Abstract
Aim
The aim of this study was to evaluate the association of genetic variants in the major genes involved in carbamazepine (CBZ) metabolism and transport with its pharmacokinetics in epilepsy patients.
Materials & methods
Twenty-five SNPs within seven CBZ pathway genes, namely CYP3A4, CYP3A5, EPHX1, NR1I2, UGT2B7, ABCB1 and ABCC2, were analyzed for association with CBZ pharmacokinetics in 90 epilepsy patients.
Results
The CYP3A4*1B SNP was significantly associated with CBZ clearance. Significant association of EPHX1 SNPs was observed with greater carbamazepine-10,11-trans dihydrodiol:carbamazepine 10-11 epoxide ratios. Among drug transporters, ABCB1 and ABCC2 SNPs were significantly associated with altered CBZ clearance.
Conclusion
SNPs within CBZ pathway genes contribute to interpatient variation in CBZ pharmacokinetics and might contribute to pharmacoresistant epilepsy. Although our results need further clinical validation in a larger patient cohort, they indicate that genetic variation in CBZ pathway genes could influence its pharmacokinetics, and hence would have clinical significance.
Keywords: ABCB1, ABCC2, carbamazepine, CYP3A4, EPHX1, pharmacogenomics, pharmacokinetics, UGT2B7
Carbamazepine (CBZ) is one of the most commonly prescribed antiepileptic agents. It is used as a first-line treatment for partial, tonic–clonic seizures, trigeminal glossopharyngeal neuralgias and bipolar disorder [1,2]. Although CBZ is a cost-effective antiepileptic drug, its use is confounded by wide interpatient variability in clinical response and unpredictable adverse events that have been reported in approximately 30–50% of patients [3,4]. CBZ-induced adverse events include CNS toxicity as well as cutaneous, hematologic, renal and hepatic disorders [5,6] as well as potential fatal allergic reactions such as Stevens–Johnson syndrome, toxic epidermal necrolysis and drug reactions with eosinophilia and systemic symptoms [7,8]. In addition, as many as 40% of patients with epilepsy experience pharmacoresistance [9,10]. When used as monotherapy, CBZ maintenance doses among responders varies from 200 to 2000 mg/day, indicating wide interpatient variation in treatment response [11]. The efficacy and toxicity of many antiepileptic drugs are related to plasma and/or CNS drug concentrations. The significant variability in clinical response coupled with unpredictable toxicity has led to the recommendation for screening of potential markers for CBZ toxicity in patients prior to initiation of CBZ therapy [12–14].
CBZ is slowly absorbed and has a low initial clearance that increases two- to three-fold owing to autoinduction [15,16]. The metabolic pathway of CBZ is shown in Supplementary Figure 1; see www.futuremedicine.com/doi/suppl/10.2217/pgs.12.180 [101]. CBZ is predominantly metabolized by hepatic CYP3A4 and CYP2C8 through formation of carbamazepine 10-11 epoxide (CBZ-E). CBZ-E is an active metabolite equipotent to CBZ [17,18]. CBZ-E is further metabolized by EPHX1 to the inactive carbamazepine-10,11-trans dihydrodiol, (CBZ-diol), which is excreted in the urine. In adults plasma levels of CBZ-E are 15–55% of CBZ [19,20] and in vitro systems CBZ-E is the major metabolite with >90% of total CBZ [21]. Approximately 15% of the dose is excreted in the urine as N-glucouronide. The major drug-metabolizing enzymes involved in CBZ biotransformation include CYP3A4, CYP3A5, CYP2C8, EPHX1 and UGT2B7. Among drug transporters, ABCB1 and ABCC2 have been implicated in transport of CBZ 10-11 epoxide [22] and CBZ [23], respectively. Studies in rats suggested P-glycoprotein transport of CBZ [24]; however, results of in vitro experiments and work in mice are conflicting [25–28]. Association of ABCB1 SNPs with drug-resistant epilepsy has been previously reported [29]. Although not significant, low plasma CBZ levels have been shown to be associated with higher intestinal ABCB1 expression [30]. In addition nuclear hormone receptors, such as NR1I2 (also known as PXR), are involved in transcriptional activation of CYPs and drug transporters. Thus, interpatient variation in expression and/or activity of genes in the CBZ pathway could alter the pharmacokinetics (PKs) and influence treatment response. In the present study we evaluated the association of SNPs in genes responsible for CBZ metabolism with CBZ PK profiles in epilepsy patients. One of the strengths of this study was the use of an intravenous, stable-labeled isotope (nonradioactive) formulation that permitted rigorous characterization of CBZ PKs in patients under steady state conditions. When administered as part of a patient’s usual oral dose, intravenous administration of stable-labeled CBZ made it possible to measure the absolute bioavailability, clearance, volume of distribution, and elimination half-life (t1/2) – parameters that cannot usually be determined from oral dose, steady-state studies in patients on CBZ maintenance therapy [31].
Materials & methods
Subjects
In the present study we included African–American or Caucasian (race self-reported) patients with epilepsy who were between 19–87 years of age taking CBZ as maintenance therapy, either as monotherapy or with other medications that do not interact with CBZ. Furthermore, patients had to be on a stable maintenance CBZ regimen, that is, receiving continuous dosing over multiple months. No dosage adjustments, even minor changes, were allowed within 2 weeks prior to the first day of the study. Enrollment was stratified with the intention of studying equal numbers of women and men, and Caucasians and African– Americans. Those with significant medical problems who might not tolerate intravenous administration or those taking medications known to affect CBZ disposition were excluded from the study, as were those who reported nonadherence to their CBZ therapy. Details of the study are published elsewhere [31].
Prior to enrollment, the project coordinator contacted subjects to discuss the protocol, confirm drug therapy and review the consent form. The study was performed at the participating institutions’ general clinical research centers or equivalent facilities. All study participants provided written consent. Institutional review boards at all sites and the University of Minnesota (MN, USA) approved the study protocols and consent forms. The study was conducted in accordance with US FDA IND #60,722: Use of an Intravenous Stable-labeled Carbamazepine Isotope in Adult and Elderly Patients (2000) [31].
Dosing & sampling
Details of the study design are published elsewhere [31]. Briefly, intravenous stable labeled CBZ (100 mg) was coadministered by replacing a portion of the patient’s usual oral dose. Immediately after receiving the stable-labeled CBZ intravenously, the patient took his/her usual morning CBZ oral dose minus 100 mg. Blood samples for determination of CBZ, CBZ-E and CBZ-diol were drawn prior to, and at 5, 15, 30 min, and 1, 2, 4, 6, 12, 24, 48, 72 and 96 h after administration of CBZ. Plasma concentrations were determined as described previously [31]. The method was comprehensively validated and had a within-assay variability of <5.0% for all standards and a between-assay variation of <5%. Accuracy ranged between 83.7 a nd 102.6% for all standards. Quality-control samples were all within ≤10% with respect to variability [31]. Both labeled and unlabeled concentrations of CBZ were measured simultaneously in all samples. Labeled CBZ concentrations were used in calculations of the PK parameters and unlabeled CBZ, CBZ-E and CBZ-diol were used in the analysis of parent to metabolite ratios. A one-time blood sample was also collected for isolation of genomic DNA.
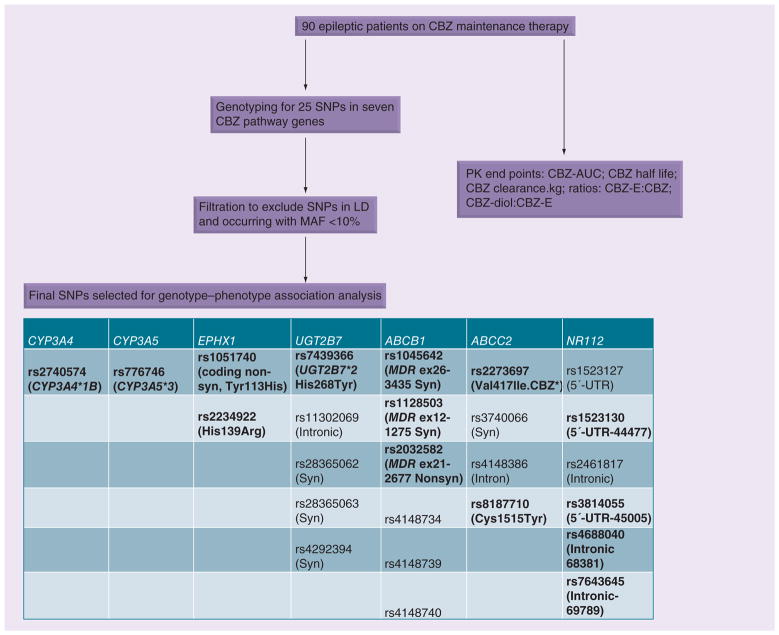
The study included 90 African–American and Caucasian patients with epilepsy. Blood samples were collected for genotyping and for determination of CBZ, CBZ-E and CBZ-diol levels as described earlier [31]. The study design is summarized in Figure 1.
Figure 1. Study design for carbamazepine pathway pharmacogenomics.
SNPs indicated in bold have been shown to be of functional relevance.
AUC: Area under the curve; CBZ: Carbamazepine; CBZ-diol: Carbamazepine-10,11-trans dihydrodiol; CBZ-E: Carbamazepine 10-11 epoxide; LD: Linkage disequilibrium; MAF: Minimum allele frequency; Nonsyn: Nonsynonymous; PK: Pharmacokinetic; Syn: Synonymous.
Selection of SNPs
Genomic DNA was extracted from 10 ml whole blood using QIAamp® DNA Blood Maxi Kit (Qiagen Inc, CA, USA). Table 1 lists genes and allele frequencies of SNPs selected for the study. Overall we genotyped 25 SNPs in seven genes (CYP3A4, CYP3A5, EPHX1, NR1I2, UGT2B7, ABCB1 and ABCC2) involved in CBZ metabolism or transport using an iPLEX® mass spectrometry-based multiplex genotyping assay (Sequenom, Inc., CA, USA) at the Bio-Medical Genomics Center, University of Minnesota. Since we did not have any information on types of seizures we did not include SNPs in the pharmacodynamic pathway. SNPs with functional and clinical significance within the candidate genes (coding region 3′-UTR and 5′-promoter SNPs) were carefully selected from previous reports in the literature and databases such as dbSNP [102] and International HapMap project [103]. We excluded SNPs occurring with the minimum allele frequency of <0.1 from the study.
Table 1.
Summary of patient demographic parameters.
| Characteristic | Values |
|---|---|
| Total patients (n) | 90 |
| M/F | 49/41 |
| Age (years) | 47 ± 15 |
| Weight (kg) | 82 ± 20 |
| CBZ dose (mg/day) | 763 ± 463 |
| Half-life (h) | 21.43 ± 12.19 (range: 7.76–114.72) |
| Clearance (l/h/kg) | 42.64 ± 17.23 (range: 10.17–95.37) |
| CBZ-E:CBZ | 0.15 ± 0.06 (range: 0.05–0.38) |
| CBZ-diol:CBZ-E | 2.73 ± 0.88 (range: 0.84–5.89) |
| Race | Caucasians: 59 (F: 23 and M: 36) African–American: 31 (F: 18 and M: 13) |
Except where indicated, values are presented as the mean ± standard deviation.
CBZ: Carbamazepine; CBZ-diol: Carbamazepine-10,11-trans dihydrodiol; CBZ-E: Carbamazepine 10-11 epoxide; F: Female; M: Male.
PK & statistical analysis
PK data analysis for CBZ concentration-time data was performed with WinNonLin® 5.1 employing nonlinear regression and a noncompartmental model assuming first order absorption and elimination as described earlier [31]. The PK end points selected for association analysis included clearance, half-life, CBZ-E:CBZ ratio (at 2 h) and CBZ-diol:CBZ-E ratio (at 2 h). Since PK end points were not normally distributed according to D’Agostino-Pearson omnibus test and Shapiro–Wilk normality test (determined using Graph pad software [Graph Pad, CA, USA]), group differences were analyzed nonparametrically by use of the Wilcoxon rank sum test to compare binary groups (e.g., GG + GT vs TT) and Kruskal–Wallis test to compare three groups of genotype for each polymorphism (e.g., GG vs GT vs TT). A p-value of <0.05 was used to indicate significance. Since we observed differences in gender and race, we performed the analysis in the combined cohort as well as in categories such as African–Americans and Caucasians separately. Similarly we also performed analysis after segregation by gender. This was carried out to identify associations that might be masked by these confounding covariates. Genotype–phenotype correlations were determined using R–statistical analysis software [104]. Since this is an exploratory study investigating the SNPs in candidate CBZ pathway gene we did not correct for multiple testing.
Results
Patient population
Ninety patients with epilepsy taking CBZ were recruited into this study, which was designed to characterize CBZ PKs. Complete genotype and PK data were available for 88 patients; 58 of these were Caucasians (23 females and 35 males) and 30 were African–American (17 females and 13 males). A summary of patient characteristics and PK end points are listed in Table 1 and the study design is shown in Figure 1. As previously reported there was a significant difference observed, with African–Americans exhibiting a lower clearance (CL; p = 0.006) and longer half-life (p = 0.01) as compared with Caucasians. Within each racial group, women had greater CBZ CL values compared to men (p = 0.02).
Genotype-CBZ PK association analysis
Association of CYP3A SNPs with CBZ PK
The CYP3A4*1B (rs2740574; A>G) SNP, as expected, occurred with greater frequency in African– Americans (0.61) as compared with Caucasians (0.08), but there was no association of this SNP with CBZ PK phenotypes within racial groups. However, in the combined cohort patients with the CYP3A4*1/*1 genotype had significantly higher CL values (AA vs AG + GG: 46.12 ± 18.79 vs 36.38 ± 11.65 l/h/kg; p = 0.027) as compared with patients with at least one CYP3A4*1B allele. This observation might be due to differences in the racial groups rather than due to the SNP. Similarly, there was an expected difference in allele frequency of the CYP3A5*3 SNP among African–Americans (0.36) and Caucasians (0.87). The CYP3A5 nonexpressor genotype (CYP3A5*3/*3) was associated with a greater half-life in African–Americans (*1/*1 + *1/*3 vs *3/*3: 21.63 ± 7.17 vs 29.66 ± 7.03 h; p = 0.037); no significant association was observed in Caucasians. No association with other PK end points was observed for the CYP3A5 SNP.
Association of EPHX1 SNPs with the ratio of CBZ-diol:CBZ-E
Since EPHX1 catalyzes the formation of CBZ-diol from CBZ-E, we evaluated the association of EPHX1 SNPs with the CBZ-diol:CBZ-E ratio determined at 2 h after CBZ administration. For the EPHX1 coding SNP (rs1051740 C>T; His113Tyr) African–American patients homozygous for the lower EPHX1 activity allele (TT) demonstrated a lower CBZ-diol:CBZ-E ratio at 2 h as compared with patients with at least one C (His) allele, CC + CT (CC + CT vs TT; 3.19 ± 1.22 vs 2.32 ± 0.62; p = 0.026; Figure 2A). However, this relationship was not observed among Caucasians. EPHX1 SNP rs2234922 (His139Arg) did not demonstrate a significant association in this patient cohort. We also performed diplotype analysis for two EPHX1 coding SNPs. Significant association was observed in patients carrying higher numbers of alleles coding for His at 113 and/or 139 (His113His and His139His or His113Tyr and His139His) with a higher CBZ-diol:CBZ-E ratio at 2 h as against those carrying lower His residues (Tyr113Tyr and His139His; Figure 2B).
Figure 2. Association of EPHX1 SNPs (rs1051740; Tyr113His) and diplotypes (rs1051740-Tyr113His and rs2234922-His139Arg) with the carbamazepine-10,11-trans dihydrodiol:carbamazepine 10-11 epoxide ratio measured at 2 h in an African–American cohort.
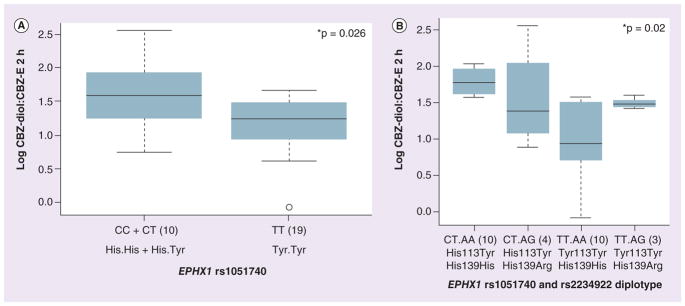
(A) Box plot for the association of the EPHX1 Tyr113His SNP (rs1051740) with the CBZ-diol:CBZ-E ratio measured at 2 h in an African–American cohort. (B) Box plot for the association of the EPHX1 for the Tyr113His (rs1051740) and His139Arg (rs2234922) diplotypes with the CBZ-diol:CBZ-E ratio measured at 2 h in African–American cohort. Box plots indicate 2nd and 3rd quartiles, with the bold line within the box representing the median value; the whiskers represent the range after excluding the outliers. The outliers are defined by the R package as data points that fall outside of the 2nd and 3rd quartiles by more than 1.5-times the interquartile range, and circles falling outside the whiskers represent outliers. The p-values from the Kruskal–Wallis nonparametric test comparing the significance of three genotypes, and the Wilcox test comparing two groups are shown.
*p < 0.05.
CBZ: Carbamazepine; CBZ-diol: Carbamazepine-10,11-trans dihydrodiol; CBZ-E: Carbamazepine 10-11 epoxide.
Association of SNPs in drug transporters with CBZ PKs
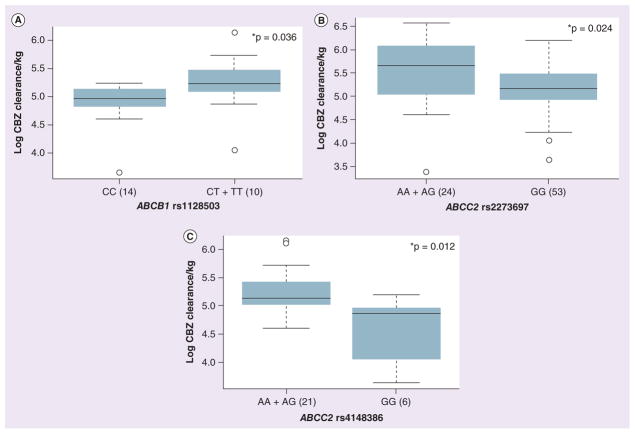
We observed a significant association between the ABCB1 synonymous SNP rs1128503 (C>T) in exon 12 and CBZ CL in African–Americans. Patients with at least one T allele demonstrated significantly higher CL (CC vs CT + TT; 30.20 ± 6.53 vs 39.85 ± 14.53 l/h/kg; p = 0.036) as compared with patients with the CC genotype (Figure 3A). ABCB1 intronic SNPs (rs4148739 and rs4148740) were significantly associated with the CBZ-diol:CBZ-E ratio in African–Americans (AA vs AG: 2.60 ± 1.02 vs 3.38 ± 0.53; p = 0.023 and CT vs TT: 3.45 ± 0.59 vs 2.53 ± 0.91; p = 0.026, respectively), but not in Caucasians.
Figure 3. Association of SNPs within drug transporters, ABCB1 and ABCC2, with carbamazepine pharmacokinetics.
(A) Box plot for the association of the ABCB1 SNP (rs1128503) with CBZ CL in African–Americans. (B) Box plot for the association of the ABCC2 SNP (rs2273697) with CBZ CL in African–Americans. (C) Box plot for the association of the ABCC2 SNP (rs4148386) with CBZ CL in African–Americans. Explanation of the box plots is the same as in Figure 2.
*p < 0.05.
CBZ: Carbamazepine.
With respect to ABCC2, a coding SNP (rs2273697 G>A; Val417Ile), demonstrated significant association with CBZ CL among Caucasians (AA + AG vs GG: 53.24 ± 23.24 vs 42.64 ± 14.39 l/h/kg; p = 0.047) but not in African– Americans. The 417Ile allele was associated with higher CBZ CL as shown in Figure 3B, this SNP also was associated with higher CBZ-E:CBZ ratio in women (AA + AG vs GG: 0.20 ± 0.06 vs 0.13 ± 0.04; p = 0.002). In Caucasian men, the ABCC2 synonymous SNP (rs3740066, A>G) was associated with lower CBZ-E:CBZ ratio (AA + AG vs GG: 0.02 ± 0.08 vs 0.12 ± 0.05; p = 0.008). In addition, in African–Americans an intronic SNP (rs4148386, A>G) was associated with CBZ CL (AA + AG vs GG: 39.00 ± 12.16 vs 25.82 ± 9.27 l/h/kg; p = 0.012, Figure 3C) and CBZ-E:CBZ ratio (AA + AG vs GG: 0.14 ± 0.06 vs 0.10 ± 0.06; p = 0.049).
Association of NR1I2 SNPs with CBZ PK end points
Although NR1I2 is not directly involved in CBZ metabolism and disposition, it is involved in transcriptional activation of CYP450s and drug transporters. We found a significant association of PXR intronic SNPs (rs7643645, rs2461817 and rs4688040) with CBZ-E:CBZ ratio in the combined cohort (p = 0.006). Among African–American patients, SNPs rs3814055 and rs2461817 were significantly associated with CBZ CL (CC + CT vs TT: 37.53 ± 13.28 vs 19.02 ± 9.28 l/h/kg; p = 0.04 and GG vs GT + TT: 29.27 ± 2.55 vs 36.99 ± 13.68 l/h/kg; p = 0.03, respectively; Figure 4A & 4B).
Figure 4. Association of SNPs in the nuclear hormone receptor PXR and the Phase II enzyme UGT2B7 with carbamazepine pharmacokinetics.
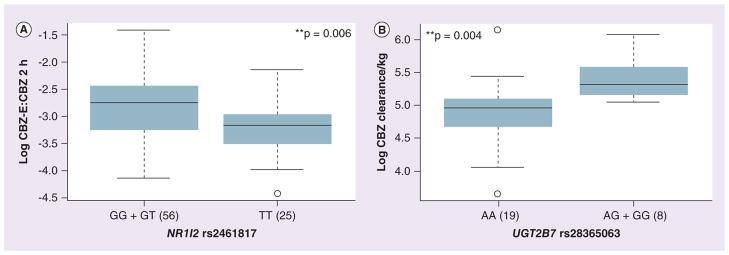
(A) Box plot for the association of the NR1I2 intronic SNP (rs2461817) with CBZ-E:CBZ ratio (B) Box plot for the association of the UGT2B7 SNP (rs28365063) with CBZ CL in African–Americans. Explanation of the box plots is the same as in Figure 2.
**p < 0.01.
CBZ: Carbamazepine; CBZ-E: Carbamazepine 10-11 epoxide.
Lastly our analysis of UGT2B7 SNPs identified significant association of rs28365063 (synonymous SNP; A>G) with greater CL within an African–American cohort (AA vs AG + GG, 31.74 ± 12.07 vs 43.55 ± 11.33 l/h/kg; p = 0.004, Figure 4B).
An overall summary of SNP associations with CBZ PK end points identified in this study are listed in Table 2.
Table 2.
Association of SNPs with carbamazepine pharmacokinetic measures in epilepsy patients receiving carbamazepine treatment.
| Gene | SNP ID | Associated allele | Half-life | Clearance l/h/kg | CBZ-E:CBZ | CBZ-diol: CBZ-E |
|---|---|---|---|---|---|---|
| CYP3A4 | rs2740574 (A>G) | A | p = 0.027↑†* (all) | |||
|
| ||||||
| CYP3A5 | rs776746 (A>G) | G | p = 0.037↑‡* | |||
|
| ||||||
| ABCB1 | rs1128503 (C>T) | T | p = 0.036↑‡* | |||
| rs4148739 (A>G) | G | p = 0.023↑‡* | ||||
| rs4148740 (T>C) | C | p = 0.026↑‡* | ||||
|
| ||||||
| ABCC2 | rs2273697 (G>A) | A | p = 0.047↑†* | p = 0.002↑†** | ||
| rs3740066 (A>G) | A | p = 0.008↓†** | ||||
| rs4148386 (A>G) | A | p = 0.012↑‡* | p = 0.049↑‡* | |||
|
| ||||||
| EPHX1 | rs1051740 (C>T) | T | p = 0.026↓‡* | |||
|
| ||||||
| NR1I2 | rs2461817 (T>G) | G | p = 0.03↓‡* | p = 0.006↑** (all) | ||
| rs7643645 (A>G) | G | p =0.04↑* (all) | ||||
| rs4688040 (G>T) | T | p =0.04↑* (all) | ||||
| rs3814055 (C>T) | T | p = 0.04↓‡* | ||||
|
| ||||||
| UGT2B7 | rs28365063 (A>G) | G | p = 0.004↑‡** | |||
Each row represents a SNP and each column represents a phenotype.
All: combined cohort of 90 patients consists of both whites and African–Americans.
p < 0.05;
p < 0.01.
Association of SNPs with pharmacokinetic phenotype in African–American population.
Association of an allele with pharmacokinetic phenotype in African–American population.
↓: Decreased levels; ↑: Increased levels; CBZ: Carbamazepine; CBZ-diol: Carbamazepine-10,11-trans dihydro-diol; CBZ-E: Carbamazepine 10-11 epoxide.
Discussion
Although CBZ is the first choice drug for several epilepsy syndromes, wide interpatient variation in treatment response and adverse drug reactions complicates its use. Genetic variation in CBZ PK pathway genes likely contributes, in part, to interpatient differences in response.
Previous studies investigating the relationship between CBZ PKs and SNPs of genes involved in CBZ disposition have been based on data derived from oral administration. Consequently, variability in bioavailability unrelated to genetic effects can affect interpretation of results. In the present study, we evaluated SNPs in seven potentially significant CBZ PK pathway genes for association with CBZ PK parameters in epilepsy patients on a stable maintenance CBZ regimen. As reported previously, the use of an intravenous stable-labeled CBZ formulation as part of patient’s usual oral dose made it possible to measure the absolute bioavailability, clearance, volume of distribution and elimination half-life – parameters that cannot usually be determined from oral dose, steady-state studies in patients on CBZ maintenance therapy [31]. Gender and race (self-reported) were associated with CBZ PK phenotypes. Women exhibited greater CBZ CL values as compared with men, which is consistent with earlier observations that CYP3A substrates are eliminated more rapidly in women than in men [31–35]. However, there was no age-related differences in the CBZ PK.
CYP3A4 is the primary enzyme responsible for CBZ-E formation [36]. Results from this study identified the CYP3A4*1B SNP to be associated with lower CBZ CL; however, this was observed in the combined cohort and not after segregation by race. CYP3A4*1B occurs with higher frequency among African–Americans (0.61) as compared with Caucasians (0.08); thus, the observed association might be due to race and not the SNP, or the reverse, where the association observed between race is driven by the SNP and differences in allele frequency are responsible for the observed racial differences.
Our study identified a significant association of EPHX1 SNPs with the CBZ-diol:CBZ-E ratio. For the EPHX1 Tyr113His SNP (rs1051740), it has been reported that presence of at least one His at 113 is associated with greater enzyme activity resulting in fast epoxide hydrolase phenotype [37]. This may result in greater CBZ-diol:CBZ-E ratio. Our study demonstrated similar findings in our epilepsy cohort, which showed that the presence of His at 113 was associated with a significantly greater ratio. In diplotype analysis (rs1051740-Tyr113His and rs2234922-His139Arg), patients with at least one His at 113 and 139 had a greater CBZ-diol:CBZ-E ratio as compared with other diplotypes. This observation is consistent with previous observations in Japanese antiepileptic patients on CBZ treatment where diol:epoxide ratios increased significantly depending on the number of haplotypes bearing His at 113 and/or His at 139 [38]. Association of EPHX1 SNPs with CBZ maintenance dose has also been reported previously [39].
There is conflicting evidence regarding the association of transporter pharmacogenetics and CBZ resistance [25]; however, low CBZ plasma levels have been associated with higher ABCB1 intestinal expression [30]. Recent evaluation of ABCB1 SNPs in Chinese patients identified significant association of the ABCB1 3435 C>T SNP (rs1045642) with lower CBZ levels [40]. However, in another cohort of 315 British patients the 3435 C allele was associated with drug-resistant epilepsy, although the antiepileptic drugs used were not specified [41]. Among other studies, few have documented an association between ABCB1 variants and CBZ resistance and others have not. This disconcordance in results could be due to different haplotype structures or racial backgrounds included in these studies. Although our results indicate association of the ABCB1 SNP rs1128503 (also known as 1236C>T) with CBZ CL in African–Americans, but not in Caucasians, future studies in larger cohorts are required to validate these observations. In addition to ABCB1, we also observed a significant association of the ABCC2 intronic SNP (rs4148386) with CBZ CL. This intronic SNP occurs in linkage disequilibrium with multiple SNPs within Caucasians (11 SNPs) and African ancestry (six SNPs) groups (Centre d’Etude du Polymorphisme Humain [CEU] samples) and with six other SNPs in African ancestry (Yoruba people in Ibadan, Nigeria [YRI] samples) from the International HapMap project [103]. The role of ABCC2 in CBZ transport and association of SNPs with altered transport activity has been recently reported. It was observed that a nonsynonymous polymorphism, 1249G>A (V417I, rs2273697), was associated with reduced CBZ transport and CBZ-induced neurological side effects [23]. A recent report also demonstrated association of ABCC2 V417I with better response in childhood epilepsy [42]. Another report has demonstrated significant association between the ABCC2 promoter SNP rs717620 and lack of response in young Caucasian epilepsy patients [9]. Our results indicate that the 417Val allele is associated with lower CBZ CL in Caucasians. Although the data is not shown, we measured volume of distribution in this patients cohort and found allelic variation in transporter, especially ABCC2 intronic (rs4148386) and non-synonymous (rs2273697) SNPs were associated with variability in the volume of distribution of CBZ.
The nonlinear [43], time and dose dependent PK of CBZ suggests the role of efflux transporters as well as of drug metabolizing enzymes in the multicompartment PK model to describe CBZ PK [44]. Although studies have suggested that here is no evidence that CBZ is a substrate for these transporters and thus this finding of a statistical difference although not direct might be due to some indirect mechanisms. It is possible that CBZ–N-glucouronide clearance is reduced thus impacting CBZ PK. Mechanistic studies on transporters suggest no direct effect on clearance would be expected. Thus observed results indicate the importance of efflux transporter variation in CBZ and warrant further investigation in a larger cohort.
Two reports in the literature have evaluated SNPs in nuclear hormone receptor (NR1I2; PXR) and reported no association of PXR SNPs (3′-UTR SNPs 11156A>C and 11193C>T and intron-5 SNP 7635 G>A) with treatment response in epilepsy [29,45]. Our previous work identified SNPs in the PXR promoter and intron 1 to be associated with CYP3A4 and MDR1 expression and/or activity [46]. In the present study we observed significant association of SNPs within NR1I2 with CBZ CL and CBZ-E:CBZ ratio. Although the role of UGT2B7 in CBZ clearance is still controversial, it has been suggested that approximately 15% of the dose is recovered as N-glucouronide. A study on N-glucuronidation of CBZ in human tissues reported that CBZ is specifically glucuronidated by human UGT2B7 [47]. It has been observed that UGT2B7*1a is associated higher mRNA expression and activity as compared with diplotypes without UGT2B7*1a in human livers [48]. This may result in higher plasma CBZ concentration and lower CBZ CL and vice versa for variant alleles. We demonstrated that UGT2B7 rs28365063 was significantly associated with greater CL within an African–American cohort. Recent study in 234 epileptic patients on CBZ demonstrated genetic variants in SCN1A, EPHX1 and UGT2B7 interactively affect the CBZ concentration:dose ratio. There is still a gap in the literature regarding comprehensive work on CBZ PK–pharmacogenomics associations, hence indicating the importance of the present study. One of the limitations of our study was the relatively small sample size within each racial group, thus our results require validation in a larger cohort. However, it is practically and economically challenging to obtain rigorously characterized PK end points in a larger cohort. In this study we utilized the largest ever epilepsy patient cohort with comprehensive PK (intravenous and per OS) data to explore the significance of pharmacogenomics factors. As this genotype– phenotype association study was exploratory in nature, no power calculations for sample size were performed a priori before statistical analysis of data, and we did not adjust for multiple testing in our analysis. However, our results provide rationale to further explore these associations in a larger population with less intense PK designs.
Conclusion & future perspective
In summary, our study evaluated the influence of genetic variations in seven genes involved in the major metabolic pathway of CBZ with its PKs. Although our results indicate that SNPs within drug-metabolizing enzymes, drug transporters and nuclear hormone receptors could influence interpatient variation in CBZ PKs and could contribute to drug-resistant epilepsy, future studies in larger patient cohorts are required to validate our findings and better understand the clinical implication of CBZ pharmacogenomics.
Supplementary Material
Executive summary.
Background
Carbamazepine (CBZ) is a widely prescribed, cost-effective antiepileptic agent.
However, its use is limited by wide interpatient variability in clinical response and unpredictable adverse events.
Genetic variation in CBZ pharmacokinetic (PK)-pathway genes likely contributes, in part, to interpatient differences in response.
Study objective
The objective of this study was to use a pathway-driven approach for the evaluation of the association of genetic variants in CBZ pathway genes with various CBZ PK end points in epilepsy patients.
Results
Significant interpatient variation in CBZ PK was observed.
Of 25 SNPs analyzed, the CYP3A4*1B SNP was significantly associated with CBZ clearance. We also observed a significant association of EPHX1 SNPs with greater carbamazepine-10,11-trans dihydrodiol:carbamazepine 10-11 epoxide ratios. Among drug transporters, ABCB1 and ABCC2 SNPs were significantly associated with altered CBZ clearance.
In this study we utilized the largest ever epilepsy patient cohort with comprehensive PK (intravenous and per OS) data to explore the significance of pharmacogenomics factors.
To the best of our knowledge, this is the first time a study has evaluated the association of genetic variants in CBZ major route of metabolism and transport pathway genes with detailed pharmacokinetics in epilepsy patients.
Conclusion
Our study evaluated the influence of genetic variation in seven genes involved in the major metabolic pathway of CBZ with its PK.
Future perspective
Future studies in larger patient cohorts are required to validate our findings and better understand the clinical implications of CBZ pharmacogenomics.
Acknowledgments
The authors would like to acknowledge W Oetting for assistance in DNA isolation, the BioMedical Genomics Center (BMGC) for genotyping facility and the Minnesota Supercomputing Institute at the University of Minnesota (MN, USA).
Footnotes
For reprint orders, please contact: reprints@futuremedicine.com
Ethical conduct of research
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
Financial & competing interests disclosure
This study was supported by NIH/National Institute of Neurological Disorder and Stroke grants P50 NS16308 (IE Leppik principal investigator and JC Cloyd coprincipal investigator) and K01NS050309 (SE Marino). AK Birnbaum, JC Cloyd and IE Leppik have a royalty agreement with Lundbeck Inc. related to the development of intravenous carbamazepine. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
▪▪ of considerable interest
- 1.Goodwin GM. Evidence-based guidelines for treating bipolar disorder: recommendations from the British Association for Psychopharmacology. J Psychopharmacol. 2003;17(2):149–173. doi: 10.1177/0269881103017002003. discussion 147. [DOI] [PubMed] [Google Scholar]
- 2.American Psychiatric Association. Practice guideline for the treatment of patients with bipolar disorder (revision) Am J Psychiatry. 2002;159(Suppl 4):S1–S50. [PubMed] [Google Scholar]
- 3.Pellock JM. Carbamazepine side effects in children and adults. Epilepsia. 1987;28(Suppl 3):S64–S70. doi: 10.1111/j.1528-1157.1987.tb05780.x. [DOI] [PubMed] [Google Scholar]
- 4.Durelli L, Massazza U, Cavallo R. Carbamazepine toxicity and poisoning. Incidence, clinical features and management. Med Toxicol Adv Drug Exp. 1989;4(2):95–107. doi: 10.1007/BF03259906. [DOI] [PubMed] [Google Scholar]
- 5.Shear NH, Spielberg SP. Anticonvulsant hypersensitivity syndrome In vitro assessment of risk. J Clin Invest. 1988;82(6):1826–1832. doi: 10.1172/JCI113798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vittorio CC, Muglia JJ. Anticonvulsant hypersensitivity syndrome. Arch Intern Med. 1995;155(21):2285–2290. [PubMed] [Google Scholar]
- 7.Ganeva M, Gancheva T, Lazarova R, et al. Carbamazepine-induced drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome: report of four cases and brief review. Int J Dermatol. 2008;47(8):853–860. doi: 10.1111/j.1365-4632.2008.03637.x. [DOI] [PubMed] [Google Scholar]
- 8.Hung SI, Chung WH, Liu ZS, et al. Common risk allele in aromatic antiepileptic-drug induced Stevens-Johnson syndrome and toxic epidermal necrolysis in Han Chinese. Pharmacogenomics. 2010;11(3):349–356. doi: 10.2217/pgs.09.162. [DOI] [PubMed] [Google Scholar]
- 9.Ufer M, Mosyagin I, Muhle H, et al. Non-response to antiepileptic pharmacotherapy is associated with the ABCC2-24C>T polymorphism in young and adult patients with epilepsy. Pharmacogenet Genomics. 2009;19(5):353–362. doi: 10.1097/fpc.0b013e328329940b. [DOI] [PubMed] [Google Scholar]
- 10.Sisodiya SM, Goldstein DB. Drug resistance in epilepsy: more twists in the tale. Epilepsia. 2007;48(12):2369–2370. doi: 10.1111/j.1528-1167.2007.01260_1.x. [DOI] [PubMed] [Google Scholar]
- 11.Kwan P, Brodie MJ. Effectiveness of first antiepileptic drug. Epilepsia. 2001;42(10):1255–1260. doi: 10.1046/j.1528-1157.2001.04501.x. [DOI] [PubMed] [Google Scholar]
- 12▪▪.Hung SI, Chung WH, Jee SH, et al. Genetic susceptibility to carbamazepine-induced cutaneous adverse drug reactions. Pharmacogenet Genomics. 2006;16(4):297–306. doi: 10.1097/01.fpc.0000199500.46842.4a. Reports on the contribution of HLA-B*1502 to the pathogenesis of carbamazepine (CBZ)-Stevens–Johnson syndrome/toxic epidermal necrolysis, and that genetic susceptibility to CBZ-induced cutaneous adverse drug reactions is phenotype specific. [DOI] [PubMed] [Google Scholar]
- 13▪▪.Lonjou C, Thomas L, Borot N, et al. A marker for Stevens-Johnson syndrome …: ethnicity matters. Pharmacogenomics J. 2006;6(4):265–268. doi: 10.1038/sj.tpj.6500356. Shows that although the HLA region may contain important genes for Stevens–Johnson syndrome, the HLA-B*1502 allele is not a universal marker for this disease and that ethnicity matters. [DOI] [PubMed] [Google Scholar]
- 14.Man CB, Kwan P, Baum L, et al. Association between HLA-B*1502 allele and antiepileptic drug-induced cutaneous reactions in Han Chinese. Epilepsia. 2007;48(5):1015–1018. doi: 10.1111/j.1528-1167.2007.01022.x. [DOI] [PubMed] [Google Scholar]
- 15.Eichelbaum M, Ekbom K, Bertilsson L, Ringberger VA, Rane A. Plasma kinetics of carbamazepine and its epoxide metabolite in man after single and multiple doses. Eur J Clin Pharmacol. 1975;8(5):337–341. doi: 10.1007/BF00562659. [DOI] [PubMed] [Google Scholar]
- 16.Rawlins MD, Collste P, Bertilsson L, Palmer L. Distribution and elimination kinetics of carbamazepine in man. Eur J Clin Pharmacol. 1975;8(2):91–96. doi: 10.1007/BF00561556. [DOI] [PubMed] [Google Scholar]
- 17.Albright PS, Bruni J. Effects of carbamazepine and its epoxide metabolite on amygdala-kindled seizures in rats. Neurology. 1984;34(10):1383–1386. doi: 10.1212/wnl.34.10.1383. [DOI] [PubMed] [Google Scholar]
- 18.Bourgeois BF, Wad N. Carbamazepine-10,11-diol steady-state serum levels and renal excretion during carbamazepine therapy in adults and children. Ther Drug Monit. 1984;6(3):259–265. doi: 10.1097/00007691-198409000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Bertilsson L, Tomson T. Clinical pharmacokinetics and pharmacological effects of carbamazepine and carbamazepine-10,11-epoxide. An update. Clin Pharmacokinet. 1986;11(3):177–198. doi: 10.2165/00003088-198611030-00001. [DOI] [PubMed] [Google Scholar]
- 20.Bu HZ, Kang P, Deese AJ, Zhao P, Pool WF. Human in vitro glutathionyl and protein adducts of carbamazepine-10,11-epoxide, a stable and pharmacologically active metabolite of carbamazepine. Drug Metab Dispos. 2005;33(12):1920–1924. doi: 10.1124/dmd.105.006866. [DOI] [PubMed] [Google Scholar]
- 21▪▪.Pelkonen O, Myllynen P, Taavitsainen P, et al. Carbamazepine: a ‘blind’ assessment of CVP-associated metabolism and interactions in human liver-derived in vitro systems. Xenobiotica. 2001;31(6):321–343. doi: 10.1080/00498250110055479. Reports that carbamazepine 10-11 epoxide is the major metabolite with >90% of total CBZ. [DOI] [PubMed] [Google Scholar]
- 22.Zhang C, Zuo Z, Kwan P, Baum L. In vitro transport profile of carbamazepine, oxcarbazepine, eslicarbazepine acetate, and their active metabolites by human P-glycoprotein. Epilepsia. 2011;52(10):1894–1904. doi: 10.1111/j.1528-1167.2011.03140.x. [DOI] [PubMed] [Google Scholar]
- 23▪▪.Kim WJ, Lee JH, Yi J, et al. A nonsynonymous variation in MRP2/ABCC2 is associated with neurological adverse drug reactions of carbamazepine in patients with epilepsy. Pharmacogenet Genomics. 2010;20(4):249–256. doi: 10.1097/FPC.0b013e328338073a. Discusses how a nonsynonymous polymorphism, 1249G>A (rs2273697) in MRP2/ABCC2, is associated with neurological adverse drug reactions of carbamazepine in patients with epilepsy. [DOI] [PubMed] [Google Scholar]
- 24.Potschka H, Fedrowitz M, Loscher W. P-glycoprotein and multidrug resistance-associated protein are involved in the regulation of extracellular levels of the major antiepileptic drug carbamazepine in the brain. Neuroreport. 2001;12(16):3557–3560. doi: 10.1097/00001756-200111160-00037. [DOI] [PubMed] [Google Scholar]
- 25.Baltes S, Gastens AM, Fedrowitz M, Potschka H, Kaever V, Loscher W. Differences in the transport of the antiepileptic drugs phenytoin, levetiracetam and carbamazepine by human and mouse P-glycoprotein. Neuropharmacology. 2007;52(2):333–346. doi: 10.1016/j.neuropharm.2006.07.038. [DOI] [PubMed] [Google Scholar]
- 26.Owen A, Pirmohamed M, Tettey JN, Morgan P, Chadwick D, Park BK. Carbamazepine is not a substrate for P-glycoprotein. Br J Clin Pharmacol. 2001;51(4):345–349. doi: 10.1046/j.1365-2125.2001.01359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luna-Tortos C, Fedrowitz M, Loscher W. Several major antiepileptic drugs are substrates for human P-glycoprotein. Neuropharmacology. 2008;55(8):1364–1375. doi: 10.1016/j.neuropharm.2008.08.032. [DOI] [PubMed] [Google Scholar]
- 28.Luna-Tortos C, Fedrowitz M, Loscher W. Evaluation of transport of common antiepileptic drugs by human multidrug resistance-associated proteins (MRP1, 2 and 5) that are overexpressed in pharmacoresistant epilepsy. Neuropharmacology. 2010;58(7):1019–1032. doi: 10.1016/j.neuropharm.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 29▪▪.Hung CC, Jen Tai J, Kao PJ, Lin MS, Liou HH. Association of polymorphisms in NR1I2 and ABCB1 genes with epilepsy treatment responses. Pharmacogenomics. 2007;8(9):1151–1158. doi: 10.2217/14622416.8.9.1151. Reports that rs2032582 and rs1045642 in ABCB1 contribute to drug-resistant epilepsy. [DOI] [PubMed] [Google Scholar]
- 30.Simon C, Stieger B, Kullak-Ublick GA, et al. Intestinal expression of cytochrome P450 enzymes and ABC transporters and carbamazepine and phenytoin disposition. Acta Neurol Scand. 2007;115(4):232–242. doi: 10.1111/j.1600-0404.2006.00761.x. [DOI] [PubMed] [Google Scholar]
- 31.Marino SE, Birnbaum AK, Leppik IE, et al. Steady-state carbamazepine pharmacokinetics following oral and stable-labeled intravenous administration in epilepsy patients: effects of race and sex. Clin Pharmacol Ther. 2012;91(3):483–488. doi: 10.1038/clpt.2011.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diczfalusy U, Miura J, Roh HK, et al. 4β-hydroxycholesterol is a new endogenous CYP3A marker: relationship to CYP3A5 genotype, quinine 3-hydroxylation and sex in Koreans, Swedes and Tanzanians. Pharmacogenet Genomics. 2008;18(3):201–208. doi: 10.1097/FPC.0b013e3282f50ee9. [DOI] [PubMed] [Google Scholar]
- 33.Harris RZ, Benet LZ, Schwartz JB. Gender effects in pharmacokinetics and pharmacodynamics. Drugs. 1995;50(2):222–239. doi: 10.2165/00003495-199550020-00003. [DOI] [PubMed] [Google Scholar]
- 34.Zhu B, Liu ZQ, Chen GL, et al. The distribution and gender difference of CYP3A activity in Chinese subjects. Br J Clin Pharmacol. 2003;55(3):264–269. doi: 10.1046/j.1365-2125.2003.01728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwartz JB. The influence of sex on pharmacokinetics. Clin Pharmacokinet. 2003;42(2):107–121. doi: 10.2165/00003088-200342020-00001. [DOI] [PubMed] [Google Scholar]
- 36.Pearce RE, Lu W, Wang Y, Uetrecht JP, Correia MA, Leeder JS. Pathways of carbamazepine bioactivation in vitro. III The role of human cytochrome P450 enzymes in the formation of 2,3-dihydroxycarbamazepine. Drug Metab Dispos. 2008;36(8):1637–1649. doi: 10.1124/dmd.107.019562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sachse C, Smith G, Wilkie MJ, et al. A pharmacogenetic study to investigate the role of dietary carcinogens in the etiology of colorectal cancer. Carcinogenesis. 2002;23(11):1839–1849. doi: 10.1093/carcin/23.11.1839. [DOI] [PubMed] [Google Scholar]
- 38▪▪.Nakajima Y, Saito Y, Shiseki K, et al. Haplotype structures of EPHX1 and their effects on the metabolism of carbamazepine-10,11-epoxide in Japanese epileptic patients. Eur J Clin Pharmacol. 2005;61(1):25–34. doi: 10.1007/s00228-004-0878-1. EPHX1 haplotypes were reported to be associated with altered CBZ 10-11 epoxide metabolism. [DOI] [PubMed] [Google Scholar]
- 39.Makmor-Bakry M, Sills GJ, Hitiris N, Butler E, Wilson EA, Brodie MJ. Genetic variants in microsomal epoxide hydrolase influence carbamazepine dosing. Clin Neuropharmacol. 2009;32(4):205–212. doi: 10.1097/WNF.0b013e318187972a. [DOI] [PubMed] [Google Scholar]
- 40.Meng H, Guo G, Ren J, Zhou H, Ge Y, Guo Y. Effects of ABCB1 polymorphisms on plasma carbamazepine concentrations and pharmacoresistance in Chinese patients with epilepsy. Epilepsy Behav. 2011;21(1):27–30. doi: 10.1016/j.yebeh.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 41.Siddiqui A, Kerb R, Weale ME, et al. Association of multidrug resistance in epilepsy with a polymorphism in the drug-transporter gene ABCB1. N Engl J Med. 2003;348(15):1442–1448. doi: 10.1056/NEJMoa021986. [DOI] [PubMed] [Google Scholar]
- 42.Ufer M, Von Stulpnagel C, Muhle H, et al. Impact of ABCC2 genotype on antiepileptic drug response in Caucasian patients with childhood epilepsy. Pharmacogenet Genomics. 2011;21(10):624–630. doi: 10.1097/FPC.0b013e3283498131. [DOI] [PubMed] [Google Scholar]
- 43.Perucca E, Johannessen SI. The ideal pharmacokinetic properties of an antiepileptic drug: how close does levetiracetam come? Epileptic Dis. 2003;5(Suppl 1):S17–S26. [PubMed] [Google Scholar]
- 44.Fagiolino P, Vazquez M, Eiraldi R, Maldonado C, Scaramelli A. Influence of efflux transporters on drug metabolism: theoretical approach for bioavailability and clearance prediction. Clin Pharmacokinet. 2011;50(2):75–80. doi: 10.2165/11539230-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 45.Haerian BS, Lim KS, Mohamed EH, et al. Lack of association of ABCB1 and PXR polymorphisms with response to treatment in epilepsy. Seizure. 2011;20(5):387–394. doi: 10.1016/j.seizure.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 46.Lamba V, Panetta JC, Strom S, Schuetz EG. Genetic predictors of interindividual variability in hepatic CYP3A4 expression. J Pharmacol Exp Ther. 2010;332(3):1088–1099. doi: 10.1124/jpet.109.160804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Staines AG, Coughtrie MW, Burchell B. N-glucuronidation of carbamazepine in human tissues is mediated by UGT2B7. J Pharmacol Exp Ther. 2004;311(3):1131–1137. doi: 10.1124/jpet.104.073114. [DOI] [PubMed] [Google Scholar]
- 48.Innocenti F, Liu W, Fackenthal D, et al. Single nucleotide polymorphism discovery and functional assessment of variation in the UDP-glucuronosyltransferase 2B7 gene. Pharmacogenet Genomics. 2008;18(8):683–697. doi: 10.1097/FPC.0b013e3283037fe4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Websites
- 101.The Pharmacogenomics Knowledgebase. www.PharmGKB.org.
- 102.dbSNP. www.ncbi.nlm.nih.gov/projects/SNP.
- 103.International HapMap Project. www.HapMap.org.
- 104.R-project for Statistical Computing. www.r-project.org.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.