Abstract
The androgen deprivation therapy (ADT) to systematically suppress/reduce androgens binding to the androgen receptor (AR) has been the standard therapy for prostate cancer (PCa); yet, most of ADT eventually fails leading to the recurrence of castration resistant PCa. Here, we found that the PCa patients who received ADT had increased PCa stem/progenitor cell population. The addition of the anti-androgen, Casodex®, or AR-siRNA in various PCa cells led to increased stem/progenitor cells, whereas, in contrast, the addition of functional AR led to decreased stem/progenitor cell population but increased non-stem/progenitor cell population, suggesting that AR functions differentially in PCa stem/progenitor vs. non-stem/progenitor cells. Therefore, the current ADT might result in an undesired expansion of PCa stem/progenitor cell population, which explains why this therapy fails. Using various human PCa cell lines and three different mouse models, we concluded that targeting PCa non-stem/progenitor cells with AR degradation enhancer ASC-J9® and targeting PCa stem/progenitor cells with 5-azathioprine and γ-tocotrienol resulted in a significant suppression of the tumors at the castration resistant stage. This suggests that a combinational therapy that simultaneously targets both stem/progenitor and non-stem/progenitor cells will lead to better therapeutic efficacy and may become a new therapy to battle the PCa before and after castration resistant stages.
Keywords: prostate cancer stem cells, androgen receptor, combination therapy
Introduction
The prostate cancer (PCa) microenvironment contains heterogeneous cells that may contribute differentially to PCa progression at either early initiation or later metastasis (Josson et al., 2010; Karlou et al., 2010; Zhang et al., 2010). The detailed mechanisms how each cell population contributes to PCa progression, especially at later castration resistant stage, remain unclear.
In general, cancer stem cells have been well-documented to play important roles in tumor initiation and metastasis (Mueller et al., 2010; Rasheed et al., 2011) and earlier reports found that prostate tumors also contained stem/progenitor cells that might play essential roles in prostate tumorigenicity and metastasis (Maitland and Collins, 2008; Klarmann et al., 2009; Pfeiffer and Schalken, 2010; Li and Tang, 2011). However, whether these PCa stem/progenitor cells also play important roles to influence androgen deprivation therapy (ADT)-treated PCa during transition into the castration resistant stage remains unclear.
It is generally agreed that PCa stem/progenitor cells (CD133+, integrin+, and CK5+) could differentiate into transit-amplifying cells/intermediate cells (CD133−, integrin+, CK5+, and CK8+) and finally into well differentiated luminal epithelial cells (CD133−, integrin−, CK5−, and CK8+; Verhagen et al., 1992; Collins et al., 2001; Litvinov et al., 2003). However, a recent report indicated that the PCa stem/progenitor cells could also originate from luminal epithelial cells and then differentiate into basal epithelial cells (Wang et al., 2009).
Here, we found that androgen receptor (AR) played opposite roles in PCa stem/progenitor vs. non-stem/progenitor cells and that castration/ADT promoted PCa stem/progenitor cell expansion. Simultaneously, targeting AR opposing signals/roles in these two types of cells resulted in a significant suppression of PCa progression before and after castration resistant stages.
Results
ADT increases PCa stem/progenitor cell population
As AR is expressed differentially in PCa stem/progenitor cells vs. non-stem/progenitor cells, we were interested in testing if the sensitivity/response to ADT treatment could also be different in these two cell populations. To test in vitro ADT effects, we used Casodex®, the currently used anti-androgen, and we found that 1 µM Casodex could suppress LNCaP-CD133− non-stem/progenitor cell growth but increase LNCaP-CD133+ stem/progenitor cell population (Figure 1Aa; gradual time-dependent increase in the CD133+ stem/progenitor cells is shown in Supplementary Figure S1A). The increase in CD133 protein expression upon Casodex® treatment was also observed (Figure 1Ab). In addition, we observed a similar, although less pronounced, increase in C4-2-CD133+ stem/progenitor cells upon Casodex® treatment (Supplementary Figure S1B).
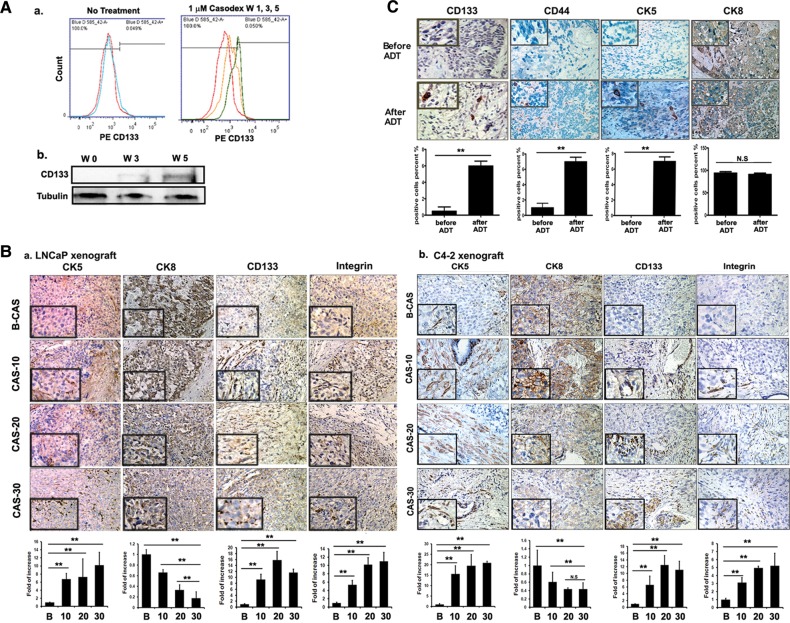
Figure 1.
Stem/progenitor cells increase after castration/ADT. (A) Cell line studies. (a) Flow cytometric analysis of CD133+ cells after 1 (red), 3 (yellow), and 5 (green) weeks of 1 µM Casodex® treatment of LNCaP cells. (b) Western blot analysis of CD133 expression after 5 weeks of Casodex® treatment. (B) Mice tumor tissue studies. Immunohistostaining (IHC) results of tumor tissues. (a) Tumors of LNCaP-xenografted mice and (b) tumor tissues of C4-2-xenografted mice. Tumor tissues were obtained before and 10, 20, and 30 days (indicated as B, 10, 20, and 30) after castration and stained with antibodies of CD133, integrin, CK5, and CK8. Quantitation is shown below the staining data (magnification, ×100; inset, ×400). (C) Human tumor tissues studies. IHC of tumor tissues with antibodies of CD133, CD44, CK5, integrin, and CK8. Human tissues were obtained from Tohoku University Hospital, Sendai, Japan, and Chang Gung Memorial Hospital, Linkou, from the same patients, before and after ADT (magnification, ×100; inset, ×400). **P< 0.01.
We then confirmed the above in vitro cell line data with in vivo mouse PCa studies. Mice were first orthotopically inoculated with LNCaP or C4-2 cells, castrated, and then sacrificed at 10, 20, and 30 days. As shown in Figure 1B, a significant increase in the expressions of the stem/progenitor cell markers, CD133 and integrin, was detected in xenografted tissues from the castrated mice when compared with the sham controls (Figure 1Ba for LNCaP xenografts and Figure 1Bb for C4-2 xenografts). We also observed the increase in CK5+ cells, but the decrease in CK8+ cells (Figure 1Ba and b), in those xenografted tissues from the castrated mice when compared with the control mice. This increase in CK5+ cells was maximal at 20 days after castration.
Importantly, we also examined stem/progenitor population changes in PCa tissues from the same patients before the ADT and after ADT when castration resistant PCa developed. A total of seven sets of paired PCa tissues were examined with antibodies of the stem/progenitor markers, such as CD133 and CD44, and cell-type markers, CK5 and CK8. The significant increase in CD133+, CD44+, and CK5+ cells, but the decrease in CK8+ cells, was detected after the ADT in all seven sets of human tissues examined (Figure 1C, only one set of data is shown, and six sets of data are shown in Supplementary Figure S1C–F), indicating the increase in the stem/progenitor cells of the basal epithelial origin, but the decrease in the differentiated luminal epithelial cells in human castration resistant PCa after ADT.
Together, results from two different in vitro PCa cell lines, two different in vivo PCa mouse models, and seven sets of human clinical PCa tissues all clearly demonstrated that ADT led to an increase in stem/progenitor cell numbers.
Isolation of stem/progenitor and non-stem/progenitor cells from various PCa tissues and cell lines
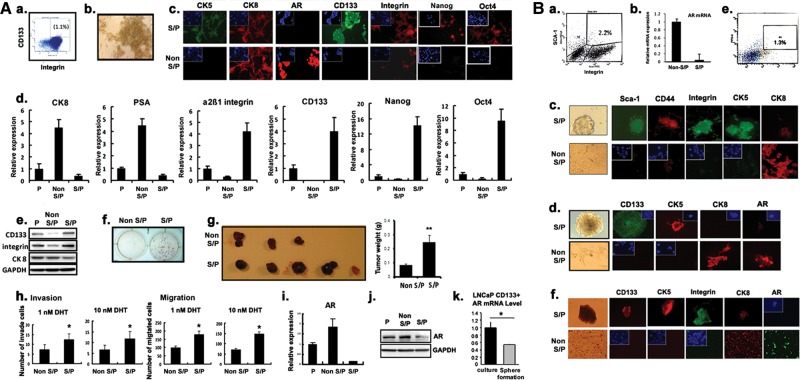
PCa tumors contain a heterogeneous mixture of multiple cell populations (Patrawala et al., 2007). Using flow cytometric or magnetic separation methods, we were able to isolate stem/progenitor cells and non-stem/progenitor cells from various PCa tissues or cell lines using antibodies of stem cell markers, CD133 (Richardson et al., 2004; Vander Griend et al., 2008; Enguita-German et al., 2010) and α2β1-integrin (Patrawala et al., 2007) for human specimens and Sca-1 (Xin et al., 2005) and CD49f (Lawson et al., 2007) for mouse specimens. Figure 2Aa demonstrates the separation of stem/progenitor (1%–1.5%) and non-stem/progenitor cells (98%–99%) from a human PCa LNCaP cell line (an androgen-sensitive human PCa cell line representing PCa before the castration resistant stage) using flow cytometry. Figure 2Ab represents the morphology of the PCa stem/progenitor cells isolated. Various stem cell-related markers (listed in Supplementary Table S1) were then applied to validate that the isolated cells were PCa stem/progenitor cells of the basal epithelial origin (Figure 2Ac–e). Interestingly, we found that the stem/progenitor cells (even at 1%) exhibited higher in vitro tumorigenicity than the remaining 99% non-stem/progenitor cells when tested by the soft agar colony formation assay (Figure 2Af). The LNCaP orthotopic xenograft mice studies further proved the higher in vivo tumorigenicity of CD133+ PCa stem/progenitor cells compared with the CD133− PCa non-stem/progenitor cells (Figure 2Ag). Moreover, the PCa stem/progenitor cells were shown to have higher potentials of migration/invasion compared with the PCa non-stem/progenitor cells (Figure 2Ah).
Figure 2.
All PCa cells/tumor tissues contain stem/progenitor and non-stem/progenitor cells. (In figure labels, stem/progenitor and non-stem/progenitor cells were abbreviated as S/P and non-S/P cells, respectively.) (A) Cell line data (LNCaP). (a) Separation of stem/progenitor (1.1%) and non-stem/progenitor (98.9%) cells of the LNCaP cell line by flow cytometry. Antibodies of CD133 and integrin were used. (b) Morphology of stem/progenitor cells in culture. (c) IF staining results of stem/progenitor and non-stem/progenitor cells of the LNCaP cell line using antibodies of markers indicated. The stem/progenitor cells stained positive for CK5, CD133, and integrin, whereas the non-stem/progenitor cells were positive for CK8 and AR (magnification, ×400; inset, 4-b-diamini-2-phenylindole (DAPI) staining). (d) qPCR analysis results showing mRNA expression levels of markers indicated. (e) Western blot analysis of CD133, integrin, and CK8 expressions using cell extracts of parental, stem/progenitor, and non-stem/progenitor cells. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody was used as a control. (f) The soft agarose colony formation assay results using stem/progenitor and non-stem/progenitor cells of LNCaP cells. (g) Results of xenograft mice study. LNCaP cells, stem/progenitor and non-stem/progenitor cells (1 × 105/site), were injected orthotopically into AP lobes of nude mice. Mice were sacrificed 4 weeks after and the size of the tumor was compared. (h) Invasion and migration assays of stem/progenitor and non-stem/progenitor cells at 1 and 10 nM DHT concentrations. (i) qPCR analysis of AR mRNA expression. (j) Western blot analysis of AR expression using the same cell extracts used in (e). (k) qPCR analysis results of AR mRNA expression when cells were grown in normal culture condition or as spheres on Matrigel coated plates. (B) Tumor tissue data. Tumor tissues were obtained, digested with collagenase (0.28%), DNase I, 10% FCS, one antibiotic/antimycotic, in RPMI. The dissociated cells were further digested with trypsin/EDTA (0.25%) and filtered through strainer. (a) Flow cytometric separation of stem/progenitor and non-stem/progenitor cells obtained from TRAMP tumor tissues (B6 background, 26 weeks old mice). Antibodies of sca-1 and integrin were used in the separation. (b) qPCR analysis result of AR mRNA expression in stem/progenitor and non-stem/progenitor cells isolated from TRAMP mice tumor tissues. (c) IF staining showing marker expressions in stem/progenitor and non-stem/progenitor cells isolated from TRAMP mice tumor tissues. The stem/progenitor cells stained positive for sca-1, CD44, and integrin, whereas the non-stem/progenitor cells were positive for CK8 (magnification, ×400; inset, DAPI staining). (d) IF staining results demonstrating marker expressions in stem/progenitor and non-stem/progenitor cells obtained from C4-2 orthotopic xenografted tumor tissues. The stem/progenitor cells stained positive for CD133 and CK5, whereas the non-stem/progenitor cells were positive for CK8 and AR (magnification, ×400; inset, DAPI staining). (e) Separation of stem/progenitor and non-stem/progenitor cells from human patient tumor tissues (age 51, prostatic adenocarcinoma, Gleason score 6) by collagenase/trypsin digestion as described earlier. (f) IF staining results of marker expressions in stem/progenitor and non-stem/progenitor cells of human patient tumor tissues. The stem/progenitor cells stained positive for CD133, CK5, and integrin and slightly positive for CK8, whereas the non-stem/progenitor cells were strongly positive for CK8 and AR (magnification, ×400; inset, DAPI staining). All experiments were done three times.
A similar amount of PCa stem/progenitor cells (1%–1.5%) as in LNCaP cell lines were also isolated from other human PCa cell lines; C4-2, an androgen-insensitive cell line representing PCa castration resistant stage, and LAPC-4, an androgen-sensitive cell line representing PCa prior to castration resistance. The C4-2 stem/progenitor cells also exhibited higher tumorigenicity and higher migration/invasion potentials when compared with the non-stem/progenitor cells (all C4-2 and LAPC-4 cell line data are shown in Supplementary Figure S2A and B, respectively).
Using similar approaches, we were also able to isolate 1% PCa stem/progenitor and 99% PCa non-stem/progenitor cells from PCa tissues obtained from human PCa patients who received radical prostatectomy (Gleason score 6) and two different types of mouse PCa tissues, the spontaneous TRAMP mouse PCa (26 weeks, B6 background) and the orthotopic xenografts of prostate tumors (castration resistant tumor). The identification/confirmation of PCa stem/progenitor vs. non-stem/progenitor cells from these three types of PCa tissues using various markers are demonstrated in Figure 2B (a–c for TRAMP PCa, d for C4-2 xenograft PCa, and e and f for human PCa tissues).
One human PCa stem cell (PCSC) line from Celprogen (Kawasaki et al., 2009; Klarmann et al., 2009; Duhagon et al., 2010; Mathews et al., 2010) was also applied and their stem/progenitor characteristics were shown in Supplementary Figure S3.
Together, results from Figure 2 and Supplementary Figures S2 and S3 demonstrated the existence of 1%–1.5% PCa stem/progenitor cells and 98.5%–99% PCa non-stem/progenitor cells with distinct characteristics in all four human PCa cell lines and three PCa tissues isolated from human and mice.
Differential AR expression in PCa stem/progenitor cells vs. non-stem/progenitor cells
We then found that AR expressions in PCa stem/progenitor cells vs. PCa non-stem/progenitor cells are quite different, with little to few expressions of AR mRNA and proteins in PCa stem/progenitor cells when compared with much higher AR expression found in PCa non-stem/progenitor cells. Figure 2Ai and j and Bb, d, and f and Supplementary Figures S2Ac and Bc and S3D–F showed very low expression of AR in the PCa stem/progenitor cells isolated from various PCa cell lines and PCa tissues, which is consistent with the earlier results (Crocoll et al., 1998; van Leenders and Schalken, 2001; Heer et al., 2007; Miki et al., 2007; Isaacs, 2008; Rajasekhar et al., 2011). When the isolated PCa stem/progenitor cells were grown in three-dimensional (3D) culture with Matrigel, which could maintain their stemness, the AR expression level was shown significantly lower (Figure 2Ak and Supplementary Figure S3G). Collectively, all these results indicate that the PCa stem/progenitor cells originally have very low AR expression when compared with those PCa non-stem/progenitor cells.
AR plays opposite roles in PCa stem/progenitor and non-stem/progenitor cells
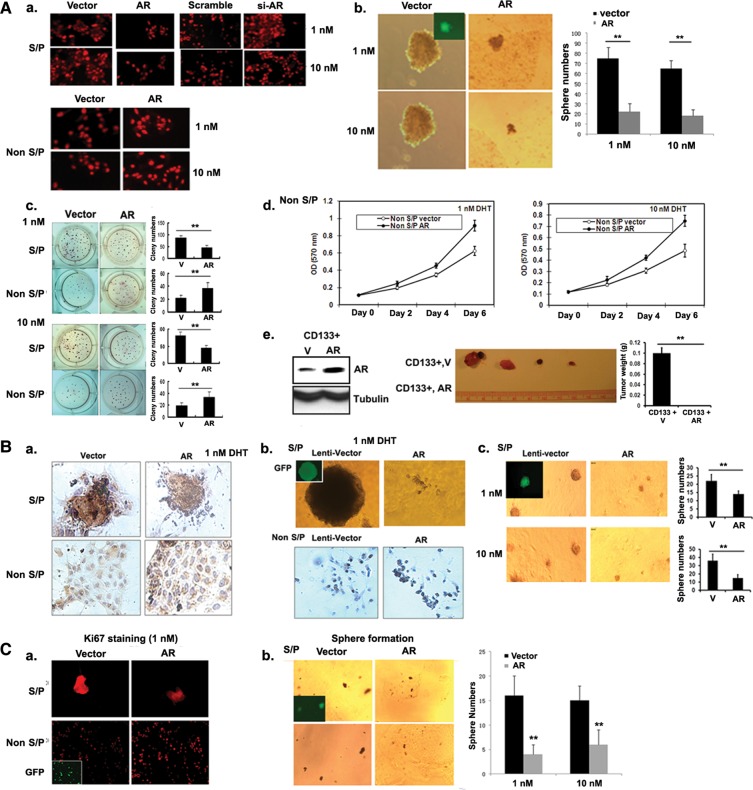
To dissect the mechanism(s) by which the ADT leads to the suppression of non-stem/progenitor cell proliferation, yet the expansion of stem/progenitor cell population, we asked if differential AR expression in stem/progenitor vs. non-stem/progenitor cells might lead to different effects on their growth. We first used lentiviral infection with AR-siRNA to silence the remaining AR in LNCaP stem/progenitor cells and found the expansion of cells as shown by increased Ki67 staining (Figure 3Aa). Similar results were also obtained when we applied a 3D sphere-forming assay on Matrigel (Figure 3Ab) and a colony formation assay (Figure 3Ac). Importantly, these results were obtained in the presence of 1 nM dihydrotestosterone (DHT) (human prostate androgen concentration after ADT) and 10 nM DHT (human prostate concentration before ADT), since recent evidence indicated that there is no ‘ligand-free AR or 0 nM androgen’ condition existing in human prostate/PCa even at ADT with surgical/medical castration conditions (Titus et al., 2005a, b; Nishiyama et al., 2007). In contrast, the lentiviral infection of LNCaP non-stem/progenitor cells with AR-cDNA led to opposite results with increased proliferating cell number (Figure 3Aa), growth (Figure 3Ad), and colony formation (Figure 3Ac).
Figure 3.
Opposite roles of AR in self-renewal/proliferation of stem/progenitor and non-stem/progenitor cells. (In figure labels, stem/progenitor and non-stem/progenitor cells were abbreviated as S/P and non-S/P cells, respectively.) All assays were performed after infection of stem/progenitor and non-stem/progenitor cells with lentivirus carrying vector, AR cDNA, or AR-siRNA. (A) Cell line studies. (a) IF staining result using Ki67 antibody (magnification, ×400). (b) The sphere formation assay. Quantitation on right. (c) The soft agar colony formation assay. Quantitation on right. (d) The MTT assay result at 1 and 10 nM DHT concentrations. (e) Left panel shows AR expression status of injected cells (105) in LNCaP xenograft mice study. Right panel represents tumors obtained from mice of two groups, inoculated with the vector expressing CD133+ or AR expressing CD133+ cells. All experiments were done three times. *P< 0.05 and **P< 0.01. (B) Mice tumor tissue data. (a) BrdU-labeling data (IHC) of stem/progenitor and non-stem/progenitor cells obtained from TRAMP mice tissues (magnification, ×400). (b) Upper two figures represent the sphere formation assay using stem/progenitor cells and lower figures are the results of BrdU-labeling experiment using non-stem/progenitor cells obtained from C4-2 xenograft tumor tissues. (c) The sphere formation assay of stem/progenitor cells obtained from TRAMP mice tissues. Quantitation on right. *P< 0.05 and **P< 0.01. (C) Human tumor tissue data. (a) Ki67 staining (IF staining) using stem/progenitor and non-stem/progenitor cells obtained from human tumor tissues (magnification, ×400; inset, DAPI staining). (b) The sphere formation assay using stem/progenitor cells of human tumor tissues. Quantitation on right.
We tested C4-2 and LAPC4 cells with both AR-siRNA and AR-cDNA infections and obtained similar results showing opposite AR effects in stem/progenitor vs. non-stem/progenitor cells (Supplementary Figures S4 and S5). Finally, we also got consistent results when we used PCSCs by showing the suppression of their growth upon the addition of AR-cDNA (Supplementary Figure S3).
We then extended these in vitro studies into in vivo xenograft mouse PCa studies by inoculating LNCaP-CD133+ stem/progenitor cells orthotopically into AP lobes of nude mice. As shown in Figure 3Ae, the addition of AR in CD133+ stem/progenitor cells suppressed their tumorigenicity significantly.
Together, results from four cell lines (LNCaP, C4-2, LAPC4, and PCSCs) with multiple growth assays all clearly demonstrated the opposite AR roles in PCa stem/progenitor vs. non-stem/progenitor cells.
AR plays opposite roles in stem/progenitor vs. non-stem/progenitor cells from human and mouse tumor tissues
We then asked if we could observe the similar opposite roles of AR in stem/progenitor vs. non-stem/progenitor cells isolated from mouse PCa tissues. The results showed that the addition of AR led to lower BrdU labeling in the stem/progenitor cells and higher BrdU labeling in the non-stem/progenitor cells isolated from either TRAMP PCa tissues (Figure 3Ba) or C4-2-xenografted PCa tissues (Figure 3Bb). Self-renewal 3D sphere formation assays also demonstrated that in the presence of 1 or 10 nM DHT, the addition of AR led to lower number of spheres in stem/progenitor cells isolated from the TRAMP PCa tissues (Figure 3Bc) or from the C4-2-xenografted PCa tissues compared with non-stem/progenitor cells (Figure 3Bb).
More importantly, consistent results were obtained from stem/progenitor and non-stem/progenitor cells isolated from human PCa tissues. The addition of AR led to lower Ki67 staining in the human PCa stem/progenitor cells, but higher Ki67 staining in the human PCa non-stem/progenitor cells (Figure 3Ca). The 3D sphere formation assay also demonstrated that the addition of AR led to a lower number of spheres in stem/progenitor cells isolated from human PCa tissues in the presence of 1 or 10 nM DHT compared with non-stem/progenitor cells (Figure 3Cb).
Taken together, the results from Figure 3 suggest that AR could function differentially to affect the populations of stem/progenitor vs. non-stem/progenitor cells regardless of the origin of the PCa samples before or after ADT treatment.
Targeting molecules that are responsible for PCa stem/progenitor cell population expansion
Results of Figures 1–3 concluded that ADT could lead to the suppression of PCa non-stem/progenitor cells, but result in an unwanted expansion of PCa stem/progenitor cells, which might explain why ADT is effective at the earlier stage, but eventually fails to cure PCa. We therefore hypothesize that a new combinational therapy that combines ADT to target PCa non-stem/progenitor cells and to target PCa stem/progenitor cells to battle PCa would be more effective.
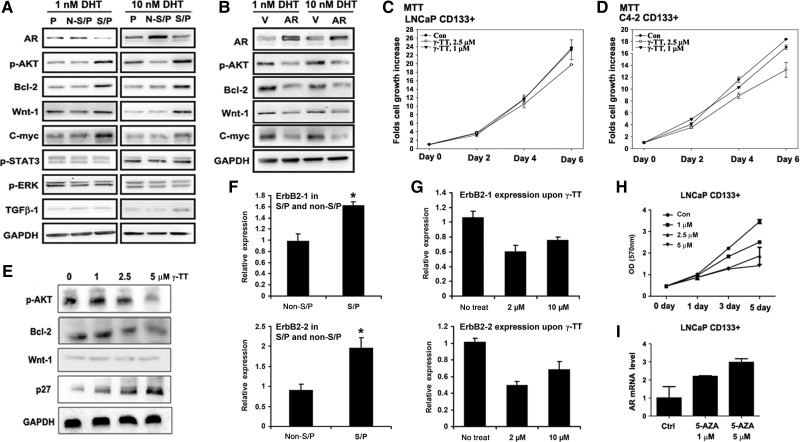
We first screened many molecules/signaling pathways that could be modulated by targeting AR and have been suggested by earlier reports to play important roles to increase the PCa stem/progenitor cell population (Bisson and Prowse, 2009; Dubrovska et al., 2009; Lee et al., 2009; Enguita-German et al., 2010). We found four such molecules/signaling pathways (activation of Akt and Wnt signaling and higher expressions of bcl-2 and c-myc) in the PCa stem/progenitor cells when compared with the PCa non-stem/progenitor cells isolated from LNCaP (Figure 4Aa), C4-2 (Supplementary Figure S6A), and PCSCs (Supplementary Figure S6D). The activation/higher expression of Akt, bcl-2, Wnt-1, and c-myc were reversed when AR was added to the LNCaP stem/progenitor cells (Figure 4Ab) and C4-2 stem/progenitor cells (Supplementary Figure S6B), indicating that the activation/high expression of these molecules could be interrupted via targeting AR or their inhibitors.
Figure 4.
The in vitro test of targeting stem/progenitor cells by inhibiting activated signaling molecules. (In figure labels, stem/progenitor and non-stem/progenitor cells were abbreviated as S/P and non-S/P cells, respectively.) (A) Western blot analysis using cell extracts of parental, stem/progenitor, and non-stem/progenitor cells of the LNCaP cell line and antibodies indicated. (B) Western blot analysis using cell extracts of stem/progenitor cells after vector (V)/AR carrying lentiviral infection. MTT analysis result of stem/progenitor cells upon γ-TT treatment is with LNCaP (C) and with C4-2 cells (D). (E) Western blot analysis of signaling molecules after treatment with γ-TT. (F) ErbB2 mRNA expressions in LNCaP stem/progenitor and non-stem/progenitor cells. (G) Decrease in ErbB2 mRNA expression in LNCaP stem/progenitor cells upon γ-TT treatment. (H) LNCaP non-stem/progenitor cells were treated with different concentrations of 5-AZA and growth was analyzed. (I) The AR mRNA level in LNCaP-CD133+ cells was analyzed by qPCR after treatment with 5-AZA.
Another strategy to suppress PCa stem/progenitor cell expansion is targeting AR via the interruption of those AR downstream targets through the use of the γ-tocotrienol (γ-TT), since it was recently showed that γ-TT suppressed PCa stem/progenitor cell population through the interruption of target molecules including bcl-2 (Luk et al., 2011). In addition, γ-TT has also been reported to inhibit PI3K/Akt signaling (Samant and Sylvester, 2006). Therefore, it is possible that γ-TT might be able to suppress PCa stem/progenitor cell population via inhibiting both PI3K/Akt signaling and bcl-2 expression. As expected, we observed such inhibition effects of γ-TT on stem/progenitor cell population of LNCaP (Figure 4C) and C4-2 (Figure 4D) with the inhibition of Akt signaling and bcl-2 expression in LNCaP stem/progenitor cells (Figure 4E), suggesting that the use of γ-TT alone might have advantage over using the two different inhibitors of Akt and bcl-2 together. It has been reported that γ-TT inhibits Akt signaling through the suppression of the ErbB2 mRNA level (Shin-Kang et al., 2011). We found a higher mRNA level of ErbB2 (both subfamilies) in the stem/progenitor cells of LNCaP cell line compared with the non-stem/progenitor cells (Figure 4F) and this level was suppressed by γ-TT treatment (Figure 4G). However, we failed to observe difference in other possible upstream molecules of Akt signal such as insulin-like growth factor 1 and focal adhesion kinase-1 (data not shown). These results suggest that the inhibitory effect of Akt signal upon γ-TT treatment was through the suppressed expression of its upstream molecule, ErbB2.
The third strategy to suppress stem/progenitor cell population is to target AR directly via reversing the low expression of AR in stem/progenitor cells that has been demonstrated to play an important role to expand the PCa stem/progenitor cell population (Figures 1 and 2). We found that the CpG islands in the promoter and exon 1 of AR gene in PCa stem/progenitor cells were highly methylated compared with the PCa non-stem/progenitor cells (Jing et al., manuscript submitted). Treatment of PCa stem/progenitor cells with the de-methylating agent, 5-aza-2′-deoxycitidine (5-AZA), led to a significant suppression of stem/progenitor cell growth (Figure 4H) via inducing AR expression (Figure 4I), but showed little effect on LNCaP non-stem/progenitor cells (Supplementary Figure S7).
Together, the results from Figure 4 and Supplementary Figures S7 and S8 suggest that we might be able to use γ-TT or 5-AZA, with their distinct mechanisms, to suppress PCa stem/progenitor cell population to battle PCa.
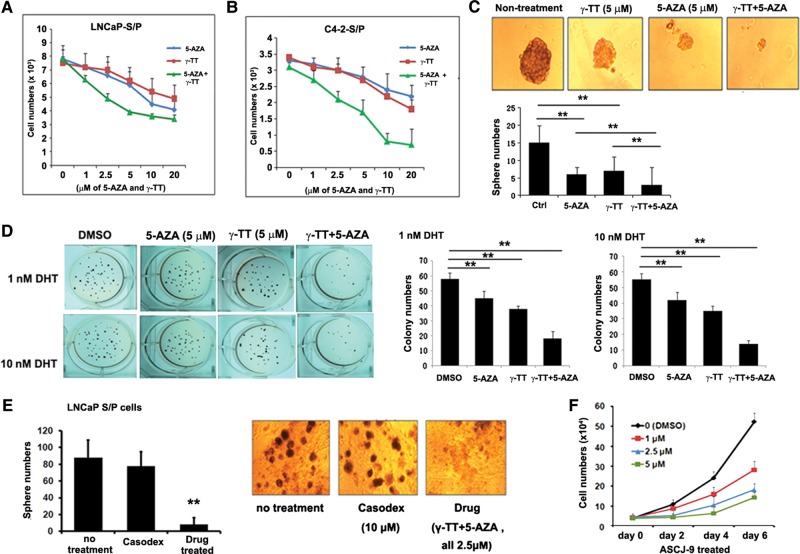
In vitro combination of γ-TT and 5-AZA to suppress stem/progenitor cell population
We found that the combinational use of γ-TT and 5-AZA indeed led to a significant suppression of PCa stem/progenitor cell population of LNCaP and C4-2 cell lines (Figure 5A and B). The IC50 values of each individual compound were significantly lowered at <2.0 µM in combination vs. >5.0 µM when used individually (Supplementary Figure S7C). Similar results were also obtained with the sphere formation (Figure 5C) and the colony formation (Figure 5D) assays. In contrast, the currently used anti-androgen, Casodex®, failed to reduce the number of spheres derived from LNCaP stem/progenitor cells (Figure 5E).
Figure 5.
Combined use of γ-TT and 5-AZA acted effectively to kill stem/progenitor cells. (In figure labels, stem/progenitor and non-stem/progenitor cells were abbreviated as S/P and non-S/P cells, respectively.) (A and B) Cell growth analysis using three drugs, γ-TT, and 5-AZA, either individually or two drugs combined. Cells (A with LNCaP stem/progenitor cells and B with C4-2 stem/progenitor cells) were treated with indicated concentrations of drugs for 4 days and cell numbers were counted at the end of incubation. (C) Effect of combined use of drugs on sphere formation. Quantitation is shown in lower panel. (D) Effect of combined use of drugs on colony formation on soft agarose. Quantitation is shown in lower panel. (E) The sphere formation assay using LNCaP stem/progenitor cells after treatment with either Casodex or Drug treatment. (F) The cell growth assay of LNCaP non-stem/progenitor cells with various concentrations of ASC-J9®.
Together, results from Figure 5 clearly demonstrated that the combinational use of two inhibitors with distinct mechanisms led to the effective suppression of PCa stem/progenitor cell population in vitro.
In vivo combinational therapy of ASC-J9® (targeting PCa non-stem/progenitor cells) with 5-AZA and γ-TT (targeting PCa stem/progenitor cells) led to the suppression of castration resistant PCa
For the PCa non-stem/progenitor cells where AR plays a stimulator role to promote PCa growth, we demonstrated that the AR degradation enhancer, ASC-J9® (Miyamoto et al., 2007; Yang et al., 2007; Ma et al., 2008; Lai et al., 2009; Wu et al., 2010), effectively suppressed LNCaP non-stem/progenitor cell growth (Figure 5F), which is in agreement with the early report showing that ASC-J9® could suppress PCa luminal epithelial cells and stromal cells (Lai et al., Am. J. Path., in revision).
To confirm these drugs' in vivo effects, we followed the similar animal system used to identify the newly developed anti-androgen, MDV3100 (Tran et al., 2009). Castration resistant PCa was obtained by orthotopic inoculation of LNCaP cells into castrated mice allowing prostate tumors to re-grow. These mice were then placed into three groups (five mice/group, two injection sites/mouse), each being treated differently: (i) vehicle only, (ii) ASC-J9® (50 mg/kg of body weight; Miyamoto et al., 2007; Yang et al., 2007; Ma et al., 2008; Lai et al., 2009), and (iii) ASC-J9® (to target PCa non-stem/progenitor cells) plus 5-AZA and γ-TT (to target PCa stem/progenitor cell population) based on our above in vitro test results (Figure 5 and Supplementary Figure S7). The doses of each drug were based on previous mouse tumor studies (Thibault et al., 1998; Hu et al., 2000; McAnally et al., 2007; Comitato et al., 2009; Xing et al., 2009), but were lowered by 25%, since our in vitro data indicate that the IC50 of each drug was lowered to that extent when used simultaneously. The final doses of the two drugs we used were: 0.25 mg/kg of 5-AZA and 6.25 mg/kg of γ-TT, which showed little toxicity (data not shown).
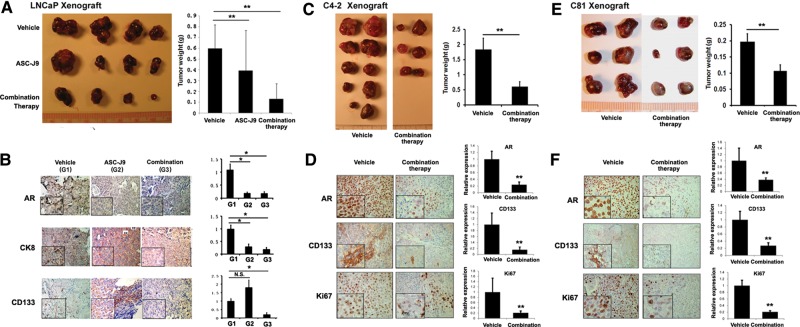
We found that the ASC-J9®-treated mice (Group 2) showed significantly smaller prostate tumor sizes when compared with the Group 1 receiving classic ADT/castration with little serum androgen (Figure 6A), suggesting that the ASC-J9® (that could suppress both androgen-mediated and non-androgen-mediated AR signals) has better efficacy than classic ADT/castration (only suppress androgen-mediated AR signals) to battle PCa. Importantly, we found that the combinational therapy group (Group 3) had the best efficacy for significantly decreased prostate tumor sizes compared with Groups 1 and 2.
Figure 6.
Targeting PCa stem/progenitor and non-stem/progenitor simultaneously blocks the growth of castration resistant tumors. (In figure labels, stem/progenitor and non-stem/progenitor cells were abbreviated as S/P and non-S/P cells, respectively.) (A) Tumors obtained from xenograft mice study. LNCaP cells were orthotopically inoculated into AP lobes of 8 weeks old nude mice. When tumors developed to palpable sizes, mice were castrated and we waited for 2 weeks for tumors to re-develop. Drug treatment started in three groups of mice as indicated (every other day, for 2 weeks). Doses of 0.25 mg/kg 5-AZA and 6.25 mg/kg γ-TT were used. (B) IHC staining of tumor tissues obtained at sacrifice (magnification, ×100; inset, ×400). Quantitation is shown at right. (C) C4-2 cells were orthotopically implanted into AP lobes of the castrated nude mice (8 weeks). When tumors developed, the mice were treated with the combination of ASC-J9® and drugs (γ-TT and 5-AZA) every other day for 3 weeks. Doses of 0.25 mg/kg 5-AZA, 6.25 mg/kg γ-TT, and 50 mg/kg of ASC-J9® were used. (D) IHC staining of tumor tissues obtained at sacrifice (magnification, ×100; inset, ×400). Quantitation at right. (E) C81 cells orthotopically implanted into AP lobes of the castrated nude mice (8 weeks). When tumors developed, the mice were treated with the combination of ASC-J9® and drugs (γ-TT and 5-AZA; same doses as in C4-2 xenograft, C) every other day for 3 weeks. Quantitation at right. (F) IHC staining of tumor tissues obtained at sacrifice (magnification, ×100; inset, ×400).
The staining results of PCa tissues (Figure 6B) obtained from each group demonstrated that the ASC-J9® treatment decreased AR+ and CK8+ non-stem/progenitor luminal epithelial cells significantly compared with the control (ADT/castration only) group 1. Importantly, we found that the combined therapy of ASC-J9® and γ-TT/5-AZA drugs to kill stem/progenitor cells led to dramatic decreases in CD133+ and AR+ cells (Figure 6B), suggesting that the combinational therapy effectively eradicated both PCa non-stem/progenitor (luminal epithelial) and PCa stem/progenitor cells. We observed no significant change in CD133+ cells after short treatment times with ASC-J9® (Figure 6B). It will be interesting to see if longer treatment times with ASC-J9® alone could reach to the similar efficacy with the combination therapy with γTT/5-AZA.
We then studied the effect of the combinational therapy of ASC-J9® combined with γ-TT/5-AZA on the suppression of castration resistant PCa with a second mouse model with xenografted C4-2 cells. As shown in Figure 6C, castrated mice treated with the combinational therapy developed significantly smaller prostate tumors than those mice castrated only. PCa tissue staining shown in Figure 6D demonstrated significant decreases in the expressions of AR and CD133, suggesting effective therapeutic effect by simultaneous targeting of PCa stem/progenitor cells and PCa non-stem/progenitor cells. Decreased Ki67 staining (Figure 6D) also supports the decrease in proliferating cells after the combinational therapy.
We thus tested the effect of the combinational therapy in a third mouse model with xenografted PCa C81 cells (another androgen-insensitive human PCa cell line representing the castration resistant stage; Igawa et al., 2002), and obtained consistent results showing the orthotopically developed prostate tumors in castrated mice had a significant reduction in tumor sizes after receiving the combinational therapy (Figure 6E). Figure 6F shows significant decreases in Ki67 staining and expressions of AR and CD133 in the PCa tumors.
Taken together, the results from the in vivo studies using three different mouse models bearing castration resistant tumors of LNCaP, C4-2, or C81 cells all concluded that the combinational therapy with γ-TT/5-AZA targeting PCa stem/progenitor cells and ASC-J9® targeting PCa non-stem/progenitor cells led to the best efficacy for significantly suppressing PCa at the castration resistant stage.
Discussion
Why does the current ADT (van der Kwast et al., 1991; Kageyama et al., 2007) using either surgical or medical castration to reduce/suppress androgens binding to AR in every cell fail to cure PCa? We believe that PCas are heterogeneous tumors containing various cell types and each cell type may have its distinct androgens/AR signals to either promote or suppress PCa progression (Niu et al., 2011). Using multiple approaches to target different PCa samples, either from human or mice tissues, or various human cell lines, we proved that AR plays distinct roles in PCa stem/progenitor vs. non-stem/progenitor cells: a suppressor in PCa stem/progenitor cells vs. a stimulator in PCa non-stem/progenitor cells, even when these two populations of cells originated from the same sources (Figure 3 and Supplementary Figures S4 and S5).
Compared with the currently used anti-androgen, Casodex®, two newly developed anti-androgens, orteronel (TAK-700), a non-steroidal inhibitor of 17,20-lyse that functions effectively to further reduce prostate intra-androgen concentration (Kaku et al., 2011), and MDV3100 (Tran et al., 2009), a much more potent anti-androgen to prevent androgens binding to AR, showed better efficacy to suppress PCa for a much longer time frame before castration resistant PCa recurs. However, both of them also failed to suppress castration resistant PCa completely and tumors recur eventually. It is possible that these two new anti-androgens are similar to the currently used anti-androgens, such as Casodex, that failed to suppress stem/progenitor cell expansion.
Among four identified AR downstream signaling pathways (activation of Akt and Wnt signaling and higher expressions of bcl-2 and c-myc) in the PCa stem/progenitor cells, we found few available small molecules in clinical trials that could be used to target Wnt and c-myc effectively. For the Akt and bcl-2, we decided to use γ-TT and not the currently available Akt inhibitors in our strategy with the following reasons: (i) γ-TT could simultaneously target bcl-2 and Akt signaling, (ii) γ-TT could overcome the problem of toxicity of the current available PI3K/Akt inhibitors, such as LY294002 (de la Pena et al., 2006; Markman et al., 2010), and (iii) those current available Akt inhibitors, such as LY294002, might result in an unwanted problem of increasing some PCa cells invasion ability (Chang et al., manuscript in preparation), even though showing better efficacy to reduce PCa sizes (Hutchinson et al., 2004; Dillon et al., 2009). We are aware of the debates about the effect of long-term supplement of α-Vit E to reduce PCa incidence, and even though γ-TT and α-Vit E share some similar structure, these two compounds have totally different functions, with γ-TT not α-Vit E, effectively inhibiting the growth of PCa stem/progenitor cells. Importantly, both γ-TT and 5-AZA used in this new combinational therapy to battle PCa are FDA approved, are currently used in various clinical trials, and shall be ready to use in PCa clinical trials soon.
We also replaced Casodex® with ASC-J9®. Earlier reports also demonstrated better efficacy when using ASC-J9® in many other AR-related diseases, such as wound healing (Lai et al., 2009), liver cancer (Ma et al., 2008), and bladder cancer (Miyamoto et al., 2007). In our mice studies, we observed a dramatic reduction in prostate tumor sizes upon ASC-J9® treatment in addition to the classic ADT/castration (Figure 6B) and the tissue staining results showed further decrease in AR positive cells, suggesting that the removal of androgen (either by castration or blocking androgen biosynthesis) might have its limitation (as shown in castrated mice that developed PCa). Therefore, using ASC-J9® to target AR [to suppress those non-androgen-mediated AR signals, such as AR3 variants and peptide growth factors (Kung and Evans, 2009) or cytokines (Lee et al., 2005, 2007) via other cellular pathways (Cabrespine et al., 2004; Hammacher et al., 2005; Desai et al., 2006; Ishiguro et al., 2009)] is better than the currently used surgical or medical castration that only targets androgen-mediated AR signals.
In summary, these studies not only provide the first direct in vivo clinical evidence demonstrating ADT will increase PCa stem/progenitor cells using the same PCa patient samples before and after ADT treatment, the data from multiple in vitro cell lines and three in vivo mouse models also proved that simultaneously targeting both PCa stem/progenitor and PCa non-stem/progenitor cells represents a novel combinational therapy to battle PCa before and after the castration resistant stages.
Materials and methods
Cell culture and growth assay
The isolated stem/progenitor cells of human PCa cell lines were grown in Celprogen PCSC media supplemented with growth supplement and the isolated stem/progenitor cells of mice tissues were grown in epithelial basal medium (PrEBM, Lonza) supplemented with growth supplement. Various concentrations of DHT (1 or 10 nM) were added in experiments. For the MTT assay, cells were incubated in 12- or 24-well culture plates (105–103 cells/well). At 2, 4, and 6 days, 0.5 mg/ml of MTT (Sigma) was added. After 2 h reaction, absorbance at 570 nm was measured. For the soft agar colony formation assay, a mixture of cell suspension (1 × 104 cells/well in 6-well culture plates, in 2 ml of 2× medium) and 1.5% agarose was overlaid onto the solidified mixture of agarose base (final agarose concentration was 0.75%). Two milliliters of normal media containing various concentrations of DHT was added on the top of the solidified agarose cell layer. All conditions were performed in triplicate. The plates were incubated at 37°C in a humidified incubator for 21 days. The colonies were stained with 1% crystal violet and those larger than 1 mm in diameter were counted.
Isolation of stem/progenitor cells from tumor tissues of mice and human patients
The protocol was followed according to the methods described previously (Vander Griend et al., 2008). Briefly, tumor tissues were obtained from xenografted mice, cut into pieces, digested overnight at 37°C with collagenase (0.28% collagenase I, Sigma), DNase I (Sigma), 10% fetal calf serum (FBS), and 1× antibiotic/anti-mycotic (Invitrogen) in RPMI 1640. The digested solution was further treated with trypsin/ethylenediaminetetraacetic acid (EDTA) (0.25%) for 30 min at 37°C. Cells were subsequently passed through a cell strainer, and stem/progenitor cells were isolated by either flow cytometric separation or magnetic sorting separation. For human tissues, primary prostate cells were isolated from patients undergoing radical prostatectomy at University of Rochester Medical Center according to an Institutional Review Board-approved protocol. Similar isolation methods were used for obtaining single-cell suspension. For culturing the isolated prostate cells from mice and human tissues, PrEBM growth medium (Lonza) was used as described earlier.
Flow cytometric separation and magnetic bead isolation of stem/progenitor cells
For the flow cytometric separation of stem/progenitor cells, 2 × 107 cells were detached with 5 mM EDTA and stained with antibodies of integrin and CD133 (for human PCa cells) and integrin and sca-1 (for mouse tissues). Cells were sorted by a BD FACS Diva cell sorter (Becton Dickinson Immunocytometry Systems) into double-positive or double-negative cell populations. For the separation using magnetic beads, cells were incubated with magnetic beads (Invitrogen) that have been conjugated with biotinylated antibodies. The positively stained cells were separated by placing tubes in the magnetic field. The unbound cells were used as non-stem/progenitor cells after washing and the bound population cells were used as stem/progenitor cells.
Infection of cells
For the infection of lentivirus, 293T cells were transfected with a mixture of DNAs consisting of lentiviral vectors (pWPI, Addgene) containing AR (vectors only were used as control), psPAX2 packaging plasmid, and pMD2G envelope plasmid, at a 4:3:2 ratio using Lipofectamine (Invitrogen). After infection, media containing the virus was replaced with the normal culture medium. Since the pWPI vector contains green fluorescent protein (GFP), transfection efficiency was monitored by detecting GFP fluorescence.
Ki67 staining
Tissue slides were incubated with anti-Ki67 (NCL-Ki67p, 1:1000) in 3% bovine serum albumin (BSA) in PBS overnight at 4°C and then incubated with 1:200 diluted biotinylated secondary antibody and ABC solution (Vector Laboratories), followed by Mayor's hematoxylin counterstaining.
Sphere formation assay
The sphere formation assay was performed as described earlier (Xin et al., 2007). Briefly, single-cell suspensions (1 × 103, in 60 μl medium) were mixed with cold 60-µl Matrigel (BD, Franklin Lakes) and the mixture was placed along the rim of the 24-well plates with a minimum of three triplicate experiments. The culture plates were placed in 37°C incubator for 10 min to let the mixture solidify and 500 µl medium was then added into the well. Sphere numbers were counted after 7–14 days under Olympus light microscope and size differences were also examined.
Migration and invasion assay
Cells (1 × 105) were added in upper well of Transwell plates (8 µm, Corning/Fisher Scientific). The medium [600 μl of 10% charcoal/dextran stripped-FBS (CS-FBS)] containing 1 or 10 nM DHT was added in the lower compartment to act as an attractant. After 24 h incubation, cells that were not migrated were removed from the upper face of the membranes with cotton swabs. Then the filters were fixed in methanol for 10 min at 4°C, stained with 1% Toluidine blue (Bio-Rad) for 5 min at room temperature, and then washed carefully in dH2O. After air drying, the membranes were observed under light microscope. The average number of cells per field of view (five random fields per membrane) was counted at ×20 magnification. For the invasion assay, before the cells were plated into the upper Transwell, 50 μl Matrigel was added onto the membrane, and then incubated for 48 h, then continue as in migration assays.
Immunofluorescence staining of cells
Cells were seeded on the four-well chamber slides and fixed with iced methanol. After fixation, cells were washed with PBS three times for 5 min and then cells were blocked with 5% BSA for 1 h. Cells were washed with PBS three times and then incubated with primary antibodies in 5% BSA in PBS overnight at 4°C. Antibodies used were: anti-AR (Santa Cruz, N20 1:250), anti-CK5 (Covance, 1:250), anti-CK8 (Abcam, 1:250), and anti-sca-1 (eBioscience, 1:250). Cells were then incubated with 1:200 diluted biotinylated secondary antibodies (Vector Laboratories) and with fluorescent secondary antibodies for immunofluorescence (IF; either Alexa 594 or Alexa 488 tagged).
Colony formation assay
LNCaP stem/progenitor cells were resuspended in 0.4% Bactoagar (BD, Franklin Labs), layered on the top of 1 ml of 0.8% agarose in six-well culture plates, and incubated with 1 ml of RPMI complete medium. After 3 weeks, the colonies were visualized by staining with 0.5% crystal violet. The experiment was analyzed in triplicate, and colonies larger than 100 mm in diameter were counted.
Development of a xenograft mouse model
To generate the xenograft mice model, LNCaP and C4-2 cells (1 × 105 cells/site, in 20 µl of medium mixed with 1:1 Matrigel) were inoculated orthotopically into anterior prostates lobes of 8-week-old male athymic nude mice. Surgical castration was performed when tumors become palpable. Tumors of non-castrated mice were used as a control. Mice were sacrificed at 10, 20, and 30 days and the tumor tissues were stained using antibodies of CD133, integrin, CK8, and CK5. TRAMP mice (B6 background) were bred and all mice experiments were under protocols approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Rochester Medical Center. For developing xenograft mice for drug study, LNCaP cells were injected first and mice castrated when tumor developed, whereas C4-2 and C81 cells were injected into castrated mice. For the injection of inhibitor molecules, two combinations or three combinations were i.p. injected into five groups of mice every other day for 2 weeks: Group 1, vehicle control; Group 2, castration + 75 mg/kg ASC-J9® (dissolved in dimethylacetamide/cremophor EL); Group 3, castration + 50 mg/kg ASC-J9® + 6.25 mg/kg γ-TT [dissolved in dimethylsulfoxide (DMSO)] and 0.25 mg/kg 5-AZA (DMSO); and Group 4, castration + 50 mg/kg ASC-J9®+ 6.25 mg/kg γ-TT and 0.25 mg/kg 5-AZA + 25 mg/kg LY294002.
Statistical analysis
Values were expressed as the mean ± standard deviation. The Student's t-test was used to calculate P-values. P-values were two-sided and considered statistically significant when *P<0.05, **P<0.01, and ***P<0.001.
Supplementary material
Supplementary material is available at Journal of Molecular Cell Biology online.
Funding
This work was supported by NIH grants (CA122840 and CA156700), George Whipple Professorship Endowment, Taiwan Department of Health Clinical Trial, Research Center of Excellence (DOH99-TD-B-111-004 to China Medical University, Taichung), and National Basic Research Program of China (2012CB518304).
Conflict of interest: ASC-J9® was patented by the University of Rochester, the University of North Carolina, and the AndroScience Corp., and then licensed to AndroScience Corp. Both the University of Rochester and C.C. own royalties and equity in AndroScience Corp.
Supplementary Material
References
- Bisson I., Prowse D.M. WNT signaling regulates self-renewal and differentiation of prostate cancer cells with stem cell characteristics. Cell Res. 2009;19:683–697. doi: 10.1038/cr.2009.43. [DOI] [PubMed] [Google Scholar]
- Cabrespine A., Guy L., Chollet P., et al. Molecular mechanisms involved in hormone resistance of prostate cancer. Bull. Cancer. 2004;91:747–757. [PubMed] [Google Scholar]
- Collins A.T., Habib F.K., Maitland N.J., et al. Identification and isolation of human prostate epithelial stem cells based on alpha(2)beta(1)-integrin expression. J. Cell Sci. 2001;114:3865–3872. doi: 10.1242/jcs.114.21.3865. [DOI] [PubMed] [Google Scholar]
- Comitato R., Nesaretnam K., Leoni G., et al. A novel mechanism of natural vitamin E tocotrienol activity: involvement of ERbeta signal transduction. Am. J. Physiol. Endocrinol. Metab. 2009;297:E427–E437. doi: 10.1152/ajpendo.00187.2009. [DOI] [PubMed] [Google Scholar]
- Crocoll A., Zhu C.C., Cato A.C., et al. Expression of androgen receptor mRNA during mouse embryogenesis. Mech. Dev. 1998;72:175–178. doi: 10.1016/s0925-4773(98)00007-0. [DOI] [PubMed] [Google Scholar]
- de la Pena L., Burgan W.E., Carter D.J., et al. Inhibition of Akt by the alkylphospholipid perifosine does not enhance the radiosensitivity of human glioma cells. Mol. Cancer Ther. 2006;5:1504–1510. doi: 10.1158/1535-7163.MCT-06-0091. [DOI] [PubMed] [Google Scholar]
- Desai S.J., Ma A.H., Tepper C.G., et al. Inappropriate activation of the androgen receptor by nonsteroids: involvement of the Src kinase pathway and its therapeutic implications. Cancer Res. 2006;66:10449–10459. doi: 10.1158/0008-5472.CAN-06-2582. [DOI] [PubMed] [Google Scholar]
- Dillon R.L., Marcotte R., Hennessy B.T., et al. Akt1 and akt2 play distinct roles in the initiation and metastatic phases of mammary tumor progression. Cancer Res. 2009;69:5057–5064. doi: 10.1158/0008-5472.CAN-08-4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubrovska A., Kim S., Salamone R.J., et al. The role of PTEN/Akt/PI3K signaling in the maintenance and viability of prostate cancer stem-like cell populations. Proc. Natl Acad. Sci. USA. 2009;106:268–273. doi: 10.1073/pnas.0810956106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duhagon M.A., Hurt E.M., Sotelo-Silveira J.R., et al. Genomic profiling of tumor initiating prostatospheres. BMC Genomics. 2010;11:324. doi: 10.1186/1471-2164-11-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enguita-German M., Schiapparelli P., Rey J.A., et al. CD133+ cells from medulloblastoma and PNET cell lines are more resistant to cyclopamine inhibition of the sonic hedgehog signaling pathway than CD133− cells. Tumour Biol. 2010;31:381–390. doi: 10.1007/s13277-010-0046-4. [DOI] [PubMed] [Google Scholar]
- Hammacher A., Thompson E.W., Williams E.D. Interleukin-6 is a potent inducer of S100P, which is up-regulated in androgen-refractory and metastatic prostate cancer. Int. J. Biochem. Cell Biol. 2005;37:442–450. doi: 10.1016/j.biocel.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Heer R., Robson C.N., Shenton B.K., et al. The role of androgen in determining differentiation and regulation of androgen receptor expression in the human prostatic epithelium transient amplifying population. J. Cell Physiol. 2007;212:572–578. doi: 10.1002/jcp.21154. [DOI] [PubMed] [Google Scholar]
- Hu L., Zaloudek C., Mills G.B., et al. In vivo and in vitro ovarian carcinoma growth inhibition by a phosphatidylinositol 3-kinase inhibitor (LY294002) Clin. Cancer Res. 2000;6:880–886. [PubMed] [Google Scholar]
- Hutchinson J.N., Jin J., Cardiff R.D., et al. Activation of Akt-1 (PKB-alpha) can accelerate ErbB-2-mediated mammary tumorigenesis but suppresses tumor invasion. Cancer Res. 2004;64:3171–3178. doi: 10.1158/0008-5472.can-03-3465. [DOI] [PubMed] [Google Scholar]
- Igawa T., Lin F.F., Lee M.S., et al. Establishment and characterization of androgen-independent human prostate cancer LNCaP cell model. Prostate. 2002;50:222–235. doi: 10.1002/pros.10054. [DOI] [PubMed] [Google Scholar]
- Isaacs J.T. Prostate stem cells and benign prostatic hyperplasia. Prostate. 2008;68:1025–1034. doi: 10.1002/pros.20763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro H., Akimoto K., Nagashima Y., et al. aPKClambda/iota promotes growth of prostate cancer cells in an autocrine manner through transcriptional activation of interleukin-6. Proc. Natl Acad. Sci. USA. 2009;106:16369–16374. doi: 10.1073/pnas.0907044106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josson S., Matsuoka Y., Chung L.W., et al. Tumor-stroma co-evolution in prostate cancer progression and metastasis. Semin. Cell Dev. Biol. 2010;21:26–32. doi: 10.1016/j.semcdb.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama Y., Hyochi N., Kihara K., et al. The androgen receptor as putative therapeutic target in hormone-refractory prostate cancer. Recent Patients Anticancer Drug Discov. 2007;2:203–211. doi: 10.2174/157489207782497172. [DOI] [PubMed] [Google Scholar]
- Kaku T., Hitaka T., Ojida A., et al. Discovery of orteronel (TAK-700), a naphthylmethylimidazole derivative, as a highly selective 17,20-lyase inhibitor with potential utility in the treatment of prostate cancer. Bioorg. Med. Chem. 2011;19:6383–6399. doi: 10.1016/j.bmc.2011.08.066. [DOI] [PubMed] [Google Scholar]
- Karlou M., Tzelepi V., Efstathiou E. Therapeutic targeting of the prostate cancer microenvironment. Nat. Rev. Urol. 2010;7:494–509. doi: 10.1038/nrurol.2010.134. [DOI] [PubMed] [Google Scholar]
- Kawasaki B.T., Hurt E.M., Kalathur M., et al. Effects of the sesquiterpene lactone parthenolide on prostate tumor-initiating cells: an integrated molecular profiling approach. Prostate. 2009;69:827–837. doi: 10.1002/pros.20931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klarmann G.J., Hurt E.M., Mathews L.A., et al. Invasive prostate cancer cells are tumor initiating cells that have a stem cell-like genomic signature. Clin. Exp. Metastasis. 2009;26:433–446. doi: 10.1007/s10585-009-9242-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung H.J., Evans C.P. Oncogenic activation of androgen receptor. Urol. Oncol. 2009;27:48–52. doi: 10.1016/j.urolonc.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai J.J., Lai K.P., Chuang K.H., et al. Monocyte/macrophage androgen receptor suppresses cutaneous wound healing in mice by enhancing local TNF-alpha expression. J. Clin. Invest. 2009;119:3739–3751. doi: 10.1172/JCI39335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai K.-P., Chang Y.-J., Huang C.-K., et al. New therapeutic approach to suppress castration-resistant prostate cancer using ASC-J9® via targeting androgen receptor in selective prostate cells. Am. J. Path. 2013;182:460–473. doi: 10.1016/j.ajpath.2012.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson D.A., Xin L., Lukacs R.U., et al. Isolation and functional characterization of murine prostate stem cells. Proc. Natl Acad. Sci. USA. 2007;104:181–186. doi: 10.1073/pnas.0609684104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.O., Lou W., Nadiminty N., et al. Requirement for NF-(kappa)B in interleukin-4-induced androgen receptor activation in prostate cancer cells. Prostate. 2005;64:160–167. doi: 10.1002/pros.20218. [DOI] [PubMed] [Google Scholar]
- Lee S.O., Chun J.Y., Nadiminty N., et al. Interleukin-6 undergoes transition from growth inhibitor associated with neuroendocrine differentiation to stimulator accompanied by androgen receptor activation during LNCaP prostate cancer cell progression. Prostate. 2007;67:764–773. doi: 10.1002/pros.20553. [DOI] [PubMed] [Google Scholar]
- Lee D.H., Szczepanski M.J., Lee Y.J. Magnolol induces apoptosis via inhibiting the EGFR/PI3K/Akt signaling pathway in human prostate cancer cells. J. Cell Biochem. 2009;106:1113–1122. doi: 10.1002/jcb.22098. [DOI] [PubMed] [Google Scholar]
- Li H., Tang D.G. Prostate cancer stem cells and their potential roles in metastasis. J. Surg. Oncol. 2011;103:558–562. doi: 10.1002/jso.21806. [DOI] [PubMed] [Google Scholar]
- Litvinov I.V., De Marzo A.M., Isaacs J.T. Is the Achilles’ heel for prostate cancer therapy a gain of function in androgen receptor signaling? J. Clin. Endocrinol. Metab. 2003;88:2972–2982. doi: 10.1210/jc.2002-022038. [DOI] [PubMed] [Google Scholar]
- Luk S.U., Yap W.N., Chiu Y.T., et al. Gamma-tocotrienol as an effective agent in targeting prostate cancer stem cell-like population. Int. J. Cancer. 2011;128:2182–2191. doi: 10.1002/ijc.25546. [DOI] [PubMed] [Google Scholar]
- Ma W.L., Hsu C.L., Wu M.H., et al. Androgen receptor is a new potential therapeutic target for the treatment of hepatocellular carcinoma. Gastroenterology. 2008;135:947–955. doi: 10.1053/j.gastro.2008.05.046. 955, e941–e945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitland N.J., Collins A.T. Prostate cancer stem cells: a new target for therapy. J. Clin. Oncol. 2008;26:2862–2870. doi: 10.1200/JCO.2007.15.1472. [DOI] [PubMed] [Google Scholar]
- Markman B., Dienstmann R., Tabernero J. Targeting the PI3K/Akt/mTOR pathway—beyond rapalogs. Oncotarget. 2010;1:530–543. doi: 10.18632/oncotarget.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews L.A., Hurt E.M., Zhang X., et al. Epigenetic regulation of CpG promoter methylation in invasive prostate cancer cells. Mol. Cancer. 2010;9:267. doi: 10.1186/1476-4598-9-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAnally J.A., Gupta J., Sodhani S., et al. Tocotrienols potentiate lovastatin-mediated growth suppression in vitro and in vivo. Exp. Biol. Med. (Maywood) 2007;232:523–531. [PubMed] [Google Scholar]
- Miki J., Furusato B., Li H., et al. Identification of putative stem cell markers, CD133 and CXCR4, in hTERT-immortalized primary nonmalignant and malignant tumor-derived human prostate epithelial cell lines and in prostate cancer specimens. Cancer Res. 2007;67:3153–3161. doi: 10.1158/0008-5472.CAN-06-4429. [DOI] [PubMed] [Google Scholar]
- Miyamoto H., Yang Z., Chen Y.T., et al. Promotion of bladder cancer development and progression by androgen receptor signals. J. Natl Cancer Inst. 2007;99:558–568. doi: 10.1093/jnci/djk113. [DOI] [PubMed] [Google Scholar]
- Mueller M.T., Hermann P.C., Heeschen C. Cancer stem cells as new therapeutic target to prevent tumour progression and metastasis. Front Biosci. (Elite Ed) 2010;2:602–613. doi: 10.2741/e117. [DOI] [PubMed] [Google Scholar]
- Nishiyama T., Ikarashi T., Hashimoto Y., et al. The change in the dihydrotestosterone level in the prostate before and after androgen deprivation therapy in connection with prostate cancer aggressiveness using the Gleason score. J. Urol. 2007;178:1282–1288. doi: 10.1016/j.juro.2007.05.138. discussion 1288–1289. [DOI] [PubMed] [Google Scholar]
- Niu Y., Wang J., Shang Z., et al. Increased CK5/CK8-positive intermediate cells with stromal smooth muscle cell atrophy in the mice lacking prostate epithelial androgen receptor. PLoS One. 2011;6:e20202. doi: 10.1371/journal.pone.0020202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrawala L., Calhoun-Davis T., Schneider-Broussard R., et al. Hierarchical organization of prostate cancer cells in xenograft tumors: the CD44+alpha2beta1+ cell population is enriched in tumor-initiating cells. Cancer Res. 2007;67:6796–6805. doi: 10.1158/0008-5472.CAN-07-0490. [DOI] [PubMed] [Google Scholar]
- Pfeiffer M.J., Schalken J.A. Stem cell characteristics in prostate cancer cell lines. Eur. Urol. 2010;57:240–254. doi: 10.1016/j.eururo.2009.01.015. [DOI] [PubMed] [Google Scholar]
- Rajasekhar V.K., Studer L., Gerald W., et al. Tumour-initiating stem-like cells in human prostate cancer exhibit increased NF-kappaB signalling. Nat. Commun. 2011;2:162. doi: 10.1038/ncomms1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasheed Z.A., Kowalski J., Smith B.D., et al. Concise review: emerging concepts in clinical targeting of cancer stem cells. Stem Cells. 2011;29:883. doi: 10.1002/stem.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson G.D., Robson C.N., Lang S.H., et al. CD133, a novel marker for human prostatic epithelial stem cells. J. Cell Sci. 2004;117:3539–3545. doi: 10.1242/jcs.01222. [DOI] [PubMed] [Google Scholar]
- Samant G.V., Sylvester P.W. gamma-Tocotrienol inhibits ErbB3-dependent PI3K/Akt mitogenic signalling in neoplastic mammary epithelial cells. Cell Prolif. 2006;39:563–574. doi: 10.1111/j.1365-2184.2006.00412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin-Kang S., Ramsauer V.P., Lightner J., et al. Tocotrienols inhibit AKT and ERK activation and suppress pancreatic cancer cell proliferation by suppressing the ErbB2 pathway. Free Radic. Biol. Med. 2011;51:1164–1174. doi: 10.1016/j.freeradbiomed.2011.06.008. [DOI] [PubMed] [Google Scholar]
- Thibault A., Figg W.D., Bergan R.C., et al. A phase II study of 5-aza-2′deoxycytidine (decitabine) in hormone independent metastatic (D2) prostate cancer. Tumori. 1998;84:87–89. doi: 10.1177/030089169808400120. [DOI] [PubMed] [Google Scholar]
- Titus M.A., Gregory C.W., Ford O.H., 3rd, et al. Steroid 5alpha-reductase isozymes I and II in recurrent prostate cancer. Clin. Cancer Res. 2005a;11:4365–4371. doi: 10.1158/1078-0432.CCR-04-0738. [DOI] [PubMed] [Google Scholar]
- Titus M.A., Schell M.J., Lih F.B., et al. Testosterone and dihydrotestosterone tissue levels in recurrent prostate cancer. Clin. Cancer Res. 2005b;11:4653–4657. doi: 10.1158/1078-0432.CCR-05-0525. [DOI] [PubMed] [Google Scholar]
- Tran C., Ouk S., Clegg N.J., et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324:787–790. doi: 10.1126/science.1168175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kwast T.H., Schalken J., Ruizeveld de Winter J.A., et al. Androgen receptors in endocrine-therapy-resistant human prostate cancer. Int. J. Cancer. 1991;48:189–193. doi: 10.1002/ijc.2910480206. [DOI] [PubMed] [Google Scholar]
- van Leenders G.J., Schalken J.A. Stem cell differentiation within the human prostate epithelium: implications for prostate carcinogenesis. BJU Int. 2001;88(Suppl 2):35–42. doi: 10.1046/j.1464-410x.2001.00117.x. discussion 49–50. [DOI] [PubMed] [Google Scholar]
- Vander Griend D.J., Karthaus W.L., Dalrymple S., et al. The role of CD133 in normal human prostate stem cells and malignant cancer-initiating cells. Cancer Res. 2008;68:9703–9711. doi: 10.1158/0008-5472.CAN-08-3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhagen A.P., Ramaekers F.C., Aalders T.W., et al. Colocalization of basal and luminal cell-type cytokeratins in human prostate cancer. Cancer Res. 1992;52:6182–6187. [PubMed] [Google Scholar]
- Wang X., Kruithof-de Julio M., Economides K.D., et al. A luminal epithelial stem cell that is a cell of origin for prostate cancer. Nature. 2009;461:495–500. doi: 10.1038/nature08361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M.H., Ma W.L., Hsu C.L., et al. Androgen receptor promotes hepatitis B virus-induced hepatocarcinogenesis through modulation of hepatitis B virus RNA transcription. Sci. Transl. Med. 2010;2:32ra35. doi: 10.1126/scitranslmed.3001143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin L., Lawson D.A., Witte O.N. The Sca-1 cell surface marker enriches for a prostate-regenerating cell subpopulation that can initiate prostate tumorigenesis. Proc. Natl Acad. Sci. USA. 2005;102:6942–6947. doi: 10.1073/pnas.0502320102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin L., Lukacs R.U., Lawson D.A., et al. Self-renewal and multilineage differentiation in vitro from murine prostate stem cells. Stem Cells. 2007;25:2760–2769. doi: 10.1634/stemcells.2007-0355. [DOI] [PubMed] [Google Scholar]
- Xing C.G., Zhu B.S., Fan X.Q., et al. Effects of LY294002 on the invasiveness of human gastric cancer in vivo in nude mice. World J. Gastroenterol. 2009;15:5044–5052. doi: 10.3748/wjg.15.5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Chang Y.J., Yu I.C., et al. ASC-J9 ameliorates spinal and bulbar muscular atrophy phenotype via degradation of androgen receptor. Nat. Med. 2007;13:348–353. doi: 10.1038/nm1547. [DOI] [PubMed] [Google Scholar]
- Zhang Q., Yang M., Shen J., et al. The role of the intravascular microenvironment in spontaneous metastasis development. Int. J. Cancer. 2010;126:2534–2541. doi: 10.1002/ijc.24979. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.