bladders is unknown. The slope of the Schild plot of p-F-HHSiD for inhibition of carbachol induced contractions of SPV SCI bladders was less than one as was the slope of the Schild plot for methoctramine inhitition of bladder contraction from DEN rats. One explanation for a Schild plot having a slope of less than one is the interaction of more than one receptor subtype mediating the response. It is possible that a combination of M2 and M3 receptors is mediating contraction in the denervated and spinal cord injured hypertrophic bladder.
In general agreement with others, we show that denervation of the rat urinary bladder induced an increase in the density of total muscarinic receptors (22, 23). The apparent change in function of the M2 receptor could be the direct result of the selective increase in M2 receptor density in DEN and NV SCI bladders (Figure 3). No increase in M2 receptor density was seen in bladders from SPV SCI rats. However, the M3 receptor density in these bladders was lower than in normal bladders.
The M2:M3 receptor ratio is similar in both NV and SPV SCI bladders. Even though this ratio is greater in SPV SCI rat bladders than in DEN bladders, M3 receptors mediate carbachol induced contractions in SPV SCI bladders while M2 receptors mediate these contractions in DEN bladders. Data from selective M3 receptor alkylation studies using 4-DAMP mustard show that even though a major proportion of M3 receptors are inactivated, carbachol induced contractions are still mediated by M3 receptors (24). Based on these finding, it appears that the absolute density of M2 receptors, is more important than the M2:M3 ratio in determining which subtype mediates contraction. However, other differences resulting from adaptation of the bladder induced by either hypertrophy or the increased mechanical stretch imposed on the hypertrophied bladders may not allow for straightforward comparison of the results between these paradigms.
In conclusion, in both DEN and NV SCI bladders unlike SPV SCI bladders, there is an increase in M2 muscarinic receptor density with no change in M3 receptor density. The bladders from these animals are severely hypertrophied and show an increased responsiveness to carbachol. The affinity of muscarinic receptor antagonists for inhibition of carbachol induced smooth muscle contraction switches corn being consistent with M3 receptor mediated contraction in control bladders to M2 mediated or a combination of M2 and M3 receptor mediated contraction in DEN and NV SCI bladders.
References
- 1.WEIN AJ, LEVIN RM, BARRET DM. Adult and Pediatric Urology. In: Gillenwater JY, Grayhack JT, Howards SS, Duckett JD, editors. Year Book Medical Publishers Inc.; Chicago: 1987. [Google Scholar]
- 2.YALLA SV, MCGUIRE EJ, ELBADAWI A, BLAVIS JG. Neurourology and Urodynamics. Macmillan Publishing Co.; New York: 1988. [Google Scholar]
- 3.LEVIN RM, SHOFFER F, WEIN AJ. J. Phannacol Exp. Ther. 1980;212:536–540. [PubMed] [Google Scholar]
- 4.HAMMER R, BERRIE CP, BIRDSALL NM, BURGER ASV, HULME EC. Nature (London) 1980;283:90–92. doi: 10.1038/283090a0. [DOI] [PubMed] [Google Scholar]
- 5.WATSON M, ROESKE WR, VICKROY TW, SMITH TL, AKIYAMA K, GULYA K, DUCKLES SP, SERRA M, ADEM A, NORDBERG A, GEHLERT DR, WAMSLEY JK, YAMAMURA HI. Trends Pharmacol. Sci. 1986;2:46–55. [Google Scholar]
- 6.BONNER TI, BUCKELY NJ, YOUNG AC, BRANN MR. Science. 1987;237:527–532. doi: 10.1126/science.3037705. [DOI] [PubMed] [Google Scholar]
- 7.CAULFIELD MP. Phartnac. Ther. 1993;58:319–379. doi: 10.1016/0163-7258(93)90027-b. [DOI] [PubMed] [Google Scholar]
- 8.DORJE F, LEVEY AI, BRANN MR. Mol. Pharm. 1991;40:459–462. [PubMed] [Google Scholar]
- 9.MAEDA A, KUBO Y, MISHINA M, NUMA S. FEBS Lett. 1988;239:339–342. doi: 10.1016/0014-5793(88)80947-5. [DOI] [PubMed] [Google Scholar]
- 10.WANG P, LUTHIN GR, RUGGIERI MR. J. Pharmacol. Exp. Ther. 1995;273:959–966. [PMC free article] [PubMed] [Google Scholar]
- 11.LATIFPOUR J, GOUSSE A, KONDO S, MORIT T, WEISS RM. J. Pharmacol. Exp. Ther. 1989;248:81–88. [PubMed] [Google Scholar]
- 12.EGLEN RM, HEGDE SS, WATSON N. Pharm. Rev. 1996;48:531–565. [PubMed] [Google Scholar]
- 13.HEGDE SS, CHOPPIN A, BONHAUS D, BRIAUD S, LOEB M, MOY TM, LOURY D, EGLEN RM. Br. J. Pharm. 1997;120:1409–1418. doi: 10.1038/sj.bjp.0701048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D’AGOSTINE G, KILBINGER H, CHIARI MC, GARNA E. J. Pharmacol. Exp. Ther. 1986;239:522–526. [PubMed] [Google Scholar]
- 15.SOMOGYI GT, TANIWITZ M, DE GROAT WC. J. Physiol. 1994;480:81–89. doi: 10.1113/jphysiol.1994.sp020342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.BRAVERMAN AS, KOHN IJ, LUTHIN GR, RUGGIERI MR. Am. J. Physiol. 1998;274:R517–R523. doi: 10.1152/ajpregu.1998.274.2.r517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.GABELLA G, UVELIUS B. Cell Tiss. Res. 1990;262:67–69. doi: 10.1007/BF00327747. [DOI] [PubMed] [Google Scholar]
- 18.EKSTROM J, ELME M. Acta Physiol. Scand. 1977;111:81–86. doi: 10.1111/j.1748-1716.1981.tb06708.x. [DOI] [PubMed] [Google Scholar]
- 19.LUTHIN GR, HARKNESS J, ARTMYSHYN RP, WOLFE BB. Mol. Pharm. 1988;34:327–333. [PubMed] [Google Scholar]
- 20.BRAVERMAN AS, LUTHIN GR, RUGGIERI MR. Am. J. Physiol. 1998 doi: 10.1152/ajpregu.1998.275.5.R1654. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.EKSTROM J, MALMBERG L. Acta Physiol. Scand. Ass. 1984:175–179. doi: 10.1111/j.1748-1716.1984.tb07495.x. [DOI] [PubMed] [Google Scholar]
- 22.NILVEBRANT L, EKSTROM J, MALMBERG L. Acta Pharmacol. et toxicol. 1986;59:306–314. doi: 10.1111/j.1600-0773.1986.tb00174.x. [DOI] [PubMed] [Google Scholar]
- 23.GUNASENA KT, NIMMO AJ, MORRISON JFB, WHITAKER EM. Br. J. Urol. 1995;76:291–296. doi: 10.1111/j.1464-410x.1995.tb07703.x. [DOI] [PubMed] [Google Scholar]
- 24.BRAVERMAN AS, LUTHIN GR, RUGGIERI MR. Soc. Neurosci. Abstr. 1997;23:2022. [Google Scholar]