Abstract
Objective
Mitochondrial dysfunction plays a key pathophysiological role in type 2 diabetes (T2DM) related endothelial dysfunction. Data delineating relationships between mitochondrial and endothelial dysfunction in humans with T2DM are lacking.
Methods and Results
In 122 human subjects, (60 with T2DM, 62 without T2DM) we measured endothelial function by brachial artery ultrasound (FMD%) and digital pulse amplitude tonometery (PAT). Endothelial function in arterioles isolated from gluteal subcutaneous adipose was measured by videomicroscopy. In arterioles and mononuclear cells, we measured inner mitochondrial membrane potential (Δψm), mitochondrial mass, and mitochondrial superoxide production using fluorophores. Endothelial function was impaired in T2DM versus non-diabetics. Δψm magnitude was larger and mitochondrial mass was lower in arterioles and mononuclear cells in T2DM. Mononuclear mitochondrial mass correlated with FMD% and PAT (r=0.38 and 0.33, P=0.001 and 0.02, respectively) and mononuclear mitochondrial superoxide production inversely correlated with FMD% (ρ=-0.58, P=0.03). Endothelial function was impaired in T2DM. Low doses of two different mitochondrial uncoupling agents (CCCP and DNP) that reduce Δψm magnitude and a mitochondrial-targeted antioxidant (MitoTEMPOL) improved endothelial function and reduced mitochondrial superoxide levels.
Conclusions
Mitochondrial dysfunction may play a central role in the origin and maintenance of endothelial dysfunction in T2DM.
Keywords: mitochondria, endothelium, diabetes mellitus type 2, nitric oxide, oxidative stress
Introduction
The worldwide incidence of type 2 diabetes is expect to exceed 350 million persons in 2030, with the incidence rate of type 2 diabetes increasing in all segments of the population worldwide.1, 2 The majority of morbidity and mortality related to diabetes is secondary to the macrovascular (myocardial infarctions, stroke) and micro-vascular complications (blindness, renal failure) of this disease.2, 3 The pathogenesis of clinically overt vascular disease in type 2 diabetes (T2DM), while not completely elucidated, appears to begin with the initial development of vascular endothelial dysfunction.4-8 Endothelial dysfunction encompasses the pro-vasoconstrictive, pro-thrombotic, and pro-inflammatory endothelial phenotype known to precede the development of clinically relevant vascular disease and portend future cardiovascular risk.9, 10
The molecular origins of insulin-resistance related vascular endothelial dysfunction include the induction of excessive oxidative stress7, 11 and the activation pro-inflammatory and pro-vasoconstrictive pathways involving NF-κB and protein kinase C beta (PKCβ).12-14 Hyperglycemia and excess circulating free fatty acids appear to be responsible for the initiation of vascular oxidative stress and inflammation.15, 16 Interestingly, in cell culture models, activation of these deleterious pathways also appear to require the development of mitochondrial dysfunction.17, 18 Based on prior cell culture and animal work, insulin-resistance related mitochondrial dysfunction is typified by two key alterations in the mitochondrial phenotype leading to excessive mitochondrial ROS production: inner mitochondrial membrane hyperpolarization19, 20 and reduced mitochondrial mass and mitochondrial network complexity.20-22 We recently observed such alterations in mitochondrial function and morphology in peripheral blood mononuclear cells and isolated venous endothelial cells from T2DM patients.20, 23
Excessive mitochondrial ROS production is known to cause deleterious vascular cell signaling and subsequent endothelial dysfunction.24 Despite cell and animal data supporting a potential role for mitochondrial dysfunction in the origins of vascular dysfunction in type 2 diabetes, there are no data linking mitochondrial dysfunction to vascular endothelial dysfunction in intact human arteries and arterioles. We hypothesized that 1) systemic measurements of arterial endothelial function would correlate with systemic measurements of mitochondrial homeostasis derived from circulating mononuclear cells 2) arterioles for T2DM patients would display phenotypical mitochondrial dysfunction with reduced mitochondrial mass and greater mitochondrial inner membrane polarization relative to non-diabetic humans and 3) mitochondrial hyperpolarization and excessive mitochondrial superoxide production would relate mechanistically to arteriolar endothelial dysfunction in T2DM patients.
Methods
Subjects
The study protocol and advertisements were approved by the Medical College of Wisconsin's Institutional Research Board. The inclusion/exclusion criteria for non-diabetic subjects and T2DM subjects were previously described.20 A total of 122 individuals between the ages of 35 and 70 years of age were consecutively enrolled. We previously reported mitochondrial membrane potential, mass, and superoxide production from 59 of these subjects (27 subjects with T2DM, 32 non-T2DM).20 For T2DM subjects, the additional cohort of 33 subjects had slightly higher systolic blood pressure (131±3 vs. 125±8, P=0.04), heart rate (70±2 vs. 64±5, P=0.046), and fasting triglycerides (151±14 vs. 128±9, P=0.02) but otherwise did not differ with respect to other baseline characteristic in Table 2. There were no significant differences in the baseline characteristics of the additional 30 non-T2DM subjects. Subjects were defined as having T2DM based on standard criteria.25 Non-diabetic control subjects were excluded if they met criteria for metabolic syndrome,26 had a history of cardiovascular disease by history or documented myocardial infarction,27 had a major chronic illness, were pregnant at the time of screening, or had smoked within 1 year of enrollment. All subjects fasted for at least 6 hours prior to their study visits, and all studies began between 7:30AM and 9:00AM. Subjects on anti-hypertensive medications were asked to hold these medications for 24 hours prior to their study visits. Subjects on oral diabetes medications were instructed to hold their morning pills until completion of the study protocol. All subjects had blood drawn for measurements of fasting glucose levels, lipid profiles, measurements of insulin resistance, and measurements of mitochondrial homeostasis. Insulin resistance was estimated using both homeostasis model assessment-insulin resistance (HOMA-IR= [(fasting insulin μIU/mL)×(fasting glucose mmol/L)]/22.5) and the quantitative insulin-sensitivity check index (QUICKI=1/[log(Insulin) + log(glucose)].
Table 2.
Subject Characteristics
| Type 2 Diabetes (N=60*) | No Diabetes (N=62†) | P-Value | |
|---|---|---|---|
| Age | 52±9 | 49±8 | 0.08 |
| Sex (% Female) | 67% | 65% | 0.80 |
| Race (% African American) | 30% | 11% | 0.009 |
| Body Mass Index (kg/m2) | 34.6±8.0 | 28.2±5.6 | <0.001 |
| Smoking Status (%Previous) | 37% | 31% | 0.48 |
| Family History of CAD (%) | 37% | 20% | 0.03 |
| Waist Circumference (cm) | 111±17 | 94±16 | <0.001 |
| Systolic Blood Pressure (mmHg) | 128±29 | 125±19 | 0.46 |
| Diastolic Blood Pressure (mmHg) | 72±10 | 73±12 | 0.29 |
| Heart Rate (beats/min) | 70±12 | 65±10 | 0.28 |
| Total Cholesterol (mg/dL) | 179±33 | 188±38 | 0.67 |
| LDL Cholesterol (mg/dL) | 98±30 | 114±30 | 0.01 |
| HDL Cholesterol (mg/dL) | 51±14 | 56±15 | 0.08 |
| Triglycerides (mg/dL) | 152±73 | 95±36 | <0.001 |
| Glucose (mg/dL) | 134±52 | 82±11 | <0.001 |
| Insulin (uU/mL) | 20±11 | 13±8 | <0.001 |
| hs-CRP (mg/dL) | 4.1±4.4 | 2.6±3.5 | 0.04 |
| HOMA-IR | 7.0±5.2 | 2.9±2.1 | <0.001 |
| QUICKI | 0.30±0.06 | 0.34±0.03 | <0.001 |
| Glycosylated Hemoglobin (%) | 6.8±1.6 | 5.6±1.0 | 0.006 |
N=59 for waist circumference, 59 for glucose, 58 for insulin, 56 for hsCRP, and 23 for glycosylated hemoglobin
N=60 for glucose, 60 for waist circumference, 59 for insulin, 59 for hsCRP, and 18 for glycosylated hemoglobin.
Materials
Acetylcholine (Ach), 2,4 dinitrophenol (DNP- reduces mitochondrial membrane potential magnitude, Δψm), carbonyl cyanide m-chlorophenyl hydrazone (CCCP- also reduces Δψm magnitude), and L-NG-nitroarginine methyl ester (L-NAME- nitric oxide synthase inhibitor) were purchased from Sigma-Aldrich (St. Louis, MO). 5,5’,6,6’-tetrachloro-1,1’3,3’-tetraethylbenzamidazol-carboncyanine (JC-1- measures Δψm magnitude ), acridine orange 10-nonyl bromide (NAO- measures mitochondrial cardiolipin content as a marker of mitochondrial mass), MitoSox™, diaminofluorescein diacetate (DAF-2DA- measures nitric oxide bioavailability) and tetramethylrhodamine methyl ester (TMRM- measures Δψm magnitude) were purchased from Invitrogen (Carlsbad, CA). MitoTEMPOL was purchased from Enzo Life Sciences International, Inc (Plymouth Meeting, Pennsylvania).
Measurements of in vivo Endothelial Function
Brachial Artery Reactivity Testing
We measured flow mediated dilation (FMD%), nitroglycerin mediated dilation(NMD%), flow velocity, and shear stress in the brachial artery as previously described.28 We recorded average diameter of the entire R-R interval and used these measurements to calculate both FMD% and NMD%. For FMD%, our intra- and inter-observer correlation coefficients are 0.99 and 0.87, respectively. The mean absolute difference measured between observers is 1.0±0.8%.
Digital Pulse Amplitude Tonometry
Digital pulse amplitude was measured using a PAT device placed on the index finger of each hand (Endo-PAT 2000, Itamar Medical, Caesarea, Israel). We measured and reported PAT as the natural log of the ratio of the ratios of baseline pulse amplitudes and post-deflation pulse amplitudes 90-120 seconds post cuff deflation as described previously.6 Greater detail on the methods for PAT can be found in the on-line supplement.
Measurement of in vitro Endothelial Function
Please see the on-line supplement for full methodological details on the procedure used to obtain human gluteal adipose arterioles and preparation of these vessels for study of endothelium-dependent and independent vasodilation by videomicroscopy. Our success rate for obtaining usable arterioles for study by biopsy was 75%. As each biopsy yielded approximately 1 usable arteriole, we could not perform all measurements on all vessels. The type of study done with each vessel was randomly selected and stratified by study group. Endothelium dependent vasodilation was determined at increasing concentrations of acetylcholine from 10-10 to 10-5 M. The eNOS dependence of vasodilation was determined by concomitant L-NAME exposure (1mM). To determine whether partial depolarization of the mitochondrial inner membrane altered endothelial function, vessels were additionally exposed (intra-luminally) to either CCCP (100 nM), DNP (50 nM) for 30 minutes prior to measurements of acetylcholine-induced vasodilation (flow rate < 5 dynes/cm2). To determine whether lowering mitochondrial superoxide levels improved endothelial function, a subset of vessels were intra-luminally exposed to MitoTEMPOL (1 mM) for 30 minutes prior to measuring acetylcholine-induced vasodilation. All vessels were exposed to 0.2 M papaverine at the end of each study to determine the level of endothelium-independent vasodilation.
Vascular Measurements of Mitochondrial Homeostasis
NAO and JC-1 Arteriolar Endothelium Measurements
Following dissection, cannulation, and equilibration, NAO (25 nM), JC-1 (100 nM), or TMRM (1 μM) was allowed to flow through the vascular lumen for 30 min at a very low rate (shear < 5 dynes/cm2). Prior to fluorophore exposure, arterioles to be exposed to NAO and TMRM were imaged by fluorescent microscopy to serve as negative controls. To verify the endothelial specificity of our studies with NAO and JC-1, we denuded several vessels with air boluses (3 for each fluorophore) prior to visualization and imaged vessel that were not exposed to fluorophores as well. Sample images and results confirming the endothelial specificity of these studies are shown in Supplemental Figures I and II. NAO and TMRM fluorescence intensities were measured by fluorescent microscopy (Eclipse TE 200, Nikon, Japan) with Ex/Em of 492/ 526nm and 549/573 nm, respectively. Reported NAO and TMRM fluorescence intensities are the net of the NAO or TMRM exposed arteriole from each individual minus the individual's matched control fluorescence intensity. Red and green JC-1 fluorescence were detected by inverted laser scanning confocal microscopy (Eclipse TE2000-U; Nikon, Tokyo, Japan) with Ex/Em of 488/520 and 578 nm. All fluorescence intensities in this study were analyzed by using MetaMorph 6.1 (Universal Imaging, West Chester, PA).
MitoSox™ and DAF2-DA Measurements on Arterioles from Subjects with and without T2DM
Arterioles were incubated in HEPES buffer under normal glucose conditions (5 mM) with either MitoSox™ (5 μM) or DAF2-DA (5 μM) for 30 minutes. MitoSox™ fluorescence intensity was measured by fluorescent microscopy (Eclipse TE 200. Nikon, Japan) at Ex/Em 510/572 nm for MitoSox™ or 492/526 nm for DAF2-DA. Negative control images from the same vessels were taken before giving fluorescent dye. Reported MitoSox™ and DAF2-DA fluorescence intensities are the net of the MitoSox™ or DAF2-DA exposed arteriole from each individual minus the individual's matched negative control vessel background fluorescence.
Systemic Measurements of Mitochondrial Homeostasis
Measurements of mitochondrial superoxide production using MitoSox™, mitochondrial membrane potential (Δψm) using JC-1, and mitochondrial cardiolipin content as a measure of mitochondrial mass using NAO were performed in mononuclear cells isolated from venous blood of subjects with and without T2DM as previously described.20 Reproducibility of both JC-1 and NAO measurements in human mononuclear cells using these assays has been previously described.20 Due to limitations on the amount of cells available from each patient for the studies presented, MitoSox™ (n=13), JC-1 (N=95), and NAO (N=71) were only performed on a subset of the full study population.
Statistical Analyses
Baseline characteristics, measurements of mitochondrial homeostasis in both mononuclear cells and arterioles, and in vivo vascular endothelial function for non-diabetics and subjects with type 2 diabetes were compared using unpaired t-tests, Wilcoxon rank sum tests, or chi-squared tests as appropriate. Correlations between mononuclear cell measurements of mitochondrial homeostasis and measures of vascular homeostasis were performed using Spearman's ρ. For significant univariate correlations, stepwise multivariable linear regressions models were constructed to determine if systemic measures of mitochondrial function predicted systemic measures of endothelial function using the following co-variables: age, sex, systolic blood pressure, LDL cholesterol, HDL cholesterol, smoking status, family history of CAD, and fasting glucose. Dose-response curves within each subject group were compared by repeated measures ANOVA and relevant between group comparisons were made using two-way mixed ANOVA analyses. Levels of mitochondrial superoxide and NO production were compared using mixed or repeated measures ANOVA as appropriate. DAF2-DA fluorescence data was log transformed for analyses to assure normal data distribution. Post-hoc testing was applied to these analyses if significant differences were noted as appropriate. P-values < 0.05 were considered significant.
Results
Subject Characteristics
A total of 136 subjects were screened and 14 potential non-diabetic participants were excluded due to screening failures, leaving 122 subjects for study. The baseline characteristics of T2DM and non-diabetic subjects are delineated in Table 2. Overall, we found no significant differences in age, sex, smoking status, blood pressure, heart rate, total cholesterol, and HDL cholesterol between the two groups. Patients with T2DM were more likely to be African American and have a family history of CAD. Patients with T2DM also had a significantly larger waist circumference, body mass index, higher fasting insulin and glucose levels, greater insulin resistance as measured by HOMA-IR and QUICKI, and higher serum C-reactive protein levels. LDL cholesterol levels were lower in patients with T2DM likely secondary to concomitant HMG CoA reductase inhibitor therapy. The breakdown of medications taken by the T2DM and non-diabetic subjects is delineated in Supplemental Table I.
Systemic Measurements of Mitochondrial Homeostasis and Their Associations with Systemic Endothelial Function
In a subset of 71 of the 122 subjects (35 non-diabetic, 36 diabetic), we measured NAO fluorescence intensity in mononuclear cells. We measured mitochondrial membrane potential in a subset of 95 of the 122 subjects (46 non-diabetic, 49 diabetic). As we previously reported in a smaller set of these subjects,20 NAO fluorescence intensity was significantly lower in patients with T2DM than control subjects, (235±19 vs. 127±17 A.U. for non-diabetic vs. subjects with T2DM, P <0.001) and mitochondrial membrane potential was significantly more polarized in T2DM subject relative to non-diabetic subjects (13.1±0.8 vs. 17.4±0.9 for non-diabetic vs. subjects with T2DM, P <0.001). We have also previously reported excess mitochondrial superoxide production in T2DM subjects relative to non-diabetic subjects in a subset of this population.20 Interestingly, we now found a strong negative correlation between paired measurements of mononuclear cell NAO fluorescence intensity and MitoSox fluorescence intensity (N=13, ρ=-0.72, p=0.006, Supplemental Figure III).
Results of systemic measures of endothelial function in both T2DM and non-T2DM subjects are summarized in Supplemental Table II. FMD% (6.2±1.6 vs. 3.7±1.7%, P<0.001) and ln PAT (0.74±0.61 vs. 0.48±0.42, P=0.02) were significantly lower in T2DM patients. There was a trend toward reduced nitroglycerin nitroglycerin-mediated dilation in T2DM (NMD%: 21.9±7.0 vs. 19.5±6.3%, P=0.07; NMDmm: 0.82±0.26 vs. 0.73±0.21, p=0.05). We found no differences in baseline peak shear (42±15 vs. 40±14 dynes/cm2, for non-T2DM and T2DM, respectively, P=0.36) or peak hyperemic shear (78±28 vs. 71±23 dynes/cm2 for non-T2DM and T2DM, respectively P=0.09) between groups.
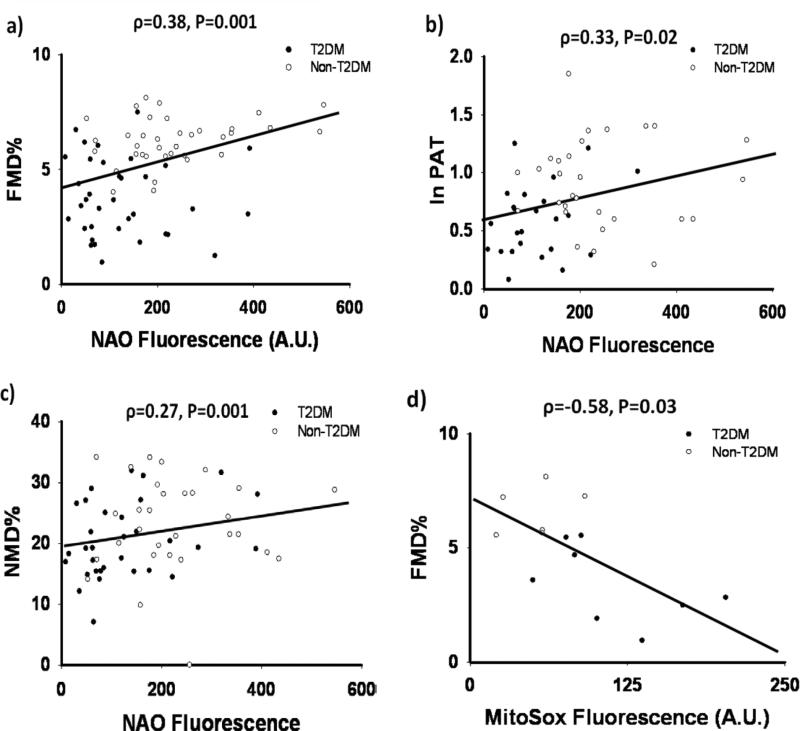
We report the significant univariate correlations between systemic measurements of endothelial function and monocyte mitochondrial homeostasis in Figure 1. NAO fluorescence intensity positively correlated with FMD%, PAT, and NMD% (Figures 1a-c). Mitochondrial superoxide production was inversely associated with FMD% (Figure 1d). We found no significant correlations between NAO, JC-1, and MitoSox™ fluorescence and resting brachial diameter (ρ=-0.17, -0.06, 0.31; P=0.15, 0.59, and 0.27, respectively), resting shear (ρ=-0.08, 0.02, 0.03; P=0.52, 0.84, and 0.91), and peak hyperemic shear (ρ=0.05, 0.01, 0.02; P=0.68, 0.94, and 0.96). There were no significant correlations between JC-1 and MitoSox™ fluorescence and NMD% (ρ=-0.10, -0.31; P=0.35, 0.31) or ln PAT (ρ=-0.18, -0.29; P=0.13, 0.33). The correlation between JC-1 fluorescence and FMD% was not significant (ρ=-0.06, P=0.59).
Figure 1. Correlations Between Systemic Measures of Endothelial Function and Mitochondrial Homeostasis.
Mitochondrial mass in human monocytes measured by NAO fluorescence intensity was positively correlated with brachial artery flow-mediated dilation (ρ=0.38, p=0.001, N=71) (a), digital PAT (ρ=0.33, p=0.02, N=53), (b) brachial artery nitroglycerin-mediated dilated, (ρ=0.27, p=0.001, N=62) (c). Mitochondrial superoxide production in human monocytes showed a strong negative correlation with FMD%, (ρ=0.58, p=0.03, N=14)(d).
In stepwise multivariable linear regression models for FMD% and ln PAT, only NAO fluorescence (β=0.22 and 0.27, P=0.047 and 0.045 for FMD% and ln PAT, respectively) and fasting glucose (β=-0.40 and -0.29, P=0.001 and 0.03 for FMD% and ln PAT, respectively) remained independent predictors of FMD% (model R2=0.24, P<0.001) and ln PAT (model R2=0.20, P=0.004). No single variable was an independent predictor of NMD%. In a multivariable analysis for independent predictors of FMD% including MitoSox™ fluorescence rather than NAO fluorescence, only MitoSox™ fluorescence (β=-0.73, P=0.003) and total cholesterol (β=-0.44,P=0.046) were independent predictors (model R2=0.60, P=0.006).
Mitochondrial Dysfunction in Arterioles from T2DM Patients
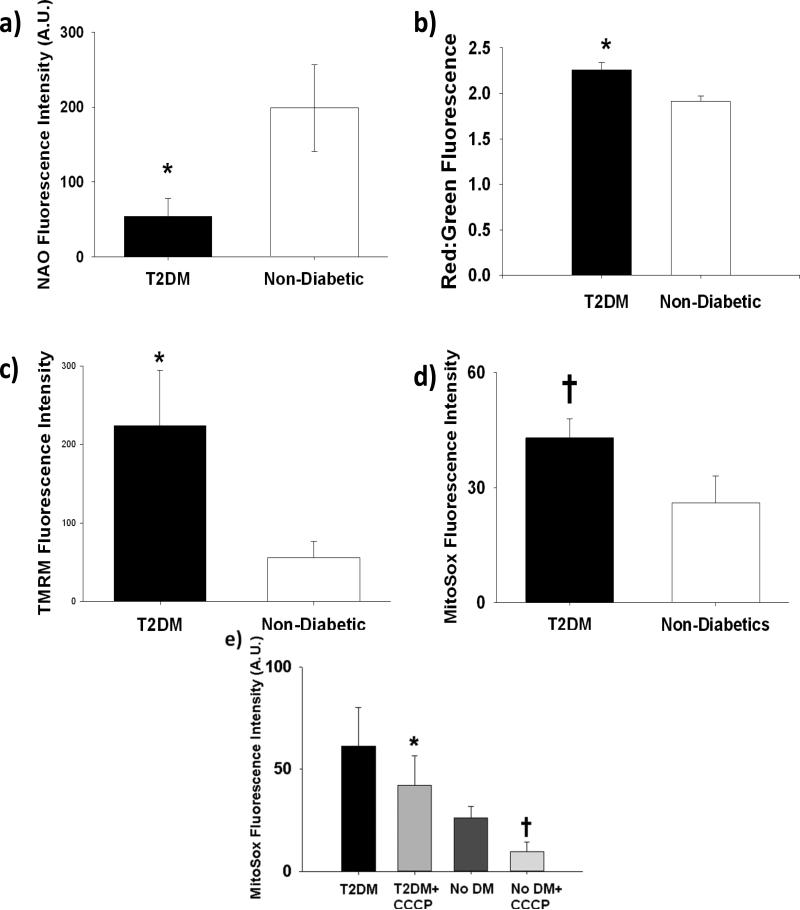
We measured mitochondrial mass, Δψm, and mitochondrial superoxide production in subcutaneous arterioles from randomly subsets of our study participants (Figure 2). Mitochondrial mass as measured by NAO fluorescence intensity was significantly lower in the arteriolar endothelium of subjects with T2DM (54±24 vs. 199±58 A.U. for T2DM (n=10) and non-diabetics (n=11), respectively, Figure 2a), while Δψm was higher in the endothelium of subjects with T2DM as measured by JC-1 (2.26±0.08 vs. 1.91±0.06 for T2DM (n=5) and non-diabetics(n=9), respectively, Figure 2b). Additional arterioles exposed to TMRM confirmed our JC-1 findings (224±70 vs. 56±21 A.U.for T2DM (n=8) and non-diabetics (n=8), respectively, Figure 2c). There was a strong trend toward increased mitochondria specific superoxide in arterioles from patients with T2DM (43±5 vs. 26±7 A.U. for T2DM (n=10) and non-diabetics (n=9), respectively P=0.05 vs. non-diabetics, Figure 2d). In an additional 5 T2DM and 4 non-T2DM subjects, treatment of arterioles with 30 minutes of 100 nM CCCP resulted in significant reductions in mitochondrial superoxide levels in both subjects with and without T2DM. (Figure 2e). Representative images for this experiment are included in Supplemental Figure IV.
Figure 2. Comparisons of Measurements of Mitochondrial Homeostasis in the Arterioles of Humans with and without T2DM.
The arteriolar endothelium of patients with T2DM had significantly reduced mitochondria mass as reflected by reduced NAO fluorescence (54±24 vs. 199±58 A.U., N=10 and 11 for T2DM and non-T2DM, respectively, *P=0.04) (a), increased Δψm by both JC-1 (2.26±0.08 vs. 1.91±0.06 , N=5 and 9 for T2DM and non-T2DM, respectively, *P=0.01) (b) and TMRM (224±70 vs. 56±21 A.U., N=8 and 8 for T2DM and non-T2DM, respectively, *P=0.04) (c). A strong trend toward an increase in mitochondrial superoxide was also seen in T2DM arterioles versus control subjects (43±5 vs. 26±7 A.U., N=10 and 9 for T2DM and non-T2DM, respectively, *P=0.05) (d). Treatment with CCCP (100nM) significantly reduced mitochondrial superoxide production in both T2DM subjects (61±42 and 42± 31 for T2DM and T2DM+CCCP, respectively, N=5) and non-T2DM subjects (37±27 and 22±10 for non-DM and non-DM +CCCP, respectively N=4) (P=0.002 overall by mixed ANOVA). *-P<0.05 vs. non-diabetics, †-P=0.05 vs. non-diabetics (e). Error bars represent ± SE.
Effect of Partial Mitochondrial Uncoupling and Mitochondrial Antioxidant Exposure on Human Arteriolar Endothelial Function in T2DM and Non-Diabetic Subjects
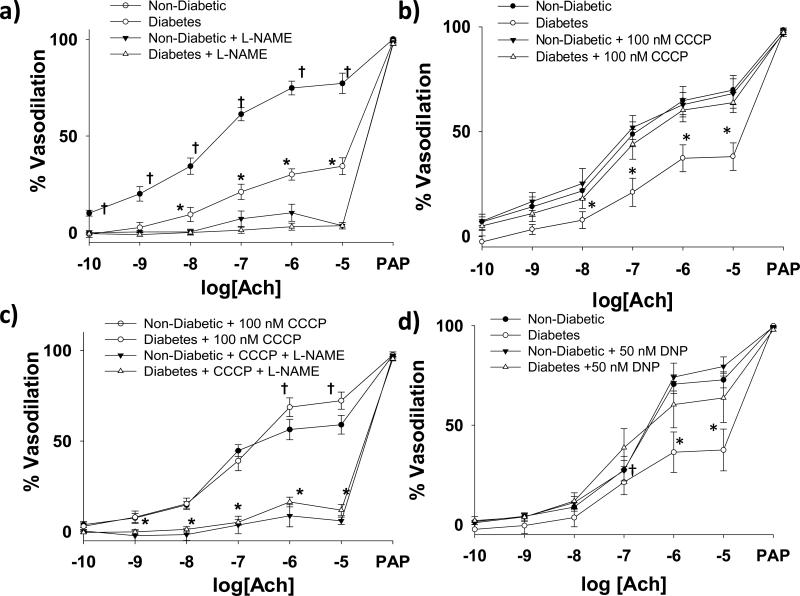
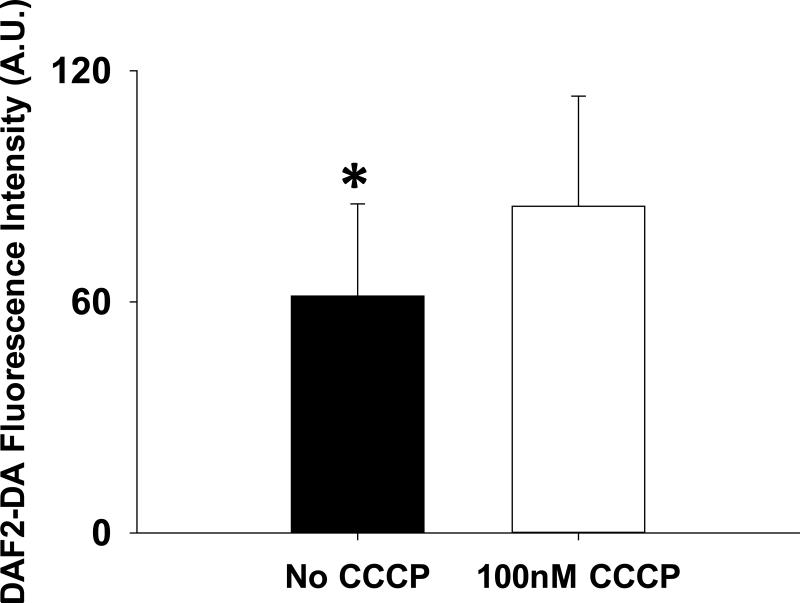
Subcutaneous arterioles isolated from patients with T2DM demonstrate impaired endothelium-dependent vasodilation compared to non-diabetic controls (P<0.001, Figure 3a). L-NAME completely inhibits acetylcholine induced endothelium dependent vasodilation in these vessels from both T2DM patients and non-T2DM controls. (Figure 3a). 30-60 minutes of exposure of T2DM arterioles to 100 nM of the mitochondrial uncoupler CCCP to reduce Δψm magnitude completely reversed endothelial dysfunction in T2DM arterioles(P=0.02, Figure 3b). Co-treatment with L-NAME abrogated the beneficial effect of CCCP (Figure 3c). A different uncoupling agent, DNP, also reversed endothelial dysfunction in T2DM vessels (Figure 3d). CCCP (P=0.94) and DNP had no effect on acetylcholine induced vasodilation of vessels from non-diabetic subjects. There were no significant differences in the acetylcholine response in T2DM vessels exposed to CCCP compared to those exposed to DNP (P=0.21). No differences in papaverine responses were seen between T2DM and non-diabetics regardless of CCCP or DNP exposure state.. In an additional 4 T2DM subjects, we found NO bioavailabilty significantly increased in arterioles from T2DM vessels following exposure to 100 nm CCCP (P=0.03, Figure 4). Representative images for these experiments are included in Supplemental Figure V.
Figure 3. eNOS-related Endothelium Dependent Vasodilation in Arterioles from Patients with T2DM.
Impairment in endothelial function in T2DM arterioles is eNOS dependent (N=8 for T2DM and N=8 for controls. P < 0.001 overall, P<0.001 between curves with and without L-NAME for both T2DM and non-T2DM, respectively. *P < 0.05 for T2DM vs. all other exposure at the indicated Ach concentrations, †- P < 0.05 vs. non-T2DM + L-NAME at the indicated Ach dose) (a). Exposure to 100 nM CCCP for 30 minutes reverses T2DM related endothelial dysfunction (N=6 controls, N=6 T2DM. P <0.001 overall and P=0.002 and 0.71 between curves with and without CCCP for T2DM and non-T2DM subjects, respectively. *P < 0.05 vs. all other exposures at the indicated Ach dose) (b). Improved endothelial dysfunction with CCCP is related to improved eNOS activity (N=5 controls, N=6 T2DM. P<0.001 between curves with and without L-NAME for both T2DM and non-T2DM subjects, *P < 0.05 for both non-diabetic + CCCP + L-NAME and diabetes + CCCP + L-NAME vs. both non-diabetic + CCCP and T2DM + CCCP. †- P < 0.05 for T2DM + CCCP vs. non-diabetic + CCCP) (c) Exposure to 50 nM DNP also improved endothelial function in T2DM arterioles (N=7 controls, N=6 T2DM. P=0.01 overall, P=0.01 and P=0.44 between curves with and without DNP for T2DM and non-T2DM subjects, respectively. *P < 0.05 vs. all other exposures at the indicated Ach dose. †- P<0.05 vs. T2DM alone only (d). Ach- Acetylcholine. PAP- Papaverine. Error bars represent ± SE.
Figure 4. CCCP Exposure Improved NO Bioavailability in Arterioles from Humans with T2DM.
61±24 vs. 85±29 for DAF2-DA fluorescence without and with CCCP, respectively. *-P=0.04 by paired t-test. Error bars represent ± SE.
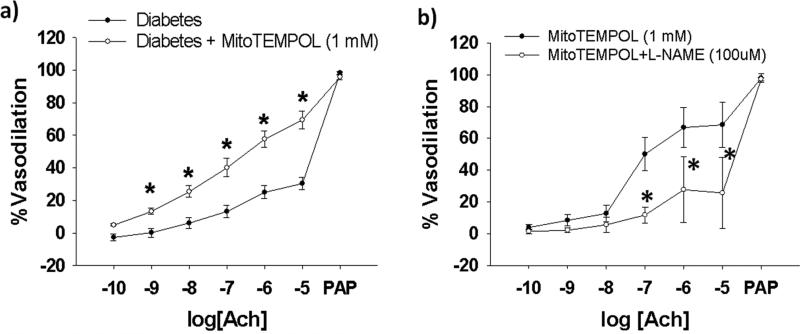
MitoTEMPOL, a mitochondrial-specific superoxide dismutase mimetic, significantly improved arteriolar endothelial function in vessels from a subset of T2DM subjects (N=7, Figure 5a). L-NAME significantly blunts the ameliorative effect of mitoTEMPOL (N=3, Figure 5b). Exposure to CCCP, DNP, or mitoTEMPOL has no significant effect on resting arteriolar diameters in any of the vasoactivity studies (Supplemental Table III).
Figure 5. Effect of MitoTEMPOL on Endothelium-Dependent Vasodilation in Human Arterioles from Patients with Type 2 Diabetes.
MitoTEMPOL (1 mM) for 30 minutes prior to vasodilator testing significantly improved arteriolar endothelial function (N=4, P < 0.001 overall for interaction of acetylcholine dose and MitoTEMPOL exposure (a). L-NAME significantly inhibits the ameliorative effect of MitoTEMPOL on T2DM acetylcholine induced vasodilation (P<0.001 overall between curves) (b). *- P <0.005 between curves at the indicated doses of acetylcholine). Ach- acetylcholine. Error bars represent ± SE.
Correlations between paired acetylcholine vasodilation data and measurements of glycosylated hemoglobin (N=18, 10 T2DM, 8 non-T2DM) demonstrate a strong negative correlation (ρ=-0.59, P=0.01) between glycosylated hemoglobin levels and vasodilation to peak dose acetylcholine (10-5 M). When stratified by T2DM status, these statistical significance of correlations likely due to small numbers but their strong negative directionality remained (ρ=-0.33 and -0.50, P=0.36 and 0.20 for T2DM and non-T2DM, respectively). For the N=42 (17 T2DM, 22 non-T2DM) paired fasting plasma glucose and peak dose Ach paired measurements, fasting glucose strongly negatively correlated with peak Ach dose vasodilation as well (ρ=-0.49, P=0.001). However, when stratified by T2DM status, these correlations were significantly weakened (ρ=-0.10 and -0.08, P=0.67 and 0.74 for T2DM and non-T2DM, respectively).
Discussion
Our studies reveal several substantial findings linking T2DM associated mitochondrial dysfunction with human arterial endothelial dysfunction. In arterioles from subjects with T2DM, we observed mitochondrial hyperpolarization, reduced mitochondrial mass, and excessive mitochondrial superoxide production relative to non-diabetic control subjects. These alterations in mitochondrial function were associated with impaired endothelium-dependent vasodilation to acetylcholine, which in limited data seems to be most associated with glycemic control. Two different treatments that reduce Δψm magnitude as well as a mitochondrial-specific superoxide scavenger improved NOS dependent endothelial function in arterioles from subjects with T2DM and improved NO bioavailability in these vessels. As we previously reported, these adverse alterations in mitochondrial function also occur in peripheral blood mononuclear cells, and we have expanded on our prior findings by showing reduced mitochondrial mass and increased mitochondrial superoxide are associated with impaired systemic measurements of human endothelial function. Taken together, our data suggest a potential mechanistic role for mitochondrial dysfunction (Δψm hyperpolarization, reduced mitochondrial mass, and excessive mitochondrial ROS production) in the development and maintenance of endothelial dysfunction in patients with T2DM. Finally, our data support the concept that mitochondrial dysfunction in T2DM is systemic and that measurements made in easily obtainable circulating cells relate to non-invasive measures of endothelial vasomotor function.
Patients with T2DM exhibit both conduit vessel and micro-vascular endothelial dysfunction.4-8 Endothelial dysfunction in this population predates the development of T2DM in humans as hyperglycemia with and without prevalent insulin resistance can induce systemic endothelial dysfunction.10 While the pathophysiological mechanisms of endothelial dysfunction in T2DM are yet to be fully elucidated, human cross-sectional studies examining other cell types strongly suggest patients with T2DM have impaired mitochondrial function with concomitant reductions in mitochondrial ATP production capacity and increased mitochondrial ROS production which predate their expression of phenotypical T2DM.20, 29, 30 Cell culture and animal work suggest exposures to high glucose and free fatty acid levels impair endothelial function through induction of excessive mitochondrial oxidative stress .10, 16, 17, 24 Our data effectively combine and extend the previous cross-sectional human studies and mechanistic cell and animal work by demonstrating 1) arterioles from T2DM patients have eNOS dependent endothelial dysfunction mechanistically related to mitochondrial ROS production and Δψm hyperpolarization and 2) alterations in mitochondrial mass and ROS production are systemic and are predictive of systemic in vivo measures of endothelial function.
Our data support the concept that Δψm is a central regulator of endothelial function in T2DM.24 Δψm hyperpolarization, occurring secondary to elevated glucose levels, increases ROS production from the mitochondrial electron transport chain due to reduced electron flux and a subsequent increase in the half-life of ROS generating intermediaries.19, 24 In cell culture, excessive mitochondrial ROS production activates endothelial inflammatory pathways through activation of both NF-κB and PKCβ with subsequent increased expression of endothelial adhesion molecules, increased expression of inflammatory cytokines, and reduced NO bioavailability.17, 24 Animal and cell culture data also demonstrate that mitochondrial uncoupling proteins (UCPs) on the inner mitochondrial membrane exert negative feedback on Δψm magnitude and are up-regulated in the setting of cellular stressors.31 Overexpression of UCPs suppresses both Δψm magnitude and mitochondrial superoxide while suppression of UCPs increases mitochondrial ROS production.16, 17 Overexpression of UCPs in an rat model is protective against free fatty acid induced endothelial dysfunction and vascular inflammation, while decreased UCP-2 expression is associated with increased atherosclerosis in mouse models.16, 32 Recent cross-sectional data suggest a human loss-of-function UCP-2 polymorphism is associated with an adverse cardiovascular risk profile.33 Our data extend these reports by demonstrating the importance of Δψm as a novel, rapid acting regulator of arteriolar endothelial function in humans with T2DM. Further, our data suggest Δψm likely alters the endothelial phenotype at least in part through modulating mitochondrial ROS production.
We observed reduced mitochondrial mass in arterioles from patients with T2DM relative to non-diabetic subjects. Further, reduced mitochondrial mass was associated with impaired systemic endothelial function while increased mitochondrial superoxide production was associated with both impaired systemic endothelial function and reduced mitochondrial mass. A growing body of work supports key links between cellular mitochondrial morphology and mass with ATP and ROS production in insulin resistant states.10, 23 Insulin resistant offspring of humans with T2DM have reduced mitochondrial density and ATP production capacity,22, 30 and mononuclear cells from patients with T2DM have smaller, less complex mitochondria.20 Interestingly, eNOS dependent NO bioavailability in cell culture and animal models has been shown to be a central regulator of transcription factor PGC-1α dependent mitochondrial biogenesis.34 NO deficient mice develop obesity and insulin resistance in concert with reduced mitochondrial mass.34 Further, in the endothelium, suppression of NO bioavailability leads to excessive mitochondrial superoxide production.35 Our data extend these findings to the human vasculature by correlating systemic reductions in mitochondrial mass with impairment of NO-dependent endothelial function as well as demonstrating a reduction in mitochondrial mass in the vascular endothelium of patients with T2DM.
Our studies have several limitations. First, the correlations between mitochondrial mass and in vivo measurements of endothelial function are relatively modest. However, the strengths of these correlations are similar to those for traditional cardiovascular risk factors and both FMD and PAT.6, 36 Due to the study size, we cannot make clear determinations as to whether T2DM or non-T2DM subjects are driving these correlations. Our correlations between glycosylated hemoglobin, fasting glucose, and acetylcholine induced vasodilation involve a small sample size and need to be repeated in a larger dataset to confirm our findings. Second, we cannot infer a causal relationship between reduced mitochondrial mass and endothelial dysfunction on the basis our data alone. Further in vitro and potentially in vivo work to determine whether manipulations of mitochondrial mass alter endothelial function in humans will be necessary to fully elucidate this potential relationship. We could not withdraw medical therapy from patients with T2DM patients for greater than 24 hours for ethical reasons. While we cannot exclude medication effects on our arteriolar measurements, the only medication to significantly influence mononuclear cell measures of mitochondrial function was ARB therapy in T2DM patients. This therapy was associated with a significantly higher mitochondrial mass by NAO fluorescence within the group with T2DM [241±106 (n=8) vs. 98±74 A.U. (n=27), P< 0.05]. We consider this finding to be hypothesis generating only. In light of the relative insulin resistance of our control group, it is possible that the differences we observed between our T2DM and non-T2DM groups would be larger if the non-T2DM group had greater insulin sensitivity. We did not collect glycosylated hemoglobin data on these subjects- therefore we cannot directly determine an association between our vessel findings and near term glycemic control. Balanced against these limitations are the novelty of the findings and their potential implications for vascular regulation and therapeutic targets in T2DM and hyperglycemia associated vascular disease.
Conclusion
Our data demonstrate that mitochondrial hyperpolarization and excessive superoxide production are directly associated with the development and maintenance of vascular endothelial dysfunction in patients with T2DM. Further, reduced mitochondrial mass is significantly associated with impaired NO dependent endothelial function. Our work suggests mitochondrial dysfunction may play a particularly key role in the development of atherosclerosis and vascular complications in patients with T2DM. Future work to determine and to evaluate the efficacy interventions targeted to reduce Δψm, mitochondrial ROS production, and/or increase mitochondrial mass in T2DM is warranted.
Supplementary Material
Table 1.
List of Abbreviations
| Δ Ψ m | mitochondrial inner membrane potential |
| Ach | acetylcholine |
| CCCP | carbonyl cyanide m-chlorophenyl hydrazone |
| DAF2-DA | diaminofluorescein diacetate |
| DNP | 2,4-dinitrophenol |
| eNOS | Endothelial nitric oxide synthase |
| FMD% | Percent flow mediated dilation |
| HOMA-IR | homeostasis model assessment-insulin resistance |
| JC-1 | 5,5′,6,6′-tetrachloro-1,1′3,3′-tetraethylbenzamidazol-carboncyanine |
| L-NAME | L-NG-nitroarginine methyl ester |
| NAO | acridine orange 10-nonyl bromide |
| NMD% | percent nitroglycerin mediated dilation |
| PAT | peripherial arterial tonometry |
| PEG-SOD | pegylated superoxide dismutase |
| PKCβ | protein kinase C beta |
| QUICKI | quantitative insulin-sensitivity check index |
| ROS | reactive oxygen species |
| T2DM | type 2 diabetes |
| TMRM | tetramethylrhodamine methyl ester |
| UCP | mitochondrial uncoupling protein |
Acknowledgements
The authors would like to thank Emily Arthur, Jennifer Mulkerin, and Dorothee Weihrauch for their help with data acquisition.
Funding Sources:
Dr Widlansky receives support from K23HL089326, HL081587, the Elsa Shoeneich Medical Research Fund (Greater Milwaukee Foundation), and a T. Franklin Williams Scholars Award provided by Atlantic Philanthropies, the American Heart Association (Grant-in-Aid 10GRNT3880044), the John A. Hartford Foundation, and the Association of Specialty Physicians. This work was supported by the Clinical Translational Research Institute at the Medical College of Wisconsin and NIH grant HL081587. Drs. Kizhakekuttu, Dharmashankar, and Wang have received supported from a Ruth L. Kirschstein NIH T32 training grant (HL007792-15). Dr. Gutterman is supported by HL094971 and HL080704. Dr. Vita is supported by HL083269, HL083801, HL081587, and HL75795. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Disclosures:
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention . National diabetes fact sheet: national estimates and general information on diabetes and prediabetes in the United States. US Department of Health and Human Services, Centers for Disease Control and Prevention; Atlanta, GA: 2011. [Google Scholar]
- 3.Morrish NJ, Wang SL, Stevens LK, Fuller JH, Keen H. Mortality and causes of death in the WHO Multinational Study of Vascular Disease in Diabetes. Diabetologia. 2001;44(Suppl 2):S14–S21. doi: 10.1007/pl00002934. [DOI] [PubMed] [Google Scholar]
- 4.McVeigh GE, Brennan GM, Johnston GD, McDermott BJ, McGrath LT, Henry WR, Andrews JW, Hayes JR. Impaired endothelium-dependent and independent vasodilation in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1992;35:771–776. doi: 10.1007/BF00429099. [DOI] [PubMed] [Google Scholar]
- 5.Lteif AA, Han K, Mather KJ. Obesity, insulin resistance, and the metabolic syndrome: determinants of endothelial dysfunction in whites and blacks. Circulation. 2005;112:32–38. doi: 10.1161/CIRCULATIONAHA.104.520130. [DOI] [PubMed] [Google Scholar]
- 6.Hamburg NM, Keyes MJ, Larson MG, Vasan RS, Schnabel R, Pryde MM, Mitchell GF, Sheffy J, Vita JA, Benjamin EJ. Cross-sectional relations of digital vascular function to cardiovascular risk factors in the Framingham Heart Study. Circulation. 2008;117:2467–2474. doi: 10.1161/CIRCULATIONAHA.107.748574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ting HH, Timimi FK, Boles KS, Creager SJ, Ganz P, Creager MA. Vitamin C improves endothelium-dependent vasodilation in patients with non-insulin-dependent diabetes mellitus. J Clin Invest. 1996;97:22–28. doi: 10.1172/JCI118394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams SB, Cusco JA, Roddy MA, Johnstone MT, Creager MA. Impaired nitric oxide-mediated vasodilation in patients with non-insulin-dependent diabetes mellitus. J Am Coll Cardiol. 1996;27:567–574. doi: 10.1016/0735-1097(95)00522-6. [DOI] [PubMed] [Google Scholar]
- 9.Widlansky ME, Gokce N, Keaney JF, Jr., Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149–1160. doi: 10.1016/s0735-1097(03)00994-x. [DOI] [PubMed] [Google Scholar]
- 10.Tabit CE, Chung WB, Hamburg NM, Vita JA. Endothelial dysfunction in diabetes mellitus: molecular mechanisms and clinical implications. Rev Endocr Metab Disord. 2010;11:61–74. doi: 10.1007/s11154-010-9134-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pieper GM, Langenstroer P, Siebeneich W. Diabetic-induced endothelial dysfunction in rat aorta: role of hydroxyl radicals. Cardiovasc Res. 1997;34:145–156. doi: 10.1016/s0008-6363(96)00237-4. [DOI] [PubMed] [Google Scholar]
- 12.Inoguchi T, Battan R, Handler E, Sportsman JR, Heath W, King GL. Preferential elevation of protein kinase C isoform beta II and diacylglycerol levels in the aorta and heart of diabetic rats: differential reversibility to glycemic control by islet cell transplantation. Proc Natl Acad Sci U S A. 1992;89:11059–11063. doi: 10.1073/pnas.89.22.11059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pieper GM, Riaz uH. Activation of nuclear factor-kappaB in cultured endothelial cells by increased glucose concentration: prevention by calphostin C. J Cardiovasc Pharmacol. 1997;30:528–532. doi: 10.1097/00005344-199710000-00019. [DOI] [PubMed] [Google Scholar]
- 14.Patel C, Ghanim H, Ravishankar S, Sia CL, Viswanathan P, Mohanty P, Dandona P. Prolonged reactive oxygen species generation and nuclear factor-kappaB activation after a high-fat, high-carbohydrate meal in the obese. J Clin Endocrinol Metab. 2007;92:4476–4479. doi: 10.1210/jc.2007-0778. [DOI] [PubMed] [Google Scholar]
- 15.Williams SB, Goldfine AB, Timimi FK, Ting HH, Roddy MA, Simonson DC, Creager MA. Acute hyperglycemia attenuates endothelium-dependent vasodilation in humans in vivo. Circulation. 1998;97:1695–1701. doi: 10.1161/01.cir.97.17.1695. [DOI] [PubMed] [Google Scholar]
- 16.Lee KU, Lee IK, Han J, Song DK, Kim YM, Song HS, Kim HS, Lee WJ, Koh EH, Song KH, Han SM, Kim MS, Park IS, Park JY. Effects of recombinant adenovirus-mediated uncoupling protein 2 overexpression on endothelial function and apoptosis. Circ Res. 2005;96:1200–1207. doi: 10.1161/01.RES.0000170075.73039.5b. [DOI] [PubMed] [Google Scholar]
- 17.Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, Brownlee M. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–790. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 18.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 19.Skulachev VP. Uncoupling: new approaches to an old problem of bioenergetics. Biochim Biophys Acta. 1998;1363:100–124. doi: 10.1016/s0005-2728(97)00091-1. [DOI] [PubMed] [Google Scholar]
- 20.Widlansky ME, Wang J, Shenouda SM, Hagen TM, Smith AR, Kizhakekuttu TJ, Kluge MA, Weihrauch D, Gutterman DD, Vita JA. Altered mitochondrial membrane potential, mass, and morphology in the mononuclear cells of humans with type 2 diabetes. Transl Res. 2010;156:15–25. doi: 10.1016/j.trsl.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstrale M, Laurila E, Houstis N, Daly MJ, Patterson N, Mesirov JP, Golub TR, Tamayo P, Spiegelman B, Lander ES, Hirschhorn JN, Altshuler D, Groop LC. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34:267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 22.Morino K, Petersen KF, Dufour S, Befroy D, Frattini J, Shatzkes N, Neschen S, White MF, Bilz S, Sono S, Pypaert M, Shulman GI. Reduced mitochondrial density and increased IRS-1 serine phosphorylation in muscle of insulin-resistant offspring of type 2 diabetic parents. J Clin Invest. 2005;115:3587–3593. doi: 10.1172/JCI25151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shenouda SM, Widlansky ME, Chen K, Xu G, Tabit CE, Hamburg NM, Frame AA, Caiano TL, Kluge MA, Duess M-A, Levit A, Joseph L, Shirihai OS, Vita J. Alterered mitochondrial dynamics contributes to endothelial dysfunction in diabetes mellitus. Circulation. 2011;124(4):444–453. doi: 10.1161/CIRCULATIONAHA.110.014506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Widlansky ME, Gutterman DD. Regulation of Endothelial Function by Mitochondrial Reactive Oxygen Species. Antioxid Redox Signal. 2011;15:1517–1530. doi: 10.1089/ars.2010.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, Kitzmiller J, Knowler WC, Lebovitz H, Lernmark A, Nathan D, Palmer J, Rizza R, Saudek C, Shaw J, Steffes M, Stern M, Tuomilehto J, Zimmet P. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–3167. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 26.Alberti KG, Zimmet P, Shaw J. The metabolic syndrome--a new worldwide definition. Lancet. 2005;366:1059–1062. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 27.Thygesen K, Alpert JS, White HD. Universal definition of myocardial infarction. J Am Coll Cardiol. 2007;50:2173–2195. doi: 10.1016/j.jacc.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 28.Kizhakekuttu TJ, Gutterman DD, Phillips SA, Jurva JW, Arthur EI, Das E, Widlansky ME. Measuring FMD in the brachial artery: how important is QRS gating? J Appl Physiol. 2010;109:959–965. doi: 10.1152/japplphysiol.00532.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, DiPietro L, Cline GW, Shulman GI. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300:1140–1142. doi: 10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med. 2004;350:664–671. doi: 10.1056/NEJMoa031314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pecqueur C, ves-Guerra MC, Gelly C, Levi-Meyrueis C, Couplan E, Collins S, Ricquier D, Bouillaud F, Miroux B. Uncoupling protein 2, in vivo distribution, induction upon oxidative stress, and evidence for translational regulation. J Biol Chem. 2001;276:8705–8712. doi: 10.1074/jbc.M006938200. [DOI] [PubMed] [Google Scholar]
- 32.Blanc J, Alves-Guerra MC, Esposito B, Rousset S, Gourdy P, Ricquier D, Tedgui A, Miroux B, Mallat Z. Protective role of uncoupling protein 2 in atherosclerosis. Circulation. 2003;107:388–390. doi: 10.1161/01.cir.0000051722.66074.60. [DOI] [PubMed] [Google Scholar]
- 33.Labayen I, Ortega FB, Sjostrom M, Nilsson TK, Olsson LA, Ruiz JR. Association of common variants of UCP2 gene with low-grade inflammation in Swedish children and adolescents; the European Youth Heart Study. Pediatr Res. 2009;66:350–354. doi: 10.1203/PDR.0b013e3181b1bd35. [DOI] [PubMed] [Google Scholar]
- 34.Nisoli E, Clementi E, Paolucci C, Cozzi V, Tonello C, Sciorati C, Bracale R, Valerio A, Francolini M, Moncada S, Carruba MO. Mitochondrial biogenesis in mammals: the role of endogenous nitric oxide. Science. 2003;299:896–899. doi: 10.1126/science.1079368. [DOI] [PubMed] [Google Scholar]
- 35.Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, Jin L, Boss O, Perni RB, Vu CB, Bemis JE, Xie R, Disch JS, Ng PY, Nunes JJ, Lynch AV, Yang H, Galonek H, Israelian K, Choy W, Iffland A, Lavu S, Medvedik O, Sinclair DA, Olefsky JM, Jirousek MR, Elliott PJ, Westphal CH. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712–716. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benjamin EJ, Larson MG, Keyes MJ, Mitchell GF, Vasan RS, Keaney JF, Jr., Lehman B, Fan S, Osypiuk E, Vita JA. Clinical correlates and heritability of endothelial function in the community: The Framingham Heart Study. Circulation. 2004;109:613–619. doi: 10.1161/01.CIR.0000112565.60887.1E. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.