Abstract
Background
Sepsis-induced organ failure is the major cause of death in critical care units, and is characterized by a massive dysregulated inflammatory response and oxidative stress. We investigated the effects of treatment with antioxidants that protect mitochondria (MitoQ, MitoE, or melatonin) in a rat model of lipopolysaccharide (LPS) plus peptidoglycan (PepG)-induced acute sepsis, characterized by inflammation, mitochondrial dysfunction and early organ damage.
Methods
Anaesthetized and ventilated rats received an i.v. bolus of LPS and PepG followed by an i.v. infusion of MitoQ, MitoE, melatonin, or saline for 5 h. Organs and blood were then removed for determination of mitochondrial and organ function, oxidative stress, and key cytokines.
Results
MitoQ, MitoE, or melatonin had broadly similar protective effects with improved mitochondrial respiration (P<0.002), reduced oxidative stress (P<0.02), and decreased interleukin-6 levels (P=0.0001). Compared with control rats, antioxidant-treated rats had lower levels of biochemical markers of organ dysfunction, including plasma alanine amino-transferase activity (P=0.02) and creatinine concentrations (P<0.0001).
Conclusions
Antioxidants that act preferentially in mitochondria reduce mitochondrial damage and organ dysfunction and decrease inflammatory responses in a rat model of acute sepsis.
Keywords: co-enzyme Q10, interleukin-6, interleukin-10, melatonin, sepsis, tocopherol
Editor's key points.
Oxidative stress and mitochondrial dysfunction are key to the pathophysiology of sepsis.
The effects of antioxidants targeted to mitochondria on inflammation, oxidative stress, and organ dysfunction were tested in a rat model of acute sepsis.
Antioxidant treatment reduced mitochondrial damage, sepsis-induced inflammation, and organ dysfunction, a positive finding that should be tested in clinical trials.
Although outcomes have improved, the overall mortality of sepsis remains at 31% overall, and over 70% in those patients who go on to develop sepsis-induced multiple organ failure.1 The hallmark of sepsis is a dysregulated and overwhelming inflammatory response, characterized by massive cytokine release, oxidative stress, and mitochondrial dysfunction. Mitochondria are both sites of reactive oxygen species (ROS) production, and targets of ROS-mediated damage, resulting in mitochondrial dysfunction.2,3 During sepsis, antioxidant defences are overwhelmed, and ROS cause cellular damage, contributing to organ dysfunction.4,5
Antioxidants acting specifically in mitochondria might be beneficial in combating the inflammation and oxidative stress seen in sepsis.6,7 MitoQ and MitoE are antioxidants attached to a lipophilic cation that accumulate several hundred-fold within mitochondria due to the negative charge inside mitochondria, delivering ubiquinol or tocopherol, respectively.8 Endogenous melatonin is primarily recognized for regulation of the sleep–wake cycle, but at high doses, it has potent antioxidant activity9 and is able to enter all cell compartments including mitochondria. The metabolites of melatonin and products from its reactions with oxidant species also have antioxidant activity.10,11
We have reported previously that in endothelial cells treated with lipopolysaccharide (LPS) and peptidoglycan G (PepG) to mimic the cellular responses seen during sepsis, MitoQ and MitoE decreased cytokine responses.12,13 Melatonin and its main metabolite 6-hydroxymelatonin also reduced cytokine responses, prevented mitochondrial dysfunction, and protected endogenous antioxidants in the same model.11 We have also shown in a small pilot study that MitoQ administration in rats given LPS/PepG appeared protective.12 We hypothesized that MitoE and melatonin may have a similar beneficial effect in rats treated with LPS and PepG. In this proof-of-concept study, we investigated the effects of treatment with MitoQ, MitoE, or melatonin on biomarkers of organ damage, cytokine responses, oxidative damage, and mitochondrial function after administration of LPS from Escherichia coli plus PepG from Staphylococcus aureus in rats. This model reproducibly creates an inflammatory response, with mitochondrial dysfunction and early changes in organ function also seen in patients with sepsis.14
Methods
Animals
These studies were performed in accordance with UK Home Office regulations and all animals remained anaesthetized to a nociceptive stimulus throughout. Adult male Sprague–Dawley rats (median weight 463 g) were used and were maintained on normal rat chow. Animals were randomly pre-assigned, using sealed envelopes, to one of the eight experimental treatment groups (n=5–8 per group). Anaesthesia was induced with 5% isoflurane in oxygen in a tank then continued using 2–3% isoflurane and a nose cone while a tracheostomy was performed. Adequate anaesthesia was ascertained by lack of a pedal withdrawal response to a painful stimulus. A pressure-controlled small animal ventilator set to pressure-assist mode (Harvard Apparatus Ltd, Kent, UK) was used to ventilate the lungs to normocapnia with 2% isoflurane. Rats were placed on a heated blanket to prevent heat loss and rectal temperature was continuously monitored. An i.v. access cannula was inserted into the tail vein. MitoQ {[10-(4,5-dimethoxy-2-methyl-3,6-dioxo-1,4-cyclohexadien-1-yl) decyl] triphenylphosphonium} coupled to β-cyclodextrin was dissolved in saline. MitoE was given as 2-[2- (triphenylphosphonio) ethyl]-3,4-dihydro-2,5,7,8-tetramethyl-2H-1-benzopyran-6-ol bromide, also in saline. Melatonin (N-acetyl-5-methoxytryptamine) was initially dissolved in a small amount of ethanol then diluted in saline.
Experimental groups
Saline control rats received a 2 ml i.v. bolus of saline followed by an infusion at 3 ml kg−1 h−1. Antioxidant control rats received a 1 ml bolus of saline i.v. followed by 1.5 μmol kg−1 MitoQ or MitoE or melatonin in 1 ml saline as a bolus and then 1 μmol kg−1 h−1 (3 ml kg−1 h−1) MitoQ, MitoE, or melatonin as an infusion. LPS/PepG control rats received a mixture of 0.1 mg kg−1 LPS plus 1 mg kg−1 PepG in saline as a 1 ml i.v. bolus followed by 1 ml saline i.v. as a bolus and then an i.v. infusion of saline at 3 ml kg−1 h−1. LPS/PepG groups with antioxidant treatment received an i.v. bolus of LPS plus PepG in 1 ml saline as above, followed by 1.5 μmol kg−1 MitoQ, MitoE, or melatonin in 1 ml saline as an i.v. bolus and then 1 μmol kg−1 h−1 MitoQ, MitoE, or melatonin as an i.v. infusion (3 ml kg−1 h−1). All rats received the same volume of fluid regardless of treatment. After 5 h, the isoflurane concentration was increased to 5%, blood was removed by cardiac puncture into a heparinized syringe, a laparotomy was performed, and lungs, heart, liver, and kidney were rapidly removed and placed immediately into ice-cold Krebs–Henseleit buffer (119 mM NaCl, 2.5 mM NaHCO3, 4.7 mM KCl, 1.2 mM MgSO4, 1.18 mM KH2PO4, 11 mM glucose, 0.027 mM EDTA, 2.5 mM CaCl2) for immediate transport to the laboratory. All subsequent analyses were undertaken by staff blinded to the experimental group. A schematic diagram showing the study design is given in Figure 1.
Fig 1.
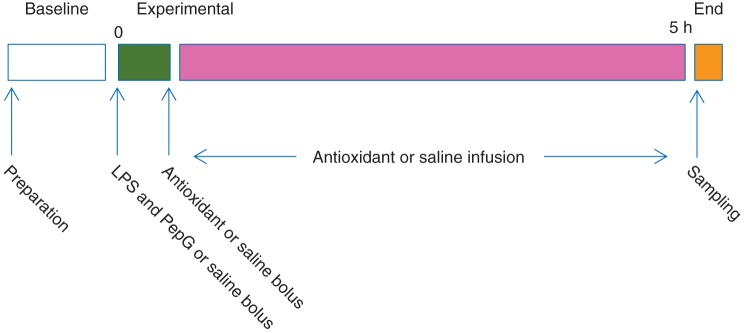
Schematic diagram of study design. Preparation=induction of anaesthesia with isoflurane in a tank, then a tracheostomy with isoflurane anaesthesia via a nose cone followed by ventilation, venous cannulation, and siting of ECG electrodes. Sampling=cardiac puncture, laparotomy, organ removal, termination.
Liver and renal function
Hepatocellular damage was assessed using plasma alanine amino transferase (ALT) and aspartate amino transferase (AST) activity. Renal function was as assessed using plasma creatinine concentration. Plasma samples were assayed in duplicate on an Advia 2400 Chemistry System (Siemens Healthcare Diagnostics, Deerfield, IL, USA). ALT and AST were measured enzymatically15 and creatinine was measured colorimetrically.16
Isolation of mitochondria from the liver
Liver pieces were placed in fresh ice-cold Krebs–Henseleit buffer to remove all traces of blood, then homogenized using a Teflon pestle in ice-cold mitochondrial isolation buffer (10 mM TRIS-MOPS, pH 7.4, 10 mM EGTA-TRIS, 200 mM sucrose). Cell debris was removed by centrifugation at 4°C. The supernatant was then further centrifuged and the pellet containing the heavy mitochondrial fraction was washed with ice-cold respiration buffer (125 mM KCl, 10 mM TRIS–MOPS pH 7.4, 1 mM EGTA–TRIS, 1 mM KH2PO4). Protein content was measured using the Bradford assay (BioRad, Herts, UK) and mitochondria were used within 3 h. Similar mitochondrial yields were seen in all samples.
Mitochondrial respiration
Mitochondrial respiration was measured using a calibrated Clark-type oxygen electrode and oxygen consumption recorded using Picolog for Windows, v5.14.6 (Pico Technology Ltd, Cambs, UK) at 37°C for 20 min in air-saturated respiration media. Respiration experiments were initiated by adding 1 mg ml−1 isolated mitochondria to respiration buffer. Oxygen consumption due to state 1 respiration was recorded for several minutes before the addition of either 5 mM glutamic acid plus 2.5 mM malic acid (complexes I, II, III, IV) or 5 mM succinic acid plus 2 mM rotenone (complexes II, III, IV), and oxygen consumption was again recorded. Then, 100 mM ADP was added to initiate state 3 respiration. State 3 respiration, followed by state 4 respiration (depletion of ADP), was recorded before the final addition of the protonophore carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP, 100 nM) to provide uncoupled respiration, independent of ADP. Respiration due to complex IV (cytochrome oxidase) activity was determined after addition of antimycin A, ascorbic acid, and N,N,N′,N′-tetramethyl-p-phenylenediamine (TMPD). Ascorbic acid maintains TMPD (an artificial electron donor) in a reduced state and the TMPD donates electrons to cytochrome c. The addition of sodium azide to inhibit cytochrome c enabled measurement of non-mitochondrial oxygen consumption.17
Oxidative damage
Plasma lipid hydroperoxides and liver protein carbonyl levels were determined in duplicate using commercially available assays (Cayman Chemical Company, Houston, TX, USA). Liver samples were washed and homogenized, centrifuged, and any contaminating nucleic acid was precipitated using streptomycin sulphate before assay. Protein levels were measured using the Bradford assay.
Cytokines
Interleukin-6 (IL-6) and IL-10 were measured in duplicate in plasma using commercially available enzyme rat immunoassay kits (R&D Systems Europe, Oxford, UK).
Statistical analysis
No assumptions were made about data distribution, and data are presented as median, inter-quartile, and full range. Statistical analysis was undertaken using the Analyse-It™ add-in for Microsoft Excel. Data sets between the LPS/PepG groups were compared using the Kruskal–Wallis with the Mann–Whitney U-test for post hoc analysis. Basal and maximal heart rate within treatment groups were compared using the Wilcoxon signed-ranks test for paired data. A P-value of <0.05 was considered to be significant.
Results
Rat model
We used a non-recovery, moderately fluid-resuscitated, anaesthetized model of acute sepsis using a combination of LPS from Gram-negative bacteria, and PepG from Gram-positive bacteria. This model has previously been shown to result in a reproducible acute inflammatory response, with increased cytokines, oxidative stress, mitochondrial dysfunction, and early biochemical changes associated with organ dysfunction,12,14 similar to the changes seen in human sepsis. Around 25% of rats given LPS/PepG without antioxidants died under anaesthesia before the end of the experimental period; these rats were not included in the analysis and were replaced by additional animals. No animals died in the other experimental groups.
Heart rate increased in the LPS/PepG control rats such that the maximum heart rate was significantly higher than the basal heart rate (Table 1). ALT and AST activity in plasma were markedly elevated in LPS/PepG control rats, compared with saline control rats, indicative of hepatocellular damage (Fig. 2). Plasma creatinine levels were also elevated by LPS/PepG treatment (Fig. 2), indicating renal damage, and liver protein carbonyl and plasma lipid hydroperoxide concentrations (Fig. 3) were increased indicating oxidative damage. Plasma IL-6 and IL-10 were also higher in LPS/PepG control rats than in saline control rats (Fig. 4) and mitochondrial respiration was decreased (Fig. 5).
Table 1.
Basal and maximum heart rates [median (range)]. *Significantly increased compared with basal heart rate (Wilcoxon signed-ranks test for paired data, P=0.016). LPS/PepG, lipopolysaccararide and peptidoglycan G
| Saline control | LPS/PepG control | LPS/PepG+MitoE | LPS/PepG+MitoQ | LPS/PepG+melatonin | |
|---|---|---|---|---|---|
| Basal heart rate (beats min−1) | 318 (314–346) | 315 (309–346) | 322 (249–350) | 355 (316–405) | 309 (300–351) |
| Max heart rate (beats min−1) | 403 (354–436) | 420* (340–509) | 364 (378–388) | 406 (329–527) | 380 (325–450) |
Fig 2.
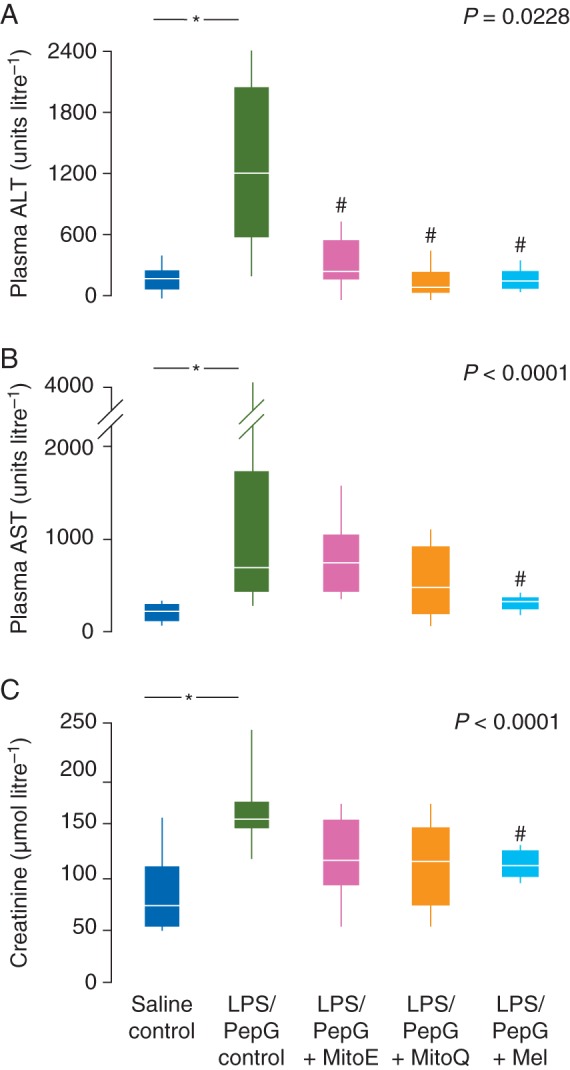
(a) Plasma ALT activity, (b) plasma AST activity, and (c) plasma creatinine levels 5 h after administration of LPs/PepG or saline showing the effects of MitoE, MitoQ or melatonin (Mel). Box and whisker plots show the median, IQR, and full range. *Significantly higher than saline control; #significantly lower than LPS/PepG control (Mann–Whitney U-test). P-value shown is Kruskal–Wallis across the LPS/PepG groups.
Fig 3.
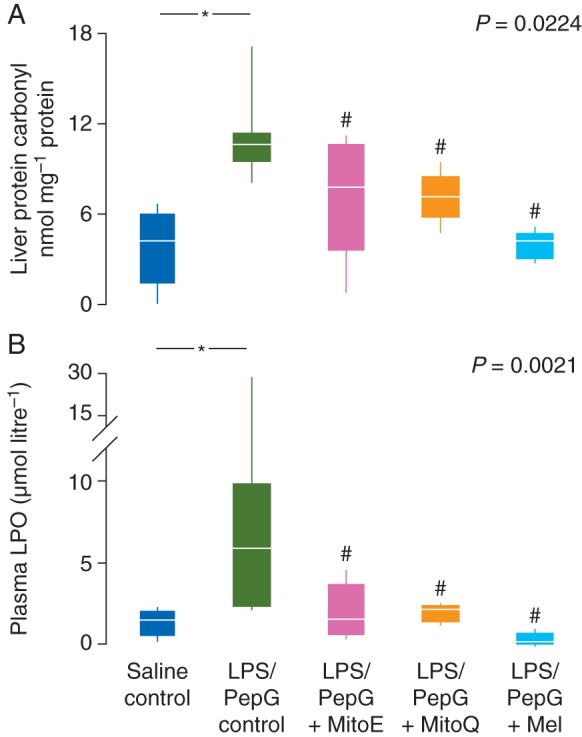
(a) Liver protein carbonyl concentrations and (b) plasma lipid hydroperoxide (LPO) concentrations 5 h after administration of saline or LPS/PepG, and the effects of treatment with MitoE, MitoQ, or melatonin (Mel). Box and whisker plots show the median, IQR, and full range. *Significantly higher than saline control; #significantly lower than LPS/PepG control (Mann–Whitney U-test). P-value shown is Kruskal–Wallis across the LPS/PepG groups.
Fig 4.
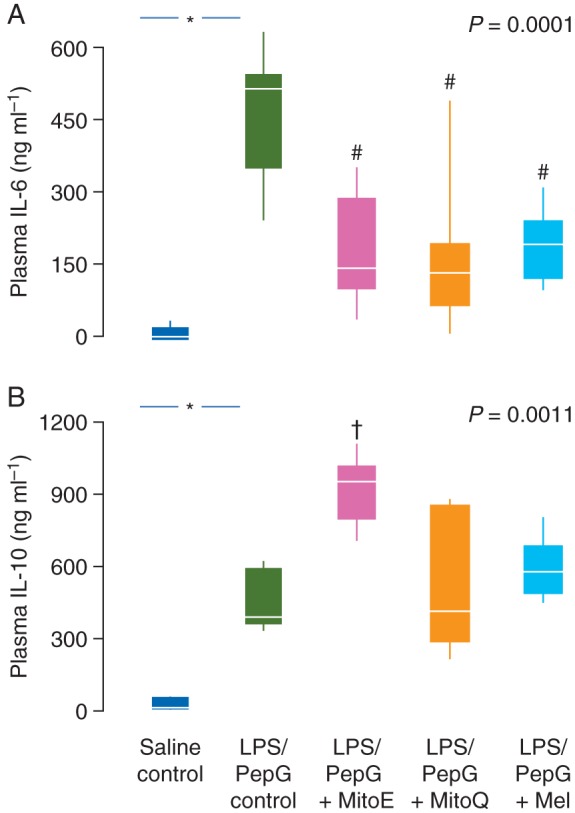
(a) Plasma IL-6 and (b) plasma IL-10 levels 5 h after administration of LPS/PepG or saline, and the effects of MitoE, MitoQ, or melatonin (Mel). Box and whisker plots show the median, IQR, and full range. *Significantly higher than saline control; #significantly lower and †significantly higher than LPS/PepG control (Mann–Whitney U-test). P-value shown is Kruskal–Wallis across the LPS/PepG groups.
Fig 5.
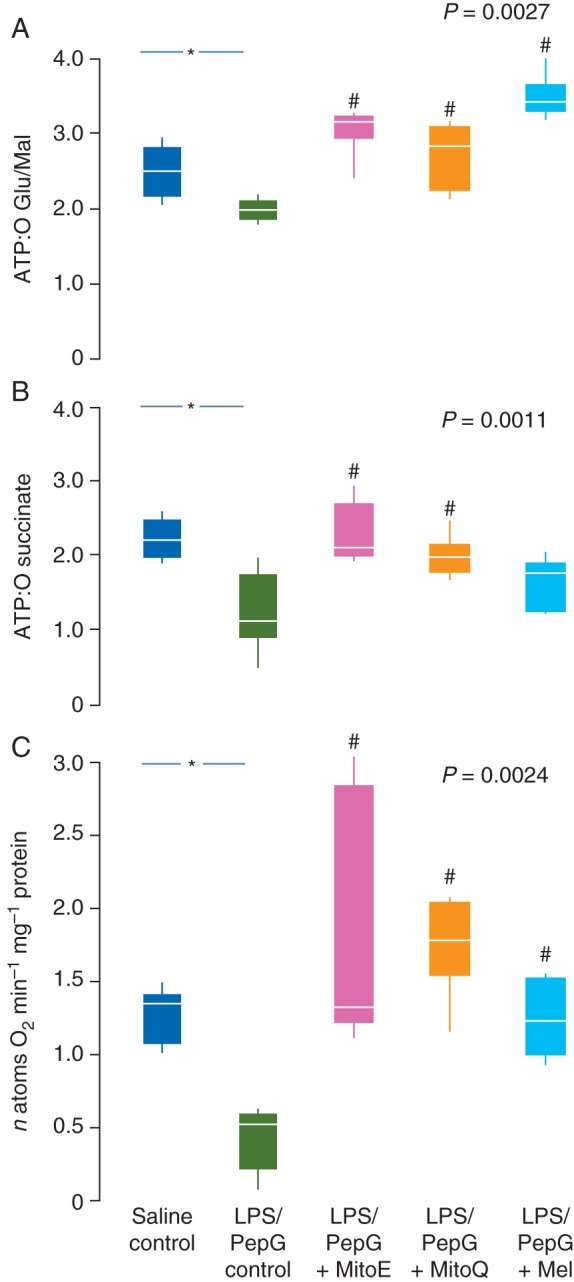
Mitochondrial ATP:O ratios with (a) glutamate and malate as substrate (complexes I, II, III, and IV) and (b) succinate (complexes II, II, and IV) and (c) complex IV activity (as oxygen used per minute) 5 h after administration of saline or LPS/PepG and the effect of treatment with MitoE, MitoQ, or melatonin (Mel). Box and whisker plots show the median, IQR, and full range. *Significantly higher than saline control; #significantly higher than the LPS/PepG control (Mann–Whitney U-test). P-value shown is Kruskal–Wallis across LPS/PepG groups.
Effects of MitoE, MitoQ, or melatonin alone
MitoE, MitoQ, or melatonin control rats had similar plasma biomarker levels to saline control rats (Table 2). Heart rates did not change in these animals (data not shown).
Table 2.
Plasma analysis of all treatment groups (median and full range). ALT, alanine amino transferase; AST, aspartate amino transferase; IL, interleukin; LPO, lipid peroxide; ND, not detected. *Significantly higher than saline control rats (Mann–Whitney U-test). #Significantly lower than LPS/PepG control rats (Kruskal–Wallis P<0.05, then Mann–Whitney U-test). ^Significantly higher than LPS/PepG control rats (Kruskal–Wallis P<0.05, then Mann–Whitney U-test)
| Saline control | LPS/PepG control | LPS/PepG+MitoE | LPS/PepG+MitoQ | LPS/PepG+melatonin | MitoE control | MitoQ control | Melatonin control | |
|---|---|---|---|---|---|---|---|---|
| ALT (units litre−1) | 93 (93–288) | 1190* (119–2360) (P=0.02) | 217# (81–637) (P=0.07) | 74# (63–418) (P=0.008) | 191# (127–247) (P=0.01) | 48 (38–104) | 95 (13–112) | 111 (102–120) |
| AST (units litre−1) | 259 (196–299) | 681* (315–3972) (P=0.006) | 734 (339–1678) | 410 (63–418) | 610 (399–653) | 274 (214–377) | 226 (145–240) | 195 (159–230) |
| Creatinine (μmol litre−1) | 73 (50–152) | 154* (120–242) (P=0.02) | 120 (67–172) | 106 (60–172) | 117# (101–129) | 75 (70–131) | 59 (39–126) | 45 (39–101) |
| IL-6 (ng ml−1) | 0.01 (0.01–0.16) | 485* (228–634) (P=0.0006) | 146# (50–350) (P=0.02) | 132# (9–476) (P=0.01) | 190# (100–300) (P=0.01) | ND | ND | ND |
| IL-10 (ng ml−1) | 0 (0.02–0.25) | 424* (337–601) (P=0.001) | 938^ (700–1108) (P=0.03) | 431 (50–365) | 570 (450–780) | ND | ND | ND |
| LPO (μM) | 1.5 (0.2–2.2) | 5.7* (2.2–29.7) (P=0.04) | 1.6# (0.5–4.8) (P=0.04) | 2.2# (1.7–2.2) (P=0.008) | 0.3# (0.2–0.8) (P=0.016) | <0.2 | 1.3 (0.2–2.0) | <0.2 |
Effect of antioxidants on organ function, oxidative stress, and cytokines
Plasma ALT activity was lower in rats given LPS/PepG that were subsequently treated with MitoE, MitoQ, or melatonin, than in LPS/PepG control rats (Fig. 2a). AST and creatinine were decreased by antioxidants but were significantly lower only in those rats treated with melatonin (Fig. 2b and c). The maximum heart rate did not increase and was not different from basal heart rate in the LPS/PepG rats treated with MitoQ, MitoE, or melatonin (Table 1). The LPS/PepG-treated rats given MitoE, MitoQ, or melatonin had significantly lower levels of liver protein carbonyls (Fig. 3a) and plasma lipid hydroperoxides (Fig. 3b). The increase in plasma IL-6 levels in rats given LPS and PepG was blunted by MitoQ, MitoE, or melatonin treatment (Fig. 4a). IL-10 levels were higher in LPS/PepG rats treated with MitoE than in those given LPS/PepG without antioxidants, but neither MitoQ nor melatonin had any effect on IL-10 (Fig. 4b).
Effect of antioxidants on mitochondrial function
Mitochondrial respiration in the liver was measured as the number of ATP molecules made as electrons pass along the respiratory chain to oxygen (expressed as the ATP:O ratio). The ATP:O ratio with glutamate and malate as substrate (complexes I, II, III, and IV) and that with succinate plus rotenone (complexes II, II, and IV) were lower in liver mitochondria from LPS/PepG control rats than saline control animals (Fig. 5a and b). Complex IV respiration was also markedly lower in the LPS/PepG control rats than saline control rats (Fig. 5c).
LPS/PepG rats treated with either MitoQ or MitoE had ATP:O ratios similar to saline control animals when either glutamate/malate or succinate was used as substrate (Fig. 5a and b). Melatonin treatment increased the ATP:O ratio with glutamate/malate but just failed to reach significance with succinate (Fig. 5b). Treatment of LPS/PepG rats with either MitoE, MitoQ, or melatonin augmented complex IV activity compared with LPS/PepG control rats (Fig. 5c). The respiratory control index (RCI), the ratio of the rate of mitochondrial oxygen consumption in respiratory state 3 to that in respiratory state 4, is a measure of the efficiency of energy coupling, used for diagnosis of mitochondrial defects. The RCI was similar in all rat groups with either glutamate/malate or succinate as substrate, suggesting that there was no difference in terms of experimentally generated mitochondrial dysfunction between treatment groups during assay and that mitochondria were not ‘uncoupled’ (data not shown).
Discussion
We have shown that in an acute model of LPS/PepG-induced sepsis, characterized by inflammation, organ dysfunction, and mitochondrial damage, treatment with MitoQ, MitoE, or melatonin had broadly similar effects, including decreased biochemical markers of early organ dysfunction, decreased oxidative stress, decreased pro-inflammatory cytokine responses, and less mitochondrial dysfunction.
Although the Surviving Sepsis Campaign, a performance improvement effort by hospitals across Europe, South America, and the USA has improved outcomes, the mortality from sepsis remains high.1 ROS contribute to inflammatory responses by activating key signalling pathways including the redox sensitive transcription factor pathway of nuclear factor κB (NFκB) and the inflammasome.18,19 Mitochondria produce more than 90% of the body's energy needs in the form of ATP, via oxidative phosphorylation. The majority of ROS production occurs within mitochondria during ATP generation. Also, being the major source of intracellular ROS, mitochondria are a major target of ROS-mediated damage.2 Mitochondrial ROS (mtROS) are important for cellular signalling but can also cause cell damage, and so are tightly regulated by endogenous antioxidant scavenging systems that limit damage. Under conditions of stress or disease, ROS production is increased, antioxidant protection is decreased, mitochondrial damage occurs, and inflammation increases, creating a vicious cycle. The loss of enzyme function in the electron transport chain, mitochondrial dysfunction, and impaired ATP generation then occurs, with peroxidation of cardiolipin, dissociation of cytochrome c, further reduction in ATP production and more ROS generation.3 This self-sustaining and amplifying cycle between ROS generation, inflammatory responses, and mitochondrial impairment contributes to organ failure in patients with sepsis.4,5,20
Since mitochondria are the main source and target of ROS, antioxidants that act in mitochondria might be more effective than antioxidants that act generally.8,9 mtROS have been shown to drive inflammatory cytokine responses via both inflammasome-dependent and -independent mechanisms acting together with NFκB to regulate several cytokines including IL-1β and IL-6.18,19 We have shown that antioxidants that act within mitochondria reduce IL-1β and IL-6 levels and decrease NFκB activation in LPS/PepG-treated endothelial cells.11–13 Decreased NFκB activation has also been shown in septic animals treated with melatonin.21
MitoQ, MitoE, and melatonin have differing antioxidant actions but all act within mitochondria. MitoQ consists of a lipophilic cation, triphenylphosphonium (TPP), attached to ubiquinone by a saturated 10-carbon alkyl chain. Once inside mitochondria, the ubiquinone is reduced to its active ubiquinol form by complex II.8,22 MitoE in contrast comprises α-tocopherol (vitamin E) attached to the TPP cation but with a shorter, 2-carbon alkyl chain. In addition to having different structures, MitoQ and MitoE also act in different parts of the mitochondrion and thus might be expected to have differing effects.
Both superoxide and nitric oxide have crucial roles in the mechanisms of mitochondrial damage in sepsis through formation of peroxynitrite, resulting in suppression of mitochondrial respiration, with reduced ATP synthesis, inhibition of complexes I and II, and damage to mitochondrial DNA.23–25 Mitochondrial antioxidants including MitoQ/E have been shown to reduce peroxynitrite formation, nitrotyrosination of proteins, and alleviate mitochondrial dysfunction in several studies.24–26 The antioxidant effects of melatonin and its metabolites occur by several mechanisms including direct effects on free radicals and interactions with other toxic species including hydrogen peroxide, nitric oxide, and peroxynitrite.9–11,26–28 The mitochondrial antioxidants used in our study might protect against mitochondrial dysfunction via effects on superoxide, nitric oxide, peroxynitrite itself, or a combination. Melatonin also up-regulates endogenous antioxidants throughout the cell,11,29,30 whereas this has not been described for MitoQ or MitoE.
Despite differing mechanisms, the effects of MitoQ, MitoE, and melatonin were broadly similar in our study. Oral, i.p., or i.v. administration of MitoQ or MitoE results in accumulation in major organs in rats and mice, including the heart, liver, and kidneys.31,32 Exogenous melatonin accumulates throughout the cell, with levels depending on the dose, highest in the cell membrane and mitochondria.33
We found that mitochondrial respiratory function was decreased in mitochondria from the livers of rats given LPS/PepG without antioxidants. The liver is highly metabolic with high yields of mitochondria and mitochondrial ultrastructural changes in the liver of patients who died from sepsis have been reported.34 Previous studies in intact peripheral leucocytes from patients in the early stages of sepsis also showed decreased mitochondrial oxygen consumption and complex activity similar to that reported here.20,35,36 The present study showed that antioxidant treatment returned mitochondrial respiration towards normal in rats given LPS and PepG. MitoE has also been shown to protect against loss of complex IV activity in hearts of septic rats32 and melatonin interacts with enzymes of the electron transport chain and oxidative phosphorylation with increased transcription of complex IV enzymes correlating with complex IV activity.35 Complex IV (cytochrome c oxidase activity) is essentially an indicator of mass and health of mitochondria and is commonly used as a predictor of ‘mitochondrial fitness’. Maj and colleagues37 recently developed a live cell screening tool and found that many compounds increase complex IV activity either via activation of peroxisome proliferator-activated (PPAR) receptors or the protein kinase A pathway. It remains speculative as to whether the antioxidants used in our study acted via these mechanisms, although melatonin is known to be a potent PPAR receptor activator.
In this study, we found that i.v. MitoQ, MitoE, or melatonin after administration of LPS/PepG resulted in improvements in measures of organ dysfunction, lower IL-6 levels, less oxidative damage, and improved mitochondrial function, concurring with our previous in vitro and in vivo studies.11–13 Early relative levels of IL-6 and IL-10 have been suggested to be crucial factors determining the severity of sepsis.36 Global mitochondrial dysfunction associated with NFκB activation, and elevated IL-6 and lipid peroxidation products in plasma, has been reported in patients, suggesting that mitochondrial dysfunction is an early event in sepsis.20 Therapies designed to interfere with signalling pathways are likely to be most effective at the early stages of sepsis.
In agreement with our study, treatment of rats with a single oral dose of MitoE was recently reported to protect the heart against damage caused by sepsis induced by intratracheal injection of Streptococcus pneumoniae, including preservation of mitochondrial structure and function, maintenance of mitochondrial membrane potential, preservation of mitochondrial respiration, and reduction in oxidative stress.32 In the same study, pro-inflammatory cytokines including IL-6 were lower in animals treated with MitoE, but IL-10 was not reported.32 We found that IL-10 levels were higher in septic rats given MitoE, but not MitoQ or melatonin, and further studies are needed to dissect the mechanism of this effect. Studies in cells, rats, and patients with sepsis show an exponential relationship between IL-6 and IL-10 levels, and it is possible that the effects of MitoE on IL-10 are mediated via effects on IL-6.36
Other studies using an acute LPS rat model have reported that IL-10 levels were higher in lung tissue, peritoneal fluid, and serum of rats treated with melatonin,21,38 although the doses of melatonin given were approximately three-fold higher than in our study. Another recent study showed decreased tissue oxidative stress in septic rats treated with slow-release melatonin delivered using nanoparticle carriers,39 but neither cytokine levels nor mitchondrial function were reported. In addition, a recent small clinical trial showed that a single oral dose of melatonin resulted in lower liver transaminase activity in patients undergoing major liver resection.40
We acknowledge several limitations of our study. Although animal models never replicate exactly what happens in patients, LPS/PepG given i.v. results in similar haemodynamic and cytokine changes as seen in acute early human sepsis. Animal models remain the only way in which proof-of-concept studies to assess the potential of novel therapies can be undertaken. We administered antioxidants after LPS/PepG, rather than pre-treating the rats, and we gave the drugs i.v. rather than i.p. to better reflect the clinical situation. LPS and PepG have been shown to synergize and amplify the inflammatory response.14 Mitochondrial dysfunction can be assessed in a variety of ways; the best is thought to be measures of respiration. Intact cells have been used in some studies, but the cell permeabilization to achieve this introduces its own problems and combinations of methods are most useful. The findings in our rat model mirror changes in mitochondria seen in cells from patients with sepsis, and so we would argue that such measures give at least a suggestion of the effects likely to be seen in vivo.20
Inhalation anaesthesia with isoflurane is an increasingly popular method for general anaesthesia for biomedical research in rats.32,41 It has some advantages over injectable agents including reduced animal handling requirements, a wide safety margin, and good control of anaesthesia. Isoflurane anaesthesia is protective against an ischaemic insult as both a pre- and post-conditioning agent. A brief period of isoflurane anaesthesia resulted in attenuated inflammatory responses and improved survival rates in septic rats.41 Longer durations of isoflurane anaesthesia have been suggested to be neurotoxic but prolonged anaesthesia of young rats with isoflurane had no effect on adult hippocampal cell proliferation.42 It is not known what effects isoflurane had in our study, but all animals did receive identical isoflurane anaesthesia.
Although MitoQ has been through phase I trials and there have also been phase II trials in other disease conditions,43 phase I trials of MitoE have not been conducted. There have been clinical studies of low doses (1–5 mg) of exogenous melatonin in critically ill patients,44 and melatonin has been administered to patients in other disease conditions at larger doses resulting in decreased cytokine and lipid peroxide levels.45 Melatonin has also been given to critically ill neonates.46
In summary, we have shown that MitoQ, MitoE, and melatonin are protective in a rat model of acute inflammation, oxidative stress, and mitochondrial dysfunction. This proof-of-concept study suggests that antioxidants that protect mitochondria might be beneficial in patients with sepsis. We propose that melatonin might be the most accessible agent for clinical studies at present.
Declaration of interest
M.P.M. holds shares in a company, Antipodean Pharmaceuticals Inc., which is commercializing MitoQ and MitoE.
N.R.W. is the Chairman and H.F.G. is an Editor of the British Journal of Anaesthesia.
Funding
This study was funded by the Medical Research Council (Grant number G0800149). Research material from this study is not available.
Acknowledgement
We are very grateful to Dr Robin A.J. Smith, Department of Chemistry, University of Otago, Dunedin, New Zealand, for the generous gifts of MitoE and MitoQ, without which this work would not have been possible.
References
- 1.Marshall JC, Vincent JL, Guyatt G, et al. Outcome measures for clinical research in sepsis: a report of the 2nd Cambridge Colloquium of the International Sepsis Forum. Crit Care Med. 2005;33:1708–6. doi: 10.1097/01.ccm.0000174478.70338.03. doi:10.1097/01.CCM.0000174478.70338.03. [DOI] [PubMed] [Google Scholar]
- 2.Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552:335–44. doi: 10.1113/jphysiol.2003.049478. doi:10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.James AM, Murphy MP. How mitochondrial damage affects cell function. J Biomed Sci. 2002;9:475–87. doi: 10.1159/000064721. doi:10.1007/BF02254975. [DOI] [PubMed] [Google Scholar]
- 4.Exline MC, Crouser ED. Mitochondrial mechanisms of sepsis induced organ failure. Frontiers Biosc. 2008;13:5031–41. doi: 10.2741/3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruggieri AJ, Levy RJ, Deutschman CS. Mitochondrial dysfunction and resuscitation in sepsis. Crit Care Clin. 2010;26:567–75. doi: 10.1016/j.ccc.2010.04.007. doi:10.1016/j.ccc.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Víctor VM, Espulgues JV, Hernández-Mijares A, Rocha M. Oxidative stress and mitochondrial dysfunction in sepsis: a potential therapy with mitochondria-targeted antioxidants. Infect Disord Drug Targets. 2009;9:376–89. doi: 10.2174/187152609788922519. [DOI] [PubMed] [Google Scholar]
- 7.Galley HF. Oxidative stress and mitochondrial dysfunction in sepsis. Br J Anaesth. 2011;107:57–64. doi: 10.1093/bja/aer093. doi:10.1093/bja/aer093. [DOI] [PubMed] [Google Scholar]
- 8.Smith RA, Porteous CM, Coulter CV, Murphy MP. Selective targeting of an antioxidant to mitochondria. Eur J Biochem. 1999;263:709–16. doi: 10.1046/j.1432-1327.1999.00543.x. doi:10.1046/j.1432-1327.1999.00543.x. [DOI] [PubMed] [Google Scholar]
- 9.Galano A. On the direct scavenging activity of melatonin towards hydroxyl and a series of peroxyl radicals. Phys Chem Chem Phys. 2011;13:7178–88. doi: 10.1039/c0cp02801k. doi:10.1039/c0cp02801k. [DOI] [PubMed] [Google Scholar]
- 10.Stetinová V, Smetanová L, Grossmann V, Anzenbacher P. In vitro and in vivo assessment of the antioxidant activity of melatonin and related indole derivatives. Gen Physiol Biophys. 2002;21:153–62. [PubMed] [Google Scholar]
- 11.Lowes DA, Almawash AM, Webster NR, Reid V, Galley HF. Role of melatonin and indole-derivatives on endothelial cells in an in vitro model of sepsis. Br J Anaesth. 2011;107:193–201. doi: 10.1093/bja/aer149. doi:10.1093/bja/aer149. [DOI] [PubMed] [Google Scholar]
- 12.Lowes DA, Thottakam BMVJ, Webster NR, Murphy MP, Galley HF. The mitochondria-targeted antioxidant MitoQ protects against organ damage in a lipopolysaccharide–peptidoglycan model of sepsis. Free Radic Biol Med. 2008;45:1559–65. doi: 10.1016/j.freeradbiomed.2008.09.003. doi:10.1016/j.freeradbiomed.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Minter BE, Lowes DA, Webster NR, Galley HF. Mitochondria targeted vitamin E in an endothelial model of sepsis (Abstract) Br J Anaesth. 2010;104:525–6P. [Google Scholar]
- 14.Wray GM, Foster SJ, Hinds CJ, Thiemermann C. A cell wall component from pathogenic and non-pathogenic gram-positive bacteria (peptidoglycan) synergises with endotoxin to cause the release of TNFα, nitric oxide production, shock, and multiple organ dysfunction in the rat. Shock. 2001;15:135–42. doi: 10.1097/00024382-200115020-00010. doi:10.1097/00024382-200115020-00010. [DOI] [PubMed] [Google Scholar]
- 15.Ozer J, Ratner M, Shaw M, Bailey W, Schomaker S. The current state of serum biomarkers of hepatotoxicity. Toxicology. 2008;245:194–205. doi: 10.1016/j.tox.2007.11.021. doi:10.1016/j.tox.2007.11.021. [DOI] [PubMed] [Google Scholar]
- 16.Peake M, Whiting M. Measurement of serum creatinine—current status and future goals. Clin Biochem Rev. 2006;27:173–84. [PMC free article] [PubMed] [Google Scholar]
- 17.Frezza C, Cipolat S, Scorrano L. Organelle isolation: functional mitochondria from mouse liver, muscle and cultured fibroblasts. Nat Protocols. 2007;2:287–95. doi: 10.1038/nprot.2006.478. doi:10.1038/nprot.2006.478. [DOI] [PubMed] [Google Scholar]
- 18.Hughes G, Murphy MP, Ledgerwood EC. Mitochondrial reactive oxygen species regulate the temporal activation of nuclear factor kappaB to modulate tumour necrosis factor-induced apoptosis: evidence from mitochondria-targeted antioxidants. Biochem J. 2005;389:83–9. doi: 10.1042/BJ20050078. doi:10.1042/BJ20050078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naik E, Dixit VM. Mitochondrial reactive oxygen species drive pro-inflammatory cytokine production. J Exp Med. 2011;208:417–20. doi: 10.1084/jem.20110367. doi:10.1084/jem.20110367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garrabou G, Morén C, López S, et al. The effects of sepsis on mitochondria. J Infect Dis. 2012;205:392–400. doi: 10.1093/infdis/jir764. doi:10.1093/infdis/jir764. [DOI] [PubMed] [Google Scholar]
- 21.Shang Y, Xu SP, Wu Y, et al. Melatonin reduces acute lung injury in endotoxemic rats. Chin Med J (Engl) 2009;122:1388–93. [PubMed] [Google Scholar]
- 22.James AM, Sharpley MS, Manas AR, et al. Interaction of the mitochondria-targeted antioxidant MitoQ with phospholipid bilayers and ubiquinone oxidoreductases. J Biol Chem. 2007;282:14708–18. doi: 10.1074/jbc.M611463200. doi:10.1074/jbc.M611463200. [DOI] [PubMed] [Google Scholar]
- 23.Radi R, Cassina A, Hodara R. Nitric oxide and peroxynitrite interactions with mitochondria. Biol Chem. 2002;383:401–9. doi: 10.1515/BC.2002.044. [DOI] [PubMed] [Google Scholar]
- 24.Apostolova N, Garcia-Bou R, Hernandez-Mijares A, Herance R, Rocha M, Victor VM. Mitochondrial antioxidants alleviate oxidative and nitrosative stress in a cellular model of sepsis. Pharm Res. 2011;28:2910–9. doi: 10.1007/s11095-011-0528-0. doi:10.1007/s11095-011-0528-0. [DOI] [PubMed] [Google Scholar]
- 25.Choumar A, Tarhuni A, Lettéron P, et al. Lipopolysaccharide-induced mitochondrial DNA depletion. Antioxid Redox Signal. 2011;15:2837–54. doi: 10.1089/ars.2010.3713. doi:10.1089/ars.2010.3713. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell T, Rotaru D, Saba H, Smith RAJ, Murphy MP, MacMillan-Crow LA. The mitochondria-targeted antioxidant mitoquinone protects against cold storage injury of renal tubular cells and rat kidneys. J Pharmacol Exp Ther. 2011;336:682–92. doi: 10.1124/jpet.110.176743. doi:10.1124/jpet.110.176743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luchetti F, Canonico B, Betti M, et al. Melatonin signaling and cell protection function. FASEB J. 2010;24:3603–24. doi: 10.1096/fj.10-154450. doi:10.1096/fj.10-154450. [DOI] [PubMed] [Google Scholar]
- 28.Gilad E, Cuzzocrea S, Zingarelli B, Salzman AL, Szabó C. Melatonin is a scavenger of peroxynitrite. Life Sci. 1997;60:PL169–74. doi: 10.1016/s0024-3205(97)00008-8. doi:10.1016/S0024-3205(97)00008-8. [DOI] [PubMed] [Google Scholar]
- 29.Limón-Pacheco JH, Gonsebatt ME. The glutathione system and its regulation by neurohormone melatonin in the central nervous system. Cent Nerv Syst Agents Med Chem. 2010;10:287–97. doi: 10.2174/187152410793429683. [DOI] [PubMed] [Google Scholar]
- 30.Mauriz JL, Collado PS, Veneroso C, Reiter RJ, González-Gallego J. A review of the molecular aspects of melatonin's anti-inflammatory actions: recent insights and new perspectives. J Pineal Res. 2013;54:1–14. doi: 10.1111/j.1600-079X.2012.01014.x. [DOI] [PubMed] [Google Scholar]
- 31.Smith RAJ, Porteous CM, Gane AM, Murphy MP. Delivery of bioactive molecules to mitochondria in vivo. Proc Natl Acad Sci USA. 2003;100:5407–12. doi: 10.1073/pnas.0931245100. doi:10.1073/pnas.0931245100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zang QS, Sadek H, Maass DL, et al. Specific inhibition of mitochondrial oxidative stress suppresses inflammation and improves cardiac function in a rat pneumonia-related sepsis model. Am J Physiol Heart Circ Physiol. 2012;302:H1847–59. doi: 10.1152/ajpheart.00203.2011. doi:10.1152/ajpheart.00203.2011. [DOI] [PubMed] [Google Scholar]
- 33.Venegas C, García JA, Escames G, et al. Extrapineal melatonin: analysis of its subcellular distribution and daily fluctuations. J Pineal Res. 2012;52:217–27. doi: 10.1111/j.1600-079X.2011.00931.x. doi:10.1111/j.1600-079X.2011.00931.x. [DOI] [PubMed] [Google Scholar]
- 34.Vanhorebeek I, De Vos R, Mesotten D, Wouters PJ, De Wolf-Peeters C, Van den Berghe G. Protection of hepatocyte mitochondrial ultrastructure and function by strict blood glucose control with insulin in critically ill patients. Lancet. 2005;365:53–9. doi: 10.1016/S0140-6736(04)17665-4. doi:10.1016/S0140-6736(04)17665-4. [DOI] [PubMed] [Google Scholar]
- 35.Davies NA, Cooper CE, Stidwill R, Singer M. Inhibition of mitochondrial respiration during early stage sepsis. Adv Exp Med Biol. 2003;530:725–36. doi: 10.1007/978-1-4615-0075-9_73. doi:10.1007/978-1-4615-0075-9_73. [DOI] [PubMed] [Google Scholar]
- 36.Novotny AR, Reim D, Assfalg V, et al. Mixed antagonist response and sepsis severity-dependent dysbalance of pro- and anti-inflammatory responses at the onset of postoperative sepsis. Immunobiology. 2012;217:616–21. doi: 10.1016/j.imbio.2011.10.019. doi:10.1016/j.imbio.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 37.Maj M, Sriskandarajah N, Hung V, et al. Identification of drug candidates which increase cytochrome c oxidase activity in deficient patient fibroblasts. Mitochondrion. 2011;11:264–72. doi: 10.1016/j.mito.2010.10.002. doi:10.1016/j.mito.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 38.Carrillo-Vico A, Lardone PJ, Naji L, et al. Beneficial pleiotropic actions of melatonin in an experimental model of septic shock in mice: regulation of pro-/anti-inflammatory cytokine network, protection against oxidative damage and anti-apoptotic effects. J Pineal Res. 2005;39:400–8. doi: 10.1111/j.1600-079X.2005.00265.x. doi:10.1111/j.1600-079X.2005.00265.x. [DOI] [PubMed] [Google Scholar]
- 39.Li Volti G, Musumeci T, Pignatello R, et al. Antioxidant potential of different melatonin-loaded nanomedicines in an experimental model of sepsis. Exp Biol Med. 2012;237:670–7. doi: 10.1258/ebm.2012.011425. doi:10.1258/ebm.2012.011425. [DOI] [PubMed] [Google Scholar]
- 40.Nickkholgh A, Schneider H, Sobirey M, et al. The use of high-dose melatonin in liver resection is safe: first clinical experience. J Pineal Res. 2011;50:381–8. doi: 10.1111/j.1600-079X.2011.00854.x. doi:10.1111/j.1600-079X.2011.00854.x. [DOI] [PubMed] [Google Scholar]
- 41.Bedirli N, Demirtas CY, Akkaya T, et al. Volatile anesthetic preconditioning attenuated sepsis induced lung inflammation. J Surg Res. 2012;178:e17–23. doi: 10.1016/j.jss.2011.12.037. doi:10.1016/j.jss.2011.12.037. [DOI] [PubMed] [Google Scholar]
- 42.Tung A, Herrera S, Fornal CA, Jacobs BL. The effect of prolonged anesthesia with isoflurane, propofol, dexmedetomidine, or ketamine on neural cell proliferation in the adult rat. Anesth Analg. 2008;106:1772–7. doi: 10.1213/ane.0b013e31816f2004. doi:10.1213/ane.0b013e31816f2004. [DOI] [PubMed] [Google Scholar]
- 43.Smith RA, Murphy MP. Mitochondria-targeted antioxidants as therapies. Discov Med. 2011;11:106–14. [PubMed] [Google Scholar]
- 44.Bourne RS, Mills GH, Minelli C. Melatonin therapy to improve nocturnal sleep in critically ill patients: encouraging results from a small randomised controlled trial. Crit Care. 2008;12:R52. doi: 10.1186/cc6871. doi:10.1186/cc6871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chahbouni M, Escames G, Venega C, et al. Melatonin treatment normalizes plasma pro-inflammatory cytokines and nitrosative/oxidative stress in patients suffering from Duchenne muscular dystrophy. J Pineal Res. 2010;48:282–9. doi: 10.1111/j.1600-079X.2010.00752.x. doi:10.1111/j.1600-079X.2010.00752.x. [DOI] [PubMed] [Google Scholar]
- 46.Gitto E, Karbownik M, Reiter RJ, et al. Effects of melatonin treatment in septic newborns. Pediatr Res. 2001;50:756–60. doi: 10.1203/00006450-200112000-00021. doi:10.1203/00006450-200112000-00021. [DOI] [PubMed] [Google Scholar]


