Abstract
Oxygen is essential for all metazoans to survive, with one known exception1. Decreased O2 availability (hypoxia) can arise during states of disease, normal development or changes in environmental conditions2-5. Understanding the cellular signaling pathways that are involved in the response to hypoxia could provide new insight into treatment strategies for diverse human pathologies, from stroke to cancer. This goal has been impeded, at least in part, by technical difficulties associated with controlled hypoxic exposure in genetically amenable model organisms.
The nematode Caenorhabditis elegans is ideally suited as a model organism for the study of hypoxic response, as it is easy to culture and genetically manipulate. Moreover, it is possible to study cellular responses to specific hypoxic O2 concentrations without confounding effects since C. elegans obtain O2 (and other gasses) by diffusion, as opposed to a facilitated respiratory system6. Factors known to be involved in the response to hypoxia are conserved in C. elegans. The actual response to hypoxia depends on the specific concentration of O2 that is available. In C. elegans, exposure to moderate hypoxia elicits a transcriptional response mediated largely by hif-1, the highly-conserved hypoxia-inducible transcription factor6-9. C .elegans embryos require hif-1 to survive in 5,000-20,000 ppm O27,10. Hypoxia is a general term for "less than normal O2". Normoxia (normal O2) can also be difficult to define. We generally consider room air, which is 210,000 ppm O2 to be normoxia. However, it has been shown that C. elegans has a behavioral preference for O2 concentrations from 5-12% (50,000-120,000 ppm O2)11. In larvae and adults, hif-1 acts to prevent hypoxia-induced diapause in 5,000 ppm O212. However, hif-1 does not play a role in the response to lower concentrations of O2 (anoxia, operational definition <10 ppm O2)13. In anoxia, C. elegans enters into a reversible state of suspended animation in which all microscopically observable activity ceases10. The fact that different physiological responses occur in different conditions highlights the importance of having experimental control over the hypoxic concentration of O2.
Here, we present a method for the construction and implementation of environmental chambers that produce reliable and reproducible hypoxic conditions with defined concentrations of O2. The continual flow method ensures rapid equilibration of the chamber and increases the stability of the system. Additionally, the transparency and accessibility of the chambers allow for direct visualization of animals being exposed to hypoxia. We further demonstrate an effective method of harvesting C. elegans samples rapidly after exposure to hypoxia, which is necessary to observe many of the rapidly-reversed changes that occur in hypoxia10,14. This method provides a basic foundation that can be easily modified for individual laboratory needs, including different model systems and a variety of gasses.
Keywords: Biochemistry, Issue 65, Molecular Biology, Cellular Biology, Genetics, Developmental Biology, C. elegans, hypoxia, hypoxia inducible factor-1 (hif-1), anoxia, oxygen
Protocol
1. Construction of Environmental Chambers
Select the smallest reasonable volume of chamber required for the scope of your project. Chamber must be made of gas (O2) impermeable material. Pyrex crystallization dishes, Anaeropack boxes, or large cast-acrylic boxes (Ellard Instrumentation), can be used. We have found that 9 50 mm plates can fit in a 100 x 50 Kimex crystallization dish. Glass plates can be used as lids for Pyrex crystallization dishes.
Drill a hole in the selected chamber and fit with a plastic male Luer to hose barb fitting (Cole Parmer). Fittings can be secured by pipe fitting or with epoxy. Install a similar fitting on the opposite side of the container to allow for gas to flow in and out of the chamber. If possible, offset holes to increase turbulent mixing.
Obtain compressed gas tanks with defined O2 concentrations (balanced with N2) that are certified standard for O2 content or, for anoxic conditions, pure N2 (<10 ppm O2). Use automatic switch-over regulators for longer-term studies to avoid disrupting the oxygen levels in the chambers.
Organismal response to hypoxia has been shown to be temperature dependent15. By placing the chamber in an incubator, different temperatures can be maintained. Temperatures within an incubator may be uneven and as such, it is prudent to make use of a temperature data logger to constantly measure the temperature inside the chamber.
2. Connecting the Gas to the Environmental Chamber
For all connections, use one-eighth-inch outer-diameter tubing connected by either snap connectors or compression fittings. Tubing should be impermeable and unreactive with O2, such as fluorinated ethylene propylene (FEP) or nylon (Cole Parmer). For a schematic of the completed setup, see Figure 1.
Connect the compressed gas tanks to a flow control device, such as a mass flow controller (Sierra Instruments) or rotameter (Aalborg). Ensure that upstream pressure from the tank is within the range of the flow device and the hose barb fittings. Two-stage regulators are generally used, with the second stage set to the desired pressure [See section three for selecting the appropriate flow rate]
Hydrate the gas by bubbling through distilled water using a gas wash bottle with fritted cylinder, then direct into one of the fittings on the environmental chamber, leaving the second fitting open for gas exhaust (see Figure 1). For short-term studies, gas hydration protects against plate dessication, but humidity monitoring may be necessary for long-term studies.
Dow Corning Vacuum Grease can be used to seal the chamber. Place weights on the lid of the chamber to ensure an airtight seal. To confirm a tight seal and adequate flow, hold a small pool of water in the palm of your gloved hand to the out fitting on the chamber and check for bubbles.
3. Selecting Flow Rate
Assuming perfect mixing, there is 90% gas exchange of the gaseous atmosphere each time the volume of the chamber is replaced (Fick's Law). For example, in a 100 cc chamber with a flow rate of 100 cc/min, the original house air in the chamber will be replaced with 90% of your desired gas after 1 minute, and will asymptotically approach complete exchange by 90% every minute thereafter.
Higher flow rates and smaller containers will reach your desired oxygen concentration more quickly. For 100 x 50 Kimex containers (400 cc), a flow rate of 120 cc/min will reach 99.9% exchange in 10 minutes (3 exchanges). This flow rate is suitable for most oxygen conditions. To our knowledge there has not been a systematic investigation of how the rate of change of O2 concentration influences the response in C. elegans.
4. Preparation of Samples for Viability Assay
Worms exposed to hypoxic conditions commonly escape the surface of agar plates. To prevent this, place a ring of palmitic acid (10 mg/ml in ethanol) around the edge of the plates. The palmitic acid will come out of solution as the ethanol evaporates, forming a physical barrier. Palmitic acid barriers do not affect rate of egg laying, fecundity or lifespan in C. elegans16. Burrowing does not occur more frequently in hypoxic conditions, so additional preventative measures are not generally required.
- Generate synchronized populations by bleaching gravid adults in a small drop of alkaline bleach solution on unseeded nematode growth media (NGM) plates17. In contrast to standard large-batch hypochlorite bleaching protocols, pick 1-100 animals in a drop of bleach solution on the surface of an NGM plate, then allow the bleach solution to absorb into the plate17. After at least 12 hours, transfer the synchronized L1 larvae to plates seeded with live OP50 bacteria. Alternatively, one can allow gravid adults to lay eggs on the plate for 2-3 hours to generate a group of worms that will develop synchronously or pick L4 larvae from a mixed population.
- Avoid exposing bleached embryos to hypoxia, as this can reduce viability13. To collect young embryos (2-4 cells), gravid adults can be chopped in a small volume of water with a razor blade and embryos moved to plates by mouth pipet for subsequent exposure to hypoxia.
Seal plates in the environmental chamber. Control animals should be kept in normoxia (house air) at the same temperature as treated worms. There is no observable difference between samples left in house air and those maintained in an identical chamber with house air flowing over them. Initiate gas flow and maintain exposure for desired time. To ensure uniformity in ramp, be sure to replace the water in the gas wash bottle before exposure.
To assay survival of embryos, allow the worms to develop for 48 h after return to room air, at which point they should be fourth-stage larvae/day one adults. Score for survival, censoring any worms that cannot be accounted for.
To visualize animals exposed to hypoxia, move worms to a drop of M9 on a 22 mm2 coverslip, and invert onto a pad of 2% agarose in M917. If necessary, levamisole (25 mM) or sodium azide (10 mM) can be used as anesthetic. Sodium azide and levamisole may confound some observations due to toxicity and should be judiciously used18.
5. Rapid Harvest of Hypoxia-exposed Worms (Example: Preparation of Samples for HIF-1 Western Analysis)
Many hypoxia-induced effects are quickly reversed upon return to room air, including the resumption of egg production12, phosphorylation of mitotic epitopes in embryogenesis13 and degradation of the HIF-1 protein9,19. Rapid isolation of animals exposed to hypoxia is required to obtain reproducible effects in these conditions. With this setup, animals can be harvested and frozen in liquid nitrogen in less than two minutes. While glove box hypoxia chambers allow for manipulation of samples in anoxic conditions, their cost and practicality for conditions other than anoxia limit their usefulness.
Grow Bristol N2 worms on 4 10 cm high growth (HG) plates until a majority of the worms are gravid adults17. Wash worms to a 15 mL conical tube containing a 1:5 alkaline bleach solution and incubate with rotation until worms begin to dissolve, not more than 5 minutes9. Wash the worms three times with M9, spinning down at 1500 x g between each wash with no braking.
Plate bleached embryos onto 8 150 mm NGM plates and allow to develop to L4 larvae (~48 hours for Bristol N2 at 22 °C). Move plates to environmental chambers and expose to hypoxic (1,000 ppm, 5,000 ppm) and anoxic (N2) conditions for 4 hours. Exposure times will vary depending on experimental design. While exposure to hypoxia has an immediate effect on rate of egg laying, two cell embryos die after 16-18 hours of exposure12. With this hypoxia chamber setup, the lower limit of exposure is constrained by the rate of atmosphere exchange necessary to reach equilibrium.
Label one 1.5 mL microfuge tube and one 15 mL conical tube for each experimental sample. Worms exposed to hypoxia are more likely to stick to the sides of the tube during harvesting. To prevent this, place 100 μL of 1% sodium dodecyl sulfate (SDS) in each 15 mL conical tube. If SDS inhibits downstream applications, bovine serum albumin (BSA) can be used to prevent sticking. Routine use of SDS or BSA does not seem to have an apparent difference. Add 50 μl of 2x protein loading dye (4% SDS, 10% 2-Mercaptoethanol and a trace of bromphenol blue in 30% glycerol (w/v)) to the 1.5 mL microfuge tube. Have a Dewar of liquid nitrogen ready.
Time the steps after removing the worms from hypoxia and record. Remove the lid to the hypoxic chamber, take one sample plate, and reseal the chamber. Use distilled water to wash the worms onto a nylon filter and then pour into the 15 mL conical tube. Spin the worms down in a desktop centrifuge at 1500 x g for 15-20 seconds with brake.
Use a vacuum to remove most of the supernatant from the tube, leaving the worm pellet untouched.
Using a pipette, move the worm pellet in 50 μL to the 1.5 mL microfuge tube. Seal the tube and immerse in liquid nitrogen.
Repeat until all samples have been isolated. Follow these procedures for house air control samples for consistency. Samples can be stored at -20 °C.
6. Representative Results
Organismal effects of hypoxia can be seen by examining the viability to adulthood of C. elegans (Figure 2). Embryos laid by wild-type Bristol (N2) and hif-1(ia04) deletion mutants are all survive in house air O2 concentrations (210,000 ppm O2). N2 worms are able to adapt and survive to adulthood in 5,000 ppm O2, while hif-1 embryos are not viable. This shows that HIF-1 is essential for adapting to the changing levels of oxygen available in the environment10. Neither N2 nor hif-1 animals can survive exposure to 1,000 ppm O2.
Visualizing worm directly in hypoxia is feasible with the use of a dissecting scope and clear container (Figure 3). By directly placing the hypoxia chamber on the dissecting scope, there is no need to remove the worms from hypoxia to observe organismal reactions. The scope can be fitted with fluorescence illumation (as in Figure 3), further extending the types of observations in hypoxia that are possible.
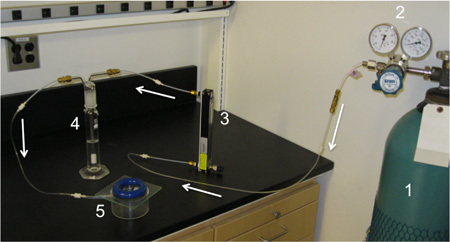 Figure 1. Example of Hypoxia chamber. Direction of gas flow is indicated by arrows. Gas is stored in compressed gas tanks with defined O2 concentrations (1) and a two stage regulator is attached (2). Gas enters the bottom of the flow tube (3), exiting the top at the correct flow rate. Gas then flows into the bubble flask (4), hydrating the gas (ensure correct connection of bubble flask by observing bubbles). Hydrated gas then passes into the hypoxia chamber at the inflow valve (5), exposing the samples to hypoxia. The gas finally vents into the room through an exhaust hole drilled in the chamber.
Figure 1. Example of Hypoxia chamber. Direction of gas flow is indicated by arrows. Gas is stored in compressed gas tanks with defined O2 concentrations (1) and a two stage regulator is attached (2). Gas enters the bottom of the flow tube (3), exiting the top at the correct flow rate. Gas then flows into the bubble flask (4), hydrating the gas (ensure correct connection of bubble flask by observing bubbles). Hydrated gas then passes into the hypoxia chamber at the inflow valve (5), exposing the samples to hypoxia. The gas finally vents into the room through an exhaust hole drilled in the chamber.
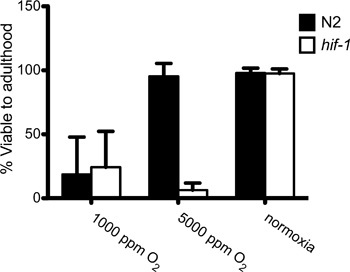 Figure 2. Viability of embryos exposed to 1,000 ppm O2, 5,000 ppm O2 and normoxia (~210,000 ppm O2). Embryos were exposed to each oxygen conditions as embryos for 24 hours in continuous flow oxygen chambers.Worms were moved to normoxic conditions, allowed to develop to adulthood for 48 hours, and then scored for viability to adulthood. n>50, N=5.
Figure 2. Viability of embryos exposed to 1,000 ppm O2, 5,000 ppm O2 and normoxia (~210,000 ppm O2). Embryos were exposed to each oxygen conditions as embryos for 24 hours in continuous flow oxygen chambers.Worms were moved to normoxic conditions, allowed to develop to adulthood for 48 hours, and then scored for viability to adulthood. n>50, N=5.
 Figure 3. Visualization of C. elegans in hypoxia with microscopy. Worms are exposed to hypoxia using the methods outlined. The transparent environmental chamber (constructed with a pyrex crystallization dish and glass plate) is placed directly on the stage of a dissecting scope. Two views are shown, one including the entire gas flow set up, the other with just the chamber on the microscope stage.
Figure 3. Visualization of C. elegans in hypoxia with microscopy. Worms are exposed to hypoxia using the methods outlined. The transparent environmental chamber (constructed with a pyrex crystallization dish and glass plate) is placed directly on the stage of a dissecting scope. Two views are shown, one including the entire gas flow set up, the other with just the chamber on the microscope stage.
Discussion
This method presents a strategy for constructing a hypoxic environment that allows for environments with precise concentrations of oxygen to be maintained in the laboratory. These chambers provide a simple method for exposing organisms to specific low concentrations of O2 and monitoring the molecular and physiological outputs. The environmental chamber described is assembled by the lab instead of commercially purchased and can thus be modified to fit the needs of the experiment.
One distinct advantage of this method is the continuous flow design. This eliminates the difficulties normally encountered with maintaining low concentrations of O2 in chambers when the external O2 concentration is much higher (210,000 ppm O2 in room air). The alternative is a stopped-flow method, in which a hypoxic environment is maintained in a sealed chamber. Even small leaks, which can be difficult to detect, prevent the maintenance of hypoxic conditions using stopped-flow methods. The continuous flow method continually exchanges the air in the chamber with the defined oxygen concentration in the compressed air tank and maintains a positive pressure that prevents leaks from disrupting the hypoxic conditions.
Obtaining exact, pre-mixed oxygen concentrations from the gas supplier solves another difficult problem with hypoxia. It is quite difficult to measure extremely low concentrations of O2. Most O2 sensors are diffusion limited and quite expensive. Because O2 diffuses slowly, measuring low O2 concentrations can be slow or inaccurate20. In contrast, it is quite easy to generate gas mixtures by measuring the weight of gasses. The mixtures we regularly purchase are certified standard to be within 2% O2 content of the desired mix.
This method can be used to elicit observable hypoxia-induced changes both at the organismal and molecular level. While this method outlines survival assays and rapid whole worm isolation for molecular experiments, there are myriad downstream readouts that could be used. For example, this design allows for direction visualization of worms in hypoxia for study of real time behavior and changes to reporter constructs. To visualize worms with a dissecting scope, assemble the chamber using transparent boxes with small volume and minimal height. The entire chamber can be placed on the dissecting scope and is easily maneuverable for optimal visualization (see Figure 3). It would also be possible to observe samples at higher magnification by using perfusion chambers with an inverted microscope. This requires some adaptation of the chambers to interface it with tubing that is normally used for gas flow, and determine an appropriate flow rate. The representative results shown only scratch the surface of experimental possibilities, as hypoxia has been shown to affect cellular systems from DNA synthesis to protein degradation21,22.
The practical nature of this method is not limited to C. elegans. As long as appropriate-sized chambers are used, this method is readily adaptable to almost any model system. For adaptation to liquid media or cell culture, oxygen diffusion constants in solution, outgassing from plastic and time to equilibrate in culture must be taken into account, and it may be most appropriate to use O2 permeable culture plates23,24.
It is possible to modify the chambers presented in this protocol for use with other gasses. For instance, chambers can be adapted to provide an anoxic environment merely by omitting the O2 in the compressed gas tanks used to create a hypoxia chamber (with the balance being filled with nitrogen). This has allowed for observation of C. elegans in suspended animation (data not shown)10,13,25. Slight modifications must be made based on the properties of the gas mixture used. The composition of the tubing used to pipe gas into and out of the chamber may have to be varied. Some plastics are permeable to CO2, while others are not compatible with corrosive gasses such has hydrogen sulfide (H2S)16,25. A list of compatible plastics can be found on the Cole-Parmer website.
For toxic gasses the gas outlet from the chamber must be vented into a certified fume hood and appropriate personal protection, such as detectors, must be employed. Additionally, EH&S officers should be consulted before beginning any experiment using potentially hazardous gasses. Corrosive gasses may also require special attention. For example, H2S can corrode many of the plastics used in standard tubing material as well as brass fitting will corrode. We generally make sure that any wetted plastic is Kalrez or equivalent in instruments used with H2S. Certain gasses may interact with impurities in tap water, so DiH20 should be used in the bubble flask. Special considerations concerning glassware may also be required; for example, H2S necessitates equipment with wetted O-rings.
Both organismal and molecular changes are observed utilizing experiments which can be completed in a day. This ability to rapidly introduce samples to hypoxia provides a valuable tool in fields from aging and cancer to development.
Disclosures
No conflicts of interest declared.
Acknowledgments
We thank members of the Miller lab for discussion and critical reading of the manuscript. This work was supported by a new investigator award from the Nathan Shock Center of Excellence in the Basic Biology of Aging to DLM and the National Institutes of Health award R00 AG030550 to DLM.
References
- Danovaro R. The first metazoa living in permanently anoxic conditions. BMC Biology. 2010;8:30. doi: 10.1186/1741-7007-8-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birner P. Overexpression of Hypoxia-inducible Factor 1 alpha Is a Marker for an Unfavorable Prognosis in Early-Stage Invasive Cervical Cancer. Cancer Research. 2000;60:4693–4696. [PubMed] [Google Scholar]
- Harris AL. Hypoxia - a key regulatory factor in tumour growth. Nat. Rev. Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- Ramirez-Bergeron DL. Hypoxia affects mesoderm and enhances hemangioblast specification during early development. Development. 2004;131:4623–4634. doi: 10.1242/dev.01310. [DOI] [PubMed] [Google Scholar]
- Staff FE. Wheel-well Stowaways Risk Lethal Levels of Hypoxia and Hypothermia. Human Factors and Aviation. 1997;44:1–5. [Google Scholar]
- Shen C, Powell-Coffman JA. Genetic Analysis of Hypoxia Signaling and Response in C. elegans. Annals of the New York Academy of Sciences. 2003;995:191–199. doi: 10.1111/j.1749-6632.2003.tb03222.x. [DOI] [PubMed] [Google Scholar]
- Shen C, Nettleton D, Jiang M, Kim SK, Powell-Coffman JA. Roles of the HIF-1 Hypoxia-inducible Factor during Hypoxia Response in Caenorhabditis elegans. Journal of Biological Chemistry. 2005;280:20580–20588. doi: 10.1074/jbc.M501894200. [DOI] [PubMed] [Google Scholar]
- Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proceedings of the National Academy of Sciences. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein ACR. C. elegans EGL-9 and Mammalian Homologs Define a Family of Dioxygenases that Regulate HIF by Prolyl Hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- Nystul TG, Goldmark JP, Padilla PA, Roth MB. Suspended Animation in C. elegans Requires the Spindle Checkpoint. Science. 2003;302:1038–1041. doi: 10.1126/science.1089705. [DOI] [PubMed] [Google Scholar]
- Gray JM. Oxygen sensation and social feeding mediated by a C. elegans guanylate cyclase homologue. Nature. 2004;430:317–322. doi: 10.1038/nature02714. [DOI] [PubMed] [Google Scholar]
- Miller DL, Roth MB. C. Elegans Are Protected from Lethal Hypoxia by an Embryonic Diapause. Current Biology. 2009;19:1233–1237. doi: 10.1016/j.cub.2009.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla PA, Nystul TG, Zager RA, Johnson ACM, Roth MB. Dephosphorylation of Cell Cycle-regulated Proteins Correlates with Anoxia-induced Suspended Animation in Caenorhabditis elegans. Molecular Biology of the Cell. 2002;13:1473–1483. doi: 10.1091/mbc.01-12-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C-J, Wang L-Y, Chodosh LA, Keith B, Simon MC. Differential Roles of Hypoxia-Inducible Factor 1{alpha} (HIF-1{alpha}) and HIF-2{alpha} in Hypoxic Gene Regulation. Molecular and Cellular Biology. 2003;23:9361–9374. doi: 10.1128/MCB.23.24.9361-9374.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treinin M. HIF-1 is required for heat acclimation in the nematode Caenorhabditis elegans. Physiological Genomics. 2003;14:17–24. doi: 10.1152/physiolgenomics.00179.2002. [DOI] [PubMed] [Google Scholar]
- Miller DL, Roth MB. Hydrogen sulfide increases thermotolerance and lifespan in Caenorhabditis elegans. PNAS. 2007;104:20618–20622. doi: 10.1073/pnas.0710191104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiernagle T. Maintenance of C. elegans. WormBook. 2006. pp. 1–11. [DOI] [PMC free article] [PubMed]
- Massie MR, Lapoczka EM, Boggs KD, Stine KE, White GE. Exposure to the metabolic inhibitor sodium azide induces stress protein expression and thermotolerance in the nematode Caenorhabditis elegans. Cell Stress Chaperones. 2003;8:1–7. doi: 10.1379/1466-1268(2003)8<1:ettmis>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salceda S, Caro J. Hypoxia-inducible Factor 1α (HIF-1α) Protein Is Rapidly Degraded by the Ubiquitin-Proteasome System under Normoxic Conditions. Journal of Biological Chemistry. 1997;272:22642–22647. doi: 10.1074/jbc.272.36.22642. [DOI] [PubMed] [Google Scholar]
- Theilacker JC, White MJ. Diffusion of Gases in Air and Its Affect on Oxygen Deficiency Hazard Abatement. AIP Conference Proceedings. 2006;823:305–312. [Google Scholar]
- Chua B, Kao RL, Rannels DE, Morgan HE. Inhibition of protein degradation by anoxia and ischemia in perfused rat hearts. Journal of Biological Chemistry. 1979;254:6617–6623. [PubMed] [Google Scholar]
- Probst G, Riedinger H, Jr, Martin P, Engelcke M, Probst H. Fast Control of DNA Replication in Response to Hypoxia and to Inhibited Protein Synthesis in CCRF-CEM and HeLa Cells. Biological Chemistry. 1999;380:1371–1382. doi: 10.1515/BC.1999.177. [DOI] [PubMed] [Google Scholar]
- Semenza GL, Sen CK. Oxygen Sensing. Vol. 381. Academic Press; 2004. [Google Scholar]
- Chan K, Roth MB. Anoxia-Induced Suspended Animation in Budding Yeast as an Experimental Paradigm for Studying Oxygen-Regulated Gene Expression. Eukaryotic Cell. 2008;7:1795–1808. doi: 10.1128/EC.00160-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nystul TG, Roth MB. Carbon monoxide-induced suspended animation protects against hypoxic damage in Caenorhabditis elegans. PNAS. 2004;101:9133–9136. doi: 10.1073/pnas.0403312101. [DOI] [PMC free article] [PubMed] [Google Scholar]


