There has been a long history of experimentation and conjecture about a potentially critical role in memory consolidation for brain processes unique to sleep (1–3). A role for sleep in memory consolidation is consistent with the fact that new memory traces are not instantly fixed but rather remain susceptible to neuromodulatory influences for several hours after acquisition and require protein synthesis to become stable long-term memories (4). And while it has been clearly demonstrated that sleep deprivation can impair later memory for recently acquired declarative and procedural memory, the precise mechanisms by which sleep may aid or mediate memory storage processes are not known (3, 5). Activation of the cholinergic system has been demonstrated to enhance attention, learning, and memory consolidation and to facilitate plasticity after physiological manipulations and during development (6–8). Acetylcholine levels are high during waking and rapid eye movement (REM; also known as paradoxical) sleep (9). These observations seem consistent with the possibility that REM sleep may play an important role in facilitating synaptic plasticity of recently acquired memory traces. However, the great similarities between the waking and REM sleep states beg the question: What about REM sleep relative to waking is privileged for memory consolidation? Explicitly, REM sleep episodes follow deep slow-wave sleep (SWS) episodes. New findings by Gais and Born (10) presented in this issue of PNAS provide compelling evidence in human subjects that SWS and the accompanying low levels of acetylcholine during SWS may mediate a critical memory consolidation process. These findings support two-stage models of memory consolidation, as will be discussed below.
Hippocampal–Neocortical Cooperation
Two-stage models of memory consolidation rest on observations that the integrity of hippocampal circuitry is necessary for the maintenance of recent memories but is no longer necessary for older, presumably better consolidated memories (11). In this scheme the hippocampus is specialized in the rapid acquisition of new information relayed from cortical circuits through the entorhinal cortex during periods of elevated cholinergic levels, namely waking and especially during arousal (9, 12–14). Buzsaki (12, 13) has suggested that sharp wave bursts initiated in the hippocampus during SWS and associated with theta and gamma oscillations may provide the mechanism by which “quanta” of information may be relayed back to the neocortex during memory consolidation. In strong support of this view, a recent study (15) demonstrated a correlation between neocortical and hippocampal activity during SWS, which suggests that these hippocampal sharp wave bursts are coupled selectively to the neocortical cell groups that participated in the triggering of the bursts. Hasselmo (14) has further postulated that the flow of information between the hippocampus and neocortex is regulated by cortical acetylcholine release. According to this model, neocortical signaling to the hippocampus predominates during waking and REM sleep periods, when hippocampal feedback to the neocortex is suppressed by acetylcholine. Memory traces encoded in and temporarily stored in hippocampal circuitry may then be relayed back to the neocortex and associated with relevant traces during SWS, when cholinergic suppression of hippocampal feedback to the neocortex is released (14).
Slow-wave sleep and low levels of acetylcholine may mediate memory consolidation.
Gais and Born (10) designed an experiment to test directly whether low levels of acetylcholine during sleep periods normally predominated by SWS were in fact necessary for normal memory consolidation of a declarative (word list) memory task. Consistent with previous findings, subjects showed improved memory for both the declarative and a procedural (mirror-tracing) task after sleep. Treatment with the acetylcholine esterase inhibitor physostigmine, however, selectively blocked the performance improvement in the declarative memory task normally observed after sleep. These results suggest that the release from elevated acetylcholine levels that occurs during normal SWS is critical in enabling sleep-associated improvement in declarative memory. This finding is consistent with the hypothesis that acetylcholine regulates the flow of information between the hippocampus and neocortex and that such shifts in information flow are necessary for effective memory consolidation (14) (Fig. 1).
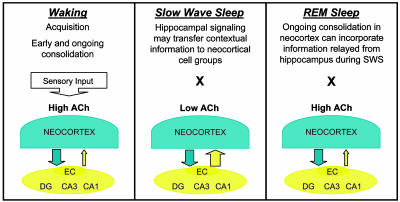
Fig. 1.
During waking, elevated acetylcholine (ACh) release from the cholinergic basal forebrain may enhance memory acquisition and consolidation (Left). The SWS release from ACh-mediated suppression of hippocampal feedback to the neocortex may then provide a privileged window for hippocampus to neocortex information transfer of memory traces recently encoded in the hippocampus (Center). A shift back to elevated ACh levels during REM sleep may facilitate savings in cortical circuits of information relayed during SWS (Right). Cortical cell groups send information to hippocampal circuitry, the dentate gyrus (DG) and area CA3, by means of the entorhinal cortex (EC). Hippocampal feedback signals to cortices are relayed back through the EC from area CA1.
Sleep and Declarative Versus Procedural Memory
These findings also highlight some as-yet-unresolved issues regarding possible differences in memory consolidation processes for different forms of memory. The physostigmine treatment did not interfere with retention of the mirror-tracing task in Gais and Born's study (10), although SWS deprivation has been reported to interfere with memory consolidation for a procedural (presumably not hippocampus-mediated) visual discrimination task (16). The lack of a physostigmine treatment-induced impairment on the mirror-tracing task was consistent with other reports that have suggested that procedural memory consolidation may depend primarily on REM sleep (17, 18). However, inconsistencies in reports indicating whether SWS or REM sleep may be more critical for memory consolidation of declarative and/or procedural tasks make such a dissociation premature. Alternatively, it has been suggested that the degree to which memory consolidation depends on REM sleep may be related to the complexity of the newly acquired memory rather than the type of memory (17). Studies attempting to examine selectively REM or non-REM sleep are further complicated by the caveat that it may not be possible to disrupt exclusively one phase of sleep without disrupting the other or at least disrupting SWS–REM transitions that may themselves be important.
SWS and REM Sleep Contributions to Consolidation
Findings such as Gais and Born's (10), which suggest a critical role for SWS in memory consolidation, raise further questions about the relationship between SWS and REM sleep. If SWS does enable feedback of information to the neocortex from the hippocampus, what could be the role of REM sleep in memory consolidation? The facilitating influence of cholinergic activation on synaptic plasticity (8) and memory consolidation in waking animals (7) suggests that REM sleep episodes may provide periods of plasticity-facilitating elevated acetylcholine levels, such that SWS modifications of neocortical memory traces by feedback from the hippocampus and perhaps other memory buffers may be saved (Fig. 1). Extensive evidence indicates that the hippocampus encodes spatial and temporal information (19, 20) and that the hippocampus, together with its associated cortices, has an especially critical role in episodic memory (21, 22). These findings, together with the above sleep data, suggest that SWS may enable the gradual relaying of critical contextual and binding information to associated neocortical traces so that those memories may become independent of the hippocampus, thereby enabling the hippocampus to continue to specialize in encoding new information.
Memory researchers have recognized acetylcholine as a local enhancer of plasticity (8, 23–25) and cognitive functions, including attention, acquisition, working memory, and consolidation of long-term memory (6, 7, 26). Also, different forms of memory have been shown to depend on different memory systems: e.g., a hippocampal dependence for declarative, episodic, or spatial memory and a striatal dependence for memory classified as procedural, skill, habit, or response (27, 28). The findings reported by Gais and Born (10) encourage us to consider more fully the importance of acetylcholine and perhaps other neuromodulators in regulating the flow of information in the brain and the cooperation of brain structures during memory consolidation (14, 29, 30).
See companion article on page 2140.
References
- 1.Stickgold, R., Hobson, J. A., Fosse, R. & Fosse, M. (2001) Science 294, 1052–1057. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman, K. L. & McNaughton, B. L. (2002) Trends Neurosci. 25, 1–2. [DOI] [PubMed] [Google Scholar]
- 3.Benington, J. H. & Frank, M. G. (2003) Prog. Neurobiol. 69, 71–101. [DOI] [PubMed] [Google Scholar]
- 4.McGaugh, J. L. (2000) Science 287, 248–251. [DOI] [PubMed] [Google Scholar]
- 5.Maquet, P. (2001) Science 294, 1048–1052. [DOI] [PubMed] [Google Scholar]
- 6.Wenk, G. L. (1997) Neurobiol. Learn. Mem. 67, 85–95. [DOI] [PubMed] [Google Scholar]
- 7.Power, A. E., Vazdarjanova, A. & McGaugh, J. L. (2003) Neurobiol. Learn. Mem. 80, 178–193. [DOI] [PubMed] [Google Scholar]
- 8.Rasmusson, D. D. (2000) Behav. Brain Res. 115, 205–218. [DOI] [PubMed] [Google Scholar]
- 9.Sarter, M. & Bruno, J. P. (2000) Neuroscience 95, 933–952. [DOI] [PubMed] [Google Scholar]
- 10.Gais, S. & Born, J. (2004) Proc. Natl. Acad. Sci. USA 101, 2140–2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bayley, P. J., Hopkins, R. O. & Squire, L. R. (2003) Neuron 38, 135–144. [DOI] [PubMed] [Google Scholar]
- 12.Buzsaki, G. (1996) Cereb. Cortex 6, 81–92. [DOI] [PubMed] [Google Scholar]
- 13.Buzsaki, G. (1998) J. Sleep Res. 7, Suppl. 1, 17–23. [DOI] [PubMed] [Google Scholar]
- 14.Hasselmo, M. E. (1999) Trends Cogn. Sci. 3, 351–359. [DOI] [PubMed] [Google Scholar]
- 15.Sirota, A., Csicsvari, J., Buhl, D. & Buzsaki, G. (2003) Proc. Natl. Acad. Sci. USA 100, 2065–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gais, S., Plihal, W., Wagner, U. & Born, J. (2000) Nat. Neurosci. 3, 1335–1339. [DOI] [PubMed] [Google Scholar]
- 17.Smith, C. (1996) Behav. Brain Res. 78, 49–56. [DOI] [PubMed] [Google Scholar]
- 18.Maquet, P., Laureys, S., Peigneux, P., Fuchs, S., Petiau, C., Phillips, C., Aerts, J., Del Fiore, G., Degueldre, C., Meulemans, T., et al. (2000) Nat. Neurosci. 3, 831–836. [DOI] [PubMed] [Google Scholar]
- 19.Burgess, N., Maguire, E. A. & O'Keefe, J. (2002) Neuron 35, 625–641. [DOI] [PubMed] [Google Scholar]
- 20.Holscher, C. (2003) Rev. Neurosci. 14, 253–284. [DOI] [PubMed] [Google Scholar]
- 21.Aggleton, J. P. & Brown, M. W. (1999) Behav. Brain Sci. 22, 425–444. [PubMed] [Google Scholar]
- 22.Eichenbaum, H. (1999) Behav. Brain Res. 103, 123–133. [DOI] [PubMed] [Google Scholar]
- 23.Sachdev, R. N., Lu, S. M., Wiley, R. G. & Ebner, F. F. (1998) J. Neurophysiol. 79, 3216–3228. [DOI] [PubMed] [Google Scholar]
- 24.Weinberger, N. M. (1998) Neurobiol. Learn. Mem. 70, 226–251. [DOI] [PubMed] [Google Scholar]
- 25.Gu, Q. (2002) Neuroscience 111, 815–835. [DOI] [PubMed] [Google Scholar]
- 26.Ellis, K. A. & Nathan, P. J. (2001) Int. J. Neuropsychopharmacol. 4, 299–313. [DOI] [PubMed] [Google Scholar]
- 27.Packard, M. G. & McGaugh, J. L. (1996) Neurobiol. Learn. Mem. 65, 65–72. [DOI] [PubMed] [Google Scholar]
- 28.White, N. M. & McDonald, R. J. (2002) Neurobiol. Learn. Mem. 77, 125–184. [DOI] [PubMed] [Google Scholar]
- 29.McGaugh, J. L., McIntyre, C. K. & Power, A. E. (2002) Neurobiol. Learn. Mem. 78, 539–552. [DOI] [PubMed] [Google Scholar]
- 30.Gold, P. E. (2003) Neurobiol. Learn. Mem. 80, 194–210. [DOI] [PubMed] [Google Scholar]