Abstract
Background
Movement disorders occur in association with stroke and may have important clinical implications.
Methods
We reviewed the medical literature regarding the clinical phenomenology, prevalence, localization and etiologic implications, and treatments for movement disorders occurring after stroke in adult patients.
Results
Movement disorders occur uncommonly after stroke and include both hyperkinetic and parkinsonian conditions. They can occur at the time of stroke or appear as a later manifestation. Stroke lesions are typically due to small vessel cerebrovascular disease in the middle or posterior cerebral artery territory, vessels supplying the basal ganglia. Hemorrhagic lesions are more likely to induce hyperkinetic movements. Movement disorders in the setting of stroke tend to resolve spontaneously over time. Medical and surgical therapies are available to treat the movement problems.
Discussion
Movement disorders after stroke can be helpful in localizing lesions after stroke, determining the etiology of stroke, may need to be a target for therapy and may importantly influence long term outcome.
Keywords: Movement disorders, stroke
Introduction
Movement disorders occur uncommonly in association with stroke in adults and tend to resolve over time. A study of 2,500 first stroke patients found that 1% developed an acute or delayed movement disorder.1 In most cases, the lesions were due to small vessel cerebrovascular disease in the middle or posterior cerebral artery territories. This is not surprising since the blood supply to the basal ganglia, the site of pathology for most movement disorders following stroke, emanates from branches of these vessels.
Hemorrhagic strokes appear to be more likely to lead to movement disorders than ischemic ones.2 Ninety percent of the acute-onset movement disorders resolved within 6 months.1 Despite the low frequency and tendency to resolve, the recognition of a movement disorder in the setting of stroke can be important in localizing the lesions and in suggesting an underlying etiology. They may need to be a target for therapy, and can importantly contribute to disability and long-term outcome. This review summarizes current knowledge regarding movement disorders following stroke.
Ballism/chorea
Hemiballism/hemichorea is the most common movement disorder reported to occur after stroke, present in 40% of cases in a case series.1 Hemiballism is characterized by vigorous, irregular, poorly patterned, high-amplitude movements of the limbs on one side of the body and is often viewed as a severe type of chorea.3 Chorea consists of brief, arrhythmic, non-repetitive movements that appear to move from one muscle to the next and is typically worsened by volitional movements. After stroke, over time as it improves, ballism often evolves into the lower amplitude chorea. Besides hemiballism, ballism can involve one limb (monoballism) or all limbs (biballism or paraballism). In one study of 25 patients with ballism, 19 had hemiballism.4 The majority of patients with hemiballism have both choreic and ballistic movement.5 Other commonly associated dyskinesias include orobuccal, oromandibular, lingual, and dystonic movements.4,5 It has been reported that 72% of cases of hemiballism are caused by stroke, with an average age at onset for stroke-induced hemiballism of 66 years.4
Among patients with post-stroke hyperkinetic movement disorders, one of the most common is the lower amplitude hemichorea, with a prevalence of more than one in every 200 stroke patients.6 After stroke, hemichorea correlates with motor weakness and mild sensory symptoms and is often accompanied by athetosis.6,7 Individuals with post-stroke chorea tend to be older than those with other post-stroke movement disorders.7
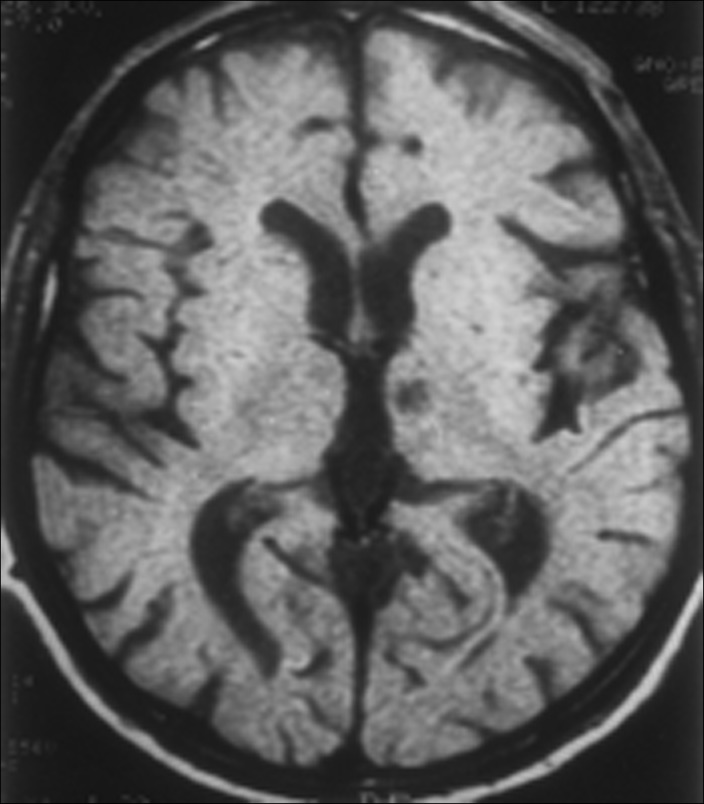
Although there are few reported cases, the most consistent neuropathological finding in patients with hemiballism after stroke is an ischemic lesion in the contralateral subthalamic nucleus (STn) (Figure 1).4 Lesions are occasionally found outside of the STn, including the caudate nucleus, putamen, and thalamus.4,8 A neuroimaging study of 22 cases of ballism found that only 4% had a lesion restricted to the STn, 59% had no evident STn lesion, and the remainder had lesions of the STn combined with other basal ganglia or midbrain structures (23%) or cortical lesions (14%).4 An unusual phenomenon of ipsilateral hemiballism-hemichorea with contralateral hemiparesis following a basal ganglia lesion has been described.9
Figure 1. Left subthalamic nucleus stroke caused contralateral hemiballism.
One study showed that patients with post-stroke hemichorea tend to have magnetic resonance imaging (MRI) hyperintensities in the basal ganglia, particularly the putamen.10 A clinical-radiological correlation study showed that STn, caudate, putamen, and cortical lesions contralateral to the affected side were the sites most commonly associated with hemichorea,6 a localization that is similar to hemiballism, which is expected given the close phenomenological relationship of the two disorders.
About 80% of patients with hemiballism experience immediate onset after stroke, whereas in the others, it is delayed by days, weeks, or months with the longest reported delay being 5 months.4 As mentioned earlier, with time, the ballistic movements become less severe and resemble chorea.11,12 While early studies described hemiballism as a grave disorder with inexorable progression to death within weeks, more recently reported cases show evidence of a more benign course and spontaneous recovery.4 Dewey et al.5 reported spontaneous recovery in 24% of cases (n = 21). Survival rate was reported to be 88% after 3 years, with stroke responsible for most deaths, particularly for deaths soon after onset of the movement disorder.3
Most patients develop the hemichorea within a few days of stroke. Although rare, hemichorea has been described as a manifestation of a transient ischemic attack. When chorea is observed in the setting of cerebrovascular disease, the clinician should consider the possibility of underlying vasculitis (e.g., systemic lupus erythematosis) or vasculopathy (e.g., paraproteinemia). Chorea has been described in the setting of stroke due to circulating antiphospholipid antibodies.13 While the choreic movement does not differ between patients with lesions in different regions, prognosis does vary depending on the location of the lesion. In one study, 85% of patients with cortical lesions and 54% of those with basal ganglia lesions recovered completely, but none of the patients with isolated lesions in the STn recovered.6
An important condition to distinguish from stroke-induced chorea is hyperglycemic chorea, which often presents acutely as hemichorea and may have basal ganglia hyperintensities on MRI. It occurs in the setting of non-ketotic hyperglycemia (usually blood glucose greater than 400 mg %) and has been attributed to hyperosmolarity.14,15 Both the involuntary movements and MRI changes are reversible with appropriate treatment of the hyperglycemia. It has been suggested that individuals of Asian descent are more likely to experience this condition.
Ballism and chorea typically respond to the same therapies. The most commonly reported pharmacological treatments have been dopamine receptor blockers, particularly haloperidol.5,6,16–17 One study reported that 56% of patients with hemiballism treated with haloperidol experienced resolution of symptoms in 3–15 days.3 Other reported treatment options include clonazepam and diazepam,6 topiramate, tetrabenazine,18 valproic acid, and, in severe and persistent cases, local intramuscular injections of botulinum toxin or ventrolateral thalamotomy.19,20
In one series of patients with post-stroke chorea, while 56% of the cases resolved within 40 days, other patients had chorea lasting as long as 41 months.6 Despite absence of published support, we generally treat disabling ballism or chorea with the atypical dopamine antagonist drug risperidone since it tends to have fewer side effects than the classical neuroleptics like haloperidol.
Dystonia
Dystonia consists of involuntary sustained muscle contractions causing twisting and repetitive movements or abnormal postures. After hemiballism-hemichorea, dystonia is the second most common movement disorder after stroke, representing about 20% of cases,1 and often taking the form of focal or hemidystonia. Stroke is the most common cause of hemidystonia, accounting for nearly 50% of all cases.21 Nearly all cases of hemidystonia with onset after the age of 50 are due to stroke.22,23 Post-stroke dystonia has been attributed to lesions of the putamen (the most common site of isolated lesions causing dystonia), caudate, pallidum, thalamus, and the midbrain.22–24 It has been suggested that dystonia can be induced by an interruption of the cortico-striato-pallido-thalamo-cortical loop.25 This disturbance, proposed to be caused by specific lesions of the sensorimotor part of the striatiopallidal complex and/or the putamen, is thought to increase thalamocortical drive, which in turn induces dystonia.25 This hypothesis is consistent with positron emission tomography (PET) studies, which have shown increased activity in cortical motor areas in patients with acquired hemidystonia from striatopallidal lesions.26 In each case, the affected cortical motor area received input from the pallidonigral thalamic territory.26
In contrast to hemiballism, which typically begins at the time of stroke, it has been shown that the time between stroke and onset of dystonia is delayed by an average of 9.5 months, with a range between 3 months and 3 years.21 Dystonia often follows hemiplegia, appearing once muscle strength begins to recover. Most patients who have onset of hemidystonia after stroke are young (below age 25), suggesting increased susceptibility in the younger brain.21 Once present after stroke, dystonia stabilizes over time, and rarely resolves completely.21
Dystonia following stroke usually has a poor response to medical therapy, typically being refractory to oral medications. Anticholinergic drugs,27 benzodiazepines,21 baclofen, and dopamine depleting/blocking agents21 have reported benefits for some patients. Local intramuscular injections of botulinium toxin can lessen stroke-induced dystonia28 and is probably the best medical approach, particularly when dystonia is focal and functionally disabling. In one study, surgical interventions (thalamotomy, pallidotomy, deep brain stimulation (DBS)) yielded the best results, showing benefit in 96% of treated patients (n = 23); however, 39% had only transient improvement.21 The recent literature suggests that DBS of either the thalamus or the internal globus pallidus appears to be more successful than lesioning approaches in producing a longer lasting response since its parameters can be altered for maximum benefit.21 It is still yet to be determined, however, which target is more effective.21
Myoclonus and asterixis
Myoclonus involves brief, involuntary twitching of muscles or muscle groups. The literature includes reported cases of myoclonus after stroke in numerous brain regions, including the frontoparietal lobes, basal ganglia, midbrain, pons, and cerebellum.29 Thus, the onset of myoclonus after stroke is not too helpful in localizing the vascular lesion. Post-stroke myoclonus can affect the arms, legs, face, or voice; however, facial myoclonus is infrequent after stroke.29
Asterixis, often referred to as negative myoclonus, is characterized by arrhythmic interruptions of sustained voluntary muscle contraction causing brief lapses of posture. Asterixis has also been described in association with stroke. It has been suggested that asterixis may be due to dysregulation of tracts from the cerebello-brainstem-thalamo-frontal lobe system.30 Kim30 reported 30 patients with unilateral stroke who displayed asterixis. Lesions in the thalamus (n = 19), frontal lobe (n = 6), lenticulocapsular area (n = 1), midbrain (n = 2), and cerebellum (n = 2) were identified.30 Another study described nine patients with asterixis, four resulting from anterior cerebral artery infarction.31
Post-stroke myoclonus often does not require treatment. When it interferes with functional abilities, like eating or writing, the two most commonly used treatments include clonazepam and sodium valproate, both GABAergic drugs.32 Other medications that may suppress myoclonus are piracetam and levitiracetam.32 In our experience, clonazepam and levitiracetam, sometimes used in combination, are the most effective medications for myoclonus. The appropriate treatment for asterixis remains unknown.
Holmes' tremor
Holmes' tremor (also called rubral, midbrain, or cerebellar outflow tremor) is characterized by a resting tremor of a limb with marked accentuation on action, intention, and goal-oriented movement. It is typically irregular, of low frequency (<4.5 Hz) and usually involves the upper extremities.33 Holmes' tremor has often been described in association with stroke. Although the most common lesions are in the brainstem, lesions of the cerebellum and thalamus have also been reported.34 These localizations suggest involvement of both the nigrostriatal and dentato-rubro-thalamic pathways,35 and this conclusion has been supported by MRI.36 In this study, all three patients with Holmes' tremor following brainstem stroke showed hypertrophy of the inferior olivary nucleus, suggesting that disruption of the denato-rubral-olivary circuitry may play a key role.36
The onset of Holmes' tremor after stroke is typically delayed by weeks to months.36 Drugs that have reported benefit include propranolol, clonazepam, levodopa, other dopaminergic agents, valproate, and levetiracetam.37 We consider levodopa as first line. However, response to drugs is usually poor in these patients37 and because of the poor response to medications, many patients with Holmes' tremor require surgical intervention,38 such as ventrointermedius thalamotomy38 and thalamic DBS.39,40
Palatal tremor
Palatal tremor consists of brief, rhythmic involuntary movements of the soft palate.41 The condition was previously described as palatal myoclonus, but was renamed since the rhythmic nature makes it more accurately classified as a tremor. Stroke is one of the most common causes of symptomatic palatal tremor (SPT).41 SPT patients have other signs of cerebellar dysfunction, abnormalities on motor learning tasks and brainstem reflex testing, and the palatal tremor persists and varies in rate during sleep.41 Imaging studies show lesions in the triangle of Guillain–Mollaret (red nucleus, inferior olive, dentate nucleus) and an enlarged inferior olivary nucleus.41 The triangle of Guillain–Mollaret functions as a feedback loop between the brainstem and cerebellar nuclei that controls spinal cord motor activity.
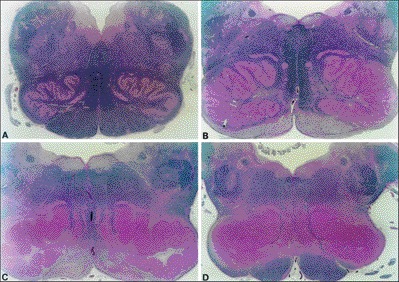
Despite the suggestion of a hypertrophied inferior olivary nucleus (Figure 2) as the cause of SPT,41 autopsy studies have shown that hypertrophic olivary degeneration (HOD) does not correlate with the presence or absence of SPT.42 Imaging studies have shown that pontine tegmental, cerebellar, and dentate nuclear lesions all cause HOD.43 The dentate nucleus is involved in control of volitional movement, suggesting that HOD is a sign of the lesion in the triangle of Guillain–Mollaret that causes the SPT, rather than the direct cause of the palatal tremor. The onset of SPT has been suggested to depend on hyperactivity of olivary neurons, while its persistence is due to a disturbance of natural rhythmicity in the circuitry because of a loss of feedback.42
Figure 2. Bilateral inferior olivary hypertrophy (post mortem).
One study showed a 2- to 49-month delay in onset of palatal tremor after acute stroke. When stroke related, SPT tends not to resolve spontaneously, particularly when associated with other cerebellar dysfunction.41 SPT generally manifests itself to the patient with an audible clicking sound. This may be tolerable and not require specific treatment. When intolerable or functionally impairing, however, the most commonly reported and our preferred treatment for SPT has been local intramuscular botulinum toxin injections.44
Tics
Tics consist of involuntary twitches (motor tics) or sounds (phonic tics). There are a few case reports of tics developing after stroke localized to the basal ganglia45–47 and one case following hemorrhage of a left frontal arteriovenous malformation.48 If disabling, tics can be treated with alpha-receptor agonists (clonidine, guanfacine) or dopamine receptor antagonists such as risperidone or fluphenazine.
Vascular parkinsonism
Stroke in critical locations, such as the midbrain and basal ganglia, can cause the acute onset of parkinsonism. It is also now appreciated that patients with chronic small vessel cerebrovascular disease can develop a progressive condition characterized by features resembling Parkinson's disease (PD). Termed “vascular parkinsonism”, this condition is now considered a distinct, although heterogeneous, clinical entity.49,50 Vascular parkinsonism is clinically manifested primarily by bilateral, symmetric bradykinesia and rigidity (idiopathic PD typically begins on one side and tends to be asymmetric), usually in the presence of a gait disorder. Presentation can be as “lower half parkinsonism”, in which there is rigidity and bradykinesia of the legs with sparing of the upper extremities, often accompanied by start hesitation (gait ignition failure) and gait freezing.51 Resting tremor may be present in vascular parkinsonism, but it is usually mild. Levodopa and other dopaminergic drugs may improve vascular parkinsonism,52 but the effects are usually modest and short-lived. On brain neuropathologic examination, patients with vascular parkinsonism show evidence of widespread, mostly subcortical small vessel cerebrovascular disease and they do not have the characteristic Lewy body (synucleinopathic) pathology of PD. Two forms of leukoencephalopathy, Binswanger's disease53 and CADASIL (cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy),54 and Moyamoya disease55 can present with vascular parkinsonism.
It can be difficult to clinically differentiate vascular parkinsonism and PD. Studies attempting to accurately classify cases rely either on clinically accepted criteria or autopsy findings. It has been shown that misdiagnosis of vascular parkinsonism as PD is common with rates of misdiagnosis of 15–30%.56,57 One reason for diagnostic confusion may be that studies of vascular parkinsonism have not carefully distinguished between true signs of parkinsonism (resting tremor, lead pipe rigidity, bradykinesia, parkinsonian gait) that reflect nigrostriatal dysfunction from the pseudoparkinsonian signs (action tremor or myoclonus, paratonic rigidity, apraxic slowness, apraxic gait) that reflect multifocal or diffuse hemispheric dysfunction that would be expected in patients with cerebrovascular disease.58
Conclusions
Movement disorders occur uncommonly in association with stroke and tend to resolve over time. Nevertheless, their recognition in the setting of stroke can be important in localizing the lesions and in suggesting an underlying etiology. Most strokes associated with movement disorders involve small vessel branches of the middle or posterior cerebral arteries since these supply the basal ganglia, the usual site of pathology. The movement disorders can appear acutely at the time of the stroke or they can have a delayed onset. The most commonly observed disorders are hemiballism-hemichorea and dystonia, but other hyperkinetic and hypokinetic disorders can occur as well. The movement disorders may need to be a target for therapy since they can contribute to disability. A variety of medications, botulinum toxin injections, and stereotactic neurosurgical procedures are available to treat these conditions. The features of movement disorders after strokes are summarized in Table 1.
Table 1. Movement Disorders after Stroke.
| Movement Disorder | Acute or Delayed Onset | Localization | Therapy |
| Ballism/chorea | Acute | STn, caudate, putamen, thalamus | Dopamine receptor antagonists, tetrabenazine, clonazepam, diazepam, topiramate, botulinum toxin, functional neurosurgery |
| Chorea | Acute | Putamen, STn, caudate, cortex | Dopamine receptor antagonists, clonazepam, diazepam, botulinum toxin, functional neurosurgery |
| Dystonia | Delayed | Putamen, caudate, pallidum, thalamus, midbrain | Anticholinergics, benzodiazepines, baclofen, tetrabenazine, botulinum toxin, functional neurosurgery |
| Myoclonus | Acute | Hemispheres, basal ganglia, midbrain, pons, spinal cord, post-anoxic | Clonazepam, valproate, piracetam, levitiracetam |
| Asterixis | Acute | Thalamus, frontal lobes, basal ganglia, midbrain, cerebellum | Clonazepam, valproate, piracetam, levitiracetam |
| Holmes' tremor | Acute | Brainstem, cerebellum, thalamus | Levodopa, clonazepam, propranolol, valproate, levitiracetam, functional neurosurgery |
| Palatal tremor | Delayed | Triangle of Guillain–Mollaret | Botulinum toxin, sumatriptan, oxitriptan, carbamazepine, clonazepam |
| Tics | Acute | Basal ganglia, frontal lobe | Alpha-agonists, dopamine receptor antagonists |
| Vascular Parkinsonism | Delayed | subcortex | Levodopa, dopamine agonists |
Footnotes
Funding: None.
Competing Interests: The authors report no conflict of interest.
References
- 1.Ghika-Schmid F, Ghika J, Regli F, et al. Hyperkinetic movement disorders after stroke. J Neurol Sci. 1997;152:109–116. doi: 10.1016/S0022-510X(96)00290-0. [DOI] [PubMed] [Google Scholar]
- 2.Kim JS. Delayed onset mixed involuntary movements after thalamic stroke. Brain. 2001;124:299–309. doi: 10.1093/brain/124.2.299. [DOI] [PubMed] [Google Scholar]
- 3.Ristic A, Marinkovic J, Dragasevic N, et al. Long-term prognosis of vascular hemiballismus. Stroke. 2002;33:2109–2111. doi: 10.1161/01.STR.0000022810.76115.C0. [DOI] [PubMed] [Google Scholar]
- 4.Vidakovic A, Dragasevic N, Kostic VS. Hemiballism: Report of 25 cases. J Neurol Neurosurg Psychiatry. 1994;57:945–949. doi: 10.1136/jnnp.57.8.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dewey RB, Jankovic J. Hemiballism-hemichorea: Clinical and pharmalogic findings in 21 patients. Arch Neurol. 1989;46:862–867. doi: 10.1001/archneur.1989.00520440044020. [DOI] [PubMed] [Google Scholar]
- 6.Chung SJ, Im J-H, Lee MC, et al. Hemichorea after stroke: Clinical-radiological correlation. J Neurol. 2004;251:725–729. doi: 10.1007/s00415-004-0412-5. [DOI] [PubMed] [Google Scholar]
- 7.Alarcon F, Zijlmans JCM, Duenas G, et al. Post-stroke movement disorders: Report of 56 patients. J Neurol Neurosurg Psychiatry. 2004;75:1568–1574. doi: 10.1136/jnnp.2003.011874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lazzarino LG, Nicolai A. Hemichorea-hemiballism and anosognosia following a contralateral infarction of the caudate nucleus and anterior limb of the internal capsule. Rev Neurol. 1991;61:9–11. [PubMed] [Google Scholar]
- 9.Krauss JK, Pohle T, Borremans JJ. Hemichorea and hemiballism associated with contralateral hemiparesis and ipsilateral basal ganglia lesions. Mov Disord. 1999;14:497–501. doi: 10.1002/1531-8257(199905)14:3<497::AID-MDS1019>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 10.Kandiah N, Tan K, Tchoyoson L, et al. Hyperglycemic choreoathetosis: Role of the putamen in pathogenesis. Mov Disord. 2009;24:915–919. doi: 10.1002/mds.22277. [DOI] [PubMed] [Google Scholar]
- 11.Hyland HH, Forman DM. Prognosis in hemiballismus. Neurology. 1957;7:381–391. doi: 10.1212/wnl.7.6.381. [DOI] [PubMed] [Google Scholar]
- 12.Pappenheim E. Therapeutic response in hemiballismus. Ann Neurol. 1979;6:139. doi: 10.1002/ana.410060215. [DOI] [PubMed] [Google Scholar]
- 13.Orzechowski NM, Wolanskyj AP, Ahlskog JE, et al. Antiphospholipid antibody-associated chorea. J Rheumatol. 2008;35:2165–2170. doi: 10.3899/jrheum.080268. [DOI] [PubMed] [Google Scholar]
- 14.Lin JJ, Chang MK. Hemiballism-hemichorea and nonketotic hyperglycemia. J Neurol Neurosurg Psychiatry. 1994;57:748–750. doi: 10.1136/jnnp.57.6.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wintermark M, Fischbein NJ, Mukherjee P, et al. Unilateral putaminal CT, MR, and diffusion abnormalities secondary to nonketotic hyperglycemia in the setting of acute neurologic symptoms mimicking stroke. Am J Neuroradiol. 2004;25:975–976. [PMC free article] [PubMed] [Google Scholar]
- 16.Shannon KM. Hemiballismus. Clin Neuropharmacol. 1990;13:413–425. doi: 10.1097/00002826-199010000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Bashir K, Manyam BV. Clozapine for the control of hemiballism. Clin Neuropharmacol. 1995;17:477–448. doi: 10.1097/00002826-199410000-00012. [DOI] [PubMed] [Google Scholar]
- 18.Jankovic J, Beach J. Long term-effects of tetrabenazine in hyperkinetic movement disorders. Neurology. 1997;48:358–362. doi: 10.1212/wnl.48.2.358. [DOI] [PubMed] [Google Scholar]
- 19.Cardoso F, Janovic J, Grossman RG, et al. Outcome following stereotactic thalamotomy for dystonia and hemiballism. Neurosurgery. 1995;36:501–508. doi: 10.1227/00006123-199503000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Krauss JK, Mundinger F. Surgical treatment of hemiballism and hemichorea. In: Krauss JK, Jankovic J, Grossman RG, editors. Surgery for Movement Disorders. Philadelphia: Lippincott Williams & Wilkins; 2000. pp. 397–403. [Google Scholar]
- 21.Chuang C, Fahn S, Frucht SJ. The natural history and treatment of acquired hemidystonia: report of 33 cases and review of the literature. J Neurol Neurosurg Psychiatry. 2002;72:59–67. doi: 10.1136/jnnp.72.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pettigrew LC, Jankovic J. Hemidystonia: A report of 22 patients and a review of the literature. J Neurol Neurosurg Psychiatry. 1985;48:650–657. doi: 10.1136/jnnp.48.7.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marsden CD, Obeso JA, Zarranz JJ, et al. The anatomical basis of symptomatic hemidystonia. Brain. 1985;108:463–483. doi: 10.1093/brain/108.2.463. [DOI] [PubMed] [Google Scholar]
- 24.Lehéricy S, Gerardin E, Poline JB, et al. Motor execution and imagination networks in post-stroke dystonia. Neuroreport. 2004;15:1887–1890. doi: 10.1097/00001756-200408260-00010. [DOI] [PubMed] [Google Scholar]
- 25.Krystowiak P, Martinat P, Defebvre L, et al. Dystonia after striatopallidal and thalamic stroke: clinicoradiological correlations and pathophysiological mechanisms. J Neurol Neurosurg Psychiatry. 1998;65:703–708. doi: 10.1136/jnnp.65.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ceballos-Baumann AO, Passingham RE, Marsden CD, et al. Motor reorganization in acquired hemidystonia. Ann Neurol. 1995;37:746–757. doi: 10.1002/ana.410370608. [DOI] [PubMed] [Google Scholar]
- 27.Fahn S. Systemic therapy of dystonia. Can J Neurol Sci. 1987;14:528–532. doi: 10.1017/s0317167100038051. [DOI] [PubMed] [Google Scholar]
- 28.Handley A, Medcalf P, Hellier K, Dutta D. Movement disorders after stroke. Age Ageing. 2009;38:260–266. doi: 10.1093/ageing/afp020. [DOI] [PubMed] [Google Scholar]
- 29.Barnes MP, Dobkin BH, Bogousslavsky J. Recovery after Stroke. Cambridge: Cambridge University Press; 2005. [Google Scholar]
- 30.Kim JS. Asterixis after unilateral stroke: Lesion location of 30 patients. Neurology. 2001;56:533–536. doi: 10.1212/wnl.56.4.533. [DOI] [PubMed] [Google Scholar]
- 31.Kim JS. Involuntary movements after anterior cerebral artery territory infarction. Stroke. 2001;32:258–261. doi: 10.1161/01.STR.32.1.258. [DOI] [PubMed] [Google Scholar]
- 32.Van Zandijcke M. Treatment of myoclonus. Acta Neurol Belg. 2003;103:66–70. [PubMed] [Google Scholar]
- 33.Baysal L, Acarer A, Celebisoy N. Post-ischemic Holmes' tremor of the lower extremities. J Neurol. 2009;256:2079–2081. doi: 10.1007/s00415-009-5273-5. [DOI] [PubMed] [Google Scholar]
- 34.Deuschl G, Krack P, Lauk M, Timmer J. Clinical neurophysiology of tremor. J Clin Neurophysiol. 1996;13:110–121. doi: 10.1097/00004691-199603000-00002. [DOI] [PubMed] [Google Scholar]
- 35.Sung YF, Hsu YD, Huang WS. (99m) TC-TRODACT-1 SPECT study in evaluation of Holmes tremor after thalamic hemorrhage. Ann Nucl Med. 2009;23:605–608. doi: 10.1007/s12149-009-0271-3. [DOI] [PubMed] [Google Scholar]
- 36.Yang YW, Chuang FC, Tsia CH, et al. Clinical and magnetic resonance imaging manifestations of Holmes tremor. Acta Neurol Taiwan. 2005;14:9–15. [PubMed] [Google Scholar]
- 37.Striano P, Elefante A, Coppola A, et al. Dramatic response to levetiracetam in post-ischaemic Holmes' tremor. J Neurol Neurosurg Psychiatry. 2007;78:438–439. doi: 10.1136/jnnp.2006.103275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim MC, Son BC, Miyagi Y, Kang JK. Vim thalamotomy for Holmes' tremor secondary to midbrain tumour. J Neurol Neurosurg Psychiatry. 2002;73:435–455. doi: 10.1136/jnnp.73.4.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nikkhah G, Prokop T, Hellwig B, et al. Deep brain stimulation of the nucleus ventralis intermedius for Holmes (rubral) tremor and associated dystonia caused by upper brainstem lesions. Report of two cases. J Neurosurg. 2004;100:1079–1083. doi: 10.3171/jns.2004.100.6.1079. [DOI] [PubMed] [Google Scholar]
- 40.Peker S, Isik U, Akgun Y, Ozek M. Deep brain stimulation for Holmes' tremor related to a thalamic abscess. Childs Nerv Syst. 2008;24:1057–1062. doi: 10.1007/s00381-008-0644-2. [DOI] [PubMed] [Google Scholar]
- 41.Deusch G, Toro C, Valles-Sole J, et al. Symptomatic essential palatal tremor. 1. Clinical, physiological and MRI analysis. Brain. 1994;117:775–788. doi: 10.1093/brain/117.4.775. [DOI] [PubMed] [Google Scholar]
- 42.Nishie M, Yoshida Y, Hirata Y, et al. Generation of symptomatic palatal tremor is not correlated with inferior olivary hypertrophy. Brain. 2002;125:1348–1357. doi: 10.1093/brain/awf126. [DOI] [PubMed] [Google Scholar]
- 43.Kitajima M, Korogi Y, Shimomura O, et al. Hypertrophic olivary degeneration: MR imaging and pathologic findings. Radiology. 1994;192:539–543. doi: 10.1148/radiology.192.2.8029428. [DOI] [PubMed] [Google Scholar]
- 44.Penney SE, Bruce IA, Saed SR. Botulinum toxin is effective and safe for palatal tremor: Report of 5 cases and review of the literature. J Neurol. 1993;253:857–860. doi: 10.1007/s00415-006-0039-9. [DOI] [PubMed] [Google Scholar]
- 45.Ward CD. Transient feelings of compulsion caused by hemispheric lesions: three cases. J Neurol Neurosurg Psychiatry. 1988;51:266–268. doi: 10.1136/jnnp.51.2.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kwak CH, Jankovic J. Tourettism and dystonia after subcortical stroke. Mov Disord. 2002;17:821–825. doi: 10.1002/mds.10207. [DOI] [PubMed] [Google Scholar]
- 47.Gomis M, Puente V, Pont-Sunyer C, et al. Adult onset simple phonic tic after caudate stroke. Mov Disord. 2008;23:765–766. doi: 10.1002/mds.21955. [DOI] [PubMed] [Google Scholar]
- 48.Yochelson MR, David RG. New-onset tic disorder following acute hemorrhage of an arteriovenous malformation. J Child Neurol. 2000;15:769–771. doi: 10.1177/088307380001501114. [DOI] [PubMed] [Google Scholar]
- 49.Demirkirn M, Bozdemir H, Sarica Y. Vascular parkinsonism: A distinct, heterogeneous clinical entity. Acta Neurol Scand. 2001;104:63–67. doi: 10.1034/j.1600-0404.2001.104002063.x. [DOI] [PubMed] [Google Scholar]
- 50.Zijlmans JC, Daniel SE, Hughes AJ, et al. Clinicopathological investigation of vascular parkinsonism, including clinical criteria for diagnosis. Mov Disord. 2004;19:630–640. doi: 10.1002/mds.20083. [DOI] [PubMed] [Google Scholar]
- 51.Fitzgerald PM, Jankovic J. Lower body parkinsonism: Evidence for vascular etiology. Mov Disord. 1989;4:249–260. doi: 10.1002/mds.870040306. [DOI] [PubMed] [Google Scholar]
- 52.Constantinescu R, Richard IH, Kurlan R. Levodopa responsiveness in disorders with parkinsonism: a review of the literature. Mov Disord. 2007;22:2141–2148. doi: 10.1002/mds.21578. [DOI] [PubMed] [Google Scholar]
- 53.Mark MH, Sage JI, Walters AS, et al. Binswanger's disease presenting as levodopa-responsive Parkinson's disease: Clinicopathological study of 3 cases. Mov Disord. 1995;10:450–454. doi: 10.1002/mds.870100408. [DOI] [PubMed] [Google Scholar]
- 54.Markus HS, Martin RJ, Simpson MA, et al. Diagnostic strategies in CADASIL. Neurology. 2002;59:1134–1138. doi: 10.1212/wnl.59.8.1134. [DOI] [PubMed] [Google Scholar]
- 55.Tan EK, Chan LL, Yu GX, et al. Vascular parkinsonism in moyamoya: Microvascular biopsy and imaging correlates. Ann Neurol. 2003;54:836–840. doi: 10.1002/ana.10783. [DOI] [PubMed] [Google Scholar]
- 56.Meara J, Bhowmick BK, Hobson P. Accuracy of diagnosis in patients with presumed Parkinson's disease. Age Ageing. 1999;28:99–102. doi: 10.1093/ageing/28.2.99. [DOI] [PubMed] [Google Scholar]
- 57.Hughes AJ, Ben-Shlomo Y, Daniel Se, et al. What features improve the accuracy of clinical diagnosis in Parkinson's disease? A clinical pathological study. Neurology. 1992;42:1142–1146. doi: 10.1212/wnl.42.6.1142. [DOI] [PubMed] [Google Scholar]
- 58.Kurlan R, Richard IH, Papka M, Marshall F. Movement disorders in Alzheimer's disease: More rigidity of definitions is needed. Mov Disord. 2000;15:24–29. doi: 10.1002/1531-8257(200001)15:1<24::AID-MDS1006>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]