Abstract
The viral integrase enzyme has recently emerged as a primary alternative target to block HIV-1 replication, and integrase inhibitors are considered a pivotal new class of antiretroviral drugs. Dolutegravir is an investigational next-generation integrase inhibitor showing some novel and intriguing characteristics, ie, it has a favorable pharmacokinetic profile with a prolonged intracellular half-life, rendering feasible once-daily dosing without the need for ritonavir boosting and without regard to meals. Moreover, dolutegravir is primarily metabolized via uridine diphosphate glucuronosyltranferase 1A1, with a minor component of the cytochrome P450 3A4 isoform, thereby limiting drug–drug interactions. Furthermore, its metabolic profile enables coadministration with most of the other available antiretroviral agents without dose adjustment. Recent findings also demonstrate that dolutegravir has significant activity against HIV-1 isolates with resistance mutations associated with raltegravir and/or elvitegravir. The attributes of once-daily administration and the potential to treat integrase inhibitor-resistant viruses make dolutegravir an interesting and promising investigational drug. In this review, the main concerns about the efficacy and safety of dolutegravir as well as its resistance profile are explored by analysis of currently available data from preclinical and clinical studies.
Keywords: antiretroviral drugs, HIV-1 integrase, integrase inhibitors, dolutegravir, once daily
Introduction
In recent years, remarkable advances have been made in clinical practice and in the development of new antiretroviral drugs for the management of patients infected with human immunodeficiency virus type-1 (HIV-1). These improvements have resulted in major advances in longevity and quality of life for infected patients.1–3 Once-daily or twice-daily regimens, in particular fixed-dose combinations of two or three drugs, have led to an improvement in tolerability and adherence with combination antiret-roviral therapy (cART). However, the era of cART is not without problems. In fact, there is still a need to improve the anti-HIV-1 armamentarium due to the persistence of concerns about the currently available drugs, by increasing tolerability, maximizing potency, improving adherence, reducing pill/dosing numbers, and enhancing resistance profiles.4,5
HIV-1 can develop resistance mutations and thus overcome the activity of several drugs, so therapies with novel mechanisms of action are needed. It has been reported that HIV-1 requires three principal steps for effective viral replication: reverse transcription of the RNA viral genome into viral cDNA by viral reverse transcriptase; integration of viral cDNA into the host cell genome using viral integrase; and, finally, cleavage of newly synthesized viral polypeptides by viral protease into single viral proteins during new virion assembly.6
Throughout the years, all these steps were approached and specific inhibitory molecules were developed and licensed to block viral enzymes, and ultimately prevent (or reverse) disease progression. Despite several reverse transcriptase and protease inhibitors being used successfully from the mid 1990s onwards in the management of HIV-infected patients, it has only been in the last decade that the viral integrase enzyme has emerged as a primary alternative target to block HIV-1 replication. Because integration is a crucial step in the retrovirus replication machinery, the viral integrase enzyme has become an attractive molecule for the treatment of subjects infected with HIV-1.7–9
The first HIV-1 integrase inhibitor, raltegravir, was approved for use in cART-experienced subjects by the US Food and Drug Administration (FDA) and European Medicines Agency at the end of 2007.10 Raltegravir is a potent inhibitor of the HIV-1 integrase enzyme and is clinically effective against viruses resistant to other classes of antiretroviral agents. The potency and efficacy of raltegravir have been tested both in treatment-naïve and experienced patients.11,12 However, raltegravir needs to be administered at the approved dose of 400 mg twice daily, with results superior to the 800 mg once daily dosing in a clinical trial.13 Twice-daily dosing could be a disadvantage when compared with the once-daily option. In addition, raltegravir has a relatively low genetic barrier to resistance, as shown by the relatively rapid onset of raltegravir-associated mutations in the setting of virologic failure.14,15
Elvitegravir is another first-generation integrase inhibitor now in advanced clinical development that has demonstrated virologic activity comparable with that of raltegravir in clinical trials. Elvitegravir can be given once daily in the presence of a pharmacokinetic booster to inhibit its metabolism by the cytochrome P450 enzyme system, thus prolonging its half-life.16–20 Although raltegravir has been associated with virologic failure and development of resistance, less is known clinically about elvitegravir resistance. Overall, both raltegravir and elvitegravir share common resistance profiles, and it has been demonstrated that subjects experiencing virologic failure during elvitegravir-based therapy do not respond to raltegravir-based treatment (and vice versa).21–24
The need to overcome these problems has driven the development of next-generation integrase inhibitor molecules25 (such as GSK1349572, dolutegravir), with improved dose administration, the potential for a higher genetic barrier to resistance, and the potential to act against integrase inhibitor-resistant viruses. Dolutegravir has been designed for low-milligram daily dosing to achieve therapeutic concentrations and with a pharmacokinetic profile that enables once-daily administration without the need for pharmacokinetic boosting, so is a genuinely stand-alone once-daily drug.26 In this review, the main concerns about the efficacy and safety of dolutegravir as well as its resistance profile will be addressed by analysis of the data available from preclinical studies and clinical trials published or currently ongoing.
Mechanism of action, in vitro studies, and metabolism
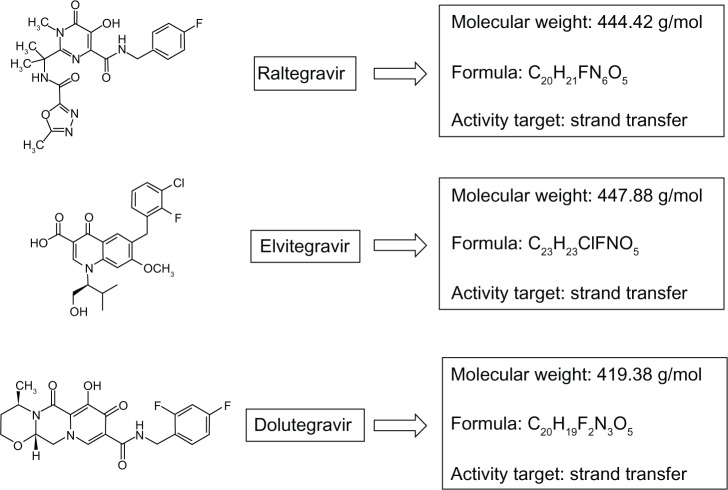
Dolutegravir is a second-generation integrase inhibitor that has been evaluated in several Phase III clinical trials. The molecule was first discovered at Shionogi Pharmaceuticals in Japan, and is now being developed as a joint venture by Shionogi-ViiV Healthcare and GlaxoSmithKline.27,28Figure 1 shows the chemical characteristics of dolutegravir in comparison with raltegravir and elvitegravir.
Figure 1.
Comparison of chemical characteristics and target of marketed (or in advanced stage of development) human immunodeficiency virus type-1 integrase inhibitors.
As a first-generation integrase inhibitor, dolutegravir blocks the strand transfer step of integration of the viral cDNA into the host genome. The integration of HIV-1- derived DNA is a two-step process mediated by the HIV-1 integrase enzyme. First, the integrase enzyme binds viral cDNA and cleaves two nucleotides, leaving it suitable for integration into cellular DNA.29 The integrase enzyme remains bound to DNA, forming the preintegration complex. The second part of integration (strand transfer) happens in the host nucleus. The mechanism of inhibition requires the integrase inhibitor molecule to chelate with two Mg2+ ions in the integrase DDE catalytic active site, so rendering the integrase enzyme unable to complete the strand transfer.30 This action of dolutegravir has been confirmed in several studies using live virus, demonstrating an accumulation of two long terminal repeat circles in treated cells at dolutegravir concentrations < 1000fold of those that cause cell toxicity.31,32 Dolutegravir has also shown potent in vitro activity, with a 50% inhibitory concentration (IC50) against HIV-1 of 2.7 nM in peripheral blood mononuclear cells and an IC90 of 2.0 nM. Moreover, the drug has shown potent antiviral activity in multiple cell types and cell-based assay formats.26
In vitro experimental studies report that dolutegravir does not increase its toxicity when used in combination, but had a synergistic effect with efavirenz, nevirapine, stavudine, abacavir, lopinavir, amprenavir, and enfuvirtide, as well as an additive effect in combination with maraviroc. Exposure to adefovir and ribavirin does not influence the efficacy of dolutegravir.26
The primary route of metabolism of dolutegravir is glucuronidation via UGT 1A1, without significant induction or inhibition of cytochrome P450 isoforms in vitro.26,30 Therefore, the interactions of dolutegravir are expected to be similar to those already known for raltegravir, because of the similar metabolic pathway shared by these two integrase inhibitors; in contrast, elvitegravir when boosted by ritonavir or cobicistat, participates in additional metabolism mediated by cytochrome P450 3A, thus having many more problems of pharmacologic interactions.
Pharmacokinetic profile, drug interactions, and safety in healthy subjects
The pharmacokinetic profile of dolutegravir was linear over the dose range studied. The geometric mean steady-state concentration at the end of the dosing interval (Ctau) for a 50 mg dose was 1.6 μg/mL, which was approximately 25-fold higher than the protein-adjusted IC90 (0.064 μg/mL). The unboosted half-life was approximately 15 hours, with low to moderate intersubject variability and a well described pharmacokinetic–pharmacodynamic relationship. The pharmacokinetic profile suggests that once-daily low doses will achieve therapeutic concentrations.30 Food intake modestly increases exposure to dolutegravir,33 but the effect of meals is not considered to be clinically significant (Table 1). The possibility of administering antiretroviral medications with or without food is an important aspect of dosing convenience.
Table 1.
Summary of interactions between dolutegravir, selected antiretrovirals, and other drugs, with effects of food on dolutegravir exposure
| Effect on DTG exposure | Comments | |||
|---|---|---|---|---|
| PI | ||||
| ATV | ↑AUC (0,τ) 91% | No dose adjustment for DTG | ||
| ↑Cmax 50%↑Cτ 180% | Increased occurrence of jaundice | |||
| ATV/r | ↑AUC (0,τ) 62% | No dose adjustment for DTG | ||
| ↑Cmax 34%↑Cτ 121% | Increased occurrence of jaundice | |||
| LPV/r | No effect on steady-state DTG pharmacokinetics | No dose adjustment for DTG | ||
| DRV/r | ↓AUC (0,τ) 22%↓Cmax 11%↓Cτ 38% | No dose adjustment for DTG | ||
| TPV/r and FVP/r | Decreased exposure to DTG | No dose adjustment for DTG | ||
| N(d)RTI-NNRTI | ||||
| TDF | No effect on steady-state DTG PK | No dose adjustment for DTG | ||
| ZDV, ABC, 3TC, FTC, DDI, d4T | No interactions expected | No dose adjustment for DTG | ||
| EFV | Decreased exposure to DTG | No dose adjustment for DTG | ||
| ETR | ↓AUC (0,τ) 71%↓C 52%↓Cτ 88% | Avoid ETR alone ETR should be coadministered only with LPV/r or DRV/r | ||
| NVP | Decreased exposure to DTG (similar to ETR) | Avoid the combination: decreased levels of DTG due to enzyme induction | ||
| Other drugs | Effect on DTG levels | Remarks | ||
|
| ||||
| Barbiturates | May cause ↓C of DTG | Caution if coadministered* | ||
| Phenytoin | May cause ↓C of DTG | Caution if coadministered* | ||
| Phenobarbital | May cause ↓C of DTG | Caution if coadministered* | ||
| Carbamazepine | May cause ↓C of DTG | Caution if coadministered* | ||
| Modafinil | May cause ↓C of DTG | Caution if coadministered* | ||
| Pioglitazone | May cause ↓C of DTG | Caution if coadministered* | ||
| Troglitazone | May cause ↓C of DTG | Caution if coadministered* | ||
| Rifampin | May cause ↓C of DTG | Caution if coadministered* | ||
| Rifabutin | May cause ↓C of DTG | Caution if coadministered* | ||
| St John’s wort | May cause ↓C of DTG | Caution if coadministered* | ||
| Multivitamins | ↓AUC (0,τ) 33% | No dose adjustment for DTG | ||
| Antacids | ↓AUC (0,τ) 77% | Administer antacids after 2 hours from DTG | ||
| Proton pump inhibitors | No effects | No dose adjustment for DTG | ||
| Foods | AUC | Cmax | Cτ | |
|
| ||||
| Low fat | 1.33 | 1.46 | 1.33 | No dose adjustments for DTG |
| Moderate fat | 1.41 | 1.52 | 1.67 | No dose adjustments for DTG |
| High fat | 1.66 | 1.67 | 1.73 | No dose adjustments for DTG |
Note: *Interactions may be relevant with other antiretrovirals included in combination antiretroviral therapy.
Abbreviations: C, concentration; DTG, dolutegravir; PI, protease inhibitor; ATV, atazanavir; ATV/r, atazanavir/ritonavir; AUC, area under the concentration-time curve; Cτ, concentration at end of dosing interval at steady state; Cmax, maximum concentration; LPV/r, lopinavir–ritonavir; DRV/r, darunavir–ritonavir; TPV/r, tipranavir–ritonavir; FVP/r, fosamprenavir–ritonavir; N(t)RTI, nucleos(t)ide reverse transcriptase inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor; TDF, tenofovir difumarate; ZDV, zidovudine; ABC, abacavir; 3TC, lamivudine; FTC, emtricitabine; DDI, didanosine; d4T, stavudine; EFV, efavirenz; ETR, etravirine; NVP, nevirapine; PK, pharmacokinetics.
Randomized, double-blind, placebo-controlled, singledose and multiple-dose dose escalation studies have evaluated the pharmacokinetics, safety, and tolerability of dolutegravir in different cohorts of healthy subjects (for a total of 50 healthy volunteers).30 In the single-dose study, two cohorts of subjects received suspension doses from 2 mg to 100 mg in an alternating panel design. In the multiple-dose study, three cohorts of subjects received suspension doses of 10 mg, 25 mg, and 50 mg once daily for 10 days.
Dolutegravir was well tolerated. During the singledose study, the most frequent adverse events described were headache and somnolence, whereas during the multiple-dose study, the most commonly reported adverse events were headache and pharyngeal/laryngeal pain. One subject developed asymptomatic lipase elevation during the single-dose study. One subject in the multiple-dose study had asymptomatic triglyceride and liver enzyme elevations. No other significant changes in lipid or liver chemistry abnormalities were observed in any of the dosing groups. The cardiotoxicity of dolutegravir, mainly the occurrence of arrhythmias due to possible drug interference with duration of the QT interval, was evaluated.34 Supratherapeutic dolutegravir exposure was generally well tolerated without the occurrence of any serious adverse events, demonstrating no relationship between plasma dolutegravir concentrations and QT (and corrected QT) interval. In vitro studies showed that dolutegravir is a potent inhibitor of the human organic cationic transporter at clinically relevant concentrations, and a study conducted in healthy subjects confirmed that dolutegravir when compared with placebo over 14 days did not significantly influence glomerular filtration rate or effective renal plasma flow.35
Table 1 summarizes the main dolutegravir drug interactions currently evaluated. In vivo pharmacokinetic studies in healthy subjects have shown that no dosage adjustments are required for dolutegravir when combined with atazanavir, atazanavir–ritonavir, darunavir–ritonavir, lopinavir–ritonavir, fosamprenavir–ritonavir, or tenofovir, whereas nevirapine should not be coadministered because it decreases dolutegravir levels as a result of enzyme induction. Entecavir and approved hormonal contraceptives can be used without dose adjustment.36–39
Given that the combination of an integrase inhibitor and a non-nucleoside reverse transcriptase inhibitor may be an attractive option as part of a two-drug or three-drug regimen, the potential pharmacokinetic interactions of etravirine alone and in combination with ritonavir-boosted protease inhibitors and the coadministration of rilpivirine have been evaluated.40,41 Etravirine alone should be avoided, but this drug may be coadministered with dolutegravir without dose adjustment when lopinavir–ritonavir or darunavir-ritonavir are involved. The combination of rilpivirine and dolutegravir was well tolerated, with no grade 3–4 adverse events or significant changes in pharmacokinetic parameters.
Multivitamins have no significant impact on dolutegravir concentrations. Antacid products containing divalent cations (ie, aluminum and magnesium) or iron supplements should be administered 2 hours before or 4 hours after a dose of dolutegravir. Proton pump inhibitors and H2 antagonists may be used with no scheduling restrictions.42
Efficacy in antiretroviral-naïve and antiretroviral-experienced subjects
The efficacy of dolutegravir in subjects infected with HIV-1 was initially evaluated in a randomized, double-blind, dose-ranging Phase IIa study, in which 35 integrase inhibitor-naïve adults currently off antiretroviral therapy were randomized to receive dolutegravir (2 mg, 10 mg, or 50 mg) or placebo once daily for 10 days. Baseline characteristics were similar across all the dose groups. Significant reductions in plasma HIV-1 RNA from baseline to day 11 were observed for all dolutegravir dose groups compared with placebo (P < 0.001), with a mean decrease of 1.51–2.46 log10 copies/mL. More than 90% of patients who received dolutegravir, irrespective of dose, had a decrease in viral load to <400 copies/mL, while 70% of those in the 50 mg arm achieved undetectable viremia. In addition, a well characterized dose-response relationship was observed for the decrease in viral load. Pharmacokinetic variability was low. There was no relationship between dolutegravir dose and adverse events.43 The dose chosen for Phase III studies in antiretroviral-naïve subjects infected with HIV-1 was 50 mg once daily.
The most important dolutegravir clinical trials which are still ongoing or have reached their primary endpoints are summarized in Table 2. In the randomized, partially blinded, dose-finding Phase IIb SPRING-1study, 205 antiretroviral-naïve patients infected with HIV-1 were enrolled. Baseline characteristics were a CD4+ T cell count > 200/μL and HIV-1 RNA > 1000 copies/mL. The subjects were randomized 1:1:1:1 to receive once-daily dolutegravir (n = 155) at 10 mg, 25 mg, or 50 mg doses, or efavirenz 600 mg (n = 50) combined with fixed doses of tenofovir-emtricitabine or abacavir-lamivudine as background therapy. This study was conducted at 34 sites in Western Europe, Russia, and the United States. The primary endpoint was the proportion of patients obtaining a viral load < 50 copies/mL at 16 weeks. In the dolutegravir arms, about 90% of participants had undetectable plasma viremia after 24 weeks, irrespective of the background nucleoside reverse transcriptase inhibitor (NRTI) combination used, thus establishing the noninferiority of dolutegravir versus efavirenz. The rate of viral decay was much faster in the dolutegravir arms than in the efavirenz arm, and was similar to that reported for raltegravir. After 48 weeks, about 90% of patients receiving dolutegravir and 82% of those receiving efavirenz achieved a viral load < 50 copies/mL. CD4+ T cells increased from baseline to week 48 in all groups and were higher in dolutegravir recipients than in efavirenz controls (+231 cells/μL versus +174 cells/μL). No relationship between dolutegravir exposure and response was observed during the study and no treatment-emergent integrase mutations were detected in the dolutegravir groups.44,45 Results at week 96 were recently presented, confirming a similar trend in the rate of virologic suppression in the dolutegravir 50 mg arm versus the efavirenz arm (Figure 2).46
Table 2.
Main clinical studies with dolutegravir: an overview
| Study (phase) | Patients (n) | DTG dose | Active comparator | Primary endpoint | Secondary endpoints |
|---|---|---|---|---|---|
| SPRING-I (IIb) | |||||
| Partially blinded (dose ranging) | ARV-naïve (205) | DTG 10 mg; 25 mg; 50 mg QD + TDF-FTC or ABC-3TC fixed doses | EFV 600 mg QD | VL < 50 copies/mL at week 16 | Safety Tolerability Efficacy |
| SPRING-2(III) | |||||
| Double-blind | ARV-naïve (822) | DTG 50 mg QD + 2 NRTIs | RAL 400 mg BID | VL < 50 copies/mL at week 48 | Safety Tolerability Efficacy |
| SINGLE (III) | |||||
| Double-blind | ARV-naïve (833) | DTG 50 mg + ABC-3TC QD Or TDF-FTC-EFV QD | TDF-FTC-EFV QD | VL < 50 copies/mL at week 48 | Safety Tolerability Efficacy |
| VIKING 1-2 (Mb) | |||||
| Single-arm | ARV-experienced RAL resistance Cohort 1 (27) | DTG 50 mg QD for 10 days then DTG 50 mg QD + OBT | None | VL < 400 copies/mL or ≥0.7 log10 decrease at day 11 | Efficacy (virological) |
| ARV-experienced RAL resistance Cohort 2 (24) | DTG 50 mg QD for 10 days then DTG 50 mg BID + OBT | None | VL < 400 copies/mL or ≥0.7 log10 decrease at day 11 | Efficacy (virological) Safety | |
| VIKING-3(IM) | |||||
| Single-arm | ARV-experienced RAL/EVG resistance (183) | DTG 50 mg BID + OBT | None | VL < 50 copies/mL at week 24 | Efficacy (virological) Safety |
| SAILING (III) | |||||
| Double-blind (ongoing) | ARV-experienced INI-naïve (688) | DTG 50 mg QD | RAL 400 mg BID | VL < 50 copies/mL at week 48 | Safety Tolerability Efficacy |
Abbreviations: ARV, antiretroviral therapy; OBT, optimized background therapy; QD, once daily; BID, twice daily; VL, viral load; INI, integrase inhibitor; TDF, tenofovir difumarate; ZDV, zidovudine; ABC, abacavir; 3TC, lamivudine; FTC, emtricitabine; DDI, didanosine; EFV, efavirenz; ETR, etravirine; NVP, nevirapine; DTG, dolutegravir; RAL, raltegravir; EVG, elvitegravir; NRTIs, nucleoside reverse transcriptase inhibitors.
Figure 2.
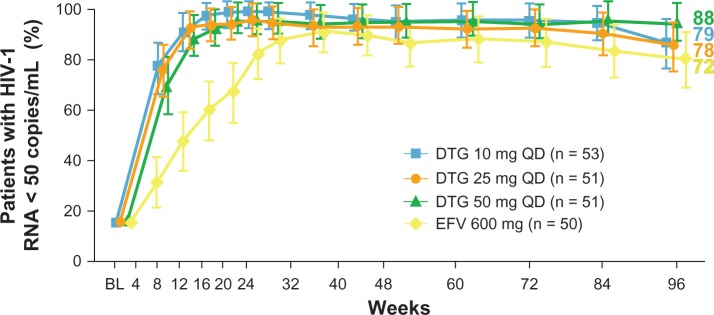
Percentage of subjects reaching human immunodeficiency virus type-1 RNA levels < 50 copies/mL at week 96 in the SPRING-1 trial.
Notes: Comparison between different dolutegravir doses and efavirenz 600 mg. The number of subjects enrolled in the study arms is shown in parentheses. Reprinted from The Lancet, 12(2), van Lunzen J, Maggiolo F, Arribas JR, et al. Once daily dolutegravir (S/GSK1349572) in combination therapy in antiretroviral-naive adults with HIV: planned interim 48 week results from SPRING-1, a dose-ranging, randomised, phase 2b trial. Lancet Infect Dis. 2012;12(2):111–118. Copyright 2012, with permission from Elsevier.45
Abbreviations: DTG, dolutegravir; EFV, efavirenz.
The 48-week results of the randomized, double-blind, double-dummy, noninferiority Phase III SPRING-2 study were reported at the Nineteenth International AIDS Conference in Washington, DC, 2012. This study compared the safety and efficacy of dolutegravir 50 mg once daily versus raltegravir 400 mg twice daily in combination with an investigator-selected NRTI backbone in 822 treatment-naïve patients infected with HIV-1 (411 patients per treatment arm). The main inclusion criteria were no previous antiretroviral therapy, HIV-1 RNA ≥ 1000 copies/mL, and no resistance mutations. The primary endpoint was HIV-1 RNA < 50 copies/mL at week 48 by FDA snapshot intent-to-treat-exposed analysis. Viral suppression was achieved in 88% of patients on dolutegravir versus 85% of those on raltegravir, thus establishing statistical noninferiority of dolutegravir versus raltegravir; the outcome was the same regardless of baseline viral load or background nucleos(t)-ide analogs. Twenty patients (5%) failed on dolutegravir and 28 (7%) failed on raltegravir, and the vast majority had HIV-1 RNA levels < 400 copies/mL. Eight of the dolutegravir failures and 18 of the raltegravir failures had integrase genotyping reported, showing that mutations were present only in the raltegravir group, one with integrase inhibitor-associated resistance (N155H) and four with NRTI mutations.47
The results of the double-blind, double-dummy, noninferiority, Phase III SINGLE study in 833 treatment-naïve patients infected with HIV-1 are now available. Enrolled subjects had HIV-1 RNA ≥ 1000 copies/mL, and were randomized 1:1 to receive dolutegravir 50 mg + fixed-dose abacavir–lamivudine once-daily or tenofovir–emtricitabine–efavirenz once daily. The primary endpoint was the proportion of patients with a viral load < 50 copies/mL at week 48 (by snapshot analysis). Other aspects analyzed were the tolerability, safety, and occurrence of viral resistance. In total, 414 patients received dolutegravir and 419 received tenofovir–emtricitabine–efavirenz; 84% were males, 32% were nonwhite, and all groups had similar characteristics at baseline (median HIV-1 RNA 4.7 log10 [>100.000 copies/mL, 32% versus 31%] in both groups, and median CD4+ T cells of 335/μL versus 339/μL). At week 48, 88% of subjects in the dolutegravir + abacavir–lamivudine arm versus 81% of those in the tenofovir–emtricitabine–efavirenz arm obtained HIV-1 RNA < 50 copies/mL, thus reaching the noninferiority endpoint. CD4+ T cells significantly increased (+267 versus +208, P < 0.001), favoring the dolutegravir arm. Virologic failure was reported in 4% of subjects in both groups. No resistance to integrase inhibition or NRTI therapy was described in the dolutegravir group, whereas one case of NRTI and four of non-NRTI resistance mutations were found in the tenofovir–emtricitabine–efavirenz arm.48
Efficacy results in experienced integrase inhibitor-resistant subjects with HIV-1 come from the VIKING trials. The VIKING study (including cohorts 1 and 2) was a single-arm Phase II trial that analyzed the feasibility of integrase inhibitor salvage therapy by replacing raltegravir 400 mg twice daily with dolutegravir 50 mg once or twice daily in two cohorts of patients infected with HIV-1 and failing current antiretroviral therapy due to the development of a raltegravir-resistant virus; 27 and 24 subjects infected with HIV-1started the trial with CD4+ T cell counts lower than those in the SPRING-1 study (<200 cells/μL), and about 60% were in Centers for Disease Control and Prevention (CDC) Class C. VIKING participants in the first cohort began the study on a dolutegravir dose of 50 mg once daily for ten days in addition to their background regimens, none of which contained active drugs. After a ten-day period, the background regimens were optimized to include active drugs, while continuing dolutegravir. Seventy-eight percent of subjects achieved a viral load < 400 copies/mL; the average decrease of HIV-1 RNA was 1.45 log10. Pre-existing resistance mutations to raltegravir caused variation in response to the study drug. The second VIKING cohort enrolled 24 subjects, also with raltegravir resistance mutations and a poor response to the current treatment; the regimens were optimized to include at least one active drug at day 11, whereas dolutegravir 50 mg was given twice daily. The primary endpoint was the proportion of subjects at day 11 with ≥0.7 log10 copies/mL plasma HIV-1 RNA reduction below baseline or <400 copies/mL. The results showed that 96% of subjects had a viral load decrease to <400 copies/mL or a reduction of at least 0.7 log10.27,49–51 Based on these findings, dolutegravir 50 mg twice-daily dosing has been chosen for the Phase III trials in HIV-1 experienced (integrase inhibitor-resistant) subjects. Results from the VIKING-3 study were recently presented. This was a multicenter, open-label, single-arm study assessing the antiviral activity and safety of dolutegravir 50 mg twice daily for 24 weeks in 183 antiretroviral-experienced adults with historical or current evidence of resistance to raltegravir or elvitegravir. Eligibility criteria included viral load > 500 copies/mL, treatment failure on a regimen containing raltegravir or elvitegravir, and documented resistance to at least one drug from three or more approved antiretroviral classes. After 7 days of open-label dolutegravir, subjects received an optimized background therapy along with the study drug. Baseline characteristics showed 124 patients with resistance to integrase inhibitors at screening and 59 with historical resistance to integrase inhibitors. The main characteristics were: median CD4+ T cell count 140 cells/μL, 13 years of prior antiretroviral therapy exposure, and CDC Class C staging in 56%. Non-R5 tropic virus was detected in 61% of patients. The proportion of subjects who had HIV-1 RNA < 50 copies/mL at week 24 (by snapshot analysis) was 63%. Virologic response varied according to the genotype pathway of integrase inhibitor resistance. In subjects with Q148 pathway mutations, the virologic response decreased with increasing number of secondary mutations. Overall background susceptibility score (number of active drugs in the optimized background therapy) was not associated with week 24 response.52
Safety and tolerability
Table 3 summarizes the main adverse events reported in the different dolutegravir studies and occurring with a frequency ≥ 5%. In the randomized Phase II trials comparing dolutegravir with efavirenz, the occurrence of adverse events (all grades) and grade 3 or 4 laboratory toxicity was similar across the treatment groups. The most common drug-related adverse events reported in the dolutegravir (10 mg, 25 mg, and 50 mg) and efavirenz arms were nausea (12% versus 6%), diarrhea (8% versus 6%), and dizziness (3% versus 18%), respectively. A large proportion of subjects in the efavirenz group had drug-related adverse events of moderate or higher severity. A grade 3–4 increase in alanine aminotransferase occurred in a recipient of dolutegravir 25 mg and in another subject who received efavirenz. Both subjects were infected with hepatitis C virus. In subjects receiving dolutegravir, a small nonprogressive increase in serum creatinine levels (+0.1 mg/dL) was reported, independent of the backbone used, which was not observed in the efavirenz arm. Serum creatinine had returned to baseline levels by week 48. Indeed, dolutegravir showed a better lipid profile.43–45 When dolutegravir was compared with efavirenz–emtricitabine–tenofovir, adverse events occurred in 2% of dolutegravir recipients versus 10% of the efavirenz group. More participants in the efavirenz arm experienced neuropsychiatric adverse events (ie, dizziness 35% versus 9%, abnormal dreams 17% versus 7%) and rash (14% versus 3%), and more frequently discontinued treatment. No grade 3–4 laboratory toxicities were reported.48 In the SPRING-2 trial (dolutegravir versus raltegravir), the most common adverse events were nausea, headache, nasopharyngitis, and diarrhea, with similar frequencies in both treatment arms. Adverse events were mild and equally distributed. A total of 29 (7%) serious adverse events were reported in dolutegravir arm, and three were considered to be drug-related (arrhythmia, hypersensitivity, and hepatitis). In the raltegravir group, 31 (8%) serious adverse events were reported; convulsions (two cases), aphasia, diarrhea, hypersensitivity, and increased creatine phosphokinase were considered to be drug-related. Dolutegravir recipients had higher serum creatinine levels and lower creatinine clearance, but changes were similar in both arms, and no participant discontinued the study drug because of changes in serum creatinine or renal function. The discontinuation rate attributable to adverse events was 11% in the dolutegravir arm and 14% in the raltegravir arm.47 When dolutegravir was administered at 50 mg twice daily in antiretroviral-experienced subjects (VIKING trials), the drug was well tolerated, with mild to moderate diarrhea being the most commonly reported adverse event. In this advanced population, the higher daily dose of dolutegravir showed a low rate of discontinuations due to adverse events (3%).27,49–51
Table 3.
Summary of adverse events described for dolutegravir with a frequency ≥ 5% in the different studies, both in treatment-naïve (50 mg once daily) and in treatment-experienced (50 mg twice daily) subjects with human immunodeficiency virus type-1
| DTG 50 mg QD (n = 980) | DTG 50 mg BID (n = 234) | |
|---|---|---|
| Diarrhea | 11%–17% | 5% |
| Nasopharyngitis | 11%–15% | – |
| Nausea | 12%–14% | 5% |
| Headache | 12%–13% | 5% |
| Fatigue | 5%–13% | – |
| Insomnia | 5%–11% | – |
| Dizziness | 6%–9% | – |
| Abnormal dreams | 7% | – |
| Upper respiratory | 5%–6% | – |
| tract infections | ||
| Pyrexia | 5% | – |
| Depression | 5% | – |
Abbreviations: DTG, dolutegravir; QD, once daily; BID, twice daily.
Dolutegravir resistance patterns
Although first-generation integrase inhibitors strongly inhibit replication of HIV-1, they have only a modest genetic barrier to resistance. Three main resistance pathways have been identified for raltegravir, involving initial mutations of the N155, Q148, and Y143 residues within the integrase enzyme.14 Both N155H and Q148HKR confer cross-resistance to elvitegravir,53 while Y143RHC has been reported to be specific for raltegravir.54 Several secondary mutations confer low levels of resistance to both these drugs. Next-generation integrase inhibitors, such as dolutegravir, showed a more robust resistance profile than raltegravir and elvitegravir.55 Dolutegravir also demonstrated efficacy against most raltegravir-resistant strains, although some viruses containing E138K, G140S, or R148H mutations had lower susceptibility.25
Exposure to dolutegravir in selection studies can cause changes in the viral genome at positions E92, L101, T124, S153, and G193.26,56 However, susceptibility fold-changes are moderate (<2.5) for all these substitutions; changes at the well characterized polymorphic positions, L101 and T124, did not increase fold-changes in dolutegravir or raltegravir.26,57 Although no major resistance mutations against dolutegravir have been identified thus far, the accumulation of multiple mutations is required to result in a fold-change > 10, confirming that next-generation integrase inhibitors possess a higher genetic barrier than raltegravir and elvitegravir. More recent in vitro selection experiments revealed R263K, followed by H51Y, as the most common mutation to emerge. Further analyses showed that R263K did confer low-level resistance to dolutegravir in culture, with an approximate 20%–30% loss in viral replication fitness. H51Y alone did not significantly affect either strand transfer activity or resistance. The presence of both mutations increased levels of resistance to dolutegravir, but this combination rarely emerged due to severe attenuation of both viral replicative capacity and integrase strand transfer activity when compared with the presence of R263K alone.58,59 It has been suggested that the high genetic barrier for dolutegravir resistance is due to tighter binding with integrase compared with the first-generation integrase inhibitors.60 Furthermore, it has been demonstrated that dolutegravir has a longer dissociative half-life from the integrase enzyme than either raltegravir or elvitegravir. The fact that first-generation integrase inhibitors have a shorter binding half-life than dolutegravir indicates that the resistance mutation affecting raltegravir binding might also be more likely to compromise its antiviral potency. As an example, Y143C/H/R substitutions have been shown to have the least effects on dissociation of dolutegravir, but compromise the interactions between integrase enzyme and raltegravir. These observations may help to explain why primary resistance to dolutegravir is infrequently observed in the clinical studies.
To evaluate the possibility of using dolutegravir as a first-line or second-line integrase inhibitor, Saladini et al61 analyzed the prevalence of dolutegravir resistance in 440 integrase inhibitor-naïve subjects and in 120 patients failing a raltegravir-containing regimen. Of the mutations selected by dolutegravir in vitro, S153FY was not detected in any isolate, whereas L101I and T124A were highly prevalent in both groups and significantly associated with the non-B subtype. Raltegravir-resistant variants most frequently detected in raltegravir-treated patients were G140S + Q148H mutants (26% of patients), resulting in lower changes in dolutegravir IC50 with respect to the isolates containing the Q148R variant. Because L101I and T124A did not exert any major effect in vivo and raltegravir rarely selected double and triple resistant mutants to dolutegravir, the drug can be effectively used in integrase inhibitor-naïve patients and may retain activity in patients failing raltegravir.
As reported above, the possibility of replacing raltegravir with dolutegravir in antiretroviral-experienced patients failing on raltegravir and harboring the Y143, Q148, and N155 mutation pathways was evaluated in VIKING trials. Patients showing N155H and Y143CR pathways did achieve successful suppression of the virus, but only a third of those with Q148H/K/R mutations had a sustained virologic response.62 These findings were supported by recent data demonstrating that dolutegravir has essentially wild-type levels of activity against N155H and T97A + Y143R mutants, whereas its susceptibility was diminished by isolates containing G140S + Q148H, and was further diminished by those carrying G140S + Q148R.50,63
Conclusion
Implementation of cART as the standard of care since the mid 1990s has substantially reduced morbidity and mortality in individuals infected with HIV-1, leading to decades of gain in life expectancy, comparable with that of the normal age-matched population in industrialized countries. During the past 10 years, knowledge of the side effects of cART has improved, and new, convenient, supposedly less toxic, and more tolerable molecules have become available, both in the oldest and in the new antiretroviral classes. Until a few years ago, standard treatment guidelines recommended cART regimens consisting of two nucleoside analogs in treatment-naïve patients and, in addition, a non-NRTI or a ritonavir-boosted protease inhibitor. Recently, the integrase strand transfer inhibitors (raltegravir and elvitegravir) have entered clinical practice. All drugs belonging to this class share an impressive ability to achieve a rapid viral decline in both treatment-naïve and treatment-experienced patients; however, this characteristic does not seem to be associated with a greater chance of long-term virologic control when compared with other cART strategies, including first-line regimens actually recommended.
The next-generation integrase inhibitor, dolutegravir, has demonstrated its safety and efficacy in both treatment-naïve and treatment-experienced patients. Ongoing clinical Phase III trials will bring more generalizable and robust information on the long-term effects of dolutegravir. Its pharmacokinetic characteristics allow once-daily administration without ritonavir boosting and a low grade of drug–drug interactions. Furthermore, dolutegravir has an interesting resistance profile, probably due to higher binding to the integrase enzyme when compared with raltegravir and elvitegravir. Pharmacokinetic studies and dose-ranging trials suggest that dolutegravir is a good candidate for a single-tablet regimen in a new coformulated pill. This possibility is actually under evaluation in a trial designed to explore the bioavailability of a fixed-dose pill containing dolutegravir 50 mg + abacavir 600 mg + lamivudine 300 mg (see the ClinicalTrials.gov website), although ongoing studies are being conducted to compare its safety and efficacy with several other NRTI combinations. Furthermore, the characteristics of dolutegravir make it a promising option in the treatment of organ transplant recipients infected with HIV-1.64 In fact, the main aspects of a cART regimen in an organ transplant recipient infected with HIV-1 should meet at least the following requirements: be potent with a high resistance barrier; have a low toxicity profile and lack of interactions with immunosuppressive agents; no (or low) impact on graft function; and easy dosing. Dolutegravir seems to meet all these conditions.
Dolutegravir is an interesting molecule with the potential to improve adherence in patients infected with HIV-1 and increase the long-term tolerability of cART. However, dolutegravir is not as yet approved by any regulatory agency, so we should not draw definitive conclusions regarding its long-term efficacy and safety, at least until Phase III data from ongoing large clinical trials become available.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338(13):853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 2.Antiretroviral Therapy Cohort Collaboration. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet. 2008;372(9635):293–299. doi: 10.1016/S0140-6736(08)61113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Sighem AI, Gras LA, Reiss P, Brinkman K, de Wolf F. ATHENA national observational cohort study. Life expectancy of recently diagnosed asymptomatic HIV-infected patients approaches that of uninfected individuals. AIDS. 2010;24(10):1527–1535. doi: 10.1097/QAD.0b013e32833a3946. [DOI] [PubMed] [Google Scholar]
- 4.Juday T, Gupta S, Grimm K, Wagner S, Kim E. Factors associated with complete adherence to HIV combination antiretroviral therapy. HIV Clin Trials. 2011;12(2):71–78. doi: 10.1310/hct1202-71. [DOI] [PubMed] [Google Scholar]
- 5.Ammassari A, Trotta MP, Shalev N, Marconi P, Antinori A. Beyond virological suppression: the role of adherence in the late HAART era. Antivir Ther. 2012;17(5):785–792. doi: 10.3851/IMP2084. [DOI] [PubMed] [Google Scholar]
- 6.Broder S, Fauci AS. Progress in drug therapies for HIV infection. Public Health Rep. 1988;103(3):224–229. [PMC free article] [PubMed] [Google Scholar]
- 7.Hazuda DJ, Felock P, Witmer M, et al. Inhibitors of strand transfer that prevent integration and inhibit HIV-1 replication in cells. Science. 2000;287(5453):646–650. doi: 10.1126/science.287.5453.646. [DOI] [PubMed] [Google Scholar]
- 8.Reinke R, Lee DJ, Robinson WE. Inhibition of human immunodeficiency virus type 1 isolates by the integrase inhibitor L-731,988, a diketo acid. Antimicrob Agents Chemother. 2002;46(10):3301–3303. doi: 10.1128/AAC.46.10.3301-3303.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pommier Y, Johnson AA, Marchand C. Integrase inhibitors to treat HIV/AIDS. Nat Rev Drug Discov. 2005;4(3):236–248. doi: 10.1038/nrd1660. [DOI] [PubMed] [Google Scholar]
- 10.[No authors listed]. FDA approves raltegravir for HIV-1 treatment naïve patients. AIDS Alert. 2009;24(9):106–107. [PubMed] [Google Scholar]
- 11.Markowitz M, Nguyen BY, Gotuzzo E, et al. Protocol 004 Part II Study Team. Rapid and durable antiretroviral effect of the HIV-1 integrase inhibitor raltegravir as part of combination therapy in treatment-naive patients with HIV-1 infection: results of a 48-week controlled study. J Acquir Immune Defic Syndr. 2007;46(2):125–133. doi: 10.1097/QAI.0b013e318157131c. [DOI] [PubMed] [Google Scholar]
- 12.Grinsztejn B, Nguyen BY, Katlama C, et al. Safety and efficacy of the HIV-1 integrase inhibitor raltegravir (MK-0518) in treatment-experienced patients with multidrug-resistant virus: a phase II randomised controlled trial. Lancet. 2007;369(9569):1261–1269. doi: 10.1016/S0140-6736(07)60597-2. [DOI] [PubMed] [Google Scholar]
- 13.Eron JJ, Rockstroh JK, Reynes J, et al. QDMRK Investigators. Raltegravir once daily or twice daily in previously untreated patients with HIV-1: a randomised, active-controlled, phase 3 non inferiority trial. Lancet Infect Dis. 2011;11(12):907–915. doi: 10.1016/S1473-3099(11)70196-7. [DOI] [PubMed] [Google Scholar]
- 14.Cooper DA, Steigbigel RT, Gatell JM, et al. BENCHMARK Study Teams. Subgroup and resistance analyses of raltegravir for resistant HIV-1 infection. N Engl J Med. 2008;359(4):355–365. doi: 10.1056/NEJMoa0708978. [DOI] [PubMed] [Google Scholar]
- 15.Steigbigel RT, Cooper DA, Teppler H, et al. Long-term efficacy and safety of raltegravir combined with optimized background therapy in treatment-experienced patients with drug-resistant HIV infection: week 96 results of the BENCHMARK 1 and 2 Phase III trials. Clin Infect Dis. 2010;50(4):605–612. doi: 10.1086/650002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeJesus E, Berger D, Markowitz M, et al. 183-001 Study Team. Antiviral activity, pharmacokinetics, and dose response of the HIV-1 integrase inhibitor GS-9137 (JTK-303) in treatment-naive and treatment-experienced patients. J Acquir Immune Defic Syndr. 2006;43(1):1–5. doi: 10.1097/01.qai.0000233308.82860.2f. [DOI] [PubMed] [Google Scholar]
- 17.Klibanov OM. Elvitegravir, an oral HIV integrase inhibitor, for the potential treatment of HIV infection. Curr Opin Investig Drugs. 2009;10(2):190–200. [PubMed] [Google Scholar]
- 18.Zolopa AR, Berger DS, Lampiris H, et al. Activity of elvitegravir, a once-daily integrase inhibitor, against resistant HIV type 1: results of a phase 2, randomized, controlled, dose-ranging clinical trial. J Infect Dis. 2010;201(6):814–822. doi: 10.1086/650698. [DOI] [PubMed] [Google Scholar]
- 19.Schrijvers R, Debyser Z. Combinational therapies for HIV: a focus on EVG/COBI/FTC/TDF. Expert Opin Pharmacother. 2012;13(13):1969–1983. doi: 10.1517/14656566.2012.712514. [DOI] [PubMed] [Google Scholar]
- 20.Ramanathan S, Mathias AA, German P, Kearney BP. Clinical pharma-cokinetic and pharmacodynamic profile of the HIV integrase inhibitor elvitegravir. Clin Pharmacokinet. 2011;50(4):229–244. doi: 10.2165/11584570-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 21.Hu Z, Kuritzkes DR. Effect of raltegravir resistance mutations in HIV-1 integrase on viral fitness. J Acquir Immune Defic Syndr. 2010;55(2):148–155. doi: 10.1097/QAI.0b013e3181e9a87a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goethals O, Clayton R, Van Ginderen M, et al. Resistance mutations in human immunodeficiency virus type 1 integrase selected with elvitegravir confer reduced susceptibility to a wide range of integrase inhibitors. J Virol. 2008;82(21):10366–10374. doi: 10.1128/JVI.00470-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson VA, Brun-Vézinet F, Clotet B, et al. Update of the drug resistance mutations in HIV-1: Dec 2010. Top HIV Med. 2010;18(5):156–163. [PubMed] [Google Scholar]
- 24.Blanco JL, Varghese V, Rhee SY, Gatell JM, Shafer RW. HIV-1 integrase inhibitor resistance and its clinical implications. J Infect Dis. 2011;203(9):1204–1214. doi: 10.1093/infdis/jir025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Quashie PK, Sloan RD, Wainberg MA. Novel therapeutic strategies targeting HIV integrase. BMC Med. 2012;10:34. doi: 10.1186/1741-7015-10-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kobayashi M, Yoshinaga T, Seki T, et al. In vitro antiretroviral properties of S/GSK1349572, a next-generation HIV integrase inhibitor. Antimicrob Agents Chemother. 2011;55(2):813–821. doi: 10.1128/AAC.01209-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eron J, Livrozet JM, Morlat P, et al. Activity of integrase inhibitor S/GSK9572 in subjects with HIV exhibiting raltegravir resistance: week 24 results of VIKING Study. J Int AIDS Soc. 2010;13( Suppl 4):O51. [Google Scholar]
- 28.Yoshinaga Y, Kanamori-Koyama M, Seki T, et al. Strong inhibition of wild-type and integrase inhibitor (INI)-resistant HIV integrase (IN) strand transfer reaction by the novel INI S/GSK1349572. International HIV and Hepatitis Virus Drug Resistance Workshop. Dubrovnik, Croatia, June 8–12, 2010. Available from: http://www.natap.org/2010/ResisWksp/ResisWksp_31.htm.
- 29.Katlama C, Murphy R. Dolutegravir for the treatment of HIV. Expert Opin Investig Drugs. 2012;21(4):523–530. doi: 10.1517/13543784.2012.661713. [DOI] [PubMed] [Google Scholar]
- 30.Min S, Song I, Borland J, et al. Pharmacokinetics and safety of S/GSK1349572, a next-generation HIV integrase inhibitor, in healthy volunteers. Antimicrob Agents Chemother. 2010;54(1):254–258. doi: 10.1128/AAC.00842-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bar-Magen T, Sloan RD, Faltenbacher VH, et al. Comparative biochemical analysis of HIV-1 subtype B and C integrase enzymes. Retrovirology. 2009;6:103. doi: 10.1186/1742-4690-6-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sloan RD, Wainberg MA. The role of unintegrated DNA in HIV infection. Retrovirology. 2011;8:52. doi: 10.1186/1742-4690-8-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song I, Borland J, Chen S, et al. Effect of food on the pharmacokinetics of the integrase inhibitor dolutegravir. Antimicrob Agents Chemother. 2012;56(3):1627–1629. doi: 10.1128/AAC.05739-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen S, Min SS, Peppercorn A, et al. Effect of a single supratherapeutic dose of dolutegravir on cardiac repolarization. Pharmacotherapy. 2012;32(4):333–339. doi: 10.1002/j.1875-9114.2012.01033.x. [DOI] [PubMed] [Google Scholar]
- 35.Koteff J, Borland J, Chen S, et al. An open label, placebo-controlled study to evaluate the effect of dolutegravir (DTG/GSK 1349572) on iohexol and para-aminohippurate clearance in healthy subjects; Abstract A1-1728 presented at the Fifty-first Interscience Conference on Antimicrobial Agents and Chemotherapy; Chicago, IL. September 17–29, 2011. [Google Scholar]
- 36.Song I, Borland J, Chen S, et al. Effect of atazanavir and atazanavir/ritonavir on the pharmacokinetics of the next-generation HIV integrase inhibitor, S/GSK1349572. Br J Clin Pharmacol. 2011;72(1):103–108. doi: 10.1111/j.1365-2125.2011.03947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Song I, Min SS, Borland J, et al. The effect of lopinavir/ritonavir and darunavir/ritonavir on the HIV integrase inhibitor S/GSK1349572 in healthy participants. J Clin Pharmacol. 2011;51(2):237–242. doi: 10.1177/0091270010371113. [DOI] [PubMed] [Google Scholar]
- 38.Song I, Borland J, Chen S, Peppercorn A, Wajima T, Piscitelli SC. Effect of fosamprenavir/ritonavir on the pharmacokinetics of the integrase inhibitor, dolutegravir, in healthy subjects; Abstract 1727 presented at the Fifty-first Interscience Conference on Antimicrobial Agents and Chemotherapy; Chicago, IL. September 17–29, 2011. [Google Scholar]
- 39.Song I, Min SS, Borland J, et al. Lack of interaction between the HIV integrase inhibitor S/GSK1349572 and tenofovir in healthy subjects. J Acquir Immune Defic Syndr. 2010;55(3):365–367. doi: 10.1097/QAI.0b013e3181e67909. [DOI] [PubMed] [Google Scholar]
- 40.Song I, Borland J, Min S, et al. Effects of etravirine alone and with ritonavir-boosted protease inhibitors on the pharmacokinetics of dolutegravir. Antimicrob Agents Chemother. 2011;55(7):3517–3521. doi: 10.1128/AAC.00073-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ford SL, Gould E, Chen S, et al. Lack of pharmacokinetic (PK) interaction between rilpivirine and the integrase inhibitors dolutegravir and S/GSK1265744; Abstract A-1249 presented at the Fifty-second Interscience Conference on Antimicrobial Agents and Chemotherapy; San Francisco, CA. September 9–12, 2012. [Google Scholar]
- 42.Patel P, Song I, Borland J, et al. Evaluation of antiacid and multivitamin effects on S/GSK1349572 pharmacokinetics in healthy volunteers. J Antimicrob Chemother. 2011;66(7):1567–1572. doi: 10.1093/jac/dkr139. [DOI] [PubMed] [Google Scholar]
- 43.Min S, Sloan L, DeJesus E, et al. Antiviral activity, safety, and pharmacokinetics/pharmacodynamics of dolutegravir as 10-day monotherapy in HIV-1-infected adults. AIDS. 2011;25(14):1737–1745. doi: 10.1097/QAD.0b013e32834a1dd9. [DOI] [PubMed] [Google Scholar]
- 44.Rockstroh J, Felizarta F, Maggiolo F, et al. Once-daily S/GSK1349572 combination therapy in antiretroviral-naïve adults: rapid and potent 24-week antiviral responses in SPRING-1 (ING112276) J Int AIDS Soc. 2010;13( Suppl 4):O50. [Google Scholar]
- 45.van Lunzen J, Maggiolo F, Arribas JR, et al. Once daily dolutegravir (S/GSK1349572) in combination therapy in antiretroviral-naive adults with HIV: planned interim 48 week results from SPRING-1, a doseranging, randomised, phase 2b trial. Lancet Infect Dis. 2012;12(2):111–118. doi: 10.1016/S1473-3099(11)70290-0. [DOI] [PubMed] [Google Scholar]
- 46.Stellbrink HJ, Reynes J, Lazzarin A, et al. Dolutegravir in combination therapy exhibits rapid and sustained antiviral response in ARV-naïve adults: 96-week results from SPRING-1 (ING112276); Abstract 102 LB presented at the Nineteenth Conference on Retroviruses and Opportunistic Infections; Seattle, WA. March 5–8, 2012. [Google Scholar]
- 47.Raffi F, Rachlis A, Stellbrink HJ, et al. Once-daily dolutegravir (DTG; S/GSK1349572) is non-inferior to raltegravir (RAL) in antiretroviralnaive adults: 48-week results from SPRING-2 (ING113086); Abstract THLBB04 presented at the Nineteenth International AIDS Conference; Washington DC. July, 22–27, 2012. [Google Scholar]
- 48.Walmsley S, Antela A, Clumeck N, et al. Dolutegravir (DTG; S/GSK1349572) + abacavir/lamivudine once daily statistically superior to tenofovir/emtricitabine/efavirenz: 48-week results – SINGLE (ING114467); Abstract H-556b presented at the Fifty-second Interscience Conference on Antimicrobial Agents and Chemotherapy; San Francisco, CA. September 9–12 2012. [Google Scholar]
- 49.Eron J, Durant J, Poizot-Martin I, et al. Activity of next generation integrase inhibitor (INI) S/GSK1349572 in subjects with HIV exhibiting raltegravir resistance: initial results of VIKING study (ING112961); Abstract MOAB0105 presented at the Eighteenth International AIDS Conference; Vienna, Austria. July 18–23, 2010. [Google Scholar]
- 50.Eron J, Kumar P, Lazzarin A, et al. DTG in subjects with HIV exhibiting RAL resistance: functional monotherapy results of VIKING study cohort II; Abstract 151 LB presented at the Eighteenth Conference on Retroviruses and Opportunistic Infections; Boston, MA. February 27–March 2, 2011. [Google Scholar]
- 51.Eron J, Clotet B, Durant J, et al. Safety and efficacy of dolutegravir (DTG) in HIV-1 treatment-experienced subjects with raltegravir-resistant virus: 24-week results of the VIKING Study J Infect DisDecember 7, 2012. [Epub ahead of print.] [DOI] [PMC free article] [PubMed]
- 52.Nichols G, Mills A, Grossberg R, et al. Antiviral activity of dolutegravir in subjects with failure on an integrase inhibitor-based regimen: week 24 phase 3 results from VIKING-3. Abstracts of the Eleventh International Congress on Drug Therapy in HIV Infection. J Int AIDS Soc. 2012;15( Suppl 4):18112. [Google Scholar]
- 53.Mouscadet JF, Delelis O, Marcelin AG, Tchertanov L. Resistance to HIV-1 integrase inhibitors: a structural perspective. Drug Resist Updat. 2010;13(4–5):139–150. doi: 10.1016/j.drup.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 54.Métifiot M, Vandegraaff N, Maddali K, et al. Elvitegravir overcomes resistance to raltegravir induced by integrase mutation Y143. AIDS. 2011;25(9):1175–1178. doi: 10.1097/QAD.0b013e3283473599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Van Wesenbeeck L, Rondelez E, Feyaerts M, et al. Cross-resistance profile determination of two second-generation HIV-1 integrase inhibitors using a panel of recombinant viruses derived from raltegravir-treated clinical isolates. Antimicrob Agents Chemother. 2011;55(1):321–325. doi: 10.1128/AAC.01733-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seki T, Kobayashi M, Wakasa-Morimoto C, et al. S/GSK1349572 is a potent next generation HIV integrase inhibitor and demonstrates a superior resistance profile substantiated with 60 integrase mutant molecular clones; Abstract 555 presented at the Seventeenth Conference on Retroviruses and Opportunistic Infections; San Francisco, CA. February 16–19, 2010. [Google Scholar]
- 57.Vavro C, Underwood M, Madsen H, et al. Polymorphisms at position 101 and 124 in the HIV-1 integrase gene: lack of effects on susceptibility to S/GSK1349572; Abstract H-935 presented at the Fiftieth Interscience Conference on Antimicrobial Agents and Chemotherapy; Boston, MA. September 12–15, 2010. [Google Scholar]
- 58.Quashie PK, Mesplède T, Han YS, et al. Characterization of the R263K mutation in HIV-1 integrase that confers low-level resistance to the second-generation integrase strand transfer inhibitor dolutegravir. J Virol. 2012;86(5):2696–2705. doi: 10.1128/JVI.06591-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mesplede T, Quashie P, Oliveira M, et al. Selection in culture of HIV resistance to dolutegravir by mutations at integrase positions R263K and H51Y that diminish viral replication fitness. Abstracts of the Eleventh International Congress on Drug Therapy in HIV Infection. J Int AIDS Soc. 2012;15( Suppl 4):18113. [Google Scholar]
- 60.Hightower KE, Wang R, Deanda F, et al. Dolutegravir (S/GSK1349572) exhibits significantly slower dissociation than raltegravir and elvitegravir from wild-type and integrase inhibitor-resistant HIV-1 integrase-DNA complexes. Antimicrob Agents Chemother. 2011;55(10):4552–4559. doi: 10.1128/AAC.00157-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saladini F, Meini G, Bianco C, et al. the ARCA Collaborative Group Prevalence of HIV-1 integrase mutations related to resistance to dolutegravir in raltegravir naïve and pretreated patients. Clin Microbiol Infect. 2012;18(10):E428–430. doi: 10.1111/j.1469-0691.2012.03917.x. [DOI] [PubMed] [Google Scholar]
- 62.Soriano V, Cox J, Eron JJ, et al. Dolutegravir (DTG, S/GSK1349572) treatment of HIV suppression at week 24 in the VIKING study; Abstract PS1/2 presented at the Thirteenth European AIDS Conference; Belgrade, Serbia. October 12–15, 2011. [Google Scholar]
- 63.Underwood MR, Johns BA, Sato A, Martin JN, Deeks SG, Fujiwara T. The activity of the integrase inhibitor dolutegravir against HIV-1 variants isolated from raltegravir-treated adults. J Acquir Immune Defic Syndr. 2012;61(3):297–301. doi: 10.1097/QAI.0b013e31826bfd02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Waki K, Sugawara Y. Implications of integrase inhibitors for HIV-infected transplantation recipients: raltegravir and dolutegravir (S/GSK 1349572) Biosci Trends. 2011;5(5):189–191. doi: 10.5582/bst.2011.v5.5.189. [DOI] [PubMed] [Google Scholar]