Abstract
A substantial proportion of HIV-1-infected individuals in sub-Saharan Africa are in stable relationships with HIV-1-uninfected partners, and HIV-1 serodiscordant couples thus represent an important target population for HIV-1 prevention. Couple-based HIV-1 testing and counseling facilitates identification of HIV-1 serodiscordant couples, counseling about risk reduction, and referrals to HIV-1 treatment, reproductive health services, and support services. Maximizing HIV-1 prevention for HIV-1 serodiscordant couples requires a combination of strategies, including counseling about condoms, sexual risk, fertility, contraception, and the clinical and prevention benefits of antiretroviral therapy (ART) for the HIV-1-infected partner; provision of clinical care and ART for the HIV-1-infected partner; antenatal care and services to prevent mother to child transmission for HIV-1- infected pregnant women; male circumcision for HIV-1-uninfected men; and, pending guidelines and demonstration projects, oral pre-exposure prophylaxis (PrEP) for HIV-1-uninfected partners.
Keywords: HIV-1 serodiscordant couples, HIV-1 prevention, Africa, antiretroviral, ART, PrEP
Introduction
Sub-Saharan Africa has the highest prevalence of HIV-1 globally and has high incidence with approximately 1.8 million new HIV-1 infections in 2009 [1]. All HIV-1 transmissions occur from an infected to an uninfected partner, but sub-Saharan Africa is unique in that a high proportion of HIV-1 transmissions may occur in stable, long-term partnerships, in which one member is HIV-1-infected (i.e., an HIV-1 serodiscordant couple). We focus this review on HIV-1 prevention in HIV-1 serodiscordant couples, with an emphasis on sub-Saharan Africa. We address testing, couples counseling, and prevention interventions, including recent evidence of the strong prevention benefits of antiretroviral therapy (ART) for the HIV-1-uninfected partner [2••] and pre-exposure prophylaxis (PrEP) for the HIV-1-uninfected partner [3••]. We also review prevention strategies based on modifiable biological risk factors (e.g., lack of male circumcision) and behavioral risk factors (e.g., lack of condom use, multiple partners) associated with HIV-1 transmission in couples (Table 1).
Table 1.
Epidemiology of HIV-1 discordance among heterosexual African couples
|
HIV-1 serodiscordance is common
| |
| ■ Half to two-thirds of HIV-1-infected adults in a cohabitating relationship in Africa have an HIV-1-uninfected partner [6-9] | |
| ■ Population-based studies indicate that the HIV-1-uninfected partner in heterosexual serodiscordant couples is equally as likely to be male as female [13] | |
|
HIV-1 transmission rates are high | |
| HIV-1 incidence in studies with HIV-1 serodiscordant couples ranges from 2.0 to 11.8/100 person-years (p-y): | |
| ■ 2/100 p-y with regular counseling and provision of free condoms [2••, 14] | |
| ■ 4/100 p-y for men and 9/100py women in couples VCT cohort study [20] | |
| ■ 8.7/100 p-y for men and 9.2/100py for women in a population-based cohort with low condom use [100] | |
| ■ 11.8/100 p-y in a retrospective cohort prior to ART scale-up [101, 102] | |
|
There are modifiable risk factors for HIV-1 transmission | |
| Plasma viral load | ■ High viral load is significantly associated with increased risk of transmission, with 2.3-2.5 increased risk per each log10 increase in plasma viral [27, 101, 103] |
| ART use by HIV-1 infected partner | ■ Early initiation of ART at CD4 350-550 cells/mL reduces risk of transmission to HIV-1-uninfected partners by 96% in context of viral load monitoring, adherence support and risk reduction counseling [14] ■ Observational studies indicate 80-92% reduced HIV-1 transmission in context of ART use by HIV-1-infected partners without virologic monitoring [43-46] ■ In one observational study, equal transmission rate from HIV-1-infected partners on & off ART in context of limited monitoring and adherence counseling [104] |
| Contraception | ■ Effective methods can prevent unintended pregnancies ■ Hormonal contraception associated with two-fold increased risk of HIV-1 acquisition and transmission among HIV-1 serodiscordant couples [76•]; thus, dual condom use and contraceptive options need to be promoted |
| Pregnancy | ■ Pregnancy in the HIV-1-infected or uninfected female associated with two-fold increased risk of male to female and female to male HIV-1 transmission [67•] |
| Condom use | ■ Low condom use among couples, especially among those unaware of their serostatus [4, 7, 20, 23, 100] ■ Condom use is highly protective with 79% lower rate of transmission among HIV-1 serodiscordant couples on a per-act basis [82] ■ Lower infection rates among couples reporting consistent condom use [20, 22, 104] ■ Alcohol use associated with lower condom use [85] |
| Outside partners | ■ Risk from outside partners of unknown HIV-1 serostatus with whom condom use is lower [15] ■ Partners in Prevention HSV/HIV Transmission study: viral linkage indicates 29% of HIV-1 infections in serodiscordant couples were acquired from outside partners [14]. Most outside partnerships reported when the HIV-1-uninfected partner did not report sex with their known HIV-1- infected partner (i.e. were not concurrent) and condom use was lower with outside partners [15] ■ HPTN 052: viral linkage indicates 25% of HIV-1 transmissions from outside partners [2••] |
| Male circumcision | ■ Lack of male circumcision is associated with increased risk of HIV-1 acquisition in men and women [101, 102] ■ Circumcision of HIV-1-uninfected men significantly reduced risk of HIV-1 acquisition [90-92] ■ Observational data indicate circumcised HIV-1 -infected men at lower risk of HIV-1 transmission to their female partners, most of whom were likely to have been circumcised prior to becoming HIV-1-infected [93], but circumcision of HIV-1-infected men did not reduce risk of transmission to female partners in randomized clinical trial [94] |
Epidemiology of HIV-1 serodiscordance among heterosexual African couples
HIV-1 serodiscordance is common in sub-Saharan Africa. National population surveys and epidemiologic studies indicate that the majority of African HIV-1-infected individuals are married or in a stable, long-term relationship [4-6], and approximately half of couples in which at least one partner is HIV-1-infected are HIV-1 serodiscordant [5, 7, 8], resulting in 2-8% of all stable couples being HIV-1 serodiscordant in some settings [5, 6, 9]. Mathematical models suggest that a substantial proportion of new HIV-1 infections occur among HIV-1 serodiscordant couples in Africa, demonstrating that HIV-1 serodiscordant couples are an important population for HIV-1 prevention [10•-12]. Women are equally likely as men to be the HIV-1-infected member in a serodiscordant couple [13].
Many HIV-1 serodiscordant couples are unaware of their serodiscordant status, primarily because one or both partners have not been tested or have tested separately and not disclosed their HIV-1 status to their partner. More than three-quarters of stable couples with at least one HIV-1-infected partner sampled in nationally representative HIV-1 serosurveys in Kenya [6], Malawi [4], and Uganda [7] were unaware of their partner's HIV-1 status. Moreover, couples often report low levels of condom use; across five African countries, fewer than 11% of cohabiting couples reported condom use at last sex [4, 5]. Finally, the term ‘stable HIV-1 serodiscordant partnerships’ does not capture the reality of dynamic partnerships, as indicated by viral linkage of transmission pairs from recent studies of HIV-1 serodiscordant couples, which documented that 25-29% of HIV-1 transmissions occur from an outside partner [2••, 14]. Some of these HIV-1 transmissions occurred after dissolution of the HIV-1 serodiscordant partnership and formation of a new partnership, often with someone of unknown HIV-1 serostatus, with whom they were less likely to use condoms [15].
Couples HIV Testing and Counseling (CHTC)
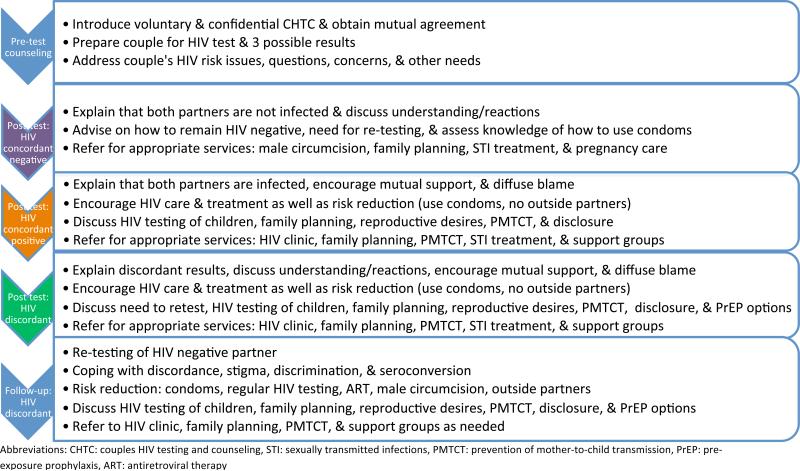
HIV-1 testing is the essential first step in the identification of HIV-1 serodiscordant couples, and disclosure is facilitated if couples test for HIV-1 together with a counselor trained in pre- and post-test counseling techniques for couples, called Couples HIV Testing and Counseling (CHTC) [16, 17]. The Centers for Disease Control and Prevention (CDC) curriculum for CHTC training has been used in many parts of sub-Saharan Africa, and has recently been adapted for brief counseling in clinical settings [18]. Couples counseling involves risk assessment with sensitivity in order to foster facilitated disclosure, avoid blame, and provide counseling messages about HIV-1 serodiscordance and referral for HIV-1 care and prevention (Figure 1) [19•-22]. Importantly, knowledge of serostatus within serodiscordant couples is likely a highly effective HIV-1 prevention strategy. African and U.S. HIV-1 serodiscordant couples who have received CHTC report increased condom use, contraceptive use, and uptake of prevention of mother-to-child transmission (PMTCT) services [20-26]. HIV-1 transmission rates were as high as 20-25% per year among HIV-1 serodiscordant couples unaware of their HIV-1 status and before ART scale-up in Africa [20, 27]. In contrast, annual HIV-1 incidence rates of 6% were observed in couples who had participated in CHTC in an observational study [20] and 2% a randomized trial of a biomedical HIV-1 intervention among HIV-1 serodiscordant couples [14]. Facilitated disclosure of serostatus within a stable partnership also fosters family support, which has been associated with improved engagement in HIV-1 care for HIV-1-infected partners and high adherence to ART [28, 29].
Figure 1.
Couples HIV testing and counseling topics [18]
The counselors who communicated with thousands of African HIV-1 serodiscordant couples in the multi-site Partners in Prevention HSV/HIV Transmission Study and Partners PrEP Study indicate that denial, disbelief, and misconceptions about discordance are frequently encountered and require additional counseling. Common reactions expressed by couples upon learning their HIV-1 serodiscordant status include disbelief in the accuracy of the test results, desire for re-testing in hope of a different outcome, anger, blaming their partner for infidelity, sadness, fear, and concerns about their children's HIV-1 status. Studies in Kenya and Uganda found that misconceptions about HIV-1 serodiscordance were common among health care providers (e.g., that HIV-1 serodiscordance reflect that the HIV-1-uninfected partner is in the ‘window period’ of seroconversion and is always already infected) as well as among the newly-identified HIV-1 serodiscordant couples (e.g., believing that the HIV-1-uninfected partner is immune or has undetectable and latent virus) [30, 31].
HIV-1 serodiscordant couples have a range of needs that emerge after the initial couples counseling and testing session and are critical to ongoing HIV-1 prevention and treatment. Couples learning of their HIV-1 serodiscordance cannot be expected to retain all messages from the first counseling session, and may need additional support to understand that HIV-1 serodiscordance is common and does not reflect inaccurate test results or immunity for the uninfected partner. Common challenges reported by HIV-1 serodiscordant couples include feeling isolated, fear of disclosing their serodiscordance, personal desires and family pressures to have more children, relationship discord over perceived infidelity and blame, union dissolution, and in some cases physical violence and threats over refusal to have sex [31-34]. Some studies have found higher rates of divorce, separation, and intimate partner violence among HIV-1 serodiscordant couples than among HIV-1 seroconcordant couples, although most reports have found intimate partner violence to be relatively rare [31, 35-37]. Counselors can identify couples at risk of violence based on a history of abuse, help ensure safety and care for those who experience violence, and make appropriate referrals, including peer support groups for HIV-1 serodiscordant couples [38].
HIV-1 re-testing is suggested for HIV-1 serodiscordant couples, as with other populations at high risk of exposure to HIV-1, in order to promote early detection of HIV-1 infection and timely referral to care and treatment [39]. The World Health Organization (WHO) recommends that individuals with a known HIV-1-infected partner re-test in four weeks to assess for recent HIV-1 transmission and annually thereafter if they continue to be sexually active [39]. The frequency of HIV-1 testing for the HIV-1-uninfected partner couple should typically be annual, and informed by the couples’ risk of transmission, fertility intentions, and symptoms suggestive of acute HIV-1. Messages about referrals for care, support in disclosure, initial relationship concerns, and methods to reduce risk of HIV-1 transmission should be reinforced at follow-up counseling and testing visits. Clinical follow-up for the HIV-1-infected partner should be emphasized and include a) CD4 cell count testing and evaluation of their eligibility for ART according to national policies to ensure timely initiation and ongoing adherence, b) correct and consistent condom use, c) discussion of family planning, safe conception, and pregnancy, and d) PMTCT for HIV-1-infected pregnant women (Figure 1) [40•]. In addition, prevention strategies should be recommended for the HIV-1 uninfected partner, including condoms, male circumcision, and PrEP, as it becomes available (Figure 1).
Antiretroviral-based strategies to reduce HIV-1 transmission in serodiscordant couples
Antiretroviral therapy for HIV-1-infected partners
ART dramatically improves the health and survival of HIV-1- infected individuals [41, 42] and also significantly reduces their infectiousness and likelihood of transmitting HIV-1 to partners (Table 2) [2••, 14, 43-45]. A recent multi-national randomized clinical trial (HPTN 052) with HIV-1 serodiscordant couples demonstrated that early initiation of ART between CD4 counts of 350-550 cells/mm3 reduced the risk of HIV-1 transmission by 96%, compared to delayed ART initiation at CD4 of 250 cells/mm3 [2••]. Importantly, HPTN 052 used intensive measures throughout the study to maximize the prevention benefits of ART: quarterly viral load monitoring and enhanced adherence counseling for those who had detectable HIV-1 RNA on ART. Thus, HPTN 052 demonstrates that ART markedly reduces HIV-1 transmission, if adherence is sufficient to achieve and sustain virologic suppression. Encouraging data from observational studies of HIV-1 serodiscordant couples, in which ART was provided following national guidelines and with less intensive adherence counseling and typically without virologic monitoring, found 80-92% reduction in HIV-1 transmission from HIV-1-infected patients who initiated ART [43-46].
Table 2.
Antiretroviral-based HIV-1 prevention strategies for HIV serodiscordant couples
| Trial | Population & Setting | Intervention | Status/Outcome |
|---|---|---|---|
| Partners PrEP Study [3••] | 4758 HIV-1 serodiscordant heterosexual couples in Kenya & Uganda | Daily oral TDF; daily oral FTC/TDF | 62% (95% CI: 34-78%) reduction in HIV-1 in TDF arm 73% (95% CI: 49-85%) reduction in HIV-1 in FTC/TDF arm |
| HPTN 052 [2••] | 1763 HIV-1 serodiscordant couples with HIV-1-infected partners’ CD4 count 350-550 in Botswana, Kenya, Malawi, South Africa, Zimbabwe, Brazil, India, Thailand, & U.S. | Immediate ART for HIV-1-infected partners with CD4 350-550 cells/mm3 vs. ART at CD4 of 250 cells/mm3 (delayed arm) | 96% (95% CI: 73-99%) reduction in HIV-1 among early ART arm |
Abbreviations: ART: antiretroviral therapy, PrEP: pre-exposure prophylaxis, TDF: tenofovir; FTC: emtricitabine
The WHO will be releasing guidelines on CHTC which will include recommending earlier ART for serodiscordant couples to reduce transmission to HIV-1-uninfected partners. Expanded use of ART for HIV-1 prevention will require careful analysis of resources and implementation strategies, including ethical, programmatic, and cost factors of earlier ART for HIV-1-infected partners who are in a serodiscordant partnership. A mathematical model predicted that treating HIV-1 serodiscordant couples in countries like Malawi and Lesotho, with high HIV-1 prevalence (7.1% and 19.5%) and where a large percentage of couples are serodiscordant (9.7% and 13.6%), could result in a substantial reduction in population-level HIV-1 incidence [47•]. Primary prevention strategies will also be needed for HIV-1 serodiscordant couples, as ART will not protect those who become infected from outside or new partners, which accounted for 25-29% of HIV-1 transmissions in HPTN 052 and the Partners in Prevention HSV/HIV Transmission Study [14, 48], but will be preventive for new partners of the HIV-1 infected partner on ART for prevention.
The success of ART for HIV-1 prevention depends on key implementation factors, including earlier identification of HIV-1-infected persons so that they can promptly initiate ART when they meet national guidelines and retention in care both pre- and post-ART, sustained adherence to ART, minimizing drug resistance, and reducing rates of treatment failure. These challenges will require expanded HIV-1 testing initiatives, strong linkages to care, and effective follow-up of HIV-1 infected persons who do not yet meet national ART guidelines. An additional consideration will be the acceptability and willingness of HIV-1-infected partners to initiate ART when they are asymptomatic. Importantly, some studies have found a substantial minority of HIV-1-infected persons who are aware of being in an HIV-1 serodiscordant partnership, are reluctant to initiate ART, as demonstrated by 37% of HIV-1-infected partners eligible for ART in a cohort of Kenyan HIV-1 serodiscordant couples who did not initiate ART within 1 year of referral for free treatment [49•]. Higher CD4 count (>100 cells/mm3) and lower socioeconomic status, measured in home ownership and rent cost, were strong predictors of non-initiation of ART [49•]. In addition to economic obstacles and structural challenges such as drug stock-outs, fear of ART side effects, stigma and disclosure, concerns over sustainability of care, food insecurity, and preference for alternative medicines pose barriers to ART initiation and ongoing adherence [50]. However, if these barriers can be overcome through counseling, other support, and strengthening of health care systems, HIV-1 infected partners who initiate ART could benefit from adherence support and involvement of their partner [28, 29].
Pre-exposure prophylaxis
Topical and oral tenofovir (TDF)-based PrEP have shown substantial efficacy in some but not all trials (Table 2). Relevant to the focus of this review, in the Partners PrEP Study, daily oral TDF and FTC/TDF were shown to have 62% and 73% efficacy, respectively, among HIV-1-uninfected partners in East African HIV-1 serodiscordant couples [3••]. Very high adherence to study drug was observed in the Partners PrEP Study [51], with qualitative interviews indicating that adherence to pill-taking was fostered by support from their partner [52]. Peri-coital use of vaginal 1% tenofovir gel showed a 39% reduction in HIV-1 acquisition and 51% reduction in HSV-2 acquisition among South African women in the CAPRISA 004 study [53]. Daily oral emtricitabine/tenofovir (FTC/TDF) was shown to have an efficacy of 44% among men who have sex with men in the multinational iPrEx study [54] and 62% in young heterosexuals in Botswana [55]. In contrast, FEM-PrEP, a trial of daily oral FTC/TDF in high-risk women was recently stopped early due to lack of efficacy [56] and the daily oral tenofovir and daily vaginal 1% tenofovir arms of the VOICE trial were stopped due to inability to demonstrate efficacy in HIV-1 prevention [57]. Further data, available in 2012, will be important to understand whether lack of efficacy in the FEM-PrEP and VOICE trials was due to insufficient adherence or other biological and behavioral factors.
A rapid review (‘rapid advice’) of the PrEP efficacy data, including guidelines about PrEP for HIV-1 serodiscordant couples, will be issued by the CDC and WHO in 2012. It is important to consider both the prevention promise and the implementation challenges of PrEP among HIV-1 serodiscordant couples, for whom the efficacy data are substantial, and interest and adherence could be high. Demonstration projects for PrEP in serodiscordant couples will need to implement CHTC with messages about ART-based prevention and evaluate targeting of PrEP to highest-risk couples, cost-effective delivery strategies, adherence support tools, and provision of a comprehensive risk reduction package [58-62]. The use of PrEP in HIV-1 serodiscordant couples should be considered as part of a combination HIV-1 prevention strategy; PrEP could be most appropriate as an effective biomedical prevention strategy under the control of the HIV-1 uninfected partner if their HIV-1 infected partner is not on ART, not adherent with ART, or if they have multiple partners or partners of unknown HIV-1 status. Mathematical modeling suggests that a cost-effective ‘staged’ use of antiretrovirals for HIV-1 prevention could involve PrEP by the HIV-1 uninfected partner in a serodiscordant relationship before the HIV-1 infected partner initiates ART, with PrEP discontinuation a few months after ART is started (to allow time to achieve viral suppression) [63•]. Based on the high levels of efficacy of PrEP observed in the Partners PrEP Study and assuming PrEP costs less than 40% that of ART, PrEP could be as cost-effective, and potentially cost-saving, if very high-risk couples are targeted, as a prevention strategy for couples compared to ART initiated before CD4 of 350 cells/μL for the HIV-1 infected partner [63•].
Family planning safe pregnancy, and prevention of mother to child transmission (PMTCT)
Many HIV-1 serodiscordant couples have high fertility; both HIV-1 infected and uninfected partners often report desires for having children with their partner [64-67•]. Over half of serodiscordant couples recruited from HIV-1 care centers in Uganda wished to have children in the future [68]. Regardless of fertility intentions, rates of highly effective contraception are often low and there are high rates of pregnancy among women of reproductive age in sub-Saharan Africa [69, 70]. Annual pregnancy rates among HIV-1 serodiscordant couples were 16.0% in the Partners in Prevention HSV/HIV Transmission Study and 9.7% in an observational cohort in Kenya [70, 71]. Pregnancy is a time of increased risk of sexual HIV-1 transmission and acquisition; pregnant women had a two-fold increased risk of male-to-female and female-to-male HIV-1 transmission in the Partners in Prevention HSV/HIV Transmission Study [67•].
Counselors and clinicians need to assess fertility intentions of HIV-1 serodiscordant couples and discuss contraceptive choices for couples not desiring children and safe conception strategies for couples who plan to have children. Less than two-thirds of women in HIV-1 serodiscordant couples surveyed in Rwanda and Zambia had ever used any contraceptive method, despite high awareness of contraception, with many citing concerns with side effects [72]. HIV-1 serodiscordant couples’ childbearing desires are not always expressed to their health care providers [65]. Health care workers’ knowledge may be incomplete and perpetuate misconceptions, and women may feel pressured by providers to terminate pregnancy or not report pregnancy intentions if they are HIV-1-infected [69, 73, 74]. An intervention that provided family planning counseling and free contraception to HIV-1 serodiscordant couples in Thika, Kenya led to significant declines in pregnancy and sustained high reports of condom use [75]. Thus, it is important to ascertain fertility desires of HIV-1 serodiscordant couples, counsel about available data on HIV-1 transmission risks during pregnancy, and encourage dual condom and contraceptive use for those not wanting to conceive [67•, 76•]. HIV-1 serodiscordant couples who do not want to conceive should be provided a choice of contraceptive options, including long-acting methods such as IUDs and implants.
HIV-1 serodiscordant couples who wish to conceive must consider options to reduce the risk of HIV-1 transmission to the HIV-1 uninfected partner. When intrauterine or intravaginal insemination is unavailable or too expensive, low technology options to reduce the risk of transmission during conception should be discussed, including delaying unprotected sex until viral load is suppressed using ART for the infected partner, screening and pre-treatment for sexually transmitted infections that might facilitate HIV-1 transmission, limited and timed unprotected sexual encounters, and male circumcision if the man is HIV-1 uninfected [77]. Safe conception may be feasible when the HIV-1 infected partner is virally suppressed on ART [78, 79]; a Swiss study of 46 HIV-1 serodiscordant couples noted no seroconversions when the HIV-1-infected male partners were on fully suppressive ART, practiced timed intercourse, and used PrEP pericoitally [80].
Further evaluation of antiretroviral-based prevention is warranted, including ART for the HIV-1 infected partner or periconception PrEP in the HIV-1 uninfected partner if their partner is not on ART or virally-suppressed [80, 81], in combination with timed unprotected intercourse during the most fertile days of the menstrual cycle [80, 81]. Advantages of periconception PrEP include autonomy of administration, which is important for HIV-1 uninfected partners, and shorter period of use which would have lower costs and may be associated with higher adherence than continuous PrEP [81].
Behavior change
Behavior change strategies available to HIV-1 serodiscordant couples to prevent HIV-1 transmission include sexual abstinence, correct and consistent condom use, and reduction of outside sexual partnerships. Couples-focused HIV-1 prevention behavioral interventions have reduced reported unprotected sex and dramatically increased reported condom use [20, 22-24]. In some studies, couples where the man was HIV-1 uninfected had higher sustainability of condom use than couples with an HIV-1 uninfected woman [20, 22]. In the Partners in Prevention HSV/HIV Transmission Study, in which HIV-1 serodiscordant couples received monthly risk reduction counseling and free condoms, self-reported unprotected sex decreased from 29% at baseline to 7% over up to 24 months of follow-up (p<0.001) [14]. Correct and consistent use of condoms is a highly effective tool for HIV-1 prevention; analyses from the Partners in Prevention HSV/HIV Transmission Study found that male condoms were associated with 79% lower risk of HIV-1 transmission on a per-contact basis [82]. However, introducing condoms into a long-term sexual relationship can be challenging. Couples often stop using condoms when they commit to a partner and if they are trying to conceive [83]. Additional challenges include perceptions that condoms interfere with sexual pleasure, spontaneity, and are a cause of sexual dysfunction [32, 83, 84]. Finally, alcohol use has been linked to unprotected sex in serodiscordant couples [85-87]. Couples should be counseled about the high efficacy of condoms for HIV-1, STIs and pregnancy [88], how condoms can enhance sexual pleasure by reducing anxiety about risk of HIV-1 transmission and pregnancy, and can become a symbol of love and commitment to the partner's well-being [88].
Sexual abstinence is an effective strategy to eliminate risk of HIV-1 transmission, but it may not be a desirable or feasible, life-long strategy for many couples [31, 83]. Cultural and social norms of sex and childbearing within marriage and long-term relationships, as well as personal desires to conceive, present a major impediment to sexual abstinence for couples.
Sexual partners outside of the marriage or primary relationship present additional risks for HIV-1 infection. HIV-1 uninfected partners may perceive new outside partners to be a safer option for unprotected sex than their infected partners. The Partners in Prevention HSV/HIV Transmission Study and HPTN 052 study found through viral linkage that 25-29% of HIV-1 infections were from outside partnerships [2••, 14]. In the Partners in Prevention HSV/HIV Transmission Study, a significant increase in reported new partnerships was observed, however, most of these were not concurrent or overlapping partnerships and most were reported after partners reported no longer having sex with their known HIV-1 infected partner [15]. Condom use was reported to be much lower with their new partners than with their known HIV-1 infected partners. Some prior studies have noted lower reported condom use during extramarital sex [22, 89]. Risk reduction counseling for HIV-1 uninfected partners in serodiscordant relationships should emphasize the risk of infection from any unprotected sex and encourage HIV-1 testing, disclosure, and protection with all sex partners.
Male circumcision
Three randomized controlled trials provide strong evidence that male circumcision reduces the risk of female-to-male HIV-1 transmission by 50-60% [90-92]. Circumcision is not generally recommended for HIV-1-infected men, given conflicting evidence between observational data and a clinical trial in terms of reduced male-to-female HIV-1 transmission [19•, 93, 94]. However, male circumcision should be strongly promoted for HIV-1 serodiscordant couples in which the male partner is HIV-1 uninfected and uncircumcised. HIV-1 discordant couples may also represent early adopters of infant male circumcision: over 90% of HIV-1 serodiscordant couples in Uganda were interested in circumcision for their male children for HIV-1 risk reduction [95].
Screening and treatment of sexually transmitted infections
The treatment of sexually transmitted infections in HIV-1 infected persons lowers genital viral load in most studies. Studies of stable HIV-1 serodiscordant couples suggest that the prevalence of curable STIs is low [96] and thus not a major driver of HIV-1 transmission within couples. However, treatment of STIs, when detected through laboratory screening or by symptomatic assessment, particularly in HIV-1 infected partners, should be done. Herpes simplex virus type 2 (HSV-2) infection is highly prevalent in HIV-1 serodiscordant couples, but acyclovir (400 mg bid) did not reduce HIV-1 transmission in three efficacy trials of HSV-2 suppression for HIV-1 prevention [14, 97, 98]. A recent cross-over study indicates that higher doses of HSV-2 suppression achieved >1 log10 reduction in plasma HIV-1 levels [99]; further consideration of higher doses of herpes suppression as a pre-ART intervention to reduce HIV-1 disease progression and infectiousness may be warranted.
Conclusion
HIV-1 prevention within HIV-1 serodiscordant couples encompasses a combination of strategies tailored to their needs and fertility intentions, including CHTC, disclosure of HIV-1 test results, condom promotion, ART for the HIV-1 infected partner, contraceptive use or safe conception strategies depending on their fertility intentions, male circumcision for HIV-1 uninfected male partners, treatment of sexually transmitted infections, and potentially, pending guidelines and demonstration projects, the use of PrEP by the HIV-1 uninfected partner. Couples HIV-1 testing and counseling is a fundamental core of HIV-1 prevention that needs to be scaled up, particularly in high prevalence settings in sub-Saharan Africa. Scale-up of counselor and clinician training in CHTC is needed to build skills in risk assessment, facilitated disclosure, discussion of HIV-1 serodiscordance and risk reduction strategies, and addressing the common challenges faced by HIV-1 serodiscordant couples. HIV-1 testing programs must ensure that knowledge of HIV-1 serostatus is coupled with strong linkages with ART and PMTCT programs and referrals for HIV-1 uninfected persons to effective prevention interventions. Partner testing should be consistently promoted and strongly encouraged in HIV-1 care settings. Forthcoming WHO guidelines will provide recommendations based on the recent HPTN 052 and Partners PrEP Study findings of significant efficacy of antiretrovirals to reduce HIV-1 risk within HIV-1 serodiscordant couples [17]. Implementation of ART for HIV-1-infected partners and PrEP for HIV-1 uninfected partners in HIV-1 serodiscordant couples must be informed by public health impact modeling, cost-effectiveness analyses, and demonstration projects. Antiretroviral-based strategies will be an important part of combination HIV-1 prevention among HIV-1 serodiscordant couples, delivered in the context of scale-up of couples HIV-1 testing and counseling to maximize the prevention impact of these effective biomedical tools.
Acknowledgments
We thank Dr. Rachel Baggaley for her review and comments on the manuscript.
Funding: the Bill and Melinda Gates Foundation (47674) and the National Institutes of Health (R01 AI083034, R21 NR012663, and R01 MH095507)
Contributor Information
Kathryn Curran, Department of Epidemiology, University of Washington, Seattle USA International Clinical Research Center, Department of Global Health University of Washington, Box 359927 325 Ninth Avenue; Seattle, WA 98104 T: 206-520-3811; F: 206-520-3831 kgcurran@uw.edu.
Jared M. Baeten, Departments of Global Health, Medicine, and Epidemiology, University of Washington, Seattle, USA International Clinical Research Center, Department of Global Health University of Washington, Box 359927 325 Ninth Avenue; Seattle, WA 98104 T: 206-520-3808; F: 206-520-3831 jbaeten@uw.edu.
Thomas J. Coates, Division of Infectious Diseases and Program in Global Health, David Geffen School of Medicine, University of California, Los Angeles, USA 9911 West Pico Blvd., Suite 955; Los Angeles, CA 90035-2703 T: 310-557-3044; F: 310-557-3679 tcoates@mednet.ucla.edu.
Ann Kurth, New York University College of Nursing, New York, USA 1006 726 Broadway; New York, NY 10003 T: 212-998-5316; F: 212-995-3143 akurth@nyu.edu.
Nelly R. Mugo, Department of Obstetrics and Gynecology, Kenyatta National Hospital/University of Nairobi, Nairobi, Kenya University of Nairobi Institute of Tropical and Infectious Diseases (UNITID) Kenyatta National Hospital, Ngong Rd, P.O. Box 19865-00202, Nairobi, Kenya T: 254-20-27376 rwamba@csrtkenya.org.
Connie Celum, Departments of Global Health, Medicine, and Epidemiology, University of Washington, Seattle, USA International Clinical Research Center, Department of Global Health University of Washington, Box 359927 325 Ninth Avenue; Seattle, WA 98104 T: 206 520-382; F: 206-520-3831 ccelum@uw.edu.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.UNAIDS [December 14, 2011];Global Report: UNAIDS Report on the Global AIDS Epidemic. 2010 Available at http://www.unaids.org/globalreport/Global_report.htm.
- 2••.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. [This is the first randomized clinical trial to demonstrate that early initiation of ART (between CD4 350-550 cells/mL) signicifanctly reduces the likelhoood of transmitting HIV-1 to sexual partners. HIV-1 serodiscordant couples initiating ART early had 96% fewer HIV-1 infections and had lower incidence of tuberculosis.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3••.Baeten J, Celum C, The Partners PrEP Study Team Antiretroviral pre-exposure prophylaxis for HIV-1 prevention among heterosexual African men and women: The Partners PrEP Study.. Presented at the VI International AIDS Society Conference on HIV Pathogenesis, Treatment and Prevention; Rome, Italy. July 17-20, 2011; [This randomized clinical trial demonstrated high efficacy of daily oral PrEP in preventing HIV-1 transmission in HIV-1 serodiscordant couples in sub-Saharan Africa: 62% with tenofovir and 73% with emtricitabine/tenofovir.] [Google Scholar]
- 4.Anand A, Shiraishi RW, Bunnell RE, et al. Knowledge of HIV status, sexual risk behaviors and contraceptive need among people living with HIV in Kenya and Malawi. AIDS. 2009;23(12):1565–73. doi: 10.1097/QAD.0b013e32832cb10c. [DOI] [PubMed] [Google Scholar]
- 5.De Walque D. Sero-discordant couples in five African countries: Implications for prevention strategies. Popul Dev Rev. 2007;33(3):501–523. [Google Scholar]
- 6.Kaiser R, Bunnell R, Hightower A, et al. Factors associated with HIV infection in married or cohabitating couples in Kenya: results from a nationally representative study. PLoS One. 2011;6(3):e17842. doi: 10.1371/journal.pone.0017842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bunnell R, Opio A, Musinguzi J, et al. HIV transmission risk behavior among HIV-infected adults in Uganda: results of a nationally representative survey. AIDS. 2008;22(5):617–24. doi: 10.1097/QAD.0b013e3282f56b53. [DOI] [PubMed] [Google Scholar]
- 8.Were WA, Mermin JH, Wamai N, et al. Undiagnosed HIV infection and couple HIV discordance among household members of HIV-infected people receiving antiretroviral therapy in Uganda. J Acquir Immune Defic Syndr. 2006;43(1):91–5. doi: 10.1097/01.qai.0000225021.81384.28. [DOI] [PubMed] [Google Scholar]
- 9.Lingappa JR, Lambdin B, Bukusi EA, et al. Regional differences in prevalence of HIV-1 discordance in Africa and enrollment of HIV-1 discordant couples into an HIV-1 prevention trial. PLoS One. 2008;3(1):e1411. doi: 10.1371/journal.pone.0001411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10•.Gray R, Ssempiija V, Shelton J, et al. The contribution of HIV-discordant relationships to new HIV infections in Rakai, Uganda. AIDS. 2011;25(6):863–5. doi: 10.1097/QAD.0b013e3283448790. [This analysis of HIV-1 infections occurring in couples in a community cohort study in Uganda found that 18% and 13% of new infections are attributable to HIV-1 serodiscordant couples, pre and post ART respectively. This study used sequential population-based HIV-1 prevalence surveys.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colvin M, Gorgens-Albino M, Kasedde S. [December 12, 2011];Analysis of HIV prevention responses and modes of HIV transmission: the UNAIDS-GAMET supported synthesis process. 2008 Available at http://www.unaidsrstesa.org/reports-and-publications/analysis-hiv-prevention-response-and-modes-hiv-transmission-unaids-gamet-su.
- 12.Coburn BJ, Gerberry DJ, Blower S. Quantification of the role of discordant couples in driving incidence of HIV in sub-Saharan Africa. Lancet Infect Dis. 2011;11(4):263–4. doi: 10.1016/S1473-3099(11)70080-9. [DOI] [PubMed] [Google Scholar]
- 13.Eyawo O, de Walque D, Ford N, et al. HIV status in discordant couples in sub-Saharan Africa: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10(11):770–7. doi: 10.1016/S1473-3099(10)70189-4. [DOI] [PubMed] [Google Scholar]
- 14.Celum C, Wald A, Lingappa JR, et al. Acyclovir and transmission of HIV-1 from persons infected with HIV-1 and HSV-2. N Engl J Med. 2010;362(5):427–39. doi: 10.1056/NEJMoa0904849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ndase P, Celum C, Thomas K, et al. Outside Sexual Partnerships and Risk of HIV Acquisition for HIV Uninfected Partners in African HIV Serodiscordant Partnerships. J Acquir Immune Defic Syndr. 2012;59(1):65–71. doi: 10.1097/QAI.0b013e318237b864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The U.S. Centers for Disease Control and Prevention [December 14, 2011];Couples HIV Counseling and Testing Intervention and Curriculum. 2007 Available at http://www.cdc.gov/globalaids/resources/prevention/chct.html.
- 17.The World Health Organization Guidance on Couples HIV Testing and Counselling and Antiretroviral Therapy for Treatment and Prevention in Serodiscordant Couples: Recommendations for a public health approach. Release date. 2012 [PubMed] [Google Scholar]
- 18.The U.S. Centers for Disease Control and Prevention (CDC) Division of Global HIV and AIDS (DGHA) Couples HIV Testing and Counseling (CHTC) in Health Care Facilities Trainer's Manual. Updated and adapted from CDC/DGHA Couples HIV Counseling and Testing Intervention and Training Curriculum (November 2009) 2011 Nov; In draft. [Google Scholar]
- 19•.Matovu JK. Preventing HIV transmission in married and cohabiting HIV-discordant couples in sub-Saharan Africa through combination prevention. Curr HIV Res. 2010;8(6):430–40. doi: 10.2174/157016210793499303. [This review paper presents various HIV-1 prevention interventions, behavioral and biomedical, to include in a combination prevention intervention for HIV-1 serodiscordant couples in sub-Saharan Africa.] [DOI] [PubMed] [Google Scholar]
- 20.Allen S, Tice J, Van de Perre P, et al. Effect of serotesting with counselling on condom use and seroconversion among HIV discordant couples in Africa. Bmj. 1992;304(6842):1605–9. doi: 10.1136/bmj.304.6842.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The Voluntary HIV-1 Counseling and Testing Efficacy Study Group Efficacy of voluntary HIV-1 counselling and testing in individuals and couples in Kenya, Tanzania, and Trinidad: a randomised trial. The Lancet. 2000;356(9224):103–12. [PubMed] [Google Scholar]
- 22.Kamenga M, Ryder RW, Jingu M, et al. Evidence of marked sexual behavior change associated with low HIV-1 seroconversion in 149 married couples with discordant HIV-1 serostatus: experience at an HIV counselling center in Zaire. AIDS. 40915(1):61–7. doi: 10.1097/00002030-199101000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Allen S, Meinzen-Derr J, Kautzman M, et al. Sexual behavior of HIV discordant couples after HIV counseling and testing. AIDS. 2003;17(5):733–40. doi: 10.1097/00002030-200303280-00012. [DOI] [PubMed] [Google Scholar]
- 24.Burton J, Darbes LA, Operario D. Couples-focused behavioral interventions for prevention of HIV: systematic review of the state of evidence. AIDS Behav. 2010;14(1):1–10. doi: 10.1007/s10461-008-9471-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farquhar C, Kiarie JN, Richardson BA, et al. Antenatal couple counseling increases uptake of interventions to prevent HIV-1 transmission. J Acquir Immune Defic Syndr. 2004;37(5):1620–6. doi: 10.1097/00126334-200412150-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.El-Bassel N, Gilbert L, Witte S, et al. Couple-based HIV prevention in the United States: advantages, gaps, and future directions. J Acquir Immune Defic Syndr. 2010;55(Suppl 2):S98–101. doi: 10.1097/QAI.0b013e3181fbf407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gray RH, Wawer MJ, Brookmeyer R, et al. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. Lancet. 2001;357(9263):1149–53. doi: 10.1016/S0140-6736(00)04331-2. [DOI] [PubMed] [Google Scholar]
- 28.Ware NC, Idoko J, Kaaya S, et al. Explaining adherence success in sub-Saharan Africa: an ethnographic study. PLoS Med. 2009;6(1):e11. doi: 10.1371/journal.pmed.1000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stirratt MJ, Remien RH, Smith A, et al. The Role of HIV Serostatus Disclosure in Antiretroviral Medication Adherence. AIDS and Behavior. 2006;10(5):483–93. doi: 10.1007/s10461-006-9106-6. [DOI] [PubMed] [Google Scholar]
- 30.Were E, Wools-Kaloustian K, Baliddawa J, et al. Stakeholders perception of HIV sero-discordant couples in western Kenya. East Afr Med J. 2008;85(7):326–33. doi: 10.4314/eamj.v85i7.9650. [DOI] [PubMed] [Google Scholar]
- 31.Bunnell RE, Nassozi J, Marum E, et al. Living with discordance: knowledge, challenges, and prevention strategies of HIV-discordant couples in Uganda. AIDS Care. 2005;17(8):999–1012. doi: 10.1080/09540120500100718. [DOI] [PubMed] [Google Scholar]
- 32.Beyeza-Kashesya J, Kaharuza F, Mirembe F, et al. The dilemma of safe sex and having children: challenges facing HIV sero-discordant couples in Uganda. Afr Health Sci. 2009;9(1):2–12. [PMC free article] [PubMed] [Google Scholar]
- 33.Porter L, Hao L, Bishai D, et al. HIV status and union dissolution in sub-Saharan Africa: the case of Rakai, Uganda. Demography. 2004;41(3):465–82. doi: 10.1353/dem.2004.0025. [DOI] [PubMed] [Google Scholar]
- 34.VanDevanter N, Thacker AS, Bass G, et al. Heterosexual couples confronting the challenges of HIV infection. AIDS Care. 1999;11(2):181–93. doi: 10.1080/09540129948072. [DOI] [PubMed] [Google Scholar]
- 35.Semrau K, Kuhn L, Vwalika C, et al. Women in couples antenatal HIV counseling and testing are not more likely to report adverse social events. AIDS. 2005;19(6):603–9. doi: 10.1097/01.aids.0000163937.07026.a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Emusu D, Ivankova N, Jolly P, et al. Experience of sexual violence among women in HIV discordant unions after voluntary HIV counselling and testing: a qualitative critical incident study in Uganda. AIDS Care. 2009;21(11):1363–70. doi: 10.1080/09540120902883077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Were E, Curran K, Delany-Moretlwe S, et al. A prospective study of frequency and correlates of intimate partner violence among African heterosexual HIV serodiscordant couples. AIDS. 2011;25(16):2009–18. doi: 10.1097/QAD.0b013e32834b005d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.The World Health Organization [December 14, 2011];Addressing violence against women in HIV testing and counselling: A meeting report. 2006 Available at www.who.int/gender/documents/VCT_addressing_violence.pdf.
- 39.The World Health Organization . Delivering HIV test results and messages for re-testing and counselling in adults. WHO Press; Geneva, Switzerland: 2010. [PubMed] [Google Scholar]
- 40•.Grabbe KL, Bunnell R. Reframing HIV prevention in sub-Saharan Africa using couple-centered approaches. JAMA. 2010;304(3):346–7. doi: 10.1001/jama.2010.1011. [This commentary underscores the importance of couples HIV-1 testing and counseling and a couple-focused approach to HIV-1 prevention in sub-Saharan Africa.] [DOI] [PubMed] [Google Scholar]
- 41.Mills EJ, Bakanda C, Birungi J, et al. Life expectancy of persons receiving combination antiretroviral therapy in low-income countries: a cohort analysis from Uganda. Annals of internal medicine. 2011;155(4):209–16. doi: 10.7326/0003-4819-155-4-201108160-00358. [DOI] [PubMed] [Google Scholar]
- 42.Cotton D. Life expectancy in Africa: back to the future? Annals of internal medicine. 2011;155(4):265–6. doi: 10.7326/0003-4819-155-4-201108160-00359. [DOI] [PubMed] [Google Scholar]
- 43.Donnell D, Baeten JM, Kiarie J, et al. Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: a prospective cohort analysis. Lancet. 2010;375(9731):2092–8. doi: 10.1016/S0140-6736(10)60705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sullivan P, Kayitenkore K, Chomba E, et al. Is the reduction of HIV transmission risk while prescribed antiretroviral therapy (ARVT) different for men and women? Results from discordant couples in Rwanda and Zambia.. V International AIDS Society Conference on HIV Pathogenesis, Treatment and Prevention.; Cape Town, South Africa. July 19-22, 2009. [Google Scholar]
- 45.Reynolds SJ, Makumbi F, Nakigozi G, et al. HIV-1 transmission among HIV-1 discordant couples before and after the introduction of antiretroviral therapy. AIDS. 2011;25(4):473–7. doi: 10.1097/QAD.0b013e3283437c2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bunnell R. HIV Prevention for a Threatened Continent: Implementing Positive Prevention in Africa. JAMA: The Journal of the American Medical Association. 2006;296(7):855–8. doi: 10.1001/jama.296.7.855. [DOI] [PubMed] [Google Scholar]
- 47•.El-Sadr WM, Coburn BJ, Blower S. Modeling the impact on the HIV epidemic of treating discordant couples with antiretrovirals to prevent transmission. AIDS. 2011;25(18):2295–9. doi: 10.1097/QAD.0b013e32834c4c22. [A mathematical model estimated the impact of ART initiation on HIV-1 incidence in HIV-1 serodiscordant couples in four African countries. HIV-1 incidence reduction and number of infections averted varied by the proportion of the population in stable partnerships, the percentage of serodiscordant couples, and HIV-1 prevalence.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hughes JP, Hudelson S, Redd A, et al. Analysis of genetic linkage of HIV from couples enrolled in the HIV Prevention Trials Network (HPTN) 052 trial.. VI International AIDS Society Conference on HIV Pathogenesis, Treatment and Prevention; Rome, Italy. July17-20, 2011. [Google Scholar]
- 49•.Guthrie BL, Choi RY, Liu AY, et al. Barriers to Antiretroviral Initiation in HIV-1-Discordant Couples. J Acquir Immune Defic Syndr. 2011;58(3):e87–e93. doi: 10.1097/QAI.0b013e31822f064e. [A cohort study of HIV-1 serodiscordant couples in Kenya evaluated time to ART initiation in HIV-1- infected partners eligible for free treatment. Socioeconomic status and CD4 count were associated with delayed ART initiation.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Unge C, Johansson A, Zachariah R, et al. Reasons for unsatisfactory acceptance of antiretroviral treatment in the urban Kibera slum, Kenya. AIDS Care. 2008;20(2):146–9. doi: 10.1080/09540120701513677. [DOI] [PubMed] [Google Scholar]
- 51.Haberer J, Baeten J, Celum C, et al. Near Perfect Early Adherence to Antiretroviral PrEP against HIV Infection among HIV Serodiscordant Couples as Determined by Multiple Measures: Preliminary Data from the Partners PrEP Study.. 18th Conference on Retroviruses and Opportunistic Infections; Boston, USA. February 27-March 2, 2011. [Google Scholar]
- 52.Ware NC, Wyatt MA, Haberer JE, et al. What's love got to do with it? A theory of adherence to oral antiretroviral pre-exposure prophylaxis (PrEP) for HIV serodiscordant couples. AIDS. 2011 doi: 10.1097/QAI.0b013e31824a060b. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Abdool Karim Q, Abdool Karim SS, Frohlich JA, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329(5996):1168–74. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grant RM, Lama JR, Anderson PL, et al. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363(27):2587–99. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thigpen MC, Kebaabetswe PM, Smith DK, et al. Daily oral antiretroviral use for the prevention of HIV infection in heterosexually active young adults in Botswana: results from the TDF2 study.. VI International AIDS Society Conference on HIV Pathogenesis, Treatment and Prevention; Rome, Italy. July 17-20, 2011. [Google Scholar]
- 56.FHI 360 [December 14, 2011];FEM-PrEP Project: FHI to initiate orderly closure of FEM-PrEP [Press release] 2011 Available at http://www.fhi.org/en/Research/Projects/FEM-PrEP.htm.
- 57.Microbicide Trials Network [December 14, 2011];Microbricide Trials Network statement on decision to discontinue use of oral tenofovir tablets in VOICE, a major HIV prevention study in women [Press release] 2011 Available at http://www.mtnstopshiv.org/node/3909.
- 58.Karim SS, Karim QA. Antiretroviral prophylaxis: a defining moment in HIV control. Lancet. 2011 doi: 10.1016/S0140-6736(11)61136-7. Epub date: July 21, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim SC, Becker S, Dieffenbach C, et al. Planning for pre-exposure prophylaxis to prevent HIV transmission: challenges and opportunities. J Int AIDS Soc. 2010;13:24. doi: 10.1186/1758-2652-13-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Underhill K, Operario D, Mimiaga MJ, et al. Implementation science of pre-exposure prophylaxis: preparing for public use. Curr HIV/AIDS Rep. 2010;7(4):210–9. doi: 10.1007/s11904-010-0062-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Underhill K, Operario D, Skeer M, et al. Packaging PrEP to Prevent HIV: An Integrated Framework to Plan for Pre-Exposure Prophylaxis Implementation in Clinical Practice. J Acquir Immune Defic Syndr. 2010;55(1):8–13. doi: 10.1097/qai.0b013e3181e8efe4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Paxton LA, Hope T, Jaffe HW. Pre-exposure prophylaxis for HIV infection: what if it works? Lancet. 2007;370(9581):89–93. doi: 10.1016/S0140-6736(07)61053-8. [DOI] [PubMed] [Google Scholar]
- 63•.Hallett TB, Baeten JM, Heffron R, et al. Optimal uses of antiretrovirals for prevention in HIV-1 serodiscordant heterosexual couples in South Africa: A modelling study. PLoS Med. 2011;8(11) doi: 10.1371/journal.pmed.1001123. [This mathematical model examined the cost-effectiveness of PrEP use before ART initiaiton, the relative cost-effectiveness of PrEP and early ART at various levels of PrEP efficacy and HIV-1 risk behavior, and optimal timing for combining PrEP and ART in HIV-1 serodiscordant couples. The model indicates that at moderate-high PrEP efficacy (>45%) and high ART efficacy (>90%), PrEP may be cost-effective until the HIV-1-infected partner begins ART (CD4 350 in the model) if high-risk couples can be targeted and PrEP delivered cost-effectively.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nattabi B, Li J, Thompson SC, et al. A systematic review of factors influencing fertility desires and intentions among people living with HIV/AIDS: implications for policy and service delivery. AIDS Behav. 2009;13(5):949–68. doi: 10.1007/s10461-009-9537-y. [DOI] [PubMed] [Google Scholar]
- 65.Cooper D, Moodley J, Zweigenthal V, et al. Fertility intentions and reproductive health care needs of people living with HIV in Cape Town, South Africa: implications for integrating reproductive health and HIV care services. AIDS Behav. 2009;13(Suppl 1):38–46. doi: 10.1007/s10461-009-9550-1. [DOI] [PubMed] [Google Scholar]
- 66.Myer L, Morroni C, Rebe K. Prevalence and determinants of fertility intentions of HIV-infected women and men receiving antiretroviral therapy in South Africa. AIDS Patient Care STDS. 2007;21(4):278–85. doi: 10.1089/apc.2006.0108. [DOI] [PubMed] [Google Scholar]
- 67.Mugo NR, Heffron R, Donnell D, et al. Increased risk of HIV-1 transmission in pregnancy: a prospective study among African HIV-1 serodiscordant couples. AIDS. 2011;25(15):1887–95. doi: 10.1097/QAD.0b013e32834a9338. [A cohort study with over 3400 HIV-1 serodiscordant couples in Africa found an increased risk of female-to-male and male-to-female HIV-1 transmission during pregnancy.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Beyeza-Kashesya J, Ekstrom AM, Kaharuza F, et al. My partner wants a child: a cross-sectional study of the determinants of the desire for children among mutually disclosed sero-discordant couples receiving care in Uganda. BMC Public Health. 2010;10:247. doi: 10.1186/1471-2458-10-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Laher F, Todd CS, Stibich MA, et al. A qualitative assessment of decisions affecting contraceptive utilization and fertility intentions among HIV-positive women in Soweto, South Africa. AIDS Behav. 2009;13(Suppl 1):47–54. doi: 10.1007/s10461-009-9544-z. [DOI] [PubMed] [Google Scholar]
- 70.Heffron R, Were E, Celum C, et al. A Prospective Study of Contraceptive Use Among African Women in HIV-1 Serodiscordant Partnerships. Sexually Transmitted Diseases. 2010;37(10):621–8. doi: 10.1097/OLQ.0b013e3181e1a162. [DOI] [PubMed] [Google Scholar]
- 71.Guthrie BL, Choi RY, Bosire R, et al. Predicting Pregnancy in HIV-1-Discordant Couples. AIDS and Behavior. 2010;14(5):1066–71. doi: 10.1007/s10461-010-9716-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Grabbe K, Stephenson R, Vwalika B, et al. Knowledge, use, and concerns about contraceptive methods among sero-discordant couples in Rwanda and Zambia. Journal of Women's Health. 2009;18(9):1449–56. doi: 10.1089/jwh.2008.1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gruskin S, Ferguson L, Omalley J. Ensuring Sexual and Reproductive Health for People Living with HIV: An Overview of Key Human Rights, Policy and Health Systems Issues. Reproductive Health Matters. 2007;15(29):4–26. doi: 10.1016/S0968-8080(07)29028-7. [DOI] [PubMed] [Google Scholar]
- 74.Gruskin S, Firestone R, Maccarthy S, et al. HIV and pregnancy intentions: do services adequately respond to women's needs? Am J Public Health. 2008;98(10):1746–50. doi: 10.2105/AJPH.2008.137232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ngure K, Heffron R, Mugo N, et al. Successful increase in contraceptive uptake among Kenyan HIV-1-serodiscordant couples enrolled in an HIV-1 prevention trial. AIDS. 2009;23(Suppl 1):S89–95. doi: 10.1097/01.aids.0000363781.50580.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76•.Heffron R, Donnell D, Rees H, et al. Use of hormonal contraceptives and risk of HIV-1 transmission: a prospective cohort study. Lancet Infect Dis. 2011 doi: 10.1016/S1473-3099(11)70247-X. Epub date: October 3, 2011. [This observational analysis of 3790 African HIV-1 serodiscordant couples found a two-fold increased risk of male to female and female to male HIV-1 transmission during periods of hormonal contraceptive use. Most women were using injectable progestins.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Matthews LT, Mukherjee JS. Strategies for harm reduction among HIV-affected couples who want to conceive. AIDS Behav. 2009;13(Suppl 1):5–11. doi: 10.1007/s10461-009-9551-0. [DOI] [PubMed] [Google Scholar]
- 78.Barreiro P, Castilla JA, Labarga P, et al. Is natural conception a valid option for HIV-serodiscordant couples? Hum Reprod. 2007;22(9):2353–8. doi: 10.1093/humrep/dem226. [DOI] [PubMed] [Google Scholar]
- 79.Barreiro P, del Romero J, Leal M, et al. Natural pregnancies in HIV-serodiscordant couples receiving successful antiretroviral therapy. J Acquir Immune Defic Syndr. 2006;43(3):324–6. doi: 10.1097/01.qai.0000243091.40490.fd. [DOI] [PubMed] [Google Scholar]
- 80.Vernazza PL, Graf I, Sonnenberg-Schwan U, et al. Pre-exposure prophylaxis and timed intercourse for HIV-discordant couples willing to conceive a child. AIDS. 2011;25(16):2049–51. doi: 10.1097/QAD.0b013e32834a36d0. [DOI] [PubMed] [Google Scholar]
- 81.Matthews LT, Baeten JM, Celum C, et al. Periconception pre-exposure prophylaxis to prevent HIV transmission: benefits, risks, and challenges to implementation. AIDS. 2010;24(13):1975–82. doi: 10.1097/QAD.0b013e32833bedeb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hughes JP, Baeten JM, Lingappa JR, et al. Determinants of per coital act HIV-1 infectivity among HIV-1 serodiscordant couples. J Infect Dis. 2011 doi: 10.1093/infdis/jir747. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Allen C, Mbonye M, Seeley J, et al. ABC for people with HIV: responses to sexual behaviour recommendations among people receiving antiretroviral therapy in Jinja, Uganda. Cult Health Sex. 2011;13(5):529–43. doi: 10.1080/13691058.2011.558593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rispel LC, Metcalf CA, Moody K, et al. Sexual relations and childbearing decisions of HIV-discordant couples: an exploratory study in South Africa and Tanzania. Reproductive Health Matters. 2011;19(37):184–93. doi: 10.1016/S0968-8080(11)37552-0. [DOI] [PubMed] [Google Scholar]
- 85.Coldiron ME, Stephenson R, Chomba E, et al. The Relationship Between Alcohol Consumption and Unprotected Sex Among Known HIV-discordant Couples in Rwanda and Zambia. AIDS and Behavior. 2007;12(4):594–603. doi: 10.1007/s10461-007-9304-x. [DOI] [PubMed] [Google Scholar]
- 86.Sarna A, Chersich M, Okal J, et al. Changes in sexual risk taking with antiretroviral treatment: influence of context and gender norms in Mombasa, Kenya. Cult Health Sex. 2009;11(8):783–97. doi: 10.1080/13691050903033423. [DOI] [PubMed] [Google Scholar]
- 87.King R, Khana K, Nakayiwa S, et al. ‘Pregnancy comes accidentally - like it did with me’: Reproductive Decisions among Women on ART and their Partners in Rural Uganda. BMC Public Health. 2011;11(1):530. doi: 10.1186/1471-2458-11-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wagner GJ, Holloway I, Ghosh-Dastidar B, et al. Factors Associated with Condom Use Among HIV Clients in Stable Relationships with Partners at Varying Risk for HIV in Uganda. AIDS and Behavior. 2010;14(5):1055–65. doi: 10.1007/s10461-010-9673-4. [DOI] [PubMed] [Google Scholar]
- 89.Mermin J, Musinguzi J, Opio A, et al. Risk factors for recent HIV infection in Uganda. JAMA. 2008;300(5):540–9. doi: 10.1001/jama.300.5.540. [DOI] [PubMed] [Google Scholar]
- 90.Auvert B, Taljaard D, Lagarde E, et al. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265 Trial. PLoS Med. 2005;2(11):e298. doi: 10.1371/journal.pmed.0020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bailey RC, Moses S, Parker CB, et al. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet. 2007;369(9562):643–56. doi: 10.1016/S0140-6736(07)60312-2. [DOI] [PubMed] [Google Scholar]
- 92.Gray RH, Kigozi G, Serwadda D, et al. Male circumcision for HIV prevention in men in Rakai, Uganda: a randomised trial. Lancet. 2007;369(9562):657–66. doi: 10.1016/S0140-6736(07)60313-4. [DOI] [PubMed] [Google Scholar]
- 93.Baeten JM, Celum C, Coates TJ. Male circumcision and HIV risks and benefits for women. Lancet. 2009;374(9685):182–4. doi: 10.1016/S0140-6736(09)61311-8. [DOI] [PubMed] [Google Scholar]
- 94.Wawer MJ, Makumbi F, Kigozi G, et al. Circumcision in HIV-infected men and its effect on HIV transmission to female partners in Rakai, Uganda: a randomised controlled trial. Lancet. 2009;374(9685):229–37. doi: 10.1016/S0140-6736(09)60998-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mugwanya KK, Whalen C, Celum C, et al. Circumcision of male children for reduction of future risk for HIV: acceptability among HIV serodiscordant couples in Kampala, Uganda. PLoS One. 2011;6(7):e22254. doi: 10.1371/journal.pone.0022254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mujugira A, Baeten JM, Donnell D, et al. Characteristics of HIV-1 Serodiscordant Couples Enrolled in a Clinical Trial of Antiretroviral Pre-Exposure Prophylaxis for HIV-1 Prevention. PLoS One. 2011;6(10):e25828. doi: 10.1371/journal.pone.0025828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Celum C, Wald A, Hughes J, et al. Effect of aciclovir on HIV-1 acquisition in herpes simplex virus 2 seropositive women and men who have sex with men: a randomised, double-blind, placebo-controlled trial. Lancet. 2008;371(9630):2109–19. doi: 10.1016/S0140-6736(08)60920-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Watson-Jones D, Weiss HA, Rusizoka M, et al. Effect of herpes simplex suppression on incidence of HIV among women in Tanzania. N Engl J Med. 2008;358(15):1560–71. doi: 10.1056/NEJMoa0800260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mugwanya K, Baeten JM, Mugo NR, et al. High-dose Valacyclovir HSV-2 Suppression Results in Greater Reduction in Plasma HIV-1 Levels Compared With Standard Dose Acyclovir Among HIV-1/HSV-2 Coinfected Persons: A Randomized, Crossover Trial. J Infect Dis. 2011;204(12):1912–7. doi: 10.1093/infdis/jir649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Serwadda D, Gray RH, Wawer MJ, et al. The social dynamics of HIV transmission as reflected through discordant couples in rural Uganda. AIDS. 1995;9(7):745–50. doi: 10.1097/00002030-199507000-00012. [DOI] [PubMed] [Google Scholar]
- 101.Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. Rakai Project Study Group. N Engl J Med. 2000;342(13):921–9. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 102.Gray RH, Kiwanuka N, Quinn TC, et al. Male circumcision and HIV acquisition and transmission: cohort studies in Rakai, Uganda. Rakai Project Team. AIDS. 2000;14(15):2371–81. doi: 10.1097/00002030-200010200-00019. [DOI] [PubMed] [Google Scholar]
- 103.Lingappa JR, Hughes JP, Wang RS, et al. Estimating the impact of plasma HIV-1 RNA reductions on heterosexual HIV-1 transmission risk. PLoS One. 2010;5(9):e12598. doi: 10.1371/journal.pone.0012598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang L, Ge Z, Luo J, et al. HIV Transmission Risk Among Serodiscordant Couples: A Retrospective Study of Former Plasma Donors in Henan, China. Jaids-J Acq Imm Def. 2010;55(2):232–8. doi: 10.1097/QAI.0b013e3181e9b6b7. [DOI] [PMC free article] [PubMed] [Google Scholar]