Abstract
Leg exercise hemodynamics during single-leg knee extensions were compared among healthy groups of early perimenopausal (n = 15), late perimenopausal (n = 12), and early postmenopausal (n = 11) women. Femoral blood flow (FBF) and vascular conductance (FVC) at rest and during very light work rates (0 and 5 W) were similar among all three menopause stage groups. Vascular responses at 10 W (FBF) and 20 W (FBF and FVC) were significantly higher (P < 0.05) in early perimenopausal compared with late perimenopausal women. At 15 and 25 W, FBF and FVC were similar between late perimenopausal and early postmenopausal groups but higher (P < 0.05) in early perimenopausal women as compared with the other two menopausal groups. In the combined sample of all three menopause stage groups, follicle-stimulating hormone was significantly correlated with vascular conductance during submaximal (15 W) exercise (R = −0.56, P < 0.001), even after adjustment for age, fitness, LDL cholesterol, and abdominal fat (R = −0.46, P = 0.005). Collectively, these findings suggest that in middle-aged women, there is an association between menopause stage and leg vascular responsiveness during exercise.
Keywords: exercise hyperemia, menopause transition, reproductive hormones
Introduction
In healthy, moderately active humans, our laboratory has previously identified a sex-specific influence of aging on local hemodynamic responses to dynamic leg exercise. Whereas leg hyperemia and vascular conductance are well preserved during submaximal exercise in older men that are neither endurance trained nor overly sedentary (Parker et al. 2008a; Proctor et al. 2003b), leg blood flow and vascular conductance responses during submaximal two-legged cycling (Proctor et al. 2003a) and single-leg knee extensor exercise (Parker et al. 2008a, 2008b) are attenuated in older versus younger women. Although the mechanisms underlying these age-associated reductions in exercise hyperemia and vasodilation in women are unknown, evidence suggests that they may be related to menopausal status (Parker et al. 2011).
The menopause transition is a critical time period for women’s health (Bittner 2009, Villablanca et al. 2010), and the menopause-associated decline in circulating estrogen has been linked with elevated cardiovascular risk (Villablanca et al. 2010). Vascular function is vulnerable to estrogen deficiency as estrogen receptors are expressed in human vascular smooth muscle (Hodges et al. 2000; Karas et al. 1994; Register and Adams 1998), as well as in endothelial cells (Venkov et al. 1996), and acute estrogen deprivation following ovariectomy (i.e., surgical menopause) impairs brachial artery flow-mediated dilation (Ohmichi et al. 2003; Takahashi et al. 2007) and forearm microvascular responses to acetyicholine (Pinto et al. 1997; Virdis et al. 2000). Likewise, estrogen is capable of decreasing the production of vasoconstrictor substances (endothelin-I and angiotensin II) (Tostes et al. 2003), blunting sympathetically mediated vasoconstrictor responses in women (Sudhir et al. 1997; Fadel et al. 2004) and increasing the production of endothelial-derived vasodilators (nitric oxide and prostaglandins) (Caulin-Glaser et al. 1997; Chambliss and Shaul 2002; Chen et al. 1999; Hayashi et al. 1995; Tostes et al. 2003), which are known to have a functional role in leg exercise hyperemia (Boushel et al. 2002; Mortensen et al. 2007; Heinonen et al. 2011). In this regard, vasodilator responsiveness in exercising muscle may be reduced during the transition into menopause.
Although there is rationale to suggest that menopause (and its associated decline in ovarian hormone production) may contribute to attenuated vascular responses during exercise, the time course for age-associated reductions in exercise-induced blood flow and vasodilation in women remains incompletely understood. Furthermore, there is little or no information available on changes in vascular responses to exercise in midlife women undergoing the menopause transition (between the early perimenopausal and early postmenopausal period) (Bittner 2009). Therefore, the purpose of the present study was to test the hypothesis that leg exercise hemodynamic responses are associated with menopause stage in healthy, middle-aged women.
Methods
Participants
Forty healthy, middle-aged (40–60 years) women were recruited from a general community-based (Centre County, Pennsylvania) sample. Two women were excluded from data analysis because of technical errors measuring blood flow velocity. The remaining 38 women were divided into early perimenopausal (n = 15), late perimenopausal (n = 12), and early postmenopausal (n = 11) groups based on self-reported bleeding history using the Stages of Reproductive Aging Workshop (STRAW) criteria (Soules et al. 2001; Harlow et al. 2007). Perimenopausal women reported experiencing irregular or infrequent menstrual cycles during the previous 12 months not associated with pathology. Early perimenopausal women reported variable cycle length (>7 days different from normal), and late perimenopausal women reported two or more skipped cycles or ≥60 days of amenorrhea (but less than 12 months). Early postmenopausal women reported not experiencing a menstrual cycle during the previous 12 months but were less than 5 years past their final menstrual period.
Only women that were considered healthy as determined by medical history questionnaire, physical examination, resting electrocardiogram, blood tests for plasma lipid concentrations, and resting blood pressure below 140/90 mm Hg were included in the present study. No women had any risk factors (excluding age) for cardiovascular disease at the time of this study. Also, no participants were taking any form of hormone therapy, blood-pressure, or lipid-lowering medications during or within 6 months of the study. All participants were asked to refrain from caffeine for ≥12 h prior to exercise testing. Participants provided written consent to participate in the study after receiving an explanation of the experimental procedures and possible risks associated with participation. This study was approved by the Office for Research Protections at The Pennsylvania State University in agreement with the guidelines set forth by the Declaration of Helsinki.
Hormone assessment
To further document menopause status, concentrations of reproductive hormones were measured from 10 h fasted blood samples (collected between 0730 and 1000 hours) and, in perimenopausal women with monthly cycles, between days 3–5 of menstrual bleeding. Blood samples were allowed to clot for 30 min at room temperature and then were centrifuged at 3000 rpm for 15 min at 4 °C. All samples were analyzed by Quest Diagnostics.
Estradiol-17β was analyzed using liquid chromatography tandem mass spectrometry. Analytical sensitivity for the estradiol assay was 2 pg·mL−1. Follicle-stimulating hormone (FSH) and luteinizing hormone (LH) were analyzed using an immunochemiluminometric assay with analytical sensitivity of 0.07 and 0.7 MIU·mL−1 for FSH and LH, respectively. Reproductive hormones were unable to be measured in four women (two for estradiol and two for FSH).
Body composition
Whole-body composition was assessed using dual X-ray absorptiometry (model QDR 4500W, Hologic, Waltham, Massachusetts) while participants were in the supine position. Total body fat and lean (fat-free) mass were measured with standard cut lines. Abdominal adiposity was calculated from manually defined specific regions of interest, specifically from the upper edge of the first lumbar vertebra to the anterior superior iliac spine.
Cardiorespiratory fitness testing
Each participant performed a modified Balke treadmill test to maximal effort (Stevenson et al. 1994). This graded test consisted of a 2-min warm-up at 2.5 mph followed by adjustment of speed to elicit 70%–75% of age-predicted maximal heart rate after which point the speed remained the same throughout the exercise test. The intensity of exercise increased every 2 min by increasing the elevation by 2.5% until the participant reached volitional fatigue, Pulmonary oxygen uptake was measured using indirect calorimetry (Parvomedics, Sandy, Utah), and V̇O2peak was determined as the highest 15-s average. Nearly all (35 of 38) women achieved heart rates greater than 95% of their age-predicted maximum, and all women achieved a peak respiratory exchange ratio of ≥1.05.
Physical activity
Daily physical activity was objectively measured using a uniaxial accelerometer (Actigraph model GT1M, Pensacola, Florida) that was positioned over the participant’s left hip with an adjustable elastic belt. Participants wore the accelerometer for 15 days as part of a daily diary study and were asked to take off the accelerometer during bathing, swimming, and (or) sexual activity. Data from four representative days (one day was a weekend day) were used to provide an estimate of daily physical activity levels. The accelerometer collected data in 1-min epochs, and the data were processed and analyzed using the ActiLife data analysis software from Actigraph (version 5.1.5). The following outcome measures were used: total physical activity counts and percentage of time spent in sedentary (0–100 counts·min−1), light (101–1951 counts·min−1), moderate (1952–5724 counts·min−1), and vigorous (5725+ counts·min−1) physical activity (Freedson et al. 1998).
Knee extensor exercise
On a separate day from the maximal treadmill test, women performed graded single-leg knee extensor exercise. Women were seated in a semireclined position with knees flexed at an angle of 90° and thighs strapped to the chair to avoid extraneous movement during exercise. The left foot was placed in a medical boot that was connected to the pedal arm of a Monark cycle ergometer that was placed behind the participant. Knee extensions were performed at 40 kicks·min−1 with the left leg, which moved through a nearly full range of motion (90°–150°). The exercise protocol consisted of the following three stages: stage one, quiet rest; stage two, knee extensions against no resistance (0 W) for 3 min; and stage three, knee extensions as resistance increased by 5 W every 3 min until the subject could no longer maintain the cadence.
During exercise, heart rate was measured using a modified three-lead electrocardiogram. Blood pressure (systolic, diastolic, and calculated mean arterial pressure) was measured continuously from a finger on the right hand using a beat-by-beat blood pressure system (Finometer MIDI, Finapress Medical Systems, Netherlands). For each subject, heart rate and blood pressure were averaged over the first 30 s of the last minute of each work rate during steady-state conditions.
Vascular responses to exercise
Diameter and blood flow velocity of the left common femoral artery were measured using Doppler ultrasound (HDI 5000, Philips, Bothell, Washington). Velocity measurements were sampled in real time (400 Hz) using a data acquisition system (Powerlab, AD Instruments, Castle Hill, Australia). Mean blood velocity was calculated from the first 30 s during the last minute of each work rate. High-resolution diameter measurements were taken during the last 30 s of each work rate, images were recorded directly to DVD, and diameter was measured across the cardiac cycle using edge-detection software (Brachial Analyzer Software, Medical Imaging Applications, Iowa City, Iowa). Femoral artery blood flow for each work rate was calculated by multiplying the cross-sectional area (πr2) of the femoral artery with mean blood velocity. Femoral vascular conductance was calculated by dividing blood flow by mean arterial blood pressure.
Data analysis
Statistical analyses were performed using SigmaStat (SigmaPlot 11, Systat Software Inc., San Jose, California). Significance was determined as P ≤ 0.05. Subject characteristics and resting hemodynamic measurements of different menopause stage groups were compared using one-way analysis of variance (ANOVA) with Tukey’s post hoc comparisons. Data beyond 25 W were excluded from graphical display and statistical analysis because of large subject dropout (~40%) by 30 W. A 20%–25% reduction in femoral blood flow from 15 to 20 W was observed in two early perimenopausal women due to a measurement error for blood velocity at 20 W; therefore, their hemodynamic responses were included up to 15 W. Menopause stage group comparisons of hemodynamic responses to graded exercise were made using two-way repeated measures ANOVA. Tukey’s post hoc analyses were performed when significant menopause stage × work rate interactions were detected.
Relations between blood reproductive hormone concentrations (estradiol and FSH), traditional cardiovascular risk factors (blood pressure, lipids, and glucose, as well as abdominal and whole-body composition), cardiorespiratory fitness (V̇O2max), age, and conductance at 15 W were determined by Pearson’s correlation coefficients. Partial correlations were used to statistically control for the influence of covariates for conductance al 15 W. A work rate of 15 W was chosen because it represented a moderate (~40%–75% peak W) exercise intensity for most subjects and was less than all subjects’ peak work rate
Results
Subject characteristics
The early postmenopausal women were older and had lower cardiorespiratory fitness than early and late perimenopausal women (Table 1). Plasma concentrations of reproductive hormones (FSH, LH, and estradiol) were similar between late perimenopausal and early postmenopausal women, but both groups had higher concentrations of FSH and LH but lower concentrations of plasma estradiol as compared with early perimenopausal women (P < 0.05). Although BMI was similar among menopause stage groups, early postmenopausal women had more total body and abdominal fat (P < 0.05). There were no differences in plasma lipid or glucose concentrations or resting blood pressure among the three groups.
Table 1.
Subject characteristics.
| Early perimenopausal | Late perimenopausal | Early postmenopausal | |
|---|---|---|---|
| n | 16 | 12 | 11 |
| Age (years) | 48±1 | 50±l | 54±l*† |
| Peak work rate (W) | 30±2 | 34±3 | 29±1 |
| Resting FAD (cm) | 0.88±0.02 | 0.84±0.04 | 0.83±0.03 |
| 15 Watts FAD (cm) | 0.91±0.02 | 0.87±0.03 | 0.85±0.03 |
| Reproductive hormones | |||
| FSH (mIU·mL−1) | 12±2 | 74±6* | 84±10* |
| Estradiol (pg·mL−1) | 158±52 | 31±5* | 25±4* |
| LH (mIU·mL−1) | 9±2 | 41±5* | 50±6* |
| Cardiorespiratory fitness | |||
| V̇O2peak (mL·kg−1·min−1) | 35±2 | 36±2 | 29±l*† |
| V̇O2peak (mL·kg FFM−1·min−1) | 51±2 | 51±2 | 45±l*† |
| Physical activity | |||
| Light (%) | 8.6±0.6 | 8.2±0.5 | 8.3±0.6 |
| Moderate (%) | 7.9±0.8 | 7.7±0.4 | 8.0±0.8 |
| Vigorous (%) | 0.3±0.1 | 0.5±0.2 | 0.1±0.04 |
| Body composition | |||
| BMI (kg·m−2) | 23±1 | 23±1 | 25±1 |
| Body fat (%) | 29±2 | 29±2 | 34±1* |
| Abdominal fat (kg) | 1.3±0.1 | 1.4±0.1 | 1.9±0.3* |
| Metabolic factors | |||
| Glucose (mg·dL−1) | 87±l | 85±2 | 89±2 |
| Cholesterol (mg·dL−1) | 164±6 | 181±8 | 186±5 |
| LDL (mg·dL−1) | 86±4 | 97±7 | 104±6 |
| HDL (mg·dL−1) | 65±4 | 70±4 | 66±6 |
| Trig (mg·dL−1) | 67±7 | 68±6 | 78±8 |
| Arterial blood pressure | |||
| Resting SBP (mm Hg) | 114±3 | 110±4 | 118±3 |
| Resting DBP (mm Hg) | 66±3 | 66±2 | 73±3 |
Note: Values are mean ± standard error of the mean (SEM); n, number of subjects; FAD, femoral artery diameter; FSH, follicle-stimulating hormone; LH, lutcinizing hormone; V̇O2peak, peak oxygen uptake; FFM, fat-free mass; BMI, body mass index; LDL, low-density lipoprotein; HDL, high-density lipoprotein; Trig, triglycerides; SBP, systolic blood pressure; DBP, diastolic blood pressure.
An asterisk (*) indicates a significant difference from early perimenopausal, P < 0.05.
A dagger (†) indicates a significant difference between early postmenopausal and late perimenopausal, P < 0.05.
Vascular responses to graded knee extensor exercise
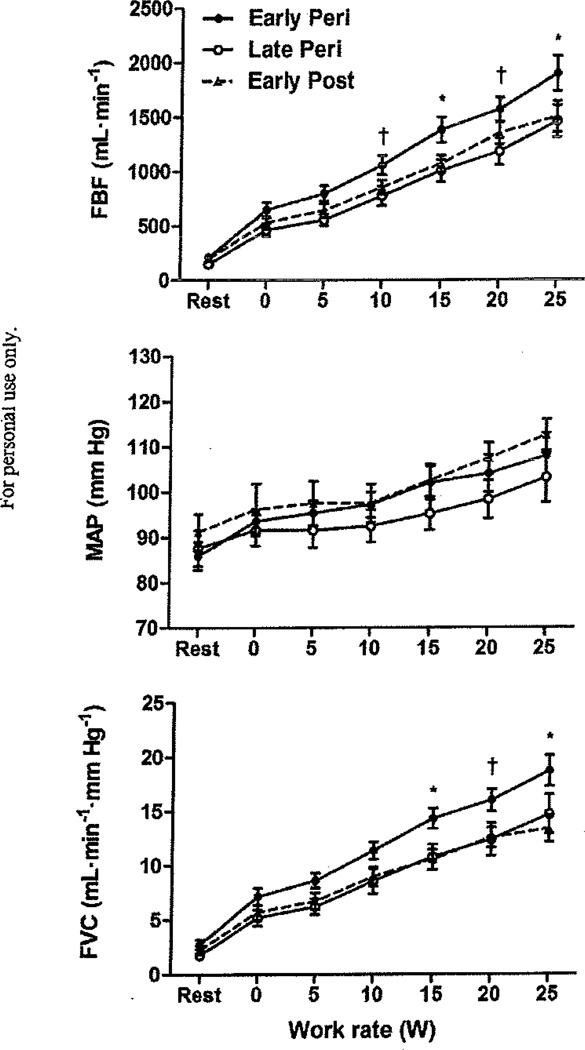
Femoral artery blood flow (FBF), mean arterial pressure (MAP), and femoral vascular conductance (FVC) increased (P < 0.001) with work rate from 0 to 25 W in all three menopause stage groups. There were no group differences in MAP during rest or graded exercise (P = 0.43). Similarly, there were no group differences in FBF or FVC at rest or during very light work rates (0 and 5 W). However, vascular responses at 10 W (FBF) and 20 W (FBF and FVC) were significantly higher (P < 0.05) in early perimenopausal compared with late perimenopausal women (Fig. 1). FBF and FVC during 20 W were higher in early perimenopausal compared with early postmenopausal women, but this difference did not reach statistical significance (P = 0.077 and 0.056, respectively). At 15 and 25 W, FBF and FVC were similar between late perimenopausal and early postmenopausal groups, but higher (P < 0.05) in early perimenopausal women as compared with the other two menopausal groups (Fig. 1).
Fig. 1.
(Top) Femoral Wood flow (FBF), (middle) mean arterial pressure (MAP), and (bottom) femoral vascular conductance (FVC) during graded knee extensor exercise in early perimenopausal (early peri), late perimenopausal (late peri), and early postmenopausal (early post) women. For early peri women, sample size (n) = 15 until 15 W, n = 13 at 20 W, and n = 11 at 25 W. For late peri women, n = 12 until 20 W, and n = 11 at 25 W. For early post women, n = 11 until 25 W. Data are group means ± SEM. A dagger (†) signifies a significant difference between early and late peri, P < 0.05. An asterisk (*) signifies that early peri is significantly different from late peri and early post, P < 0.05.
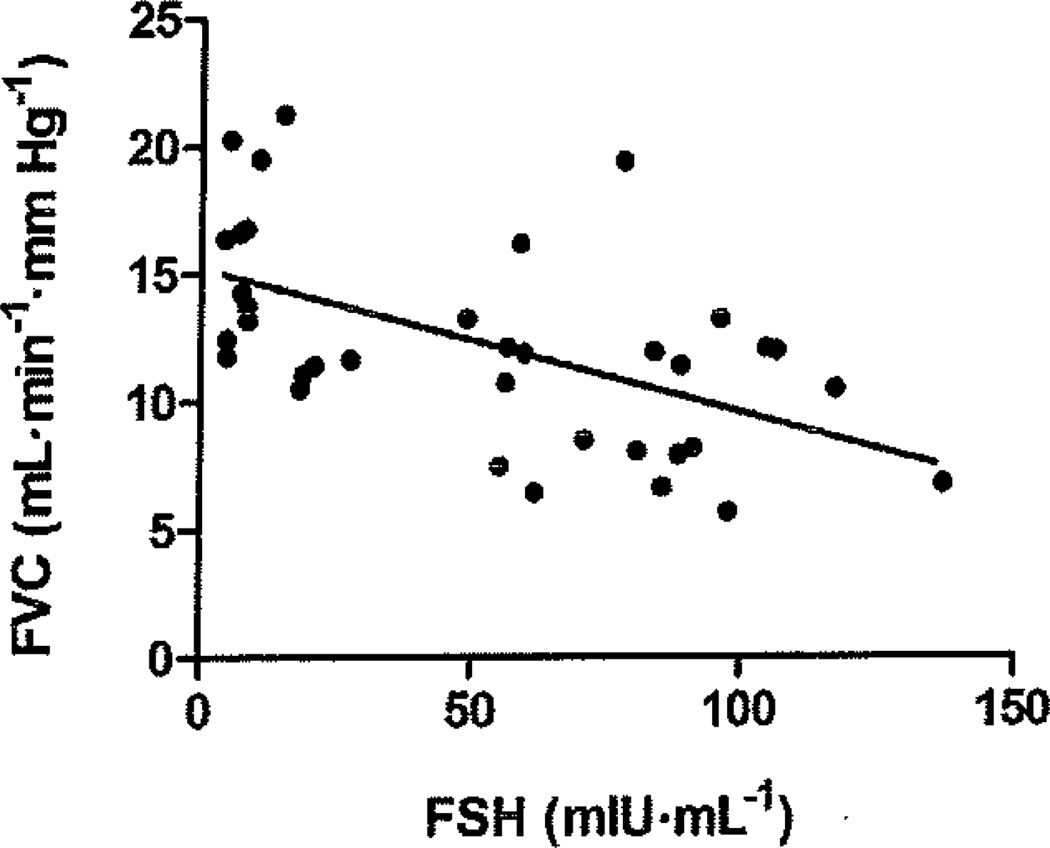
In the combined sample of all three menopause stage groups, FVC during submaximal (15 W) exercise was significantly (P < 0.05) correlated with FSH (R = −0.56; Fig. 2), estradiol (R = 0.44), V̇O2peak (R = 0.44), age (R = −0.33), abdominal fat (R = −0.34), and LDL cholesterol (R = −0.39). The relation between FSH and FVC at 15 W remained significant (R = −0.46, P = 0.005) after controlling for age, abdominal fat, V̇O2peak (normalized to fat-free mass), and LDL cholesterol. Furthermore, the relation between FVC at 15 W and age was abolished after controlling for FSH (R = −0.011, P = 0.95).
Fig. 2.
Relation between femoral vascular conductance (FVC) during submaximal (15 W) exercise and follicle-stimulating hormone (FSH) in the combined sample (n = 36) of early perimenopausal, late perimenopausal, and early postmenopausal women, R = −0.56, P < 0.001.
Discussion
The new major finding of the present study is that leg blood flow and vascular conductance responses to graded submaximal exercise appear to be associated with menopause stage in healthy, middle-aged women such that vasodilation and functional hyperemia are attenuated in late perimenopausal and early postmenopausal women. Additionally, the vascular conductance response during exercise does not appear to be influenced by chronological age, after controlling for the influence of FSH, a marker of reproductive age in middle-aged women.
Menopause status and leg vascular responses to exercise
The changing hormonal milieu during menopause has been suspected to underlie the age-related reductions in leg vascular responses during exercise (Proctor and Parker 2006). However, most studies on aging and active leg blood flow in women have been limited to two age groups: young (20–30 years) and old (60–75 years) (Parker et al. 2008a, 2008b; Proctor et al. 2003a). Parker et al. (2011) recently provided evidence that leg blood flow and conductance during knee extensor exercise are influenced by menopausal status, independent of age, in middle-aged and older women. However, the exercise protocol used was limited to a single, light work intensity (~10 W), and no perimenopausal women were included and reproductive hormones were not measured. The results of our present study provide additional support for an association between menopause stage and leg hemodynamic responses to exercise. Furthermore, we have extended the results of Parker et al. (2011) by providing evidence that the onset of menopause stage associated alterations in leg exercise vasodilation occur within the perimenopausal period (between early and late perimenopause) and across multiple exercise intensities.
To our knowledge, the findings of the present study also provide the first evidence that there is a significant relation between a marker of reproductive age (plasma FSH) and the vascular conductance response to exercise. Despite the wide-spread vascular actions of estrogen, plasma FSH was a stronger predictor of FVC at 15 W (R = 0.44 vs. R = −0.56, respectively; both P < 0.01). Interestingly, these findings are consistent with those concerning menopause stage dependent changes in bone metabolism in which FSH, but not estradiol, was correlated with bone resorption rates in middle-aged women (Ebeling et al. 1996). It is important to note that this should not exclude estradiol as a potential mediator of menopause stage differences in exercise hemodynamics as FSH may simply provide a more reliable marker of mean exposure to ovarian hormones, including estrogen, than single measurements of circulating estradiol concentrations (Khosla et al. 2011; Sowers et al. 2006). However, FSH may also have a direct influence on vascular responses to exercise, independent of estradiol. The potential for FSH to independently influence extragonadal tissue is supported by its ability to directly regulate the activity and production of cells involved in bone metabolism (Iqbal et al. 2010). Furthermore, FSH has been shown to enhance the secretion of inflammatory cytokines (tumor necrosis factor-α and interleukin-6) that can impair vascular reactivity (Rajsheker et al. 2010; Yudkin et al. 2005) from cultured monocytes (Cannon et al. 2010). Although this suggests an interesting potential mechanism for menopause stage dependent alterations in vascular function, we did not measure circulating cytokine concentrations in our present study. Taken together, further investigation of the specific influences of FSH and estradiol as they relate to menopause stage dependent alterations in vascular function is clearly warranted.
Experimental considerations
Despite strict inclusion criteria to limit the confounding influences of age, medication use, and metabolic factors, interpretation of our data may be limited by the study’s cross-sectional design. As such, a direct causal effect of menopausal stage on attenuated exercise vascular responses cannot be made. However, the findings of the present study suggest that exercise hemodynamics may differ at different points along the menopause transition and provide rationale for conducting future studies that make longitudinal assessments of vascular function during exercise in midlife women, with careful attention to reproductive stage. Lower femoral blood flow and conductance responses in late perimenopausal and early postmenopausal women could also potentially reflect differences in muscle blood flow kinetics, which may be slowed in older humans (DeLorey et al. 2004), Therefore, future studies should also focus on the kinetics of the exercise hyperemic response as they relate to the menopause transition. Lastly, we did not manipulate circulating reproductive hormone concentrations in this study. Therefore, their role in mediating reproductive stage associated changes in vascular responses during exercise remains speculative.
In contrast to previous studies on healthy aging from our laboratory that have focused primarily on normally active women (20th–80th percentile for age-predicted V̇O2max), the present study included women with an even wider range of cardiorespiratory fitness. Consequently, we deemed it important to control for the potential influence of fitness on vascular responses during exercise. After controlling for V̇O2peak, FSH was still significantly correlated with exercise-induced vasodilation in our overall sample. Thus menopause stage group differences in exercise-induced hemodynamic responses are not likely explained by differences in cardiorespiratory fitness in our sample of middle-aged women.
Conclusions
The results of the present study suggest that healthy aging in women is associated with a menopause stage specific attenuation of functional vasodilation and hyperemia during dynamic leg exercise. Specifically, these hemodynamic alterations in the peripheral vasculature appear to occur within the perimenopausal period (from early to late perimenopause). Although leg exercise hyperemia and vasodilation were similar between late perimenopausal and early postmenopausal groups, the possibility that additional years beyond menopause may result in further reductions in active limb blood flow and vasodilation cannot be excluded.
Acknowledgments
We thank the participants for their willingness to volunteer for our study. We also acknowledge the GCRC clinical staff and Sandra Smithmyer for assisting with subject screening and scheduling, as well as with data collection. The following funding is greatly appreciated: K12 HD055882 (PI: Weisman; Mentee: Elavsky); M01 RR-10732 (General Clinical Research Center); and the Social Sciences Research Institute and Center on Population Health and Aging at Pennsylvania State University (S. Elavsky).
Contributor Information
David J. Moore, Noll Laboratory, Department of Kinesiology, The Pennsylvania State University, University Park, PA 16802, USA; Intercollege Graduate Degree Program in Physiology, The Pennsylvania State University, University Park, PA 16802, USA
Joaquin U. Gonzales, Noll Laboratory, Department of Kinesiology, The Pennsylvania State University, University Park, PA 16802, USA; Department of Health, Exercise & Sport Sciences, Texas Tech University, Lubbock, Texas, USA
Steven H. Tucker, Noll Laboratory, Department of Kinesiology, The Pennsylvania State University, University Park, PA 16802, USA
Steriani Elavsky, Noll Laboratory, Department of Kinesiology, The Pennsylvania State University, University Park, PA 16802, USA.
David N. Proctor, Noll Laboratory, Department of Kinesiology, The Pennsylvania State University, University Park, PA 16802, USA; General Clinical Research Center, The Pennsylvania State University, University Park, PA 16802, USA; Intercollege Graduate Degree Program in Physiology, The Pennsylvania State University, University Park, PA 16802, USA
References
- Bittner V. Menopause, age, and cardiovascular risk: a complex relationship. J. Am. Coll. Cardiol. 2009;54(25):2374–2375. doi: 10.1016/j.jacc.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Boushel R, Langberg H, Gemmer C, Olesen J, Crameri R, Scheede C, et al. Combined inhibition of nitric oxide and prostaglandins reduces human skeletal muscle blood flow during exercise. J. Physiol. 2002;543(2):691–698. doi: 10.1113/jphysiol.2002.021477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon JG, Cortez-Cooper M, Meaders E, Stallings J, Haddow S, Kraj B, et al. Follicle-stimulating hormone, interleukin-1, and bone density in adult women. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010;298(3):R790–R798. doi: 10.1152/ajpregu.00728.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulin-Glaser T, Garcia-Cardena G, Sarrel P, Sessa WC, Bender JR. 17 beta-estradiol regulation of human endothelial cell basal nitric oxide release, independent of cytosolic Ca2+ mobilization. Circ. Res. 1997;81(5):885–892. doi: 10.1161/01.res.81.5.885. [DOI] [PubMed] [Google Scholar]
- Chambliss KL, Shaul PW. Estrogen modulation of endothelial nitric oxide synthase. Endocr. Rev. 2002;23(5):665–686. doi: 10.1210/er.2001-0045. [DOI] [PubMed] [Google Scholar]
- Chen Z, Yuhanna IS, Galcheva-Gargova Z, Karas RH, Mendelsohn ME, Shaul PW. Estrogen receptor alpha mediates the nongenomic activation of endothelial nitric oxide synthase by estrogen. J. Clin. Invest. 1999;103(3):401–406. doi: 10.1172/JCI5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLorey DS, Kowalchuck JM, Paterson DH. Effect of age on O2 uptake kinetics and the adaptation of muscle deoxygentation at the onset of moderate-intensity cycling exercise. J. Appl. Physiol. 2004;97(1):165–172. doi: 10.1152/japplphysiol.01179.2003. [DOI] [PubMed] [Google Scholar]
- Ebeling PR, Atley LM, Guthrie JR, Burger HG, Dennerstein L, Hopper JL, Wark JD. Bone turnover markers and bone density across the menopausal transition. J. Clin. Endocrinol. Metab. 1996;81(9):3366–3371. doi: 10.1210/jcem.81.9.8784098. [DOI] [PubMed] [Google Scholar]
- Fadel PJ, Wang Z, Watanabe H, Arbique D, Vongpatanasin W, Thomas GD. Augmented sympathetic vasoconstriction in exercising forearms of postmenopausal women is reversed by oestrogen therapy. J. Physiol. 2004;561(3):893–901. doi: 10.1113/jphysiol.2004.073619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med. Sci. Sports Exerc. 1998;30(5):777–781. doi: 10.1097/00005768-199805000-00021. [DOI] [PubMed] [Google Scholar]
- Harlow SD, Crawford S, Dennerstein L, Burger HG, Mitchell ES, Sowers MF. Recommendations from a multi-study evaluation of proposed criteria for staging reproductive aging. Climacteric. 2007;10(2):112–119. doi: 10.1080/13697130701258838. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Yamada K, Esaki T, Kazuya M, Satake S, Ishikawa T, et al. Estrogen increases endothelial nitric oxide by a receptor-mediated system. Biochem. Biophys. Res. Commun. 1995;214(3):847–855. doi: 10.1006/bbrc.1995.2364. [DOI] [PubMed] [Google Scholar]
- Heinonen I, Saltin B, Kemppainen J, Sipila HT, Oikonen V, Nuutila P, et al. Skeletal muscle blood flow and oxygen uptake at rest and during exercise in humans: a pet study with nitric oxide and cyclooxygenase inhibition. Am. J. Physiol, Heart Circ. Physiol. 2011;300(4):H1510–H1517. doi: 10.1152/ajpheart.00996.2010. [DOI] [PubMed] [Google Scholar]
- Hodges YK, Tung L, Yan XD, Graham JD, Horwitz KB, Horwitz LD. Estrogen receptors alpha and beta: prevalence of estrogen receptor beta mRNA in human vascular smooth muscle and transcriptional effects. Circulation. 2000;101(15):1792–1798. doi: 10.1161/01.cir.101.15.1792. [DOI] [PubMed] [Google Scholar]
- Iqbal J, Sun L, Zaidi M. Commentary — FSH and bone 2010: evolving evidence. Eur. J. Endocrinol. 2010;163(1):173–176. doi: 10.1530/EJE-10-0397. [DOI] [PubMed] [Google Scholar]
- Karas RH, Patterson BL, Mendelsohn ME. Human vascular smooth muscle cells contain functional estrogen receptor. Circulation. 1994;89(5):1943–1950. doi: 10.1161/01.cir.89.5.1943. [DOI] [PubMed] [Google Scholar]
- Khosla S, Melton LJ, III, Riggs BL. The unitary model for estrogen deficiency and the pathogenesis of osteoporosis: is a revision needed? J. Bone Miner. Res. 2011;26(3):441–451. doi: 10.1002/jbmr.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen SP, GonzaJez-Alonso J, Damsgaard R, Saltin B, Hellsten Y. Inhibition of nitric oxide and prostaglandins, but not endothelial-derived hyperpolarizing factors, reduces blood flow and aerobic energy turnover in the exercising human leg. J. Physiol. 2007;581(2):853–861. doi: 10.1113/jphysiol.2006.127423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmichi M, Kanda Y, Hisamoto K, Morishige K, Takahashi K, Sawada K, et al. Rapid changes of flow-mediated dilatation after surgical menopause. Maturitas. 2003;44(2):125–131. doi: 10.1016/s0378-5122(02)00320-1. [DOI] [PubMed] [Google Scholar]
- Parker BA, Smithmyer SL, Pelberg JA, Mishkin AD, Proctor DN. Sex-specific influence of aging on exercising leg blood flow. J. Appl. Physiol. 2008a;104(3):655–664. doi: 10.1152/japplphysiol.01150.2007. [DOI] [PubMed] [Google Scholar]
- Parker BA, Smithmyer SL, Ruiout SJ, Ray CA, Proctor DN. Age and microvascular responses to knee extensor exercise in women. Eur. J. Appl. Physiol. 2008b;103(3):343–351. doi: 10.1007/s00421-008-0711-0. [DOI] [PubMed] [Google Scholar]
- Parker B, Capizzi J, Augeri A, Grimaldi A, Proctor D, Thompson P. Sex-specific effect of aging on submaximal leg exercise hemodynamics in middle-aged and older adults. Eur. J. Appl. Physiol. 2011;111(7):1369–1379. doi: 10.1007/s00421-010-1766-2. [DOI] [PubMed] [Google Scholar]
- Pinto S, Virdis A, Ghiadoni L, Bernini G, Lombardo M, Petraglia R, et al. Endogenous estrogen and acetylcholine-induced vasodilation in normotensive women. Hypertension. 1997;29(1, Part 2):268–273. doi: 10.1161/01.hyp.29.1.268. [DOI] [PubMed] [Google Scholar]
- Proctor DN, Parker BA. Vasodilation and vascular control in contracting muscle of the aging human. Microcirculation. 2006;13(4):315–327. doi: 10.1080/10739680600618967. [DOI] [PubMed] [Google Scholar]
- Proctor DN, Koch DW, Newcomer SC, Le KU, Leuenberger UA, et al. Impaired leg vasodilation during dynamic exercise in healthy older women. J Appl. Physiol. 2003a;95(5):1963–1970. doi: 10.1152/japplphysiol.00472.2003. [DOI] [PubMed] [Google Scholar]
- Proctor DN, Newcomer SC, Koch DW, Le KU, MacLean DA, Leuenberger UA, et al. Leg blood flow during submaximal cycle ergometry is not reduced in healthy older normally active men. J. Appl. Physiol. 2003b;94(5):1859–1869. doi: 10.1152/japplphysiol.00898.2002. [DOI] [PubMed] [Google Scholar]
- Rajsheker S, Manka D, Blomkalns AL, Chatterjee TK, Stoll LL, Weintraub NL. Crosstalk between perivascular adipose tissue and blood vessels. Curr. Opin. Pharmacol. 2010;10(2):191–196. doi: 10.1016/j.coph.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Register TC, Adams MR. Coronary artery and cultured aortic smooth muscle cells express mRNA for both the classical estrogen receptor and the newly described estrogen receptor beta. J. Steroid Biochem. Mol. Biol. 1998;64(3–4):187–191. doi: 10.1016/s0960-0760(97)00155-6. [DOI] [PubMed] [Google Scholar]
- Soules MR, Sherman S, Parrott B, Rebar R, Santoro N, Utian W, Woods N. Executive summary: Stages of Reproductive Aging Workshop (STRAW) Fertil. Steril. 2001;76(5):874–878. doi: 10.1016/s0015-0282(01)02909-0. [DOI] [PubMed] [Google Scholar]
- Sowers MR, Jannausch M, McConnell D, Little R, Greendale GA, Finkelstein JS, et al. Hormone predictors of bone mineral density changes during the menopausal transition. J. Clin. Endocrinol. Metab. 2006;91(4):1261–1267. doi: 10.1210/jc.2005-1836. [DOI] [PubMed] [Google Scholar]
- Stevenson ET, Davy KP, Seals DR. Maximal aerobic capacity and total blood volume in highly trained middle-aged and older female endurance athletes. J. Appl. Physiol. 1994;77(4):1691–1696. doi: 10.1152/jappl.1994.77.4.1691. [DOI] [PubMed] [Google Scholar]
- Sudhir K, Elscr MD, Jennings GL, Komesaroff PA. Estrogen supplementation decreases norepinephrine-induced vasoconstriction and total body norepinephrine spillover in perimenopausal women. Hypertension. 1997;30(6):1538–1543. doi: 10.1161/01.hyp.30.6.1538. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Mori-Abe A, Takata K, Ohta T, Kawagoe J, Tsutsumi S, et al. Raloxifene improves the ovariectomy-induced impairment in endothelium-dependent vasodilation. Menopause. 2007;14(4):656–661. doi: 10.1097/01.gme.0000248704.30204.33. [DOI] [PubMed] [Google Scholar]
- Tostes RC, Nigro D, Fortes ZB, Carvaiho MH. Effects of estrogen on the vascular system. Braz. J. Med. Biol. Res. 2003;36(9):1143–1158. doi: 10.1590/s0100-879x2003000900002. [DOI] [PubMed] [Google Scholar]
- Venkov CD, Rankin AB, Vaughan DE. Identification of authentic estrogen receptor in cultured endothelial cells. A potential mechanism for steroid hormone regulation of endothelial function. Circulation. 1996;94(4):727–733. doi: 10.1161/01.cir.94.4.727. [DOI] [PubMed] [Google Scholar]
- Villablanca AC, Jayachaadran M, Banka C. Atherosclerosis and sex hormones: current concepts. Clin. Sci. (Lond.) 2010;119(12):493–513. doi: 10.1042/CS20100248. [DOI] [PubMed] [Google Scholar]
- Virdis A, Ghiadoni L, Pinto S, Lombardo M, Petraglia F, Gennazzani A, et al. Mechanisms responsible for endothelial dysfunction associated with acute estrogen deprivation in normotensive women. Circulation. 2000;101(19):2258–2263. doi: 10.1161/01.cir.101.19.2258. [DOI] [PubMed] [Google Scholar]
- Yudkin JS, Eringa E, Stehonwer CD. “Vasocrine” signalling from perivascular fat: a mechanism linking insulin resistance to vascular disease. Lancet. 2005;365(9473):1817–1820. doi: 10.1016/S0140-6736(05)66585-3. [DOI] [PubMed] [Google Scholar]