Abstract
Transmitter release at synapses ensures faithful chemical coding of information that is transmitted in the sub-second time frame. The brain, the central unit of information processing, depends upon fast communication for decision making. Neuronal and neurosensory cells are equipped with the molecular machinery that responds reliably, and with high fidelity, to external stimuli. However, neuronal cells differ markedly from neurosensory cells in their signal transmission at synapses. The main difference rests in how the synaptic complex is organized, with active zones in neuronal cells and ribbon synapses in sensory cells (such as photoreceptors and hair cells). In exocytosis/neurosecretion, SNAREs (soluble N-ethylmaleimide-sensitive fusion protein attachment protein receptors) and associated proteins play a critical role in vesicle docking, priming, fusion and synchronization of neurotransmitter release. Recent studies suggest differences between neuronal and sensory cells with respect to the molecular components of their synaptic complexes. In this review, we will cover current findings on neuronal and sensory-cell SNARE proteins and their modulators. We will also briefly discuss recent investigations on how deficits in the expression of SNARE proteins in humans impair function in brain and sense organs.
Keywords: SNARE, Exocytosis, Vesicle fusion, Active zone, Ribbon synapse, Porosome
Introduction
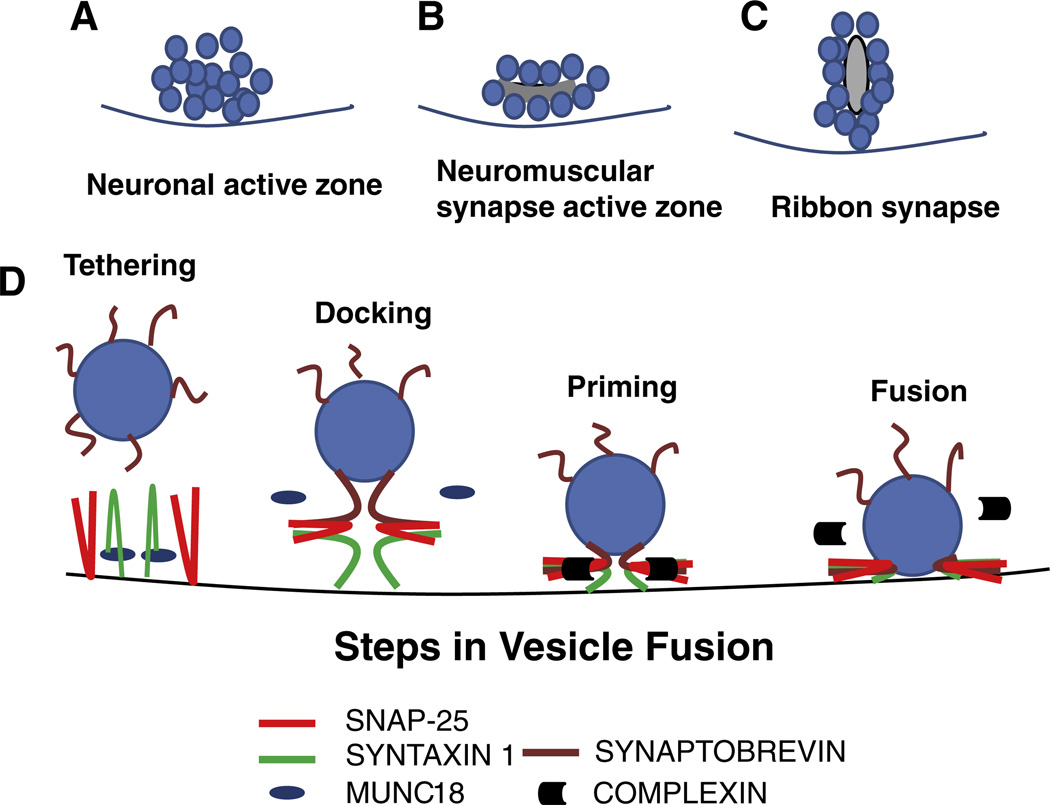
In vertebrates, neurotransmitter release at neuronal synapses is directed by action potentials (Schmitt et al., 1976), whereas in sensory synapses, release is driven by receptor potentials that direct graded exocytosis (Kreft et al., 2003; Parsons et al., 1994). Neurotransmitter release allows fast communication between neurons in higher organisms. In neurons, release is restricted to specialized, electron-dense regions called active zones that appear as disk-like structures covered with synaptic vesicles (Walrond and Reese, 1985) (Fig. 1). At neuromuscular junctions, active zones resemble elongated ridges with synaptic vesicles arranged on both sides (Harlow et al., 2001). In sensory cells, such as hair cells and photoreceptor cells, active zones are manifested as spheres or ribbons surrounded by vesicles (Matthews and Fuchs, 2010). Despite differences in shape and structure, all active zones contain voltage-gated calcium channels and proteins that mediate and regulate exocytosis and endocytosis. An array of structural proteins, such as piccolo and bassoon, are set in the cytoskeletal framework and form the backbone of the active-zone cytomatrix that organizes a dynamic pool of vesicles around the zone (Kantardzhieva et al., 2012; Siksou et al., 2007).
Fig. 1.
Vesicle organization across different types of synapses and steps in synaptic vesicle fusion. (A) Neuronal active zone at the presynaptic terminal, where synaptic vesicles are clustered in the presynaptic area. (B) Neuromuscular junction synapse, where vesicles are organized in a ridge-shaped structure. (C) Sensory cell ribbon synapse, where vesicles are organized around a ribbon-like or spherical structure at the presynaptic terminal. (D) Steps in vesicle fusion include vesicle tethering, docking, priming and finally, fusion. These events are driven by high-affinity interaction between v-SNARE and t-SNARE proteins, regulated by calcium and calcium-binding proteins through their interaction with the SNARE complexes.
Synaptic vesicles are lipid-bilayer structures, 40–100 nm in diameter, filled with neurotransmitter molecules (De Robertis and Franchi, 1956; Di Carlo, 1967). The membranes of neuronal (Takamori et al., 2006) and sensory-cell vesicles (Uthaiah and Hudspeth, 2010) are packed with proteins that are essential for vesicle regeneration, trafficking and exocytosis/neurosecretion. Exocytosis at fast synapses, such as those of neurons, photoreceptors and hair cells, occurs within sub-milliseconds after calcium influx (0.5 ms or less), probably due to a readily-releasable pool of vesicles in close proximity (within 100 nm) to the calcium channels that cluster around the active zone (Beaumont et al., 2005; Sabatini and Regehr, 1999). Synaptic vesicles, before the release of their contents, dock at the pre-synaptic membrane of active zones or ribbon synapses and undergo a priming reaction that prepares them for exocytosis/neurosecretion. Secretion is induced when voltage-gated Ca2+ channels open in response to membrane depolarization, arising from action potentials in neuronal cells and neuromuscular junctions, and from receptor potentials in sensory cells.
Vesicle fusion and pore formation are facilitated by high-affinity interaction of a group of highly-conserved proteins, collectively called SNAREs (soluble N-ethylmaleimide-sensitive fusion protein attachment protein receptors). SNARE proteins associated with the vesicles are termed vesicle-SNAREs (v-SNAREs) and those on the presynaptic plasma membrane are called target-SNAREs (t-SNAREs). Vesicles are released synchronously as well as asynchronously, and their mode of release is determined by proteins such as synaptotagmins and complexins that interact with and regulate conformational changes within the SNARE proteins (Krishnakumar et al., 2011). Homologs and orthologs of SNARE proteins have been found to govern membrane trafficking in different cellular compartments of organisms ranging from yeast to humans, supporting the contention that most types of membrane fusion events share a common mechanism (Jahn et al., 2003). Some of the best-studied proteins crucial to regulated vesicle fusion are Sec18/N-ethylmaleimide-sensitive fusion proteins (Sec18/NSF) (Zhao et al., 2007), Sec17/soluble N-ethylmaleimide sensitive fusion protein attachment proteins (Sec17/SNAPs), SNAP receptors (SNAREs), Sec1/Munc-18 homologs (also known as SM proteins; Verhage et al., 2000) and the Rab family of small GTPases (Pavlos and Jahn, 2011). In addition, synaptotagmins (Hui et al., 2011), complexins (Yang et al., 2010), DoC2 proteins (Groffen et al., 2010) and snapin (Pan et al., 2009) are recognized for their involvement in the regulation of Ca2+-triggered exocytosis. While there is an underlying similarity in exocytosis/neurosecretion across different systems, the process appears to be uniquely controlled in each case to meet the spatial and temporal activities of the given cell type. In the present review, we examine some of the recent advances in our understanding of the SNARE proteins in higher organisms, particularly their regulation and role in neuronal and neurosensory release. We also examine some human disorders caused by deficits in SNARE expression.
Steps in neurotransmitter release
Synaptic vesicles are generated from the endoplasmic reticulum or presynaptic plasma membrane and transported by cellular trafficking to the presynaptic active zones. Vesicles undergo repeated recycling, and this process requires an ordered and sequential participation of many different proteins. Mass spectrometry of isolated rat brain synaptic vesicles reveals the presence of an array of proteins, such as SNARE proteins, transporters, ion channels, signaling proteins, cytoskeletal proteins and trafficking proteins. Synaptobrevin is the most abundant protein of neuronal synaptic vesicles, with an estimated 70 protein molecules per vesicle (Takamori et al., 2006).
In both neuronal and sensory cells, there are three distinct pools of vesicles: (1) a readily-releasable pool (RRP) characterized by a small cluster of vesicles (10+) at the active zone, ready for release, (2) a recycling pool (100+ vesicles) that supplies vesicles to the RRP, and (3) a large reserve pool (several hundred vesicles) that supplies vesicles to the recycling pool (Rizzoli and Betz, 2004). The average number of vesicles in each pool may differ, depending on the tissue type and state. In general, most tissue types show 1–2% of the total vesicles at the active zone, 10–20% in the recycling pool, and 80–90% in the reserve pool (Rizzoli and Betz, 2005). Clustering and mobilization of synaptic vesicles in each pool, at least in neuronal cells, requires proteins such as synapsin, actin, and synaptotagmin 4. Hippocampal synapses of synaptotagmin 4 knockout mice show a defect in trafficking of synaptic vesicles and an accumulation of small vesicles near the trans-Golgi network (Arthur et al., 2010). These defective synapses exhibit a five-fold reduction in docked vesicles after depolarization, suggesting a deficit in vesicle replenishment. In fast synapses, such as those of hair cells and photoreceptors, the RRPs are replenished rapidly from the adjacent reserve pool of vesicles in a calcium-dependent manner. This activity requires a continuous supply of vesicles from the reserve pool in a process that is thought to be driven by stored calcium in the hair cells (Schnee et al., 2011). In hair cells, otoferlin, a protein with six calcium-binding C2 domains, is thought to play a role in the fast replenishment of vesicles in the readily-releasable pool (Pangrsic et al., 2010).
The first step in vesicle fusion is “tethering,” where the vesicles are brought to the active zone to be attached to protein complexes at the presynaptic membrane (Fig. 1) (Whyte and Munro, 2002), facilitating contact between v-SNARE and t-SNARE proteins. At the presynaptic membrane of neurons, syntaxin 1A is attached to Munc-18, forming a closed structure (Smyth et al., 2010). The tethering process is thought to detach this complex and open syntaxin 1A for SNARE interaction. Munc-18 activates exocytosis/neurosecretion (Gracheva et al., 2010) with its dissociation from the syntaxin 1A closed form, freeing the SNARE motif for complex formation (Dulubova et al., 1999; Shi et al., 2011). However, there is evidence for a continued association of Munc-18 with the amino terminus of syntaxin 1A in the assembled SNARE complex. Munc-18 may also be involved in chaperoning syntaxin 1A to the membrane (Shi et al., 2011). The tethering step is followed by docking of vesicles, where SNARE proteins come in contact with each other via the SNARE motifs in a calcium-independent manner. Docking is followed by vesicle priming, where the SNARE proteins form a stable complex via their SNARE motifs, rendering the vesicles competent for fusion (Fig. 1).
The final step – the fusion of vesicles – is triggered by high calcium surrounding the active zone. Active zones possess clusters of voltage-gated calcium channels, and the t-SNARE proteins are thought to surround these channels via direct interaction. During generation of the action potential or receptor potential, the cells undergo depolarization, prompting the voltage-gated calcium channels to open, resulting in an influx of calcium and formation of a calcium micro-domain surrounding the presynapse. Calcium is thought to mediate several molecular interactions between the vesicle proteins and the presynaptic plasma-membrane proteins, resulting in structural changes in the SNARE complex (Han and Jackson, 2006). In neurons, synaptotagmins, the vesicle-bound calcium sensors, also play an important role in vesicle fusion by their direct interaction with the lipid membrane. The structural changes lead to a “puncturing” of the membrane, thus forcing fusion and creating a pore for release of transmitters. Synaptotagmins sense calcium, and complexins act as calcium-dependent switches, facilitating synchronized vesicle fusion via their interaction with SNARE proteins (Bai et al., 2004; Dai et al., 2007; Giraudo et al., 2006). After releasing transmitters, the vesicles are detached by dissociation of the SNARE complex in a process that requires the ATPase in the NSF complex. It is thought that fusion-competent conformation of SNARE molecules is maintained by molecular chaperone complexes composed of cysteine string protein α (CSPα), Hsc70 (heat shock 70 kDa protein 8) and SGT (small glutamine-rich tetratricopeptide repeat-containing protein). Deletion of CSPα leads to degradation of SNAP-25 and decreased SNARE complex assembly (Sharma et al., 2011).
The SNARE complex
The SNARE hypothesis describes a mechanistic model of membrane fusion based on the characteristics of plasma membranes and exocytosis/neurosecretion. Most of the mechanistic steps necessary for transmitter release occur at the presynaptic region. It is assumed that the SNARE proteins present in the acceptor (plasma membrane) and donor (vesicle) membranes mediate the spatial specificity of the interaction between the vesicle and presynaptic membrane preceding fusion (Sollner et al., 1993). Extensive studies have shown that the SNARE complex comprises two classes of components: (1) the v-SNAREs, the SNARE proteins present in the vesicles (predominantly synaptobrevin; Schoch et al., 2001) and (2) the t-SNAREs, the proteins present on the target presynaptic plasma membrane (predominantly syntaxin and synaptosomal-associated proteins such as SNAP-25). Interaction between these two groups of proteins occurs through the highly-conserved SNARE motifs present in these molecules that form an extremely stable four-helix bundle and bring together the vesicle and plasma membranes, thereby facilitating their fusion and release of the vesicle contents. Because of the characteristic complex formed by the three core proteins synaptobrevin, syntaxin, and SNAP-25/23 (the latter contributes two helices), SNARE proteins are thought to catalyze the steps involved in the release by reducing the energy barrier (Li et al., 2007) and increasing the specificity of vesicle fusion, as well as by directly facilitating pore formation by inducing distortion in the membranes.
SNARE core proteins: molecular structure and function
Synaptobrevins
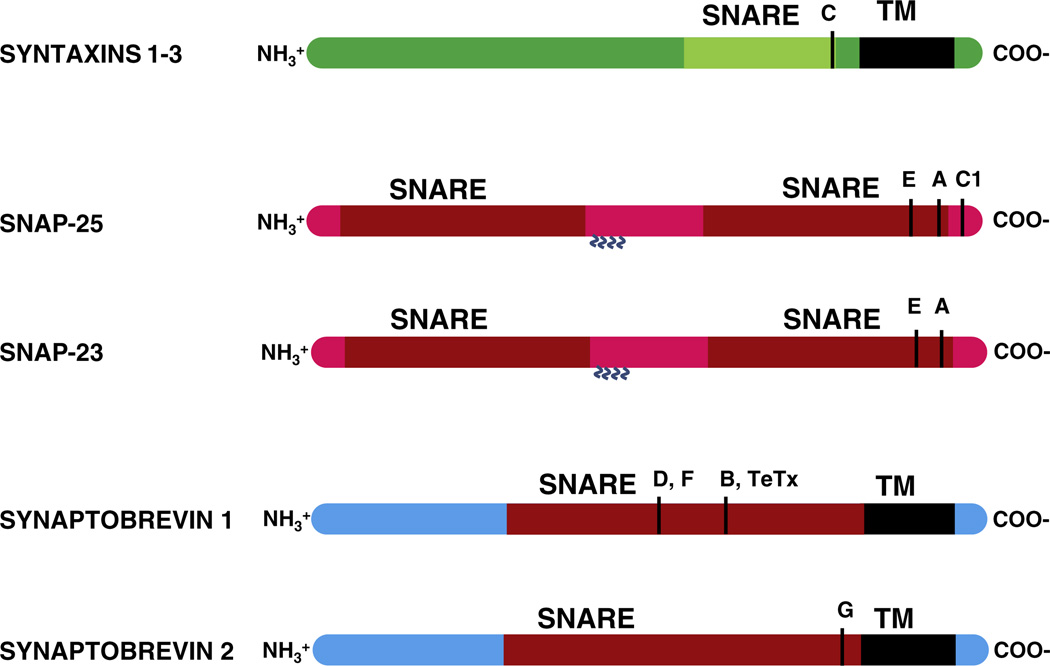
SNARE proteins have been sequenced and their role in synaptic exocytosis/neurosecretion studied extensively (Bennett et al., 1992; Oyler et al., 1989; Trimble et al., 1988). Of the SNAREs, the v-SNARE synaptobrevins are a group of small proteins of 19 kDa molecular mass that are integral to the vesicle membrane and are required for calcium-dependent vesicle fusion (Schoch et al., 2001). Synaptobrevins facilitate pore formation by perturbing the vesicle membrane through their C-terminal trans membrane domains during SNARE “zippering” activity (Ngatchou et al., 2010). Synaptobrevins are cleaved by Clostridium botulinum neurotoxin (BoNT) serotypes B, D, F and G, with each serotype specific for a given peptide bond (Table 1; Fig. 2), resulting in inhibition of exocytosis/neurosecretion (Blasi et al., 1994). Synaptobrevins 1 and 2 are expressed in eukaryotic neurons, neuromuscular junctions, and sensory cells such as hair cells and photoreceptors. Deficiency of synaptobrevin impairs overall vesicular exocytosis and completely inhibits the calcium-triggered portion of exocytosis (Schoch et al., 2001).
Table 1.
Expression of SNARE proteins and their regulators in brain and sensory cells of retina and cochlea.
| SNARE protein | Brain | Retina | Hair Cell | Neurotoxin |
|---|---|---|---|---|
| Syntaxin 1 | Yes | Yes | Yes | BoNT/C |
| Syntaxin 2 | Yes | No | No | BoNT/C |
| Syntaxin 3 | Yes | Yes | Yes | BoNT/C |
| SNAP-23 | Yes | Yes | Yes | BoNT/A,E |
| SNAP-25 | Yes | Yes | Yes | BoNT/A,C,E |
| Synaptobrevin 1 | Yes | Yes | Yes | BoNT/B,D,F,G TeTx |
| Synaptobrevin 2 | Yes | Yes | Yes | BoNT/D,F |
| SNARE regulator proteins | ||||
| Complexin 1 | Yes | No | No | |
| Complexin 2 | Yes | No | No | |
| Complexin 3 | Yes | Yes | No | |
| Complexin 4 | Yes | Yes | No | |
| Synaptotagmin 1 | Yes | Yes | No | |
| Synaptotagmin 2 | Yes | Yes | No | |
| Synaptotagmin 4 | Yes | Yes | Yes/No | |
| Otoferlin | Yes | Yes? | Yes |
The t-SNAREs syntaxins 1, 2 and 3 are expressed in brain tissue, whereas in retina they are expressed differentially, with syntaxin 3 in the photoreceptor ribbon synapses. Syntaxins 1 and 3 are expressed in the ribbon synapses of hair cells (Uthaiah and Hudspeth, 2010). Although the t-SNAREs, SNAP-25 and SNAP-23, are expressed in brain, the distribution of each in brain follows a distinct pattern, with a suggested role for SNAP-23 in the postsynaptic distribution of glutamate receptors (Suh et al., 2010). SNAP-25 and SNAP-23 are expressed in retina as well as in hair cells (SNAP-23 BoNT/A,E cleavage occurs in mouse, not human), but their role in exocytosis has yet to be ascertained. The v-SNAREs synaptobrevins 1 and 2 are expressed in brain, retina and hair cells. Of the SNARE regulators, the complexin isoforms 1, 2, 3 and 4 are expressed in brain. Only complexins 3 and 4 are expressed in the photoreceptor ribbon synapse. None of these four isoforms is expressed in hair cells. The neuronal SNAREs synaptotagmins 1, 2 and 4 are expressed in brain and photoreceptor ribbon synapses. Expression of synaptotagmin 1 is developmentally regulated in hair cells, with little or no expression postnatally. Expression of synaptotagmin 4 has been immunodetected in postnatal hair cells (Johnson et al., 2010); however synaptotagmin 4 protein has not been detected in hair cells by proteomic approaches (Uthaiah and Hudspeth, 2010). Otoferlin, a putative hair-cell calcium sensor, is expressed in brain, hair cells and probably retina (Goodyear et al., 2010). However, of these cell sources, only hair cells exhibit an inhibition of exocytosis when otoferlin is in deficit (Roux et al., 2006).
Fig. 2.
Isoforms of SNARE proteins and the sites of action of neurotoxins. Syntaxins 1, 2 and 3 share a common functional domain arrangement, with a C-terminal transmembrane domain (TM) and a preceding SNARE motif. Clostridium botulinum neurotoxin (BoNT) serotype C cleaves the C-terminus of all three isoforms of the syntaxin SNARE motif. SNAP-25, a neuronal SNARE protein, and its non-neuronal isoform, SNAP-23, share common features of molecular domains while showing dissimilar sensitivity to neurotoxins. SNAP-25 is cleaved by BoNT/A, C, and E, while SNAP-23 is cleaved by BoNT/A and E. Similarly, the v-SNARE proteins synaptobrevins 1 and 2 share common features. However, synaptobrevin 1 is cleaved by BoNT/B, D and F as well as tetanus toxin (TeTx). Synaptobrevin 2, on the other hand, is cleaved by BoNT/G. Examples above are given for mouse sequences.
Syntaxins
Syntaxins are t-SNARE transmembrane proteins present at most target plasma membranes. Different syntaxin functional domains take part in different steps during membrane fusion and calcium-triggered exocytosis (Kee et al., 1995; Wu et al., 1999). Syntaxins possess a single transmembrane domain and a cytoplasmic region consisting of a SNARE domain (H3) and a regulatory domain (Habc). The SNARE domain of syntaxin forms a stable core complex with specific domains of synaptobrevin and SNAP-25 (McMahon and Südhof, 1995). Recent studies have shown that syntaxin cleavage by the neurotoxin BoNT/C (Table 1; Fig. 2) inhibits calcium-dependent secretion from neuronal and neuroendocrine cells (Wang et al., 2011). The Habc domain is characterized by three alpha-helices that fold to form a closed configuration, and unfold to expose the SNARE motif for interaction during vesicle fusion. Syntaxin interacts with a number of regulatory proteins, such as synaptotagmin, calcium channels, and otoferlin (latter present in hair cells; Ramakrishnan et al., 2009), leading to a fine-tuning of the fusion process as required by specific cells. Syntaxin 1A and syntaxin 1B are the major syntaxin isoforms in brain, whereas syntaxins 3 and 3A are important for retinal exocytosis/neurosecretion (Curtis et al., 2010). Mammalian and avian hair cells express syntaxin 1A and syntaxin 3 (Uthaiah and Hudspeth, 2010); however, their exact role in hair-cell SNARE complex formation has yet to be determined (Nouvian et al., 2011).
Synaptosomal-associated proteins
SNAP-25, a member of the family of SNAP proteins widely expressed in prokaryotes and eukaryotes, plays an important role, as a t-SNARE, in membrane fusion. SNAP proteins, or synaptosomal-associated proteins (not to be confused with soluble NSF attachment proteins bearing the same acronym) are cytoplasmic proteins which lack a transmembrane domain and attach to the presynaptic membrane via palmitoyl side chains formed through thioester linkages to cysteine residues located around the center of the molecule (Gonzalo et al., 1999). SNAP-25 contributes two helices to the SNARE core complex (Sørensen et al., 2002) which is necessary for calcium-triggered exocytosis. SNAP-25 interacts with proteins such as synaptotagmin (Zhang et al., 2002), calcium channels (Condliffe et al., 2010), and assumedly, snapin (Pan et al., 2009) in the regulation of exocytosis in neuronal cells. A SNAP-25 knockout mouse shows severe inhibition of calcium-triggered exocytosis, indicating the importance of this t-SNARE in neurosecretion (Washbourne et al., 2002). SNAP-25 is cleaved by the botulinum neurotoxin BoNT/A, thus making SNAP-25 incompetent for SNARE formation, and inhibiting exocytosis. SNAP-23, an isoform of SNAP-25, is involved in inserting glutamate receptor proteins into the postsynaptic membrane (Suh et al., 2010). Both of these SNAP isoforms share common molecular features and are attached to the membrane via palmitoyl side chains. However, they show different sensitivity to BoNT toxins. SNAP-25 is cleaved by BoNT/A, C and E, whereas SNAP-23 is cleaved by BoNT/A and E (Table 1; Fig. 2).
Voltage-gated calcium channels
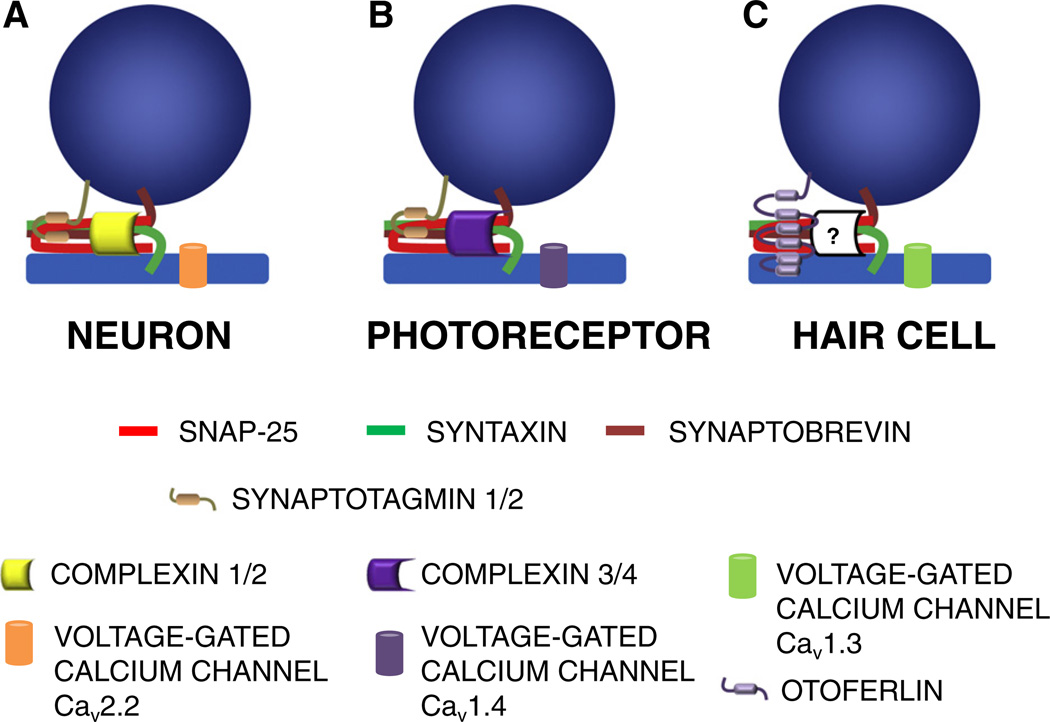
Voltage-gated calcium channels, localized around neuronal active zones and ribbon synapses, open in response to membrane depolarization and give rise to an influx of calcium. The N-type channel, Cav 2.2, mediates calcium conductance typically in neurons, whereas the L-type channels Cav1.3 and Cav1.4 are important for exocytosis in hair cells and photoreceptor cells, respectively (Fig. 3). One of the major differences, pertinent to exocytosis, for the L-type vs. the N-type calcium channels is that certain L-type channels show little or no calcium-dependent inactivation and also undergo only slow voltage-dependent inactivation. This means that the channels remain open longer with depolarization, allowing a continuous calcium flow to the cell. In hair cells, it is estimated that more than 90 percent of the calcium current is carried by Cav1.3 (Platzer et al., 2000). Recently it has been suggested that at the ribbon synapses of hair cells, Cav1.3 channels form nano-domains of calcium responsible for triggering vesicle fusion. These nano-domains are resistant to the slow calcium chelator EGTA, reflecting the highly rapid response to calcium within the nano-domain (Graydon et al., 2011). It is observed that diffuse localization of pre-synaptic calcium channels weakens synaptic activity. About 20% of the Cav1.3 channels are co-localized with the ribbon synapse marker CtBP (RibEYE) (Zampini et al., 2010). Ribbon synapses of photoreceptor cells employ Cav1.4 and these channels display a varying and dynamic distribution during the exocytotic process (Mercer et al., 2011). Voltage-gated calcium channels directly interact with the t-SNAREs syntaxin 1A and SNAP-25 (Wiser et al., 1996), as well as with the presumptive calcium-sensor proteins synaptotagmin 1 (Sheng et al., 1997) and otoferlin (Ramakrishnan et al., 2009). Syntaxin 1A and SNAP-25, either alone or in combination, produce strong inhibition of L-type and N-type calcium channels (Wiser et al., 1996). In addition to channel modulation, interactions between SNAREs and the voltage-gated calcium channel are thought to couple these molecules tightly together at the site of release (Catterall, 1999). A similar function has been attributed to the calcium sensor synaptotagmin 1 in positioning vesicles at the release site at the calyx of Held (Young and Neher, 2009).
Fig. 3.
Vesicle fusion complex in neurosecretory cells from different sources. (A) In neurons, the complex is composed of the core SNARE proteins syntaxin 1A and SNAP-25 (t-SNAREs) and synaptobrevin1 (v-SNARE). The fusion is synchronized by reversible inhibition of SNARE complex formation by complexin 1/2 that interferes in the SNARE helix formation. Voltage-gated calcium channel Cav2.2 opens during depolarization of the cell membrane, allowing calcium entry that is sensed by synaptotagmin1. Synaptotagmin 1 in turn replaces complexin and allows the SNARE formation that brings vesicle and plasma membrane together. Synaptotagmin 1 interacts with PIP2 on the plasma membrane and forces the membranes to fuse, creating a pore for the release of the neurotransmitters. (B) In photoreceptor cells, the basic mechanism of fusion remains the same as for neurons, except that syntaxin 3, paired with SNAP-25, forms the SNARE complex. Synchronous release in photoreceptor cells is believed to be regulated by complexin 3/4. Synaptotagmin 1/2 is thought to be the calcium sensor. Importantly, the L-type channel isoform Cav1.4 mediates calcium current in the ribbon synapses of photoreceptor cells. (C) In mechanosensory auditory and vestibular hair cells, a similar neuronal SNARE model is thought to be operative, as described above. However, there are divergent opinions regarding the identity of the calcium sensors. So far, otoferlin is the only candidate that seems to fit the qualifications required for a calcium sensor in this setting. Differing views of otoferlin's hair-cell function are probably due to themolecule's multiple roles in vesicle recycling/transport and/or in maintaining membrane integrity. Hair cells exhibit synchronous release, similarly to neuronal and photoreceptor cells. However, the nature of the protein that regulates this pivotal step in hair-cell secretion has yet to be identified. The L-type channel isoform Cav1.3 is the major voltage-gated calcium channel expressed in hair cells.
The core complex structure and fusion pore formation
The SNARE core complex is a three-molecule, extremely stable four-helix complex, also termed the “SNAREpin” (Li et al., 2007; Sutton et al., 1998; Weber et al., 1998), that bridges the vesicle membrane and the plasma membrane. The SNARE core complex is formed by SNARE helical motifs, each of approximately 60 amino acids, with synaptobrevin and syntaxin individually contributing one helix and SNAP-25 two helices from the same molecule (Sutton et al., 1998). The high-resolution structure of the complex reveals parallel alpha-helices that twist around each other and create a leucine-zipper-like assembly with an embedded ionic layer consisting of repeating modules of an arginine residue and three glutamine residues (Sutton et al., 1998). Grooves containing distinct hydrophobic, hydrophilic and charged regions are also present in the core that may facilitate interaction with regulatory factors. In the neuronal SNARE core complex, SNARE proteins form a continuous helical bundle that is stabilized by side-chain interactions in the linker regions (Stein et al., 2009). The structural extension of the core complex into the lipid bilayer (Stein et al., 2009) is taken as direct evidence of the involvement of SNARE complexes in vesicle fusion.
“Zippering” of the SNAREpin, in which the SNAREpin coils tighten and come together (see Südhof and Rothman, 2009), forces the vesicle and plasma membranes close to each other (Hanson et al., 1997; Weber et al., 1998), and the conformational changes and specific amino acid sequences of the transmembrane domains of syntaxin and synaptobrevin mechanistically drive membrane fusion (Stelzer et al., 2008). It is estimated that more than three SNAREpins should provide sufficient energy for the fusion of vesicle and plasma membrane (Hua and Scheller, 2001; Li et al., 2007; Mohrmann et al., 2010). Consistent with this idea, it has been proposed that 5–7 syntaxin transmembrane domains form the outer rim of the fusion pore (Han et al., 2004). Alternatively, it is hypothesized that a stable hemi-fusion product leads to fusion pore formation (Wong et al., 2007). It is believed that conformational changes in the SNARE complex and the zipper-tightening activity of the core complex force the membranes on each side to distort and form a pore (Jahn et al., 2003).
Complexin, a calcium-dependent regulator of neuronal exocytosis (see also below), has been shown to bind to the groove between the synaptobrevin and syntaxin helices in the SNARE complex in an anti-parallel alpha-helical conformation, thus stabilizing the interphase between the two helices. Because of their apposing positions, the vesicle and presynaptic membranes exert mutually-repulsive forces that are counteracted by the v-SNARE and t-SNARE helices bound to complexin (Chen et al., 2002). Synaptotagmin, along with calcium and phospholipids, binds SNARE proteins (Dai et al., 2007) to facilitate calcium-dependent exocytosis (Fernández-Chacón et al., 2001). Complexins are thought to function in association with synaptotagmin as a molecular switch in “clamping” the vesicle fusion complex prior to calcium-dependent activation via synaptotagmin (Krishnakumar et al., 2011).
In chromaffin cells expressing a SNAP-25 d9 mutant with the last 9C-terminal residues deleted, exocytosis, as measured by membrane capacitance, is characterized by reduced fusion pore conductance as well as a lower rate of fusion pore expansion, probably because this SNAP-25 mutant forms a loose SNARE complex (Fang et al., 2008). The d9 deletion in the C-terminal SNARE motif of SNAP-25 abolishes spontaneous neurotransmission while reducing evoked exocytosis, and deletion of the N-terminal SNARE motif of SNAP-25 delays vesicle priming (Weber et al., 2010), indicating involvement of SNAP-25 at different stages of vesicle fusion.
Evidence in support of the role of SNAREs in fusion-pore formation is also provided by studies on the v-SNARE, synaptobrevin 2. It has been suggested that perturbation of the vesicle membrane caused by the C-terminal transmembrane domain of synaptobrevin 2 during zippering causes the vesicle to open (Ngatchou et al., 2010). Engineered addition of two charged amino acids (lysine or glutamate) to the C-terminus of synaptobrevin has been reported to inhibit fusion and exocytosis. The latter finding suggests that the SNARE complex provides both the energy (in the zippering process) as well as physical means to perturb the lipid bilayer during membrane fusion. Although synaptobrevin is the most abundant protein on the vesicle surface (as stated, ~70 molecules per vesicle) (Takamori et al., 2006), a recent study, utilizing cultured hippocampal neurons deficient in synaptobrevin, showed that only two synaptobrevin molecules, and thus two SNARE complexes, are minimally sufficient for exocytosis (Sinha et al., 2011). Thus, it is not clear why the synaptic vesicle membrane needs to be packed with such a large number of synaptobrevin molecules. One possibility is that multiple synaptobrevin molecules increase the probability that at least some of the synaptobrevin will be readily available for fast exocytosis at any given time.
Calcium control of vesicle function
Calcium is thought to control two main processes in the functioning of synaptic vesicles (Hosoi et al., 2007; Lou et al., 2005). One is the recruitment of vesicles to the presynaptic membrane in preparation for release. This activity involves the association of the vesicles with the SNARE complex as part of priming process, prior to release. The recruitment is dependent upon calcium in the 0–500 nM range, where the recruitment rate will be greater than the release rate since calcium dependence of release is minimal in this range. The second calcium-dependent process, release itself, predominates at calcium concentrations greater than 5 µM. In the latter range, calcium-dependent release obeys a high-power exponential function of calcium concentration. Here the calcium-dependent release rate will be greater than the recruitment rate, particularly for high frequencies of stimulation (Neher and Sakaba, 2008).
Current physical models of vesicle neurosecretion (Fig. 1) are important subjects of ongoing deliberation. Neher and Sakaba (2008) have defined two kinds of synaptic-vesicle priming, “molecular priming” and “positional priming.” Molecular priming involves the association of the vesicle with the SNARE complex. Positional priming describes the process in which primed vesicles situate themselves optimally near the voltage-gated calcium channels (Wadel et al., 2007). Both these modes of priming are calcium-dependent, as discussed above for the overall recruitment process. Molecular priming is rate-limiting in “tonic” synapses and neuroendocrine cells, but not in “phasic” synapses, where high-frequency activity would first be limited by the positional priming (Neher and Sakaba, 2008).
Once the vesicle is primed, and positioned optimally near active-zone calcium channels, release can occur (Fig. 1). Classically the release of vesicle contents is thought to be followed by the fusion of the synaptic vesicle with the cell plasma membrane and incorporation into the membrane. According to this view, the membrane bilayer is recycled by endocytosis of vesicles re-created from the cell membrane (Dresbach et al., 2001) and returned to a recycling pool to undergo clustering (Haucke et al., 2011). However, between priming and the postulated endocytic step, the remaining protein cargo of the synaptic membrane and the SNARE complex must be accounted for. A “site-clearing” step has been postulated to involve patch diffusion or declustering-reclustering of vesicle cargo proteins toward the endocytic retrieval site (Haucke et al., 2011). An alternative model postulates the existence of a fusion pore which opens and closes (“kiss and run” hypothesis) (Fesce et al., 1994). Until recently, a physical correlate of the fusion pore for kiss-and-run had not been identified. However, a mechanism involving the porosome, a stable fusion pore structure (see below), offers an energetically-efficient model for support of rapid and continuous neurosecretion, which could replace or supplement the process of classical exocytosis (Fig. 4).
Fig. 4.
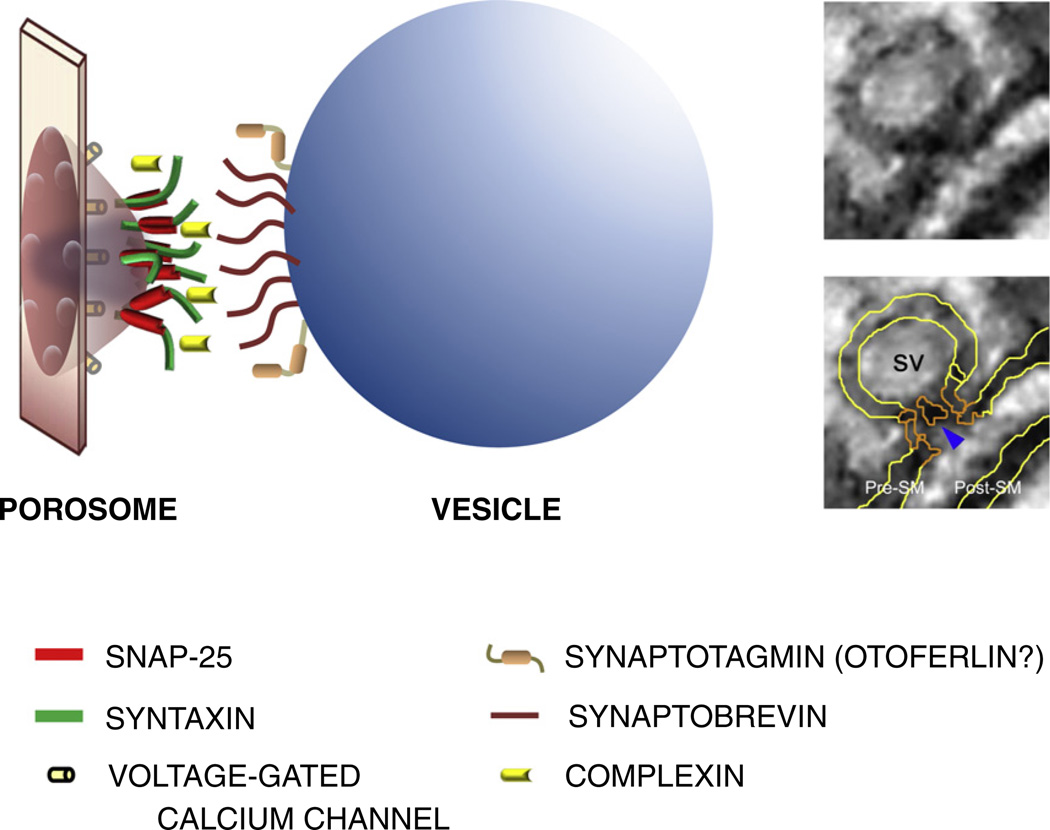
Hypothetical model of the porosome and synaptic vesicle, showing involvement of multiple SNARE molecules in vesicle fusion. Porosomes, nano-scale stable membrane invaginations observed at active zones and ribbon synapses, consist of cup-shaped structures with the base of the “cup” facing the vesicle (Jena, 2009b). The outer rim of the cup opens towards the outside and bears eight to twelve protein knobs. The cavity of the porosome is occupied by a mobile “plug” that is thought to move in and out during neurosecretion. Multiple t-SNAREs required for vesicle fusion are shown localized at the cup's base, ready to pair with the v-SNAREs of the vesicle. Voltage-gated calcium channels are thought to be localized close to the fusion machinery via their protein–protein interaction with t-SNARE proteins. Close proximity of channels is required for the formation of calcium nano-domains that support fast exocytosis. It is hypothesized that vesicles attach to the porosome, probably via SNARE formation and are recycled after release. Insets show transmission electron micrograph photos (same micrograph unlabeled and labeled) of hair-cell porosome (orange) with central plug (blue arrowhead) from the sacculus of the rainbow trout. SV, synaptic vesicle; SM, synaptic membrane (cf. Drescher et al., 2011).
Porosomes
We indicated previously that more than three SNARE complexes are estimated to be required for fusion of a single vesicle, even though only two appear to be sufficient to support exocytosis, as a minimum requirement under controlled experimental conditions (Sinha et al., 2011). A recent paper shows, with in vitro studies, that more than three SNARE molecules are required for maintaining an open fusion pore to facilitate synchronized vesicle fusion in reaching a physiological release rate (Shi et al., 2012). We stated that synaptic vesicles are packed with the v-SNARE protein synaptobrevin. Further questions are: How are t-SNARE proteins of the complex distributed? What regulates the clustering of syntaxin 1A and SNAP-25 at the active zones? Is the distribution random or is it organized? Fast synapses, such as hair-cell ribbon synapses, exhibit sustained, evoked exocytosis/neurosecretion. This situation begs a question: Is there a stable structural component, or module, that organizes multiple SNAREs in the pre-synaptic area to facilitate uninterrupted vesicle fusion and neurosecretion? Recent findings suggest that such a module, in fact, exists. This structure, called the “porosome,” has been identified in neurons (Cho et al., 2010), pancreatic cells, and recently, in teleost saccular hair cells (Drescher et al., 2011; Fig. 4). Porosomes are specialized target membranes that invaginate in the active zone in an inverted-cup-shaped structure. Cytoskeletal proteins such as actin and vimentin, and voltage-gated calcium channel subunits, as well as NSF and SNAREs, are considered to be part of the structure. It is believed that SNARE proteins surround the porosome cup, ready for vesicle docking and fusion (Jena, 2009a,b). Many in vitro and in vivo studies show that 3–15 SNAREs are required for exocytosis/neurosecretion (Mohrmann et al., 2010). While these numbers vary, and are compiled from studies on a variety of cells, it is clear that one or few SNAREs may not efficiently catalyze membrane fusion. It has yet to be seen how many SNARE complexes do participate in fast exocytosis/neurosecretion from sensory cells, such as hair cells or photoreceptors. Multiple SNARE complexes may be necessary for the fast, sustained and high-fidelity exocytosis required by these cells in response to evoked receptor potentials. Thus, because of the need for tight regulation and fast dynamics, each ribbon synapse may employ at least several SNAREs to meet the turnover of hundreds of vesicle fusions per second. In the latter scenario, the utilization of a more stable structure like the porosome may be the most efficient way to accomplish fast exocytosis/neurosecretion.
SNARE regulators and exocytosis/neurosecretion
Spatial and temporal precision in neurosecretion is critical for accurate transmission of information to the brain. Several protein and non-protein regulators are known to act in tandem to attain the specificity and accuracy of vesicle fusion. In general, v-SNARE and t-SNARE protein interactions determine the specificity of this fusion. From a minimalistic point of view, merely the SNARE motifs of the SNARE proteins would be needed to elicit membrane fusion in vitro (Fasshauer et al., 1998; Weber et al., 1998). However, recent studies have demonstrated the involvement of other proteins that regulate the functions of the SNARE complex, and thus of vesicle fusion.
Complexins
Complexins act to modulate SNARE-mediated exocytosis by their calcium-dependent interaction with the assembled SNARE core complex (SNAREpin), thus “clamping” the SNARE and temporarily arresting exocytosis (Bracher et al., 2002; Chen et al., 2002; Giraudo et al., 2006). Clamping of SNAREpins by complexins 1 and 2 is necessary for synchronous exocytosis. This step is especially important at graded synapses, such as ribbon synapses (Martin et al., 2011). However, none of the four known isoforms of complexin (complexins 1–4) has been detected in hair cells (Strenzke et al., 2009), and only complexins 3 and 4 have been detected in photoreceptors (Fig. 3) (Reimet al., 2009). Interestingly, complexin 1 knockout mice are hearing-deficient, possibly because of diminished release from the synapses of the endbulb of Held of auditory nerve fibers (Strenzke et al., 2009). Hair-cell synchronous release thus remains an enigma. Complexins also potentiate fusogenicity of vesicles by interacting with SNAREs through their N-termini (Xue et al., 2010). Further, it has been suggested that the complexin-SNARE interaction regulates the force that trans-SNARE complexes apply to the fusing membranes (Maximov et al., 2009).
Synaptotagmins
In neurons and sensory cells, calcium acts as a trigger in the exocytotic process. Calcium does not directly bind and modify the SNARE complex, but calcium-binding proteins, known to be located near active zones and ribbon synapses, act as intermediaries. In neurons and photoreceptors, synaptotagmin isoforms 1 and 2 are major calcium-sensing proteins that regulate SNARE nucleation (van den Bogaart et al., 2011) and vesicle docking, priming and fusion (Kuo et al., 2011). Synaptotagmins are vesicle-bound proteins with a single transmembrane domain at the N-terminus followed by two calcium-binding C2 domains, C2A and C2B. C2A binds three calcium ions, whereas C2B binds two. C2 domains are also known to bind phospholipids, such as phosphoserine and phosphoinositol phosphates, and to facilitate fusion of vesicle and plasma membrane. Phosphatidylinositol 4,5-bisphosphate (PIP2) is an important component of the plasma membrane necessary for SNARE-mediated membrane fusion. Synaptotagmin 1 has been shown to interact directly with PIP2 to facilitate C2 calcium sensing by inserting a portion of the calcium-bound C2 domain into the target membrane during vesicle fusion. It has been suggested that synaptotagmins, in conjunction with complexin, may directly play a role in synchronous release, with the C2B domain mediating the process (Yoshihara et al., 2010). In auditory and vestibular hair cells, expression of synaptotagmin is developmentally regulated, with the synaptotagmin isoforms 1 and 2 expressed prenatally and little or no synaptotagmin expressed postnatally, at the onset of hearing (Johnson et al., 2010) . Many lines of evidence suggest that otoferlin may substitute for synaptotagmin as a calcium-sensing protein interacting with SNAREs in adult hair cells. Although the role of otoferlin as a calcium sensor has been questioned, there is ample experimental evidence showing its direct interaction with syntaxin 1A, SNAP-25, and the voltage-gated calcium channel, Cav1.3 (Ramakrishnan et al., 2009; Roux et al., 2006). Further, there is an absolute requirement for otoferlin in calcium-dependent exocytosis/neurosecretion at the hair cell ribbon synapse (Roux et al., 2006).
Munc-18
Even though the t-SNARE syntaxin 1A is distributed throughout the plasma membrane, exocytosis occurs mainly at the active zones (Garcia et al., 1995), and Sec/Munc-18 proteins play a role in the localization of syntaxin at the active zone. A key regulator in the early stages of vesicle docking is Munc-18-1, which binds tightly to the closed conformation of syntaxin 1A, preventing its interaction with other SNARE proteins (Dulubova et al., 1999). Munc-18-1 is crucial for vesicle exocytosis at presynaptic terminals (Verhage et al., 2000) and undergoes rapid PKC-catalyzed phosphorylation during the depolarization stage (Craig et al., 2003; de Vries et al., 2000). Phosphorylation of Munc-18-1 reduces its affinity for its presynaptic binding partner syntaxin 1A (Barclay et al., 2003), making syntaxin available for SNARE complex formation.
Non-protein regulators
During the last several years, non-protein regulators of SNARE assembly and exocytsis have emerged. Sphingosine, an 18-carbon amino alcohol with an unsaturated hydrocarbon chain, is best known for its role in forming the backbone of sphingolipids, which are important components of cell membranes. Sphingosine has been shown to activate synaptobrevin in the assembly of the SNARE complex. Resulting positive regulation of exocytosis is observed in isolated nerve terminals, neuroendocrine cells, and neuromuscular junctions. This upregulation is not observed in the synaptobrevin 2 knockout mouse (Darios et al., 2009). Another study has revealed a role of phospholipase-C-mediated diacylglycerol synthesis and priming of syntaxin in SNARE-mediated vesicle fusion (Wierda et al., 2007). Diacylglycerol is known transiently to enhance both evoked exocytosis (Malenka et al., 1986) and spontaneous release from presynaptic terminals (Lou et al., 2005).
SNAREs at the hair-cell ribbon synapse
In higher vertebrates, specialized, sensitive mechanoreceptor cells called hair cells convert the energy of sound and motion into graded potentials that are communicated to the primary afferent nerve during neurotransmission. Two types of hair cells, inner and outer, are present in the hearing organs of higher vertebrates. Inner hair cells are innervated by twenty or more unbranched afferent neurons, each one receiving signals from a single ribbon synapse. In contrast, each outer hair cell connects to a highly-branched afferent neuron. As in many neuronal cells, neurotransmission in hair cells is synchronous, and the ribbon synapses are thought to play an important role in this process (Khimich et al., 2005). Is the synaptic release machinery in hair cells similar to that in neuronal cells? It is speculated that hair-cell and neuronal SNARE complexes are indeed similar, even though there is as yet no direct proof that identical neurosecretory proteins are involved in both kinds of cells. A recent study, utilizing a proteomic approach to unravel the identity of hair-cell synaptic proteins, clearly demonstrated the presence in the hair cell of syntaxin 1, SNAP-25 and synaptobrevin, along with the ribbonsynapse-specific structural protein, RIBEYE (Uthaiah and Hudspeth, 2010). SNAP-25, syntaxin 1A and synaptobrevin were also immunolocalized to hair cells, with dense labeling in the presynaptic basolateral region. Other SNARE isoforms such as syntaxin 3 and SNAP-23 were labeled, but at low intensity. These findings confirm the results of a previous study in which syntaxin 1, SNAP-25 and synaptobrevin were found to be expressed in hair cells, as detected by RT-PCR, western analysis, in situ hybridization and immunohistochemistry (Safieddine and Wenthold, 1999). However, in a recent study, it was reported that neurotoxin proteases that cleave specific SNARE proteins, and thus inhibit neurotransmission, did not inhibit hair-cell exocytosis (Nouvian et al., 2011). What is intriguing about the latter study is that the hair cells examined expressed transcripts for the classic SNARE proteins syntaxin 1A, SNAP-25 and synaptobrevin, but no corresponding SNARE protein products could be detected (Nouvian et al., 2011). Moreover, a resistance to known synaptic inhibitors that cleave SNARE proteins (Table 1; Fig. 2) was reported. Thus, further investigations will be required to clarify the precise protein composition of SNARE complexes in hair cells.
Exocytosis/neurosecretion is a tightly-regulated process, and hair cells are capable of maintaining fast, graded, and sustained release of transmitters in encoding complex sound signals. This characteristic mode of hair-cell exocytosis may be correlated with a number of molecular mechanisms and properties specific to this sensitive mechanosensory cell. First, in hair cells, exocytosis is driven by the voltage-gated calcium channel isoform Cav1.3, the major calcium channel present in vertebrate hair cells (Kollmar et al., 1997; Platzer et al., 2000; Ramakrishnan et al., 2002). Cav1.3 is capable of opening with little inactivation in the presence of high calcium within the microdomain surrounding the ribbon synapse, and is absolutely required for hair-cell exocytosis (Platzer et al., 2000). Second, post-natal hair cells express little or no synaptotagmins 1 or 2 (Johnson et al., 2010). Third, hair cells do not express any of the known complexin isoforms (Strenzke et al., 2009). Fourth, otoferlin, a multi-C2 domain-containing protein of the ferlin family, is absolutely required for hair-cell exocytosis and hearing (Roux et al., 2006). Even though otoferlin splice variants are expressed in several tissues, including brain, otoferlin deficiency specifically impairs hearing, in human as well as mouse. Recent studies suggest that synaptotagmin 1 cannot rescue hair-cell exocytosis in hair cells lacking otoferlin (Reisinger et al., 2011), suggesting that otoferlin plays a specific role in regulating hair-cell exocytosis/neurosecretion. Further, otoferlin has been shown to interact with the SNARE proteins syntaxin 1A and SNAP-25 (Ramakrishnan et al., 2009; Roux et al., 2006), as well as with Cav1.3 (Ramakrishnan et al., 2009). Moreover, otoferlin also mediates calcium-dependent vesicle fusion in vitro in the presence of neuronal SNARE proteins (Johnson and Chapman, 2010). Otoferlin in hair cells is also thought to be associated with the calcium-dependent vesicle replenishment required to meet the sustained, high turnover of vesicles at the hair-cell ribbon synapse. These lines of evidence point to a possible non-neuronal character of SNARE complexes that participate in hair-cell exocytosis. Complexins 3 and 4, distinctly different from complexins 1 and 2, are expressed in the retinal sensory neurons and mediate high-efficiency exocytosis at retinal ribbon synapses (Reim et al., 2005). Implications of the absence of complexins 3 and 4 in hair cells (Strenzke et al., 2009) may be clarified in future studies.
Hair-cell synapses: unresolved issues
The characteristic ribbon synapses of hair cells likely play an important role in supporting the hair cell's remarkable ability for rapid, sustained, and graded exocytosis/neurosecretion, but the exact cellular and molecular mechanisms have yet to be elucidated. Vesicle recruitment at hair-cell ribbons appears to be fast and essentially inexhaustible (Griesinger et al., 2005). A rapid burst of transmitter release occurs at depolarization onset, followed by sustained release with continued depolarization. Interestingly, knockout of the protein bassoon, which normally holds the ribbon complex in place, abolishes the rapid release component but retains the sustained release component (Khimich et al., 2005). The question of how our present models of vesicle priming, docking, and fusion exactly fit these release kinetics remains to be answered. There is also a need to explain the micro-anatomical correlates of the huge amount of hair-cell membrane turnover, as measured by capacitance changes, observed during prolonged depolarization (Parsons et al., 1994; Schnee et al., 2005). A precise quantitation of single-vesicle contribution to the capacitance change for classical exocytosis, vs. “kiss and run” neurosecretion, may help clarify the mechanism of high membrane turnover. As stated previously, we also do not know what molecules perform the function of complexins in the postulated hair-cell SNARE activity, since no complexins have yet been identified in hair cells. Further, the evidence for a possible role of otoferlin replacing synaptotagmin as the calcium sensor in SNARE-mediated neurosecretion in hair cells needs to be solidified. Finally, the observation that t-SNARE and v-SNARE proteins (Safieddine and Wenthold, 1999; Uthaiah and Hudspeth, 2010), as well as their RNA messages (Nouvian et al., 2011), are present in hair cells, needs to be reconciled with the seemingly contradictory finding that hair-cell exocytosis is not inhibited by SNARE-specific neurotoxins.
The SNARE complex in retina
Conventional synapses, such as those of neurons, release neurotransmitters transiently, whereas photoreceptors and retinal bipolar cells, containing ribbon synapses, release neurotransmitter continuously with modulation of release rate in response to stimulus. Both modes of release are mediated by synaptic vesicles, but probably differ in the regulation of docking and fusion of the vesicles with the plasma membrane. Expression of syntaxin 3 has been detected in photoreceptors and retinal bipolar cells (Morgans et al., 1996). In retinal horizontal cells, components of synaptic vesicles and SNAREs have been identified by immunostaining (Lee and Brecha, 2010). Neuronal SNARE core-complex proteins syntaxin 1A, syntaxin 4 and SNAP-25, along with complexins 1 and 2, are strongly expressed in the soma of horizontal cells. Moreover, the vesicular calcium-sensing protein, synaptotagmin 2, has been immunolocalized to processes and somata of horizontal cells. However, it has been reported that syntaxin 3B (detected by molecular techniques, immunohistochemistry, and membrane capacitance measurements) is also expressed in bipolar cells of the goldfish retina and is essential for exocytosis in ribbon synapses of retina. Syntaxin 3B peptides inhibit exocytosis as measured by changes in membrane capacitance after stimulation (Curtis et al., 2010). These results show that exocytosis in retinal cells may be fine-tuned by the action of a combination of different SNARE-protein isoforms. Syntaxin isoforms show distinct patterns of expression in the mouse retina. Syntaxins 1 and 2 are expressed in amacrine cell bodies and in conventional presynaptic terminals of the inner plexiform layer, and are not co-localized. Syntaxin 3 is present in photoreceptor and bipolar ribbon synapses, while syntaxin 4 expression is limited to horizontal cell processes in the ribbon synaptic complexes of photoreceptor terminals (Sherry et al., 2006). Recently, studies on a syntaxin 1A knockout mouse suggested a role for syntaxin in the formation of the outer plexiform layer and inner nuclear layer in retina (Kaneko et al., 2011). However, the neuronal SNARE protein syntaxin 1A has not been immunolocalized in photoreceptor ribbon synapses (Sherry et al., 2006). SNAP-25 expression in mammalian retinal horizontal cells in both inner and outer plexiform layers confirms the presence of a neuronal-type exocytotic machinery in retina (Hirano et al., 2011). Involvement of SNAP-25 in exocytosis at the ribbon synapse of retina is still poorly understood. Complexins 3 and 4 are specifically immunolocalized to retinal ribbon synapses, and are possibly involved in the regulation of exocytosis at the synapse. A complexin 3 plus complexin 4 double mutant shows a disorganized outer plexiform layer, disoriented spherically-shaped ribbons (Reim et al., 2009) and impairment of light perception. Complexin may thus play roles in regulation of retinal exocytosis/neurosecretion as well as the organization of ribbon synapses.
SNAREs and human disorders
Communication within the brain occurs between neurons numbering in the billions. Recent advancement in mapping of the brain points to the roles played by the different brain areas in cognitive functions. Synaptic connections between neurons integrate the nervous system into a complex neural network responsible for information processing, memory storage, learning, and spatial-temporal coordination of all body functions. Synaptic communication occurs via release of neurotransmitters, and impairment in any of the release steps may lead to neurodegenerative diseases (Alzheimer's disease, Parkinsonism), as well as neurodevelopmental (autism) and psychiatric disorders (schizophrenia, depression, bipolar disease). Proteins at the synapse engage in highly dynamic interactions which, when disturbed, can cause hypo- or hyper-activity in neurotransmitter release, leading to dysfunction.
SNARE proteins and other proteins related to exocytosis may exert a direct impact on mental well-being. In fact, many clinical cases have been reported that connect SNARE genes to neural disorders. As we continue to progress in our understanding of how mutations or regulation of genes cause certain diseases, our chance of developing novel methods to remedy such diseases increases. Numerous investigations have been prompted by the analysis of humans with genetic disorders. Genetic knockout of suspected genes in animal models, such as mouse, has also proven to be very useful in understanding the effect of these genes on behavior, in addition to revealing cellular and molecular mechanisms.
Deficiency in SNAP-25 mRNA has been observed in the hippocampus of certain schizophrenic patients (Young et al., 1998). Further, a single-nucleotide polymorphismin SNAP-25 was reported to be related to hyperactivity in autism-spectrum disorders (Guerini et al., 2011). Overexpression of the SNAP-25B isoform, due to a variant promoter, leads to early onset of bipolar disorder (Etain et al., 2010). It has been suggested that formaldehyde inhalation in mice, producing a decrease in hippocampal expression of SNAP-25 and synaptobrevin 2 (but not of syntaxin 1A), leads to learning and memory impairments (Liu et al., 2010). Although it was previously thought that syntaxin 1A expression is unchanged in schizophrenic patients, recent reports suggest that Ser-14-phosphorylated syntaxin 1A is reduced by 25% in schizophrenics, correlating with a corresponding decrease in protein kinase CK2 (Castillo et al., 2010). Over expression of syntaxin 1A has been implicated in high-functioning autism (Nakamura et al., 2008). A recent study in a Japanese population showed that syntaxin 1A expression was reduced in the anterior cingulate gyrus, a region of the brain associated with rational cognitive functions, in autistic patients compared to non-autistic controls (Nakamura et al., 2008). Western blot analysis of tissues of patients with Huntington's disease, an autosomal-dominant neurodegenerative disorder, showed that SNAP-25 and rabphilin-3A expression are remarkably reduced in the Huntington tissues, pointing to a possible impairment of synaptic release in these patients (Smith et al., 2007). In the latter study, expression of other SNARE proteins, such as syntaxin 1A and synaptobrevin 1, was found to be unaffected. Studies also show that expression of complexins 1 and 2, expressed in hippocampal neurons, is decreased in schizophrenic patients. Reduction of mRNA of complexins 1 and 2 in schizophrenics was found to be restricted to select areas of the hippocampus (Begemann et al., 2010). Similarly, in patients with bipolar disorder and unipolar mood disorders, reduction in complexins 1 and 2 was detected in several subsections of hippocampus (Eastwood and Harrison, 2000). Further studies may reveal how a combination of different isoforms and splice variants of SNARE proteins fine-tunes neural and sensorineural functions.
Concluding remarks
Membrane fusion, mediated by SNAREs and resulting in transmitter release, is an important step in cellular communication. Impressive progress has been made in the identification and characterization of SNARE proteins, leading to the conclusion that the SNARE mechanism is probably universal. The diversity of SNARE proteins and SNARE regulatory factors allows cellular communication to meet the spatial-temporal precision, accuracy, and speed required for shaping sensory perception in diverse systems. An in-depth knowledge of SNAREs, related proteins, and the mechanisms of their regulation should yield a better understanding of neuronal and sensory plasticity as well as of disorders arising from defects in cellular communication.
Acknowledgments
This work was supported by grants from the National Institutes of Health (R01 DC000156 to DGD and R01 DC004076 to MJD) and the Hearing Health Foundation, formerly Deafness Research Foundation (to NAR).
Abbreviations
- SNARE
soluble N-ethylmaleimide-sensitive fusion protein attachment protein receptor
- v-SNARE
vesicle SNARE
- t-SNARE
target SNARE
- NSF
N-ethylmaleimide sensitive fusion protein
- SNAP
soluble N-ethylmaleimide sensitive fusion protein attachment protein
- SNAP-25
synaptosomal-associated protein of 25 kDa molecular mass
- SNAP-23
synaptosomal-associated protein of 23 kDa molecular mass
- SM
Sec1/Munc-18 proteins
- DoC2
double C2 domain protein
- RRP
readily-releasable pool of vesicles
- C2
Ca2+ binding motif targeting proteins to cell membranes
- H3
syntaxin SNARE domain
- Habc
syntaxin regulatory domain
- CSP-α
cysteine string protein α
- Hsc70
heat shock 70 kDa protein 8
- SGT
small glutamine-rich tetratricopeptide repeat-containing protein
- BoNT
botulinum neurotoxin
- SNAREpin
SNARE core complex
- Cav1.3
neuroendocrine L-type voltage-gated calcium channel
- PKC
protein kinase C
- TM
transmembrane domain
- TeTx
tetanus toxin
Contributor Information
Neeliyath A. Ramakrishnan, Email: neelramakrishnan@gmail.com.
Marian J. Drescher, Email: mdresche@med.wayne.edu.
Dennis G. Drescher, Email: ddresche@med.wayne.edu.
References
- Arthur CP, Dean C, Pagratis M, Chapman ER, Stowell MH. Loss of synaptotagmin IV results in a reduction in synaptic vesicles and a distortion of the Golgi structure in cultured hippocampal neurons. Neuroscience. 2010;167:135–142. doi: 10.1016/j.neuroscience.2010.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai J, Wang CT, Richards DA, Jackson MB, Chapman ER. Fusion pore dynamics are regulated by synaptotagmin t-SNARE interactions. Neuron. 2004;41:929–942. doi: 10.1016/s0896-6273(04)00117-5. [DOI] [PubMed] [Google Scholar]
- Barclay JW, Craig TJ, Fisher RJ, Ciufo LF, Evans GJ, Morgan A, Burgoyne RD. Phosphorylation of Munc18 by protein kinase C regulates the kinetics of exocytosis. J. Biol. Chem. 2003;278:10538–10545. doi: 10.1074/jbc.M211114200. [DOI] [PubMed] [Google Scholar]
- Beaumont V, Llobet A, Lagnado L. Expansion of calcium microdomains regulates fast exocytosis at a ribbon synapse. Proc. Natl. Acad. Sci. U.S.A. 2005;102:10700–10705. doi: 10.1073/pnas.0501961102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begemann M, Grube S, Papiol S, Malzahn D, Krampe H, Ribbe K, Friedrichs H, Radyushkin KA, El-Kordi A, Benseler F, Hannke K, Sperling S, Schwerdtfeger D, Thanhäuser I, Gerchen MF, Ghorbani M, Gutwinski S, Hilmes C, Leppert R, Ronnenberg A, Sowislo J, Stawicki S, Stödtke M, Szuszies C, Reim K, Riggert J, Eckstein F, Falkai P, Bickeböller H, Nave KA, Brose N, Ehrenreich H. Modification of cognitive performance in schizophrenia by complexin 2 gene polymorphisms. Arch. Gen. Psychiatry. 2010;67:879–888. doi: 10.1001/archgenpsychiatry.2010.107. [DOI] [PubMed] [Google Scholar]
- Bennett MK, Calakos N, Scheller RH. Syntaxin: a synaptic protein implicated in docking of synaptic vesicles at presynaptic active zones. Science. 1992;257:255–259. doi: 10.1126/science.1321498. [DOI] [PubMed] [Google Scholar]
- Blasi J, Binz T, Yamasaki S, Link E, Niemann H, Jahn R. Inhibition of neurotransmitter release by clostridial neurotoxins correlates with specific proteolysis of synaptosomal proteins. J. Physiol. Paris. 1994;88:235–241. doi: 10.1016/0928-4257(94)90086-8. [DOI] [PubMed] [Google Scholar]
- Bracher A, Kadlec J, Betz H, Weissenhorn W. X-ray structure of a neuronal complexin-SNARE complex from squid. J. Biol. Chem. 2002;277:26517–26523. doi: 10.1074/jbc.M203460200. [DOI] [PubMed] [Google Scholar]
- Castillo MA, Ghose S, Tamminga CA, Ulery-Reynolds PG. Deficits in syntaxin 1 phosphorylation in schizophrenia prefrontal cortex. Biol. Psychiatry. 2010;67:208–216. doi: 10.1016/j.biopsych.2009.07.029. [DOI] [PubMed] [Google Scholar]
- Catterall WA. Interactions of presynaptic Ca2+ channels and snare proteins in neurotransmitter release. Ann. N. Y. Acad. Sci. 1999;868:144–159. doi: 10.1111/j.1749-6632.1999.tb11284.x. [DOI] [PubMed] [Google Scholar]
- Chen X, Tomchick DR, Kovrigin E, Arac D, Machius M, Südhof TC, Rizo J. Three-dimensional structure of the complexin/SNARE complex. Neuron. 2002;33:397–409. doi: 10.1016/s0896-6273(02)00583-4. [DOI] [PubMed] [Google Scholar]
- Cho WJ, Lee JS, Jena BP. Conformation states of the neuronal porosome complex. Cell Biol. Int. 2010;34:1129–1132. doi: 10.1042/CBI20100510. [DOI] [PubMed] [Google Scholar]
- Condliffe SB, Corradini I, Pozzi D, Verderio C, Matteoli M. Endogenous SNAP-25 regulates native voltage-gated calcium channels in glutamatergic neurons. J. Biol. Chem. 2010;285:24968–24976. doi: 10.1074/jbc.M110.145813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig TJ, Evans GJ, Morgan A. Physiological regulation of Munc18/nSec1 phosphorylation on serine-313. J. Neurochem. 2003;86:1450–1457. doi: 10.1046/j.1471-4159.2003.01955.x. [DOI] [PubMed] [Google Scholar]
- Curtis L, Datta P, Liu X, Bogdanova N, Heidelberger R, Janz R. Syntaxin 3B is essential for the exocytosis of synaptic vesicles in ribbon synapses of the retina. Neuroscience. 2010;166:832–841. doi: 10.1016/j.neuroscience.2009.12.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai H, Shen N, Arac D, Rizo J. A quaternary SNARE-synaptotagmin-Ca2+- phospholipid complex in neurotransmitter release. J. Mol. Biol. 2007;367:848–863. doi: 10.1016/j.jmb.2007.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darios F, Wasser C, Shakirzyanova A, Giniatullin A, Goodman K, Munoz-Bravo JL, Raingo J, Jorgacevski J, Kreft M, Zorec R, Rosa JM, Gandia L, Gutiérrez LM, Binz T, Giniatullin R, Kavalali ET, Davletov B. Sphingosine facilitates SNARE complex assembly and activates synaptic vesicle exocytosis. Neuron. 2009;62:683–694. doi: 10.1016/j.neuron.2009.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Robertis E, Franchi CM. Electron microscope observations on synaptic vesicles in synapses of the retinal rods and cones. J. Biophys. Biochem. Cytol. 1956;2:307–318. doi: 10.1083/jcb.2.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries KJ, Geijtenbeek A, Brian EC, de Graan PN, Ghijsen WE, Verhage M. Dynamics of munc18-1 phosphorylation/ dephosphorylation in rat brain nerve terminals. Eur. J. Neurosci. 2000;12:385–390. doi: 10.1046/j.1460-9568.2000.00931.x. [DOI] [PubMed] [Google Scholar]
- Di Carlo V. Ultrastructure of the membrane of synaptic vesicles. Nature. 1967;213:833–835. doi: 10.1038/213833b0. [DOI] [PubMed] [Google Scholar]
- Dresbach T, Qualmann B, Kessels MM, Garner CC, Gundelfinger ED. The presynaptic cytomatrix of brain synapses. Cell. Mol. Life Sci. 2001;58:94–116. doi: 10.1007/PL00000781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drescher DG, Cho WJ, Drescher MJ. Identification of the porosome complex in the hair cell. Cell Biol. Int. Rep. 2011;18:31–34. doi: 10.1042/CBR20110005. (art:e00012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulubova I, Sugita S, Hill S, Hosaka M, Fernandez I, Südhof TC, Rizob J. A conformational switch in syntaxin during exocytosis: role of Munc18. EMBO J. 1999;18:4372–4382. doi: 10.1093/emboj/18.16.4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastwood SL, Harrison PJ. Hippocampal synaptic pathology in schizophrenia, bipolar disorder and major depression: a study of complexin mRNAs. Mol. Psychiatry. 2000;5:425–432. doi: 10.1038/sj.mp.4000741. [DOI] [PubMed] [Google Scholar]
- Etain B, Dumaine A, Mathieu F, Chevalier F, Henry C, Kahn JP, Deshommes J, Bellivier F, Leboyer M, Jamain S. A SNAP25 promoter variant is associated with early-onset bipolar disorder and a high expression level in brain. Mol. Psychiatry. 2010;15:748–755. doi: 10.1038/mp.2008.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Q, Berberian K, Gong L, Hafez I, Sorensen JB, Lindau M. The role of the C terminus of the SNARE protein SNAP-25 in fusion pore opening and a model for fusion pore mechanics. Proc. Natl. Acad. Sci. U.S.A. 2008;105:15388–15392. doi: 10.1073/pnas.0805377105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasshauer D, Eliason WK, Brunger AT, Jahn R. Identification of a minimal core of the synaptic SNARE complex sufficient for reversible assembly and disassembly. Biochemistry. 1998;37:10354–10362. doi: 10.1021/bi980542h. [DOI] [PubMed] [Google Scholar]
- Fernández-Chacón R, Königstorfer A, Gerber SH, García J, Matos MF, Stevens CF, Brose N, Rizo J, Rosenmund C, Südhof TC. Synaptotagmin I functions as a calcium regulator of release probability. Nature. 2001;410:41–49. doi: 10.1038/35065004. [DOI] [PubMed] [Google Scholar]
- Fesce R, Grohvaz F, Valtorta F, Meldolesi J. Neurotransmitter release: fusion or ‘kiss-and-run’? Trends Cell Biol. 1994;4:1–4. doi: 10.1016/0962-8924(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Garcia EP, McPherson PS, Chilcote TJ, Takei K, De Camilli P. rbSec1A and B colocalize with syntaxin 1 and SNAP-25 throughout the axon, but are not in a stable complex with syntaxin. J. Cell Biol. 1995;129:105–120. doi: 10.1083/jcb.129.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraudo CG, Eng WS, Melia TJ, Rothman JE. A clamping mechanism involved in SNARE-dependent exocytosis. Science. 2006;313:676–680. doi: 10.1126/science.1129450. [DOI] [PubMed] [Google Scholar]
- Gonzalo S, Greentree WK, Linder ME. SNAP-25 is targeted to the plasma membrane through a novel membrane-binding domain. J. Biol. Chem. 1999;274:21313–21318. doi: 10.1074/jbc.274.30.21313. [DOI] [PubMed] [Google Scholar]
- Goodyear RJ, Legan PK, Christiansen JR, Xia B, Korchagina J, Gale JE, Warchol ME, Corwin JT, Richardson GP. Identification of the hair cell soma-1 antigen, HCS-1, as otoferlin. J. Assoc. Res. Otolaryngol. 2010;11:573–586. doi: 10.1007/s10162-010-0231-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracheva EO, Maryon EB, Berthelot-Grosjean M, Richmond JE. Differential regulation of synaptic vesicle tethering and docking by UNC-18 and TOM-1. Front. Synaptic Neurosci. 2010;2:1–12. doi: 10.3389/fnsyn.2010.00141. (Art. 141) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graydon CW, Cho S, Li GL, Kachar B, von Gersdorff H. Sharp Ca2+ nanodomains beneath the ribbon promote highly synchronous multivesicular release at hair cell synapses. J. Neurosci. 2011;31:16637–16650. doi: 10.1523/JNEUROSCI.1866-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griesinger CB, Richards CD, Ashmore JF. Fast vesicle replenishment allows indefatigable signalling at the first auditory synapse. Nature. 2005;435:212–215. doi: 10.1038/nature03567. [DOI] [PubMed] [Google Scholar]
- Groffen AJ, Martens S, Díez Arazola R, Cornelisse LN, Lozovaya N, de Jong AP, Goriounova NA, Habets RL, Takai Y, Borst JG, Brose N, McMahon HT, Verhage M. Doc2b is a high-affinity Ca2+ sensor for spontaneous neurotransmitter release. Science. 2010;327:1614–1618. doi: 10.1126/science.1183765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerini FR, Bolognesi E, Chiappedi M, Manca S, Ghezzo A, Agliardi C, Sotgiu S, Usai S, Matteoli M, Clerici M. SNAP-25 single nucleotide polymorphisms are associated with hyperactivity in autism spectrum disorders. Pharmacol. Res. 2011;64:283–288. doi: 10.1016/j.phrs.2011.03.015. [DOI] [PubMed] [Google Scholar]
- Han X, Jackson MB. Structural transitions in the synaptic SNARE complex during Ca2+-triggered exocytosis. J. Cell Biol. 2006;172:281–293. doi: 10.1083/jcb.200510012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Wang CT, Bai J, Chapman ER, Jackson MB. Transmembrane segments of syntaxin line the fusion pore of Ca2+-triggered exocytosis. Science. 2004;304:289–292. doi: 10.1126/science.1095801. [DOI] [PubMed] [Google Scholar]
- Hanson PI, Roth R, Morisaki H, Jahn R, Heuser JE. Structure and conformational changes in NSF and its membrane receptor complexes visualized by quick-freeze/deep-etch electron microscopy. Cell. 1997;90:523–535. doi: 10.1016/s0092-8674(00)80512-7. [DOI] [PubMed] [Google Scholar]
- Harlow LH, Ress D, Stoschek A, Marshall RM, McMahan UJ. The architecture of active zone material at the frog's neuromuscular junction. Nature. 2001;409:479–484. doi: 10.1038/35054000. [DOI] [PubMed] [Google Scholar]
- Haucke V, Neher E, Sigrist SJ. Protein scaffolds in the coupling of synaptic exocytosis and endocytosis. Nat. Rev. Neurosci. 2011;12:127–138. doi: 10.1038/nrn2948. [DOI] [PubMed] [Google Scholar]
- Hirano AA, Brandstätter JH, Morgans CW, Brecha NC. SNAP25 expression in mammalian retinal horizontal cells. J. Comp. Neurol. 2011;519:972–988. doi: 10.1002/cne.22562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoi N, Sakaba T, Neher E. Quantitative analysis of calcium-dependent vesicle recruitment and its functional role at the calyx of Held synapse. J. Neurosci. 2007;27:14286–14298. doi: 10.1523/JNEUROSCI.4122-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua Y, Scheller RH. Three SNARE complexes cooperate to mediate membrane fusion. Proc. Natl. Acad. Sci. U.S.A. 2001;98:8065–8070. doi: 10.1073/pnas.131214798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui E, Gaffaney JD, Wang Z, Johnson CP, Evans CS, Chapman ER. Mechanism and function of synaptotagmin-mediated membrane apposition. Nat. Struct. Mol. Biol. 2011;18:813–821. doi: 10.1038/nsmb.2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn R, Lang T, Südhof TC. Membrane fusion. Cell. 2003;112:519–533. doi: 10.1016/s0092-8674(03)00112-0. [DOI] [PubMed] [Google Scholar]
- Jena BP. Membrane fusion: Role of SNAREs and calcium. Protein Pept. Res. 2009a;16:712–717. doi: 10.2174/092986609788681869. [DOI] [PubMed] [Google Scholar]
- Jena BP. Porosome: The secretory portal in cells. Biochemistry. 2009b;48:4009–4018. doi: 10.1021/bi9002698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CP, Chapman ER. Otoferlin is a calcium sensor that directly regulates SNARE-mediated membrane fusion. J. Cell Biol. 2010;191:187–197. doi: 10.1083/jcb.201002089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Franz C, Kuhn S, Furness DN, Rüttiger L, Münkner S, Rivolta MN, Seward EP, Herschman HR, Engel J, Knipper M, Marcotti W. Synaptotagmin IV determines the linear Ca2+ dependence of vesicle fusion at auditory ribbon synapses. Nat. Neurosci. 2010;13:45–52. doi: 10.1038/nn.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko Y, Suge R, Fujiwara T, Akagawa K, Watanabe S. Unusual retinal layer organization in HPC-1/syntaxin 1A knockout mice. J. Mol. Histol. 2011;42:483–489. doi: 10.1007/s10735-011-9346-2. [DOI] [PubMed] [Google Scholar]
- Kantardzhieva A, Peppi M, Lane WS, Sewell WF. Protein composition of immunoprecipitated synaptic ribbons. J. Proteome Res. 2012;11:1163–1174. doi: 10.1021/pr2008972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kee Y, Lin RC, Hsu SC, Scheller RH. Distinct domains of syntaxin are required for synaptic vesicle fusion complex formation and dissociation. Neuron. 1995;14:991–998. doi: 10.1016/0896-6273(95)90337-2. [DOI] [PubMed] [Google Scholar]
- Khimich D, Nouvian R, Pujol R, Tom Dieck S, Egner A, Gundelfinger ED, Moser T. Hair cell synaptic ribbons are essential for synchronous auditory signalling. Nature. 2005;434:889–894. doi: 10.1038/nature03418. [DOI] [PubMed] [Google Scholar]
- Kollmar R, Montgomery LG, Fak J, Henry LJ, Hudspeth AJ. Predominance of the alpha1D subunit in L-type voltage-gated Ca2+ channels of hair cells in the chicken's cochlea. Proc. Natl. Acad. Sci. U.S.A. 1997;94:14883–14888. doi: 10.1073/pnas.94.26.14883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreft M, Krizaj D, Grilc S, Zorec R. Properties of exocytotic response in vertebrate photoreceptors. J. Neurophysiol. 2003;90:218–225. doi: 10.1152/jn.01025.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnakumar SS, Radoff DT, Kümmel D, Giraudo CG, Li F, Khandan L, Baguley SW, Coleman J, Reinisch KM, Pincet F, Rothman JE. A conformational switch in complexin is required for synaptotagmin to trigger synaptic fusion. Nat. Struct. Mol. Biol. 2011;18:934–940. doi: 10.1038/nsmb.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo W, Herrick DZ, Cafiso DS. Phosphatidylinositol 4,5-bisphosphate alters synaptotagmin 1 membrane docking and drives opposing bilayers closer together. Biochemistry. 2011;50:2633–2641. doi: 10.1021/bi200049c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Brecha NC. Immunocytochemical evidence for SNARE protein-dependent transmitter release from guinea pig horizontal cells. Eur. J. Neurosci. 2010;31:1388–1401. doi: 10.1111/j.1460-9568.2010.07181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Pincet F, Perez E, Eng WS, Melia TJ, Rothman JE, Tareste D. Energetics and dynamics of SNAREpin folding across lipid bilayers. Nat. Struct. Mol. Biol. 2007;14:890–896. doi: 10.1038/nsmb1310. [DOI] [PubMed] [Google Scholar]
- Liu Y, Ye Z, Yang H, Zhou L, Fan D, He S, Chui D. Disturbances of soluble Nethylmaleimide- sensitive factor attachment proteins in hippocampal synaptosomes contribute to cognitive impairment after repetitive formaldehyde inhalation in male rats. Neuroscience. 2010;169:1248–1254. doi: 10.1016/j.neuroscience.2010.05.061. [DOI] [PubMed] [Google Scholar]
- Lou X, Scheuss V, Schneggenburger R. Allosteric modulation of the presynaptic Ca2+ sensor for vesicle fusion. Nature. 2005;435:497–501. doi: 10.1038/nature03568. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Madison DV, Nicoll RA. Potentiation of synaptic transmission in the hippocampus by phorbol esters. Nature. 1986;321:175–177. doi: 10.1038/321175a0. [DOI] [PubMed] [Google Scholar]
- Martin JA, Hu Z, Fenz KM, Fernandez J, Dittman JS. Complexin has opposite effects on two modes of synaptic vesicle fusion. Curr. Biol. 2011;21:97–105. doi: 10.1016/j.cub.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews G, Fuchs P. The diverse roles of ribbon synapses in sensory neurotransmission. Nat. Rev. Neurosci. 2010;11:812–822. doi: 10.1038/nrn2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maximov A, Tang J, Yang X, Pang ZP, Südhof TC. Complexin controls the force transfer from SNARE complexes to membranes in fusion. Science. 2009;323:516–521. doi: 10.1126/science.1166505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer AJ, Chen M, Thoreson WB. Lateral mobility of presynaptic L-type calcium channels at photoreceptor ribbon synapses. J Neurosci. 2011;31:4397–4406. doi: 10.1523/JNEUROSCI.5921-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon HT, Südhof TC. Synaptic core complex of synaptobrevin, syntaxin, and SNAP25 forms high affinity α-SNAP binding site. J. Biol. Chem. 1995;270:2213–2217. doi: 10.1074/jbc.270.5.2213. [DOI] [PubMed] [Google Scholar]
- Mohrmann R, deWit H, Verhage M, Neher E, Sorensen JB. Fast vesicle fusion in living cells requires at least three SNARE complexes. Science. 2010;330:502–505. doi: 10.1126/science.1193134. [DOI] [PubMed] [Google Scholar]
- Morgans CW, Brandstätter JH, Kellerman J, Betz H, Wässle H. A SNARE complex containing syntaxin 3 is present in ribbon synapses of the retina. J. Neurosci. 1996;16:6713–6721. doi: 10.1523/JNEUROSCI.16-21-06713.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Anitha A, Yamada K, Tsujii M, Iwayama Y, Hattori E, Toyota T, Suda S, Takei N, Iwata Y, Suzuki K, Matsuzaki H, Kawai M, Sekine Y, Tsuchiya KJ, Sugihara G, Ouchi Y, Sugiyama T, Yoshikawa T, Mori N. Genetic and expression analyses reveal elevated expression of syntaxin 1A (STX1A) in high functioning autism. Int. J. Neuropsychopharmacol. 2008;11:1073–1084. doi: 10.1017/S1461145708009036. [DOI] [PubMed] [Google Scholar]
- Neher E, Sakaba T. Multiple roles of calcium ions in the regulation of neurotransmitter release. Neuron. 2008;59:861–872. doi: 10.1016/j.neuron.2008.08.019. [DOI] [PubMed] [Google Scholar]
- Ngatchou AN, Kisler K, Fang Q, Walter AM, Zhao Y, Bruns D, Sørensen JB, Lindau M. Role of the synaptobrevin C terminus in fusion pore formation. Proc. Natl. Acad. Sci. U.S.A. 2010;107:18463–18468. doi: 10.1073/pnas.1006727107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouvian R, Neef J, Bulankina AV, Reisinger E, Pangršič T, Frank T, Sikorra S, Brose N, Binz T, Moser T. Exocytosis at the hair cell ribbon synapse apparently operates without neuronal SNARE proteins. Nat. Neurosci. 2011;14:411–413. doi: 10.1038/nn.2774. [DOI] [PubMed] [Google Scholar]
- Oyler GA, Higgins GA, Hart RA, Battenberg E, Billingsley M, Bloom FE, Wilson MC. The identification of a novel synaptosomal-associated protein, SNAP-25, differentially expressed by neuronal subpopulations. J. Cell Biol. 1989;109:3039–3052. doi: 10.1083/jcb.109.6.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan PY, Tian JH, Sheng ZH. Snapin facilitates the synchronization of synaptic vesicle fusion. Neuron. 2009;61:412–424. doi: 10.1016/j.neuron.2008.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangrsic T, Lasarow L, Reuter K, Takago H, Schwander M, Riedel D, Frank T, Tarantino LM, Bailey JS, Strenzke N, Brose N, Müller U, Reisinger E, Moser T. Hearing requires otoferlin-dependent efficient replenishment of synaptic vesicles in hair cells. Nat. Neurosci. 2010;13:869–876. doi: 10.1038/nn.2578. [DOI] [PubMed] [Google Scholar]
- Parsons TD, Lenzi D, Almers W, Roberts WM. Calcium-triggered exocytosis and endocytosis in an isolated presynaptic cell: capacitance measurements in saccular hair cells. Neuron. 1994;13:875–883. doi: 10.1016/0896-6273(94)90253-4. [DOI] [PubMed] [Google Scholar]
- Pavlos NJ, Jahn R. Distinct yet overlapping roles of Rab GTPases on synaptic vesicles. Small GTPases. 2011;2:77–81. doi: 10.4161/sgtp.2.2.15201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platzer J, Engel J, Schrott-Fischer A, Stephan K, Bova S, Chen H, Zheng H, Striessnig J. Congenital deafness and sinoatrial node dysfunction in mice lacking class D L-type Ca2+ channels. Cell. 2000;102:89–97. doi: 10.1016/s0092-8674(00)00013-1. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan NA, Green GE, Pasha R, Drescher MJ, Swanson GS, Perin P, Lakhani RS, Ahsan SF, Hatfield JS, Khan KM, Drescher DG. Voltage-gated Ca2+ channel CaV1.3 subunit expressed in the hair cell epithelium of the sacculus of the trout Oncorhynchus mykiss: cloning and comparison across vertebrate classes. Mol. Brain Res. 2002;109:69–83. doi: 10.1016/s0169-328x(02)00522-3. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan NA, Drescher MJ, Drescher DG. Direct interaction of otoferlin with syntaxin 1A, SNAP-25, and the L-type voltage-gated calcium channel Cav1.3. J. Biol. Chem. 2009;284:1364–1372. doi: 10.1074/jbc.M803605200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reim K, Wegmeyer H, Brandstätter JH, Xue M, Rosenmund C, Dresbach T, Hofmann K, Brose N. Structurally and functionally unique complexins at retinal ribbon synapses. J. Cell Biol. 2005;169:669–680. doi: 10.1083/jcb.200502115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reim K, Regus-Leidig H, Ammermüller J, El-Kordi A, Radyushkin K, Ehrenreich H, Brandstätter JH, Brose N. Aberrant function and structure of retinal ribbon synapses in the absence of complexin 3 and complexin 4. J. Cell Sci. 2009;122:1352–1361. doi: 10.1242/jcs.045401. [DOI] [PubMed] [Google Scholar]
- Reisinger E, Bresee C, Neef J, Nair R, Reuter K, Bulankina A, Nouvian R, Koch M, Bückers J, Kastrup L, Roux I, Petit C, Hell SW, Brose N, Rhee JS, Kügler S, Brigande JV, Moser T. Probing the functional equivalence of otoferlin and synaptotagmin 1 in exocytosis. J. Neurosci. 2011;31:4886–4895. doi: 10.1523/JNEUROSCI.5122-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzoli SO, Betz WJ. The structural organization of the readily releasable pool of synaptic vesicles. Science. 2004;303:2037–2039. doi: 10.1126/science.1094682. [DOI] [PubMed] [Google Scholar]
- Rizzoli SO, Betz WJ. Synaptic vesicle pools. Nat. Rev. Neurosci. 2005;6:57–69. doi: 10.1038/nrn1583. [DOI] [PubMed] [Google Scholar]
- Roux I, Safieddine S, Nouvian R, Grati M, Simmler MC, Bahloul A, Perfettini I, Le Gall M, Rostaing P, Hamard G, Triller A, Avan P, Moser T, Petit C. Otoferlin, defective in a human deafness form, is essential for exocytosis at the auditory ribbon synapse. Cell. 2006;127:277–289. doi: 10.1016/j.cell.2006.08.040. [DOI] [PubMed] [Google Scholar]
- Sabatini BL, Regehr WG. Timing of synaptic transmission. Annu. Rev. Physiol. 1999;61:521–542. doi: 10.1146/annurev.physiol.61.1.521. [DOI] [PubMed] [Google Scholar]
- Safieddine S, Wenthold RJ. SNARE complex at the ribbon synapses of cochlear hair cells: analysis of synaptic vesicle- and synaptic membrane-associated proteins. Eur. J. Neurosci. 1999;11:803–812. doi: 10.1046/j.1460-9568.1999.00487.x. [DOI] [PubMed] [Google Scholar]
- Schmitt RO, Dev P, Smith BH. Electrotonic processing of information by brain cells. Science. 1976;193:114–120. doi: 10.1126/science.180598. [DOI] [PubMed] [Google Scholar]
- Schnee ME, Lawton DM, Furness DN, Benke TA, Ricci AJ. Auditory hair cell-afferent fiber synapses are specialized to operate at their best frequencies. Neuron. 2005;47:243–254. doi: 10.1016/j.neuron.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Schnee ME, Santos-Sacchi J, Castellano-Muñoz M, Kong JH, Ricci AJ. Calcium-dependent synaptic vesicle trafficking underlies indefatigable release at the hair cell afferent fiber synapse. Neuron. 2011;70:326–338. doi: 10.1016/j.neuron.2011.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch S, Deak F, Konigstorfer A, Mozhayeva M, Sara Y, Südhof TC, Kavalali ET. SNARE function analyzed in synaptobrevin/VAMP knockout mice. Science. 2001;294:1117–1122. doi: 10.1126/science.1064335. [DOI] [PubMed] [Google Scholar]
- Sharma M, Burré J, Südhof TC. CSPα promotes SNARE-complex assembly by chaperoning SNAP-25 during synaptic activity. Nat. Cell Biol. 2011;13:30–39. doi: 10.1038/ncb2131. [DOI] [PubMed] [Google Scholar]
- Sheng ZH, Yokoyama CT, Catterall WA. Interaction of the synprint site of N-type Ca2+ channels with the C2B domain of synaptotagmin I. Proc. Natl. Acad. Sci. U.S.A. 1997;94:5405–5410. doi: 10.1073/pnas.94.10.5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherry DM, Mitchell R, Standifer KM, du Plessis B. Distribution of plasma membrane-associated syntaxins 1 through 4 indicates distinct trafficking functions in the synaptic layers of the mouse retina. BMC Neurosci. 2006;7:54. doi: 10.1186/1471-2202-7-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Kümmel D, Coleman J, Melia TJ, Giraudo CG. Dual roles of Munc18-1 rely on distinct binding modes of the central cavity with Stx1A and SNARE complex. Mol. Biol. Cell. 2011;22:4150–4160. doi: 10.1091/mbc.E11-02-0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Shen QT, Kiel A, Wang J, Wang HW, Melia TJ, Rothman JE, Pincet F. SNARE proteins: one to fuse and three to keep the nascent fusion pore open. Science. 2012;335:1355–1359. doi: 10.1126/science.1214984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siksou L, Rostaing P, Lechaire JP, Boudier T, Ohsuka T, Fejtová A, Kao HT, Greengard P, Gundelfinger ED, Triller A, Marty S. Three-dimensional architecture of presynaptic terminal cytomatrix. J. Neurosci. 2007;27:6868–6877. doi: 10.1523/JNEUROSCI.1773-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Ahmed S, Jahn R, Klingauf J. Two synaptobrevin molecules are sufficient for vesicle fusion in central nervous system synapses. Proc. Natl. Acad. Sci. U.S.A. 2011;108:14318–14323. doi: 10.1073/pnas.1101818108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R, Klein P, Koc-Schmitz Y, Waldvogel HJ, Faull RL, Brundin P, Plomann M, Li JY. Loss of SNAP-25 and rabphilin 3a in sensory-motor cortex in Huntington's disease. J. Neurochem. 2007;103:115–123. doi: 10.1111/j.1471-4159.2007.04703.x. [DOI] [PubMed] [Google Scholar]
- Smyth AM, Duncan RR, Rickman C. Munc18-1 and syntaxin1: unraveling the interactions between the dynamic duo. Cell. Mol. Neurobiol. 2010;30:1309–1313. doi: 10.1007/s10571-010-9581-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollner T, Whiteheart SW, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P, Rothman JE. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- Sørensen JB, Matti U, Wei SH, Nehring RB, Voets T, Ashery U, Binz T, Neher E, Rettig J. The SNARE protein SNAP-25 is linked to fast calcium triggering of exocytosis. Proc. Natl. Acad. Sci. U.S.A. 2002;99:1627–1632. doi: 10.1073/pnas.251673298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein A, Weber G, Wahl MC, Jahn R. Helical extension of the neuronal SNARE complex into the membrane. Nature. 2009;460:525–528. doi: 10.1038/nature08156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelzer W, Poschner BC, Stalz H, Heck AJ, Langosch D. Sequence-specific conformational flexibility of SNARE transmembrane helices probed by hydrogen/ deuterium exchange. Biophys. J. 2008;95:1326–1335. doi: 10.1529/biophysj.108.132928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strenzke N, Chanda S, Kopp-Sscheinpflug C, Khimich D, Reim K, Bulankina AV, Neef A, Wolf F, Brose N, Xu-Friedman MA, Moser T. Complexin-I is required for high-fidelity transmission at the endbulb of held auditory synapse. J. Neurosci. 2009;24:7991–8004. doi: 10.1523/JNEUROSCI.0632-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof TC, Rothman JE. Membrane fusion: grappling with SNARE and SM proteins. Science. 2009;323:474–477. doi: 10.1126/science.1161748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh YH, Terashima A, Petralia RS, Wenthold RJ, Isaac JT, Roche KW, Roche PA. A neuronal role for SNAP-23 in postsynaptic glutamate receptor trafficking. Nat. Neurosci. 2010;13:338–343. doi: 10.1038/nn.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton RB, Fasshauer D, Jahn R, Brünger AT. Crystal structure of a SNARE complex involved in synaptic exocytosis at 2.4 A resolution. Nature. 1998;395:347–353. doi: 10.1038/26412. [DOI] [PubMed] [Google Scholar]
- Takamori S, Holt M, Stenius K, Lemke EA, Grønborg M, Riedel D, Urlaub H, Schenck S, Brügger B, Ringler P, Müller SA, Rammner B, Gräter F, Hub JS, De Groot BL, Mieskes G, Moriyama Y, Klingauf J, Grubmüller H, Heuser J, Wieland F, Jahn R. Molecular anatomy of a trafficking organelle. Cell. 2006;127:831–846. doi: 10.1016/j.cell.2006.10.030. [DOI] [PubMed] [Google Scholar]
- Trimble WS, Cowan DM, Scheller RH. VAMP-1: a synaptic vesicle-associated integral membrane protein. Proc. Natl. Acad. Sci. U.S.A. 1988;85:4538–4542. doi: 10.1073/pnas.85.12.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uthaiah RC, Hudspeth AJ. Molecular anatomy of the hair cell's ribbon synapse. J. Neurosci. 2010;30:12387–12399. doi: 10.1523/JNEUROSCI.1014-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bogaart G, Thutupalli S, Risselada JH, Meyenberg K, Holt M, Riedel D, Diederichsen U, Herminghaus S, Grubmüller H, Jahn R. Synaptotagmin-1 may be a distance regulator acting upstream of SNARE nucleation. Nat. Struct. Mol. Biol. 2011;18:805–812. doi: 10.1038/nsmb.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhage M, Maia AS, Plomp JJ, Brussaard AB, Heeroma JH, Vermeer H, Toonen RF, Hammer RE, van den Berg TK, Missler M. Synaptic assembly of the brain in the absence of neurotransmitter secretion. Science. 2000;287:864–869. doi: 10.1126/science.287.5454.864. [DOI] [PubMed] [Google Scholar]
- Wadel K, Neher E, Sakaba T. The coupling between synaptic vesicles and Ca2+ channels determines fast neurotransmitter release. Neuron. 2007;53:563–575. doi: 10.1016/j.neuron.2007.01.021. [DOI] [PubMed] [Google Scholar]
- Walrond JP, Reese TS. Structure of axon terminals and active zones at synapses on lizard twitch and tonic muscle fibers. J. Neurosci. 1985;5:1118–1131. doi: 10.1523/JNEUROSCI.05-05-01118.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Zhang Z, Dong M, Sun S, Chapman ER, Jackson MB. Syntaxin requirement for Ca2+-triggered exocytosis in neurons and endocrine cells demonstrated with an engineered neurotoxin. Biochemistry. 2011;50:2711–2713. doi: 10.1021/bi200290p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washbourne P, Thompson PM, Carta M, Costa ET, Mathews JR, Lopez-Benditó G, Molnár Z, Becher MW, Valenzuela CF, Partridge LD, Wilson MC. Genetic ablation of the t-SNARE SNAP-25 distinguishes mechanisms of neuroexocytosis. Nat. Neurosci. 2002;5:19–26. doi: 10.1038/nn783. [DOI] [PubMed] [Google Scholar]
- Weber T, Zemelman BV, McNew JA, Westermann B, Gmachl M, Parlati F, Söllner TH, Rothman JE. SNAREpins: Minimal machinery for membrane fusion. Cell. 1998;92:759–772. doi: 10.1016/s0092-8674(00)81404-x. [DOI] [PubMed] [Google Scholar]
- Weber JP, Reim K, Sørensen JB. Opposing functions of two sub-domains of the SNARE-complex in neurotransmission. EMBO. J. 2010;29:2477–2490. doi: 10.1038/emboj.2010.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte JRC, Munro S. Vesicle tethering complexes in membrane traffic. J. Cell Sci. 2002;115:2627–2637. doi: 10.1242/jcs.115.13.2627. [DOI] [PubMed] [Google Scholar]
- Wierda KD, Toonen RF, de Wit H, Brussaard AB, Verhage M. Interdependence of PKC-dependent and PKC-independent pathways for presynaptic plasticity. Neuron. 2007;54:275–290. doi: 10.1016/j.neuron.2007.04.001. [DOI] [PubMed] [Google Scholar]
- Wiser O, Bennett MK, Atlas D. Functional interaction of syntaxin and SNAP-25 with voltage-sensitive L- and N-type Ca2+ channels. EMBO J. 1996;15:4100–4110. [PMC free article] [PubMed] [Google Scholar]
- Wong JL, Koppel DE, Cowan AE, Wessel GM. Membrane hemifusion is a stable intermediate of exocytosis. Dev. Cell. 2007;12:653–659. doi: 10.1016/j.devcel.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MN, Fergestad T, Lloyd TE, He Y, Broadie K, Bellen HJ. Syntaxin 1A interacts with multiple exocytotic proteins to regulate neurotransmitter release in vivo . Neuron. 1999;23:593–605. doi: 10.1016/s0896-6273(00)80811-9. [DOI] [PubMed] [Google Scholar]
- Xue M, Craig TK, Xu J, Chao HT, Rizo J, Rosenmund C. Binding of the complexin N terminus to the SNARE complex potentiates synaptic-vesicle fusogenicity. Nat. Struct. Mol. Biol. 2010;17:568–575. doi: 10.1038/nsmb.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Kaeser-Woo YJ, Pang ZP, Xu W, Südhof TC. Complexin clamps asynchronous release by blocking a secondary Ca2+ sensor via its accessory α helix. Neuron. 2010;68:907–920. doi: 10.1016/j.neuron.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara M, Guan Z, Littleton JT. Differential regulation of synchronous versus asynchronous neurotransmitter release by the C2 domains of synaptotagmin 1. Proc. Natl. Acad. Sci. U.S.A. 2010;107:14869–14874. doi: 10.1073/pnas.1000606107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SM, Jr, Neher E. Synaptotagmin has an essential function in synaptic vesicle positioning for synchronous release in addition to its role as a calcium sensor. Neuron. 2009;63:482–496. doi: 10.1016/j.neuron.2009.07.028. [DOI] [PubMed] [Google Scholar]
- Young CE, Arima K, Xie J, Hu L, Beach TG, Falkai P, Honer WG. SNAP-25 deficit and hippocampal connectivity in schizophrenia. Cereb. Cortex. 1998;8:261–268. doi: 10.1093/cercor/8.3.261. [DOI] [PubMed] [Google Scholar]
- Zampini V, Johnson SL, Franz C, Lawrence ND, Münkner S, Engel J, Knipper M, Magistretti J, Masetto S, Marcotti W. Elementary properties of CaV1.3 Ca2+ channels expressed in mouse cochlear inner hair cells. J. Physiol. 2010;588:187–199. doi: 10.1113/jphysiol.2009.181917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Kim-Miller MJ, Fukuda M, Kowalchyk JA, Martin TF. Ca2+-dependent synaptotagmin binding to SNAP-25 is essential for Ca2+-triggered exocytosis. Neuron. 2002;34:599–611. doi: 10.1016/s0896-6273(02)00671-2. [DOI] [PubMed] [Google Scholar]
- Zhao C, Slevin JT, Whiteheart SW. Cellular functions of NSF: not just SNAPs and SNAREs. FEBS Lett. 2007;581:2140–2149. doi: 10.1016/j.febslet.2007.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]