Abstract
In mammals, odorant molecules are thought to activate only a few glomeruli, leading to the hypothesis that odor representation in the olfactory bulb is sparse. However, the studies supporting this model used anesthetized animals or monomolecular odorants at limited concentration range. In this study, using optical imaging and 2-photon microscopy, we show that natural odorants at their native concentrations can elicit dense representations in the olfactory bulb. Both anesthesia and odorant concentration are shown to modulate the representation density of natural odorants.
Keywords: In vivo imaging, functional imaging, glomerulus, intrinsic signals, natural stimuli
In the olfactory bulb, odorant molecules evoke complex spatio-temporal patterns of activated glomeruli1-10 that substantially vary in density with stimulus concentration1,6-7,10-11. To better understand how odorants are normally coded in the glomeruli array, it is important to use concentrations that are behaviorally relevant. Mixed odorants from natural products come by definition in behaviorally relevant concentrations and ratios. Such complex odorants activate only a few glomeruli12, providing evidence for a general theory of sparse glomerular coding12-13. However, the studies supporting the model of sparse coding were performed on anesthetized animals or used monomolecular odorants within a limited range of concentrations.
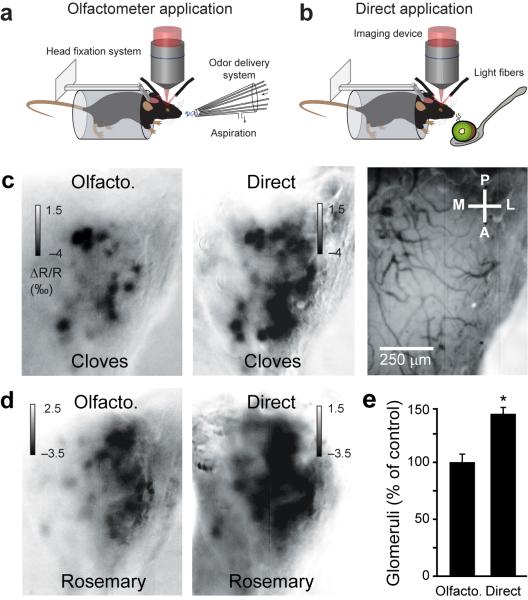
Here we investigated, using intrinsic optical signal imaging, how natural odorants are represented in awake mice (Fig. 1a,b). We presented the stimuli either directly to the mouse snout (Fig. 1b) or using an olfactometer (Fig. 1a). In the latter case, the concentrations were reduced at least two fold compared to the concentrations that a mouse would experience when being close to the odor source (Supplementary Methods and Fig. 1c,d), leading to a reduced number of activated glomeruli (Fig. 1e, n = 6 odorant/mouse pairs, paired t-test P < 0.005).
Figure 1.
Intrinsic optical signal imaging of glomerular evoked activity in awake mice. (a,b) Schema showing natural odorants delivered by an olfactometer in a and natural products drawn up to the mouse snout in a spoon in b. (c,d) Glomeruli maps evoked by two natural odorants presented either by an olfactometer or directly. The blood vessel pattern is shown in c (right image). (e) Comparison of the number of glomeruli activated in the two applications. The number of glomeruli activated by the olfactometer application served as control (ctrl; 100%) for each natural odorant/mouse pairs (n = 6). Data shown as mean ± s.e.m.
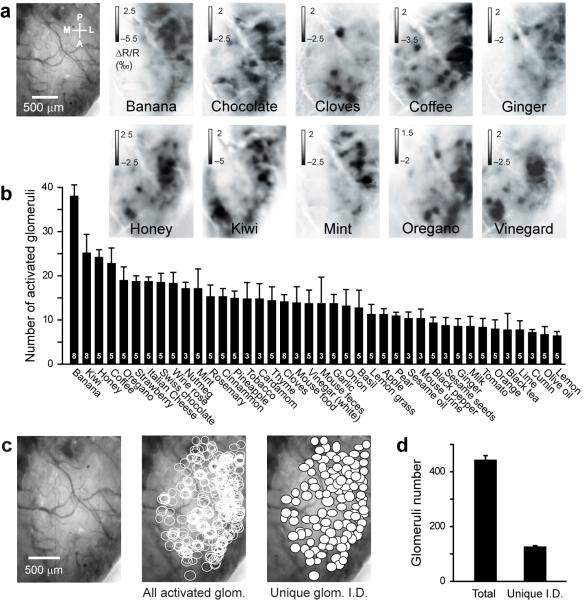
We then applied forty different natural odorants using the olfactometer in order to minimize the animal stress and to have a better temporal control of the odorant application. In all mice tested (n = 8), every odorant evoked a specific dense pattern of glomerular activity (Fig. 2a and Supplementary Fig. 1). For each odorant, we analyzed the image sequence (Supplementary Methods and Supplementary Fig. 2) and quantified the number of activated glomeruli (Fig. 2b and Supplementary Fig. 3). We found the number of activated glomeruli for each stimulus ranged between 10 and 40 glomeruli (Fig. 2b). For the same set of 30 natural stimuli, the total number of activated glomeruli per OB was 443 ± 15 glomeruli (n = 5 mice; Fig. 2c,d). By merging the glomeruli activated by several odorants (Supplementary Methods), we obtained a map composed of glomeruli with a unique identity showing that the natural odorants used activated a large portion of the dorsal OB (Fig. 2c,d; 127 ± 3 glomeruli).
Figure 2.
Dense representation of natural stimuli in the olfactory bulb of awake mice. (a) Glomerular activity patterns evoked by a variety of natural odorants in a single mouse. The images represent the average of all frames during odor application. (b) Number of glomeruli activated by each natural odorant tested (number of mice tested in white). (c) Total number (middle image; glomeruli activated by multiple odorants and plotted several times) and unique I.D. number (right image; i.e. each activated glomerulus is only plotted once) of glomeruli activated by thirty natural odorants presented to one mouse and overlaid on the blood vessel pattern (left image) after realignment with the blood vessels. (d) Quantification of the total and unique ID numbers of activated glomeruli (n = 5 mice that shared the same thirty natural odorants). Data shown as mean ± s.e.m.
We then addressed the issue of the fraction of the total number of glomeruli activated by the odorants. Using two-photon imaging of Omp-GFP14 mice, we estimated the dorsal olfactory bulb accessible to imaging contains ~200 glomeruli (Supplementary Fig. 4). Therefore, natural odorants activate 10-30 % of the total glomeruli number (including the correction factor 1.5 calculated in Fig. 1e), though this value may have been underestimated (Supplementary Methods).
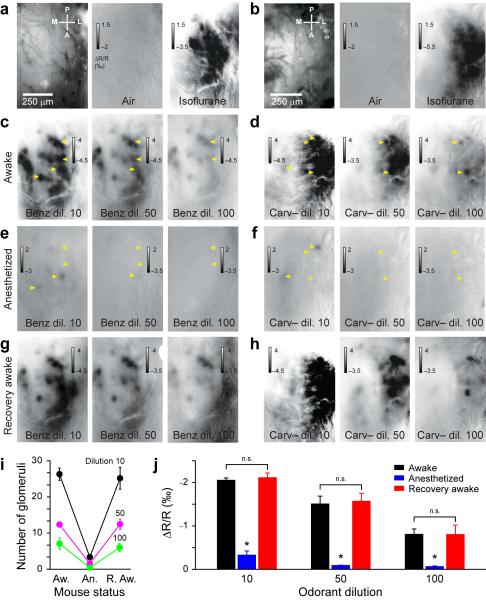
Our observations differ considerably from a previous report in isoflurane anesthetized animals12. We tested the hypothesis that isoflurane may alter odorant evoked patterns. Used as a regular smell, isoflurane specifically and stereotypically activated glomeruli in the posterior-lateral portion of the OB in awake mice (Fig. 3a,b and Supplementary Fig. 5a,b). We then assessed the potential impact of constant isoflurane application on odorant maps. All the odorants tested consistently activated more glomeruli in the awake condition than during isoflurane anesthesia (Fig. 3c-h and Supplementary Fig. 5c-f). The effect was independent of odorant concentration (Fig. 3i,j), reversible after ending gas anesthesia (Fig. 3i,j) and not homogenous across the OB surface (Supplementary Fig. 5d,f). Therefore, gas anesthesia led to previously underestimate12 the number of glomeruli normally activated by natural odorants (Supplementary Figs. 6).
Figure 3.
Gas anesthesia reversibly suppresses odorant-evoked response. (a,b) Blood vessel patterns of the OB in two mice (left images). Glomerular maps induced by air and isoflurane when applied as an odorant. (c,d) Glomerular patterns induced by benzaldehyde (Benz) and carvone-(-) (Carv-) during wakefulness at three odorant dilutions. (e,f) Constant isoflurane anesthesia suppresses glomerular responses. Yellow arrowheads indicate some glomeruli that disappear or display a reduced response following anesthesia. (g,h) Recovery of the glomerular patterns when animals are awake again (10 minutes after ending the anesthesia). (i) Quantification in different conditions (Aw.: awake, An. Anesthetized, R. Aw.: awake after anesthesia) of the average number of glomeruli activated by different odorants (n = 24 odorant/animal pairs) at three dilutions (10, 50, 100 dilutions: black, magenta and green curves, respectively). Aw. vs. An.: for all paired t-test, at least P < 0.05. Aw. vs. R.Aw.: for all paired t-test, P > 0.05. (j) Quantification of the glomeruli responses amplitude. Gas anesthesia mediates a suppression of activity at all dilutions used (paired t-test, P < 0.05). The effect of the gas anesthesia is reversible (paired t-test, P > 0.05). Data shown as mean ± s.e.m.
We identified two mechanisms possibly explaining the isoflurane mediated glomeruli suppression. First, long-term exposure to the anesthetic may alter normal neuronal functioning (pharmacological effect). Second, constant application of isoflurane may also induce adaptation and/or desensitization of a large number of OSNs, as a non-anesthetic odorant constantly applied could also do (odorant effect; Supplementary Fig. 7). Further work will be necessary to identify the exact mechanisms underlying the isoflurane mediated glomeruli suppression.
Natural odorants evoked denser patterns than previously reported for monomolecular odorants13. In this regard, one important parameter is the odorant concentration used6-7,9-11. Natural odorants are complex mixtures of monomolecular components, each of which can bind different olfactory receptors. A monomolecular odorant could either activate as many glomeruli as a multimolecular odorant or only few glomeruli. In the latter case, natural odorant evoked patterns could represent the summation of multiple monomolecular odorant evoked patterns12. We compared natural odorants with concentrations of monomolecular odorants that are perceived by humans to smell the same and as strong as the natural odorants (Supplementary Fig. 8a-c). The number of activated glomeruli was comparable between natural and monomolecular odorants (Supplementary Fig. 8d). Therefore, even a monomolecular odorant can activate for some concentrations a large number of glomeruli in the OB. This result differs with the sparse patterns reported in previous studies that used weaker concentrations of monomolecular odorants13. However, decreasing the concentrations of odorants (up to similar concentrations previously used) led to a sparsening of activated glomerular patterns and a decrease of glomerular response amplitude (Supplementary Fig. 9).
We therefore conclude that the odorant representation can vary in density with concentration and anesthesia. Natural odorants at their native concentrations are evoking dense patterns of activated glomeruli in awake mice. Since the coactivation of multiple glomeruli is required to fire a pyramidal neuron in the piriform cortex in vivo15, a dense representation should improve processing of odorants from the OB to the olfactory cortex.
Supplementary Material
Acknowledgements
We thank Ivan Rodriguez for kindly providing the Omp-GFP mice. We thank Ivan Rodriguez, Anthony Holtmaat, Sidney Simon, Michael Patterson and members of the laboratory for helpful discussions and comments on the manuscript. This work was supported by the Swiss National Science Foundation (SNF professor grant number PP0033_119169 and NCCR Synapsy), the University of Geneva, the European Research Council (contract number ERC-2009-StG-243344-NEUROCHEMS), the Novartis foundation for medical research, the Leenaards foundation and the European Molecular Biology Organization (young investigator program).
Footnotes
Competing financial interests
The authors declare no competing financial interests
REFERENCES
- 1.Abraham NM, et al. Neuron. 2004;44:865–876. doi: 10.1016/j.neuron.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 2.Bathellier B, Van De Ville D, Blu T, Unser M, Carleton A. Neuroimage. 2007;34:1020–1035. doi: 10.1016/j.neuroimage.2006.10.038. [DOI] [PubMed] [Google Scholar]
- 3.Bathellier B, Buhl DL, Accolla R, Carleton A. Neuron. 2008;57:586–598. doi: 10.1016/j.neuron.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 4.Belluscio L, Katz LC. J Neurosci. 2001;21:2113–2122. doi: 10.1523/JNEUROSCI.21-06-02113.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bozza T, McGann JP, Mombaerts P, Wachowiak M. Neuron. 2004;42:9–21. doi: 10.1016/s0896-6273(04)00144-8. [DOI] [PubMed] [Google Scholar]
- 6.Meister M, Bonhoeffer T. J Neurosci. 2001;21:1351–1360. doi: 10.1523/JNEUROSCI.21-04-01351.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rubin BD, Katz LC. Neuron. 1999;23:499–511. doi: 10.1016/s0896-6273(00)80803-x. [DOI] [PubMed] [Google Scholar]
- 8.Spors H, Grinvald A. Neuron. 2002;34:301–315. doi: 10.1016/s0896-6273(02)00644-x. [DOI] [PubMed] [Google Scholar]
- 9.Uchida N, Takahashi YK, Tanifuji M, Mori K. Nature neuroscience. 2000;3:1035–1043. doi: 10.1038/79857. [DOI] [PubMed] [Google Scholar]
- 10.Wachowiak M, Cohen LB. Neuron. 2001;32:723–735. doi: 10.1016/s0896-6273(01)00506-2. [DOI] [PubMed] [Google Scholar]
- 11.Fried HU, Fuss SH, Korsching SI. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:3222–3227. doi: 10.1073/pnas.052658399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin DY, Shea SD, Katz LC. Neuron. 2006;50:937–949. doi: 10.1016/j.neuron.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 13.Fantana AL, Soucy ER, Meister M. Neuron. 2008;59:802–814. doi: 10.1016/j.neuron.2008.07.039. [DOI] [PubMed] [Google Scholar]
- 14.Potter SM, et al. J Neurosci. 2001;21:9713–9723. doi: 10.1523/JNEUROSCI.21-24-09713.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davison IG, Ehlers MD. Neuron. 2011;70:82–94. doi: 10.1016/j.neuron.2011.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.